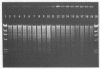
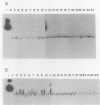
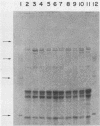
Abstract
We examined a representative collection of Salmonella typhi strains from Chile, Peru, Mexico, India, and England for the presence of several properties. All strains had a conserved pattern of outer membrane proteins, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The electrophoresis profiles of chromosomal DNA digested with EcoRI and PstI restriction enzymes were similar for all the strains. A conserved pattern of hybridization was observed when digested chromosomal DNA was hybridized with DNA probes for the 36-kilodalton porin, enterobactin synthesis, and enterobactin receptor genes. All the strains produced enterobactin but not aerobactin in bioassays. None of the strains produced heat-labile toxin, as measured by an enzyme-linked immunosorbent assay. Colony and Southern hybridizations with DNA probes for aerobactin synthesis and its receptor and heat-labile toxin genes were negative. These results indicate that S. typhi strains from different origins have similar phenotypic and genetic properties and, as has been suggested, constitute a clone.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agüero J., Mora G., Mroczenski-Wildey M. J., Fernandez-Beros M. E., Aron L., Cabello F. C. Cloning, expression and characterization of the 36 KDal Salmonella typhi porin gene in Escherichia coli. Microb Pathog. 1987 Dec;3(6):399–407. doi: 10.1016/0882-4010(87)90010-6. [DOI] [PubMed] [Google Scholar]
- Agüero M. E., Reyes L., Prado V., Orskov I., Orskov F., Cabello F. C. Enterotoxigenic Escherichia coli in a population of infants with diarrhea in Chile. J Clin Microbiol. 1985 Oct;22(4):576–581. doi: 10.1128/jcm.22.4.576-581.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altwegg M., Hickman-Brenner F. W., Farmer J. J., 3rd Ribosomal RNA gene restriction patterns provide increased sensitivity for typing Salmonella typhi strains. J Infect Dis. 1989 Jul;160(1):145–149. doi: 10.1093/infdis/160.1.145. [DOI] [PubMed] [Google Scholar]
- Beltran P., Musser J. M., Helmuth R., Farmer J. J., 3rd, Frerichs W. M., Wachsmuth I. K., Ferris K., McWhorter A. C., Wells J. G., Cravioto A. Toward a population genetic analysis of Salmonella: genetic diversity and relationships among strains of serotypes S. choleraesuis, S. derby, S. dublin, S. enteritidis, S. heidelberg, S. infantis, S. newport, and S. typhimurium. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7753–7757. doi: 10.1073/pnas.85.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello F., Agüero M. E., Fernández M. E. Epidemia de fiebre tifoidea en Chile: aspectos ecológicos y microbiológicos. Rev Med Chil. 1984 Aug;112(8):826–828. [PubMed] [Google Scholar]
- Calderón I., Lobos S. R., Rojas H. A., Palomino C., Rodríguez L. H., Mora G. C. Antibodies to porin antigens of Salmonella typhi induced during typhoid infection in humans. Infect Immun. 1986 Apr;52(1):209–212. doi: 10.1128/iai.52.1.209-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Gill D. M., Falkow S. Cistrons encoding Escherichia coli heat-labile toxin. J Bacteriol. 1979 Sep;139(3):850–858. doi: 10.1128/jb.139.3.850-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman R., Levine M. M. Summary of an international workshop on typhoid fever. Rev Infect Dis. 1986 May-Jun;8(3):329–349. doi: 10.1093/clinids/8.3.329. [DOI] [PubMed] [Google Scholar]
- Faundez G., Figueroa G., Troncoso M., Cabello F. C. Characterization of enteroinvasive Escherichia coli strains isolated from children with diarrhea in Chile. J Clin Microbiol. 1988 May;26(5):928–932. doi: 10.1128/jcm.26.5.928-932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Beros M. E., Gonzalez C., McIntosh M. A., Cabello F. C. Immune response to the iron-deprivation-induced proteins of Salmonella typhi in typhoid fever. Infect Immun. 1989 Apr;57(4):1271–1275. doi: 10.1128/iai.57.4.1271-1275.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández M., Sierra-Madero J., de la Vega H., Vázquez M., López-Vidal Y., Ruíz-Palacios G. M., Calva E. Molecular cloning of a Salmonella typhi LT-like enterotoxin gene. Mol Microbiol. 1988 Nov;2(6):821–825. doi: 10.1111/j.1365-2958.1988.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Johnson W. M., Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Escherichia coli isolates from clinical material. Microb Pathog. 1988 Feb;4(2):103–113. doi: 10.1016/0882-4010(88)90052-6. [DOI] [PubMed] [Google Scholar]
- Maher K. O., Morris J. G., Jr, Gotuzzo E., Ferreccio C., Ward L. R., Benavente L., Black R. E., Rowe B., Levine M. M. Molecular techniques in the study of Salmonella typhi in epidemiologic studies in endemic areas: comparison with Vi phage typing. Am J Trop Med Hyg. 1986 Jul;35(4):831–835. doi: 10.4269/ajtmh.1986.35.831. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Heuzenroeder M. W., Yeadon J., Leavesley D. I., Reeves P. R., Rowley D. Molecular cloning and expression in Escherichia coli K-12 of the O antigens of the Inaba and Ogawa serotypes of the Vibrio cholerae O1 lipopolysaccharides and their potential for vaccine development. Infect Immun. 1986 Aug;53(2):272–277. doi: 10.1128/iai.53.2.272-277.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahlik M. S., Fleming T. P., McIntosh M. A. Cluster of genes controlling synthesis and activation of 2,3-dihydroxybenzoic acid in production of enterobactin in Escherichia coli. J Bacteriol. 1987 Sep;169(9):4163–4170. doi: 10.1128/jb.169.9.4163-4170.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves M. W., Evins G. M., Heiba A. A., Plikaytis B. D., Farmer J. J., 3rd Clonal nature of Salmonella typhi and its genetic relatedness to other salmonellae as shown by multilocus enzyme electrophoresis, and proposal of Salmonella bongori comb. nov. J Clin Microbiol. 1989 Feb;27(2):313–320. doi: 10.1128/jcm.27.2.313-320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. D., Robbins J. B. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984 Sep;150(3):436–449. doi: 10.1093/infdis/150.3.436. [DOI] [PubMed] [Google Scholar]
- Silva B. A., Gonzalez C., Mora G. C., Cabello F. Genetic characteristics of the Salmonella typhi strain Ty21a vaccine. J Infect Dis. 1987 May;155(5):1077–1078. doi: 10.1093/infdis/155.5.1077. [DOI] [PubMed] [Google Scholar]