Abstract
Cajal-Retzius cells, located in layer I of the cortex, synthesize and secrete the glycoprotein reelin, which plays a pivotal role in neuronal migration during embryonic development. Cajal-Retzius cells persist after birth, but their postnatal role is unknown. Here we show that Cajal-Retzius cells receive a major excitatory synaptic input via serotonin 5-HT3 receptors. Blocking this input using pharmacological tools or neutralization of reelin signaling results in hypercomplexity of apical, but not basal, dendrites of cortical layer II/III pyramidal neurons. A similar hypercomplexity is observed in the cortex of the 5-HT3A receptor knockout mouse. The increased dendritic complexity can be rescued by application of recombinant full-length reelin or its N-terminal fragment, but not by the central fragment of reelin, and involves a signal transduction pathway independent of the activation of the canonical reelin receptors. Taken together, our results reveal a novel role of serotonin, Cajal-Retzius cells, and reelin in the postnatal maturation of the cortex.
Keywords: 5-HT3 receptor, Cajal-Retzius cells, postnatal development
Cajal-Retzius cells are among the earliest generated and most prevalent neurons in the marginal zone, the later layer I of the cortex. During embryonic corticogenesis, Cajal-Retzius cells orchestrate the typical inside-out layering of the cortex by releasing the glycoprotein reelin, which is critical for neuronal migration and cell positioning by activating the very-low-density lipoprotein (VLDL) receptor and the apolipoprotein E receptor 2 (ApoER2) (1–6). In vivo, reelin is cleaved by extracellular metalloproteinases into three fragments of which only the central fragment R3–6 is able to bind to the ApoER2 and VLDL receptors (7–9). The N-terminal fragment N–R2 has been shown to interact with α3β1 integrin receptors (10, 11).
Upon completion of the cortical layer formation, the density of Cajal-Retzius cells decreases during the first two postnatal weeks (in rodents) as a consequence of progressive “dilution,” apoptosis, or differentiation into an interneuron-like phenotype (12–14). During that period, neurons expressing reelin remain confined to layer I (9, 15). The functional role of Cajal-Retzius cells during the first 2 postnatal weeks is still obscure. It has been suggested that, in addition to their role in migration and positioning of postmitotic neurons, reelin-secreting neurons are also involved in the formation of vertical cortical columns. For example, the reeler mouse, an autosomal recessive mutant in which reelin is defective, displays abnormalities in presubicular columns, which led Nishikawa et al. (16) to postulate that reelin may act as a stop signal for dendritic extensions of cortical neurons. In addition, Janusonis et al. (17) showed that serotonergic input on Cajal-Retzius cells is important for proper corticogenesis, as disruption of the serotonergic system during embryonic development results in lower levels of whole-brain reelin and a disturbed formation of cortical columns in the presubicular cortex. However, it is not clear whether the manifestations of these postnatal abnormalities are a mere consequence of the absence of reelin during the embryonic stage, or whether a different postnatal mechanism is responsible for the changes in cortical column formation.
In rodents, serotonergic innervation of the cortex starts during late embryogenesis and persists during life (18, 19). Among the first targets of the serotonergic afferents in the cortex are the Cajal-Retzius cells. They receive serotonergic projections as early as E15 in mice and E17 in rats, primarily through axo-dendritic synapses (17). There are no reports on the expression of serotonin receptors on Cajal-Retzius cells, although it has been suggested that they may express 5-HT1A and/or 5-HT3 receptors (17, 20). In this study, we made use of a transgenic mouse line which expresses enhanced green fluorescent protein (EGFP) under the control of the 5-HT3A promotor (21) to show that Cajal-Retzius cells indeed express functional 5-HT3 receptors. Because Cajal-Retzius cells are strategically located in layer I of the cortex, where the apical dendrites of pyramidal neurons develop and branch after birth, we tested the hypothesis that serotonergic input on Cajal-Retzius cells controls the postnatal maturation of the apical dendritic trees.
Results
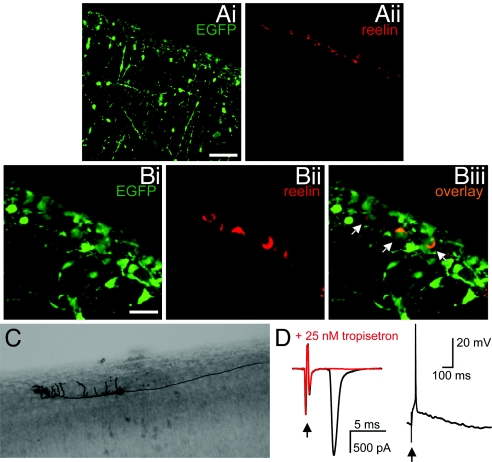
Reelin-expressing Cajal-Retzius cells were identified in layer I of the cortex of newborn (P0) 5-HT3/EGFP transgenic mice. The expression of reelin was restricted exclusively to layer I (Fig. 1A). From the population of reelin-positive cells (n = 339), 80% expressed EGFP (Fig. 1B), and their morphology conformed with the typical morphology of Cajal-Retzius cells, i.e., a fusiform, horizontally oriented soma and extended horizontal dendrites parallel to the pia and confined to layer I (Fig. 1C). The presence of functional 5-HT3 receptors was confirmed by electrical stimulation of layer I which resulted in a large and fast 5-HT3 receptor-mediated synaptic current of 2.29 ± 0.45 nA (n = 15; Fig. 1D), which was blocked by the selective 5-HT3 receptor antagonists tropisetron (25 nM, n = 5) and MDL-72222 (100 nM, n = 3). Under current clamp conditions, the synaptic activation of 5-HT3 receptors was sufficient to induce action potential firing in Cajal-Retzius cells (Fig. 1D). The rapid kinetics of the current are reminiscent of the fast 5-HT3 receptor-mediated synaptic current in cortical interneurons (22). Local, somatic application of 100 μM 5-HT to Cajal-Retzius cells resulted in a small 5-HT3 receptor-mediated inward current of 41 ± 11 pA (n = 11) in only 44% of the cells tested (n = 25; not shown), corroborating the previous finding that serotonergic afferents preferentially innervate the distal dendrites of Cajal-Retzius cells (17).
Fig. 1.
Cajal-Retzius cells express 5-HT3 receptors. (A) Expression of EGFP (Ai) and reelin (Aii) in saggital sections of the cortex of 5-HT3A/EGFP mice at P0, shown at low magnification. Scale bar, 50 μm. (B) Saggital sections of the cortex taken from 5-HT3A/EGFP mice at P0, showing expression of EGFP (Bi), of reelin (Bii) and the co-expression (Biii). Arrows indicate cells that co-express EGFP and reelin. Scale bar, 20 μm. (C) Biocytin-filled EGFP-positive cell in layer I of the cortex showing the typical morphology of a Cajal-Retzius cell. (D) Whole-cell patch clamp recordings of 5-HT3 receptor-mediated currents under voltage clamp (left trace) and 5-HT3 receptor-mediated depolarization under current clamp (right trace) by electrical stimulation of layer I. Bath application of 25 nM tropisetron resulted in a complete block of the 5-HT3 receptor-mediated synaptic current (red trace). Arrows indicate stimulation artifact.
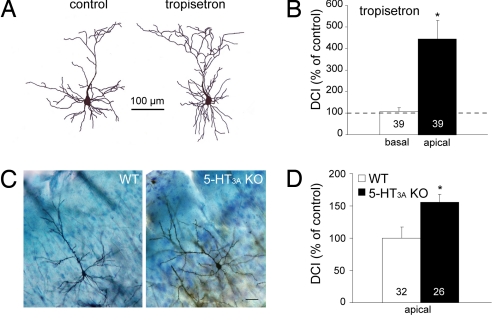
To test the hypothesis that Cajal-Retzius cells are involved in postnatal dendritic maturation of cortical neurons, we made cortical organotypic slice cultures from newborn (P0) mice, manipulated either the (serotonergic) input or the output (reelin) of these cells, and assessed the effects on dendritic maturation of layer II/III pyramidal neurons. We quantified the morphology of layer II/III pyramidal neurons by determining the dendritic complexity index (DCI; see Materials and Methods). Figures 2A and 2B show that overall block of 5-HT3 receptor activity using the selective 5-HT3 receptor antagonist tropisetron (100 nM) results in an increase in complexity of the apical, but not the basal, dendrites, to 444 ± 94% (n = 39, P < 0.0001) of control [see supporting information (SI) Table S1 for absolute values of the DCI]. We also analyzed the morphology of layer II/III pyramidal neurons from adult 5-HT3A receptor knockout mice (23). Figures 2C and 2D show that the complexity of the apical dendrites in adult 5-HT3A receptor knockout mice was significantly higher (155 ± 12%, n = 26, P = 0.014) as compared with wild-type mice. In summary, in 5-HT3A receptor knockout mice as well as in organotypic slice cultures of wild-type mice where 5-HT3 receptors were blocked pharmacologically, we observed a hypercomplexity of the apical dendrites of layer II/III cortical pyramidal neurons.
Fig. 2.
Role of 5-HT3 receptors in postnatal dendritic maturation. (A) Examples of reconstructed layer II/III pyramidal neurons from organotypic slices cultured in the absence (left) and presence (right) of the 5-HT3 receptor antagonist tropisetron. (B) DCI, expressed as percentage of control, of the basal and apical dendrites of layer II/III pyramidal neurons cultured in the presence of tropisetron. (C) Golgi-Cox stainings of adult (P90) wild-type (WT) and 5-HT3A receptor knockout (KO) mice. (D) DCI calculated for the apical dendrites of layer II/III pyramidal neurons is significantly higher in 5-HT3A receptor KO mice as compared with WT mice. Numbers in bars indicate the number of cells. Asterisks indicate P < 0.05.
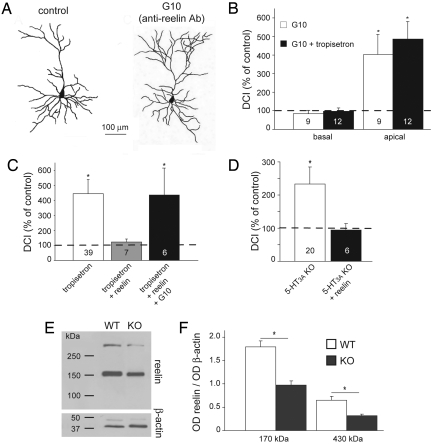
Given that Cajal-Retzius cells are the only cells that express and secrete reelin at early postnatal ages (9, 15; see also Fig. 1B), we tested whether reelin secreted by Cajal-Retzius cells is involved in the control of the maturation of layer II/III pyramidal neurons. Neutralization of reelin with the specific anti-reelin antibody G10 resulted in an increase in the complexity of the apical dendrites to 402 ± 109% (n = 9, P = 0.014, Figs. 3 A and B), whereas the complexity of basal dendrites remained unchanged (85 ± 17%, n = 9, P = 0.396). Co-application of tropisetron (100 nM) and the G10 antibody did not further increase the complexity of the apical dendrites (486 ± 94%, n = 12, P = 0.356 against G10 alone, Fig. 3C), suggesting that 5-HT3 receptors and reelin are key components of a common pathway regulating dendritic maturation. This was confirmed by adding recombinant full-length reelin to the organotypic slices while blocking the 5-HT3 receptor with tropisetron. Under these conditions, reelin rescued the increase in dendritic complexity induced by tropisetron (DCI = 122 ± 20%, n = 7, P = 0.317, Fig. 3C). Rescue of the tropisetron-induced hypercomplexity by recombinant reelin was prevented by neutralization of the recombinant reelin with the anti-reelin antibody G10 (Fig. 3C).
Fig. 3.
Role of reelin in postnatal dendritic maturation. (A) Examples of reconstructed layer II/III pyramidal neurons from organotypic slice cultures in the absence and presence of the anti-reelin antibody G10. (B) DCI, expressed as percentage of control, of the basal and apical dendrites of layer II/III pyramidal neurons cultured in the presence of G10 alone and in the presence of G10 and tropisetron. (C) DCI, expressed as percentage of control, of the apical dendrites of layer II/III pyramidal neurons. Slices were cultured in the presence of the 5-HT3 receptor antagonist tropisetron and with either recombinant reelin or recombinant reelin and the anti-reelin antibody G10. Leftmost bar is identical to that in Fig. 2B and is displayed for comparison. (D) Rescue of the apical dendritic morphology in 5-HT3A receptor knockout mice. DCI, expressed as percentage of control, of apical dendrites of layer II/III pyramidal neurons from slices cultured in the absence and presence of recombinant reelin. (E) Western blot analysis of the levels of whole-cortex reelin in wild-type and 5-HT3A knockout mice. (F) Quantification, expressed as optical density (OD) of the levels of reelin in the cortex of wild-type and 5-HT3 knockout mice (n = 4) at P0. Numbers in bars indicate the number of cells. Asterisks indicate P < 0.05.
The increased dendritic complexity of layer II/III pyramidal neurons in the cortex of adult 5-HT3 receptor knockout mice was also evident in organotypic slice cultures from newborn knockout mice (Fig. 3D). DCI values of the apical dendrite of layer II/III pyramidal neurons, but not of the basal dendrites, were increased to 233 ± 50% (n = 20, P = 0.004) as compared with wild-type mice. Addition of recombinant reelin rescued the increase in dendritic complexity in the knockout mice, with the DCI value being close to control values (95 ± 18%, n = 6, P = 0.768, Fig. 3D). It was shown previously that disruption of the serotonergic innervation in the cortex results in lower levels of whole-brain reelin (17). In concordance with this finding we found that the levels of reelin in the cortex of the 5-HT3A receptor knockout mouse are reduced (Figs. 3E, 3F). Taken together, our results show that 5-HT3 receptor activity modulates the levels of reelin, and that addition of recombinant reelin is neccesary and sufficient to rescue the phenotypes induced by block or absence of 5-HT3 receptors.
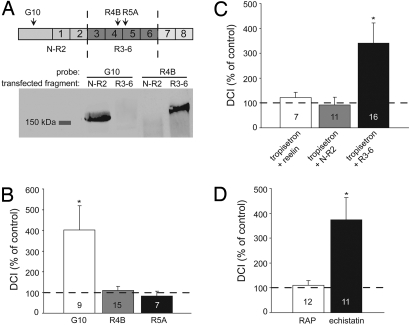
The functional block of the anti-reelin antibody G10, which specifically targets the N-terminal fragment of reelin (Fig. 4A), suggests that the control of postnatal dendritic maturation is mediated by the N-terminal fragment of reelin, N–R2. To test this, we first cultured slices in the presence of the antibodies R4B or R5A, which target the central reelin fragment of reelin (R3–6, Fig. 4A) and thus prevent binding of reelin to the ApoER2 and VLDL receptors (9). In contrast to the G10 antibody, which induced an increase in the DCI (see also Fig. 3B), antibodies R4B and R5A had no effect on the complexity of apical dendrites (Fig. 4B). We then tested whether the N-terminal or central fragments of reelin could mimic the effect of the full-length reelin in rescuing the tropisetron-induced increase in dendritic complexity. In the presence of conditioned culture medium from HEK293 cells transfected with a plasmid encoding the N-terminal reelin fragment N–R2, the complexity of the apical dendrites reverted to control values (91 ± 31%, n = 11, P = 0.251, Fig. 4C), similar to the effect of full-length reelin (DCI = 122 ± 20%, n = 7, P = 0.317, Figs. 3C and 4C). In contrast, application of the central reelin fragment R3–6 did not rescue the tropisetron-induced increase in dendritic complexity (DCI = 341 ± 80%, n = 16, P = 0.002, Fig. 4C). Taken together, these results show that the N-terminal fragment of reelin mediates the control over the postnatal maturation of the apical dendritic tree of cortical layer II/III pyramidal neurons. The fact that the N-terminal fragment does not bind to and activate the ApoER2 and VLDL receptors (8, 9) implies the involvement of a different receptor system such as integrin receptors (10, 11). Indeed, when blocking the ApoER2 and VLDL receptor using receptor-associated protein (RAP) (2, 3, 24), the dendritic complexity was not affected (DCI = 110 ± 19%, n = 12, P = 0.08, Fig. 4D). In contrast, when brain slices were cultured in the presence of echistatin, a potent inhibitor of β1- and β3-containing integrin receptors (25), the DCI of the apical dendritic tree was increased to 374 ± 88% (n = 11, P = 0.0004), confirming the notion that the N-terminal fragment of reelin acts independently of the canonical reelin receptors.
Fig. 4.
N-terminal fragment of reelin mediates control of dendritic maturation. (A) (Top) Schematic representation of the reelin protein consisting of an N-terminal fragment followed by eight repeats. Cleavage by metalloproteinases (dashed lines) results in two main diffusible fragments: N–R2 (180 kDa) and R3–6 (190 kDa). Arrowheads indicate regions recognized by the specific anti-reelin antibodies G10, R4B, and R5B. (Bottom) Western blot analysis of conditioned media from HEK293 cells transfected with plasmids encoding either the N-terminal fragment (N–R2) or the central fragment (R3–6). Blots were probed with anti-reelin antibodies directed against the N–R2 fragment (G10) and against the R3–6 fragment (R4B). (B) Slices were cultured in the presence of anti-reelin antibodies directed against the N-terminal fragment (G10) or directed against the central fragment (R4B and R5A). (C) Rescue of the hypercomplexity of apical dendrites induced by pharmacological block of 5-HT3 receptors. DCI, expressed as percentage of control, of apical dendrites of layer II/III pyramidal neurons from slices cultured in the presence of the following recombinant proteins: full-length reelin, the N-terminal fragment (N–R2) and the central fragment (R3–6). (D) Role of integrin receptors in the dendritic maturation. Slices were cultured either in the presence of RAP (50 μg/ml), a blocker of the ApoER2 and VLDL receptors or in the presence of echistatin (10 μM), an inhibitor of β1/β3-containing integrin receptors. DCI values are expressed for the apical dendrites as a percentage of control. Leftmost bars in (B) and (C) are identical to those in Figs. 3B and 3C, respectively, and are displayed for comparison. Numbers in bars indicate numbers of cells. Asterisks indicate P < 0.05.
Discussion
In this study we provide evidence that the N-terminal fragment of reelin, produced by Cajal-Retzius cells, controls the postnatal maturation of apical dendrites of cortical layer II/III pyramidal neurons, and that this maturational process is driven by serotonergic activity via 5-HT3 receptors. The expression of 5-HT3 receptors by Cajal-Retzius cells was first suggested by Tecott et al. (20), who showed a dense labeling in layer I of the juvenile rat cortex using autoradiography. In the transgenic 5-HT3A/EGFP mouse line, we found a similar high density of 5-HT3 receptor-expressing cells in layer I, which we identified as Cajal-Retzius cells by the expression of reelin, their developmentally regulated decrease, and their morphology.
In addition to the well-documented role of reelin in neuronal migration during cortical embryogenesis, which requires the binding of the central reelin fragment R3–6 to ApoER2 and VLDL receptors, we propose here a new role for reelin in the postnatal maturation of apical dendrites of cortical neurons. In the present study, apical dendritic complexity was not affected by preventing the binding of R3–6 fragment to ApoER2 and VLDL receptors using the antibodies R4B and R5A, which otherwise act as function-blocking antibodies (8, 9) or by culturing slices in the presence of an inhibitor of ApoER2 and VLDL receptors (2, 3, 24). Moreover, the G10 antibody, directed against the N-terminal reelin fragment N–R2, induced an increase in apical dendritic complexity. Furthermore, the N–R2 fragment, which does not bind to ApoER2 and VLDL receptors (9), is sufficient to rescue the hypercomplexity of apical dendrites induced by block of 5-HT3 receptors. Given that the N-terminal fragment is able to bind α3β1 integrin receptors (11) and that blocking β1/β3-containing integrin receptors increases the dendritic complexity in the same order of magnitude as does the block of reelin (compare Figs. 4B and 4D), we propose that the signaling pathway requires binding of the N–R2 fragment to integrin receptors. It is yet unclear how the activation of integrin receptors by reelin leads to changes in dendritic morphology. It has been shown that dendritic maturation can be modulated by neuronal activity (26) and that reelin affects neuronal activity in hippocampus by modulation of NMDA receptors (27), albeit that these effects are mediated by an interaction with the canonical reelin receptors which are not involved in the presently reported effects of reelin (Fig. 4D). In addition, it has been reported that the expression of integrin receptors seem to be restricted to the apical dendrites of cortical layer II/III pyramidal neurons (28, 29). Whether reelin initiates its action directly on pyramidal neurons or indirectly via modulation of activity levels of interneurons remains to be determined.
In conclusion, from our work and from other reports it appears that reelin plays a dual role in cortical development: (i) reelin orchestrates neuronal migration at embryonic stages via the central reelin fragment R3–6 and the ApoER2/VLDL receptor signaling pathway, and (ii) reelin regulates dendritic maturation at early postnatal stages via the N-terminal reelin fragment N–R2 and an integrin receptor-mediated signaling pathway that remains to be further characterized. From observations of the reeler mouse brain and from in utero silencing of Dab1 signaling, reelin activation of the ApoER2/VLDL receptor signaling pathway results in a decreased dendritic complexity in both hippocampal (30, 31) and cortical pyramidal neurons (32), opposite to the effect of reelin on postnatal dendritic maturation that we report here. The most parsimonious explanation for this apparent discrepancy is that in both the reeler mouse and in the in utero silenced Dab1 studies, the effects on postnatal dendritic development are secondary to the absence of reelin signaling at embryonic stages.
The intimate relation between serotonin and neuronal development has been firmly established by a number of studies that show that serotonin regulates mechanisms of proliferation, migration, and differentiation (18, 19). Our results suggest that 5-HT3 receptors may, either directly or indirectly, control the amount of release of reelin. It should be noted that the exact mechanism of reelin release by Cajal-Retzius cells is not known. It has been suggested that Cajal-Retzius cells contain axonal reelin reservoirs (33). In addition, it has been reported that in rat cerebellar granule neurons release of reelin is independent of Ca2+-induced exocytosis, and is probably related to a constitutively active pathway involving Golgi secretory vesicles (34). It remains to be determined whether the release of reelin from Cajal-Retzius cells is directly controlled by 5-HT3 receptors. Nevertheless, the fact that the effects of 5-HT3 receptor block and functional block of reelin on the dendritic morphology of layer II/III pyramidal neurons are not additive (Fig. 3B), in addition to the observations that reelin levels are reduced in the 5-HT3A knockout mice and that reelin fully rescues the phenotype induced by either pharmacological block of 5-HT3 receptors or in the 5-HT3A receptor knockout mouse (Figs. 3D and 3E), strongly suggests that 5-HT3 receptors and reelin are key components of a common pathway by which Cajal-Retzius cells control the postnatal maturation of the dendritic arbors of layer II/III pyramidal neurons.
Considering the prominent role of both serotonin and Cajal-Retzius cells in the development of the cortex, the results of this study suggest that fast serotonergic signaling to Cajal-Retzius cells in early postnatal life is important for shaping the cortical microcircuitry. Both serotonin and Cajal-Retzius cells have been implicated in the formation of cortical columns (16, 17). Nishikawa et al. (16) postulated that reelin regulation of early postnatal maturation of dendritic arborization is involved in the development of vertical columnar structures in the mouse presubicular cortex. They hypothesized that reelin may act as a stop signal for growth and branching of apical dendrites. Further extension of the distal dendrites of pyramidal neurons reaching the marginal zone is then stopped by reelin thus preventing hyperarborization. Moreover, disruption of serotonergic innervation to Cajal Retzius cells results in decreased reelin levels and aberrant column formation in mouse presubicular cortex (17). In addition, some of the neurological disorders in which serotonin has been implicated to play a prominent role, such as autism and schizophrenia, are also characterized by abnormalities in cortical columns (35). The changes in dendritic morphology of layer II/III pyramidal neurons upon manipulation of Cajal-Retzius cells that we report here may partially underlie the previously reported changes in columnar structure, thereby providing new insights in the intricate process of postnatal cortical circuit formation and its pathological consequences.
Materials and Methods
Animals.
Experiments were performed using the 5-HT3A/EGFP transgenic mouse line (21) and the 5-HT3A knockout mouse line (23). The 5-HT3A knockout mice were maintained on the C57/BL6 background and back-crossed for at least 35 generations. For electrophysiological and culture experiments, mice were killed by cervical dislocation, and 250 to 400 μm-thick coronal or saggital brainslices were cut on a vibroslicer (Leica VT1000S). All experiments were approved by the ethical committees of the participating institutes.
Organotypic Slice Cultures.
Coronal brainslices of newborn (P0) mice were cultured on cell culture inserts (Falcon, 1-μm pore size) for 6–7 days at 37 °C in a humidified atmosphere containing 5% CO2. Slices were maintained in Neurobasal medium (Invitrogen) supplemented with B27 (1:50, vol/vol), 2 mM L-glutamine, and 100 μg/ml penicillin/streptomycin. Experimental treatments and controls were carried out simultaneously on parallel cultures. Substances were added to the culture medium starting at the day of culture, and half of the culture medium was refreshed every 2 days. Added substances were: 100 nM of the selective 5-HT3 receptor antagonist tropisetron (ICS 205–930; Sigma), the anti-reelin antibodies G10 (Abcam, Cambridge, UK), and R4B and R5A (gift from André Goffinet, Louvain, Belgium) at a dilution of 1/1000, recombinant full-length or cleaved reelin (see below), receptor-associated protein (RAP, GST-cleaved, Calbiochem), echistatin (Sigma).
Preparation of Recombinant Reelin.
Human Embryonic Kidney 293 (HEK293) cells were maintained in Minimum Essential Medium supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, and 100 μg/ml penicillin/streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. Cells were passaged weekly and medium was refreshed every 2–3 days. Cells were plated in 24-well plates and were transiently transfected with a vector encoding either mouse full-length reelin (gift of Tom Curran, Philadelphia, PA), the N-terminal fragment (N–R2), or the central fragment of reelin (R3–6) (gift of André Goffinet, Louvain, Belgium) using the calcium phosphate precipitation method. After 24 hours, the culture medium was changed to Neurobasal medium (Invitrogen) supplemented with B27 (1:50, vol/vol), 2 mM l-glutamine and 100 μg/ml penicillin/streptomycin. After 2 days, the conditioned Neurobasal culture medium was harvested once a day for 3 days, pooled, and stored at + 4 °C until the following day for culturing the organotypic slices (see above). For determination of reelin levels in mouse cortex, cortices of P0 wild-type and 5-HT3A knockout mice were rapidly dissected out in ice-cold homogenization buffer (320 mM sucrose, 10 mM Hepes, and protease inhibitor mixture (Complete, Roche), pH = 7.4), homogenized and centrifuged at 10,000 g (4 °C, 10 minutes). Supernatant were collected and stored at −80 °C until use.
For detection of the reelin fragments from either transfected HEK293 cells or mouse cortex, 24 μl of conditioned culture medium or cortex homogenate was mixed with 6 μl 5X sample buffer and boiled for 5 minutes. Proteins (20 μl per lane) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 5% Tris-glycine gel and transferred onto nitrocellulose membranes by electrophoresis using a TransBlot Semi Dry (BioRad Laboratories). The membranes were allowed to dry overnight and then processed for immunodetection of reelin. Nonspecific binding sites on the nitrocellulose were blocked by immersion of the membranes in 4% nonfat dry milk (BioRad Laboratories) in Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl, pH 7.4) containing 0.1% (vol/vol) Tween-20 (TBST). Membranes were then incubated for 2 hours at room temperature with the following antibodies: mouse anti-reelin G10 antibody (Abcam, diluted 1:1000 in the blocking solution), mouse anti-R4B and mouse anti-R5A antibodies (1/500), and mouse anti-β-actin (1/4000, Sigma). Blots were then rinsed five times in TBST and incubated for 1 hour at room temperature with horseradish peroxidase–conjugated goat anti-mouse IgG (1:3000 in blocking solution). The reelin bands were detected using the ECL Plus Western Blotting detection reagents (Amersham) and were visualized with Hyperfilm ECl (Amersham).
Electrophysiology.
Acute slices and cultured orgaotypic slices were kept submerged at room temperature (20–22 °C) and were continuously superfused with artificial cerebrospinal fluid (ACSF) containing the following (in mM): NaCl (120), KCl (3.5), CaCl2 (2.5), MgSO4 (1.3), NaH2PO4 (1.25), NaHCO3 (25), glucose (25), continuously bubbled with 95% O2-5% CO2 (pH = 7.4). EGFP-positive neurons in layer I of the cortex and layer II/III pyramidal neurons were visualized using differential interference contrast videomicroscopy on a Zeiss FS2 microscope equipped with standard epifluorescence. Patch pipettes were pulled from boroscilicate glass and had a resistance of 3–6 MΩ when filled with internal solution containing the following (in mM): K-gluconate (110), KCl (30), CaCl2 (0.5), EGTA (5), Hepes (10), and Mg-ATP (2), pH = 7.3 with KOH. Whole-cell recordings were made using an EPC9 patch-clamp amplifier and PULSE software (HEKA Electronic, Lambrecht, Germany). Signals were filtered at 1–5 kHz and sampled at 2–10 kHz. Series resistance ranged from 5–20 MΩ and was compensated for at least 80%. Cells were voltage clamped at −60 mV (corrected for liquid junction potential). A second pipette, connected to a picospritzer (General Valve, Fairfield, NJ), and containing 100 μM 5-HT (Sigma) in ACSF was positioned in the vicinity of the cell soma. 5-HT was applied for 500 ms at 35–100 kPa. Synaptic 5-HT3 currents were evoked by a bipolar stimulation electrode placed in layer 1 of the cortex and stimuli (100–400 μA, 200-μs duration) were delivered using a custom-made current stimulator. During the recordings, cells were filled with either biocytin (4 mg/ml; Sigma) or the fluorescent dye Alexa 568 (0.2 mg/ml; Molecular Probes) which were dissolved in internal solution. Drugs were applied via the superfusion system.
Golgi Stainings.
We used a modified method initially described by Gibb and Kolb (36). Briefly, animals were transcardially perfused with a 0.9% saline solution. Whole brains were dissected out, immersed in the Golgi-Cox solution (10.4 g/l K2Cr2O7, 10.4 g/l HgCl2, 8.3 g/l K2Cr2O4 in distilled water) in the dark for 5 days, immersed in a 30% sucrose solution in distilled water for 5 additional days, and processed. Brains were cut on a vibratome (Leica VT 1000S), and 300-μm thick sections were collected on gelatin-coated slides. Slides were rapidly dried by applying blotting paper on the surface of the sections and were immersed in distilled water until processing. Slides were sequentially immersed in the following solutions: NH4OH (30′), water (1′), D19 (30′), water (1′), 0.5% toluidine blue (15′), ethanol 70% (4′), ethanol 95% (4′), ethanol absolute (10′), and a solution containing (1:3 xylene, 1:3 ethanol, 1:3 chloroform; 15′) and were cleared in xylene. Slides were cover-slipped with Depex. Except D19 (Kodak), all products were purchased from Sigma.
Morphological Analysis.
Organotypic slices with Alexa 568-filled layer II/III pyramidal neurons were fixed o/n in 4% paraformaldehyde (PFA)/phosphate buffered saline (PBS) and scanned on a confocal microscope (Zeiss LSM 510), equipped with a dry Plan Neofluor 20x/0.75 objective, using the 568-nm line of an ArKr laser. The morphological analysis was performed using Image J (National Institute of Health, Bethesda, MD; http://rsb.info.nih.gov/nih-image/), and the Neuron Morpho plug-in for reconstruction and the LMeasure software to define the parameters used to determine the dendritic complexity index (DCI). The DCI was calculated as previously described (37). Briefly, we assigned each branch tip an order value that equaled the number of branch points between the tip and the base of its primary dendrite. The DCI was subsequently calculated according to the following formula:
 |
The DCI of each neuron was normalized to the mean DCI of at least six control neurons cultured and filled during the same culturing- and recording session. Values are expressed as mean ± standard error of the mean and compared with control values using a Mann-Whitney U test. Values are considered significant at P < 0.05.
Immunohistochemistry.
Newborn (P0), 7-day-old (P7), and adult transgenic 5-HT3/EGFP mice were used to analyze cortical coexpression of EGFP and reelin. After fixation with 4% PFA/PBS (pH 7.4), 100 μm-thick coronal brain sections were obtained using a vibratome (Leica VT1000S). After extensive washes in PBS, the sections were permeabilized by incubation in PBS plus 0.4% Triton X-100 (PBST) (30 minutes) and blocked in 10% normal goat serum/PBST (30 min). Slices were then incubated overnight with the rabbit polyclonal Alexa Fluor 488-conjugated anti-EGFP antibody, 1:400 (Molecular Probes) in combination with the mouse G10 anti-reelin antibody, 1:1000 (Abcam). Slices were washed extensively and incubated 2 hours at room temperature with Alexa Fluor 568–conjugated anti-mouse antibody, 1/500 (Molecular Probes). The distribution of EGFP- and reelin-expressing cells was analyzed using a confocal microscope (Zeiss LSM 510), equipped with a dry Plan Neofluor 40x/1.2W objective, using the 488- and the 568-nm lines of an ArKr laser.
Supplementary Material
Acknowledgments.
We thank André Goffinet (Université Catholique de Louvain, Belgium) for providing the N-R2 and R3–6 plasmids and the R4B and R5A antibodies, and for his comments on the manuscript; David Julius (University of San Francisco, San Fransisco, CA) for providing the 5-HT3A knockout mice; Tom Curran (St. Jude Children's Hospital of Philadelphia, Philadelphia, PA) for providing the full-length reelin plasmid; Erik Manders of the Center for Advanced Microscopy at the University of Amsterdam for support with the confocal microscopy; and Yoav Noam, Marlies Oostland, and Laura Smit-Rigter for their comments on the manuscript. D.I. was supported by the Graduate College 791 of the Deutsche Forschungsgemeinschaft, H.M. by the Schilling Foundation, and J.A.v.H. by the Royal Netherlands Academy of Arts and Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810764106/DCSupplemental.
References
- 1.D'Arcangelo G, et al. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 2.Hiesberger T, et al. Direct binding of reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of Disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 3.Trommsdorff M, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;7:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 4.Magdaleno S, Keshvara L, Curran T. Rescue of ataxia and preplate splitting by ectopic expression of reelin in reeler mice. Neuron. 2002;33:573–586. doi: 10.1016/s0896-6273(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 5.Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- 6.Hack I, et al. Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development. 2007;134:3883–3891. doi: 10.1242/dev.005447. [DOI] [PubMed] [Google Scholar]
- 7.Lambert de Rouvroit C, et al. Reelin, the extracellular matrix protein deficient in reeler mutant mice, is processed by a metalloproteinase. Exp Neurol. 1999;156:214–217. doi: 10.1006/exnr.1998.7007. [DOI] [PubMed] [Google Scholar]
- 8.Jossin Y, et al. The central fragment of reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci. 2004;24:514–521. doi: 10.1523/JNEUROSCI.3408-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jossin Y, Gui L, Goffinet AM. Processing of reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J Neurosci. 2007;27:4243–4252. doi: 10.1523/JNEUROSCI.0023-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulabon L, et al. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 11.Schmid RS, Jo R, Shelton S, Kreidberg JA, Anton ES. Reelin, integrin and DAB1 interactions during embryonic cerebral cortical development. Cereb Cortex. 2005;15:1632–1636. doi: 10.1093/cercor/bhi041. [DOI] [PubMed] [Google Scholar]
- 12.Del Río JA, et al. Differential survival of Cajal-Retzius cells in organotypic cultures of hippocampus and neocortex. J Neurosci. 1996;16:6896–6907. doi: 10.1523/JNEUROSCI.16-21-06896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- 14.Meyer G, Soria JM, Martinez-Galan JR, Martin-Clemente B, Fairen A. Different origins and developmental histories of transient neurons in the marginal zone of the fetal and neonatal rat cortex. J Comp Neurol. 1998;397:493–518. [PubMed] [Google Scholar]
- 15.Alcántara S, et al. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa S, Goto S, Hamasaki T, Yamada K, Ushio Y. Involvement of reelin and Cajal-Retzius cells in the developmental formation of vertical columnar structures in the cerebral cortex: Evidence from the study of mouse presubicular cortex. Cereb Cortex. 2002;12:1024–1030. doi: 10.1093/cercor/12.10.1024. [DOI] [PubMed] [Google Scholar]
- 17.Janusonis S, Gluncic V, Rakic P. Early serotonergic projections to Cajal-Retzius cells: Relevance for cortical development. J Neurosci. 2004;24:1652–1659. doi: 10.1523/JNEUROSCI.4651-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 19.Vitalis T, Parnavelas JG. The role of serotonin in early cortical development. Dev Neurosci. 2003;25:245–256. doi: 10.1159/000072272. [DOI] [PubMed] [Google Scholar]
- 20.Tecott L, Shtrom S, Julius D. Expression of a serotonin-gated ion channel in embryonic neural and nonneural tissues. Mol Cell Neurosci. 1995;6:43–55. doi: 10.1006/mcne.1995.1005. [DOI] [PubMed] [Google Scholar]
- 21.Inta D, et al. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci USA. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Férézou I, et al. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeitz KP, et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991;266:21232–21238. [PubMed] [Google Scholar]
- 25.Thibault G. Sodium dodecyl sulfate-stable complexes of echistatin and RGD-dependent integrins: A novel approach to study integrins. Mol Pharmacol. 2000;58:1137–1145. doi: 10.1124/mol.58.5.1137. [DOI] [PubMed] [Google Scholar]
- 26.Whitford KL, Dijkhuizen P, Polleux F, Ghosh A. Molecular control of cortical dendrite development. Annu Rev Neurosci. 2002;25:127–149. doi: 10.1146/annurev.neuro.25.112701.142932. [DOI] [PubMed] [Google Scholar]
- 27.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez MA, et al. Colocalization of integrin receptors and reelin in dendritic spine postsynaptic densities of adult nonhuman primate cortex. Proc Natl Acad Sci USA. 2000;97:3550–3555. doi: 10.1073/pnas.050589797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi X, Lynch G, Zhou J, Gall CM. Polarized distribution of alpha5 integrin in dendrites of hippocampal and cortical neurons. J Comp Neurol. 2001;435:184–193. doi: 10.1002/cne.1201. [DOI] [PubMed] [Google Scholar]
- 30.Niu S, Renfro A, Quattrocchi CC, Sheldon M, D'Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- 31.Zhao S, et al. Rescue of the reeler phenotype in the dentate gyrus by wild-type coculture is mediated by lipoprotein receptors for reelin and Disabled 1. J Comp Neurol. 2006;495:1–9. doi: 10.1002/cne.20846. [DOI] [PubMed] [Google Scholar]
- 32.Olson EC, Kim S, Walsh CA. Impaired neuronal positioning and dendritogenesis in the neocortex after cell-autonomous Dab1 suppression. J Neurosci. 2006;26:1767–1775. doi: 10.1523/JNEUROSCI.3000-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derer P, Derer M, Goffinet AM. Axonal secretion of reelin by Cajal-Retzius cells: Evidence from comparison of normal and relnOrl mutant mice. J Comp Neurol. 2001;440:136–143. doi: 10.1002/cne.1375. [DOI] [PubMed] [Google Scholar]
- 34.Lacor PN, et al. Reelin secretion from glutamatergic neurons in culture is independent from neurotransmitter regulation. Proc Natl Acad Sci USA. 2000;97:3556–3561. doi: 10.1073/pnas.050589597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buxhoeveden DP, Casanova MF. The minicolumn hypothesis in neuroscience. Brain. 2002;125:935–951. doi: 10.1093/brain/awf110. [DOI] [PubMed] [Google Scholar]
- 36.Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- 37.Lom B, Cohen-Cory S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J Neurosci. 1999;19:9928–9938. doi: 10.1523/JNEUROSCI.19-22-09928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.