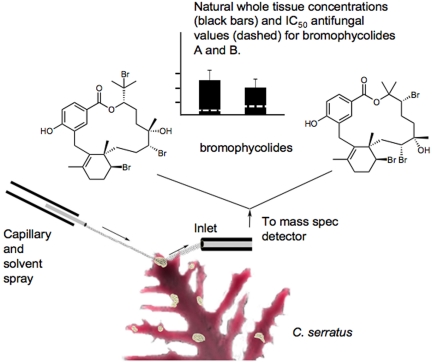
To demonstrate that a toxin or other natural product serves as a predator or pathogen deterrent requires that (i) the defined compound in question have the requisite biological activity, (ii) the compound is present at levels capable of eliciting an effect in the target organism, (iii) there is a physical mechanism for the substance to interact with its target before death of the producer, and (iv) the avoidance behavior in the target organism actually occurs. Although many highly-unique natural products have been defined from the marine environment, and they appear to serve as predator and pathogen deterrents, it has been extremely challenging to establish their quantity and physical location in the producing organism's tissues, thus leaving uncertainty about their true natural function. However, in this issue of PNAS, Lane et al. (1) have used a novel mass spectrometric approach [desorption electrospray ionization (DESI)] (Fig. 1) to sensitively, yet precisely, measure the physical location and quantities of antifungal natural products in the surface tissues of a tropical red alga. This advance, combined with other analytical MS developments as described below, is allowing more definitive identification of the physical location of natural products within biological tissues, cells, or even complex mixed-species assemblages (2, 3) and represents a dramatic technological advance for studying such phenomena.
Fig. 1.
An overview graphic of the DESI MS approach for determining the heterogenous distribution of 2 antifungal natural products across the surface of the marine alga C. serratus. The capillary on the left emits a solvent stream aimed at one of the light-colored patches found across ≈5% of the surface of the algae sample. Molecules contained in the targeted area are ionized and absorbed by the MS inlet. In this case, m/z values consistent with bromophycolides A (Left) and B (Right) are detected only in the patches. In separate experiments, antifungal IC50 values and naturally-occurring whole tissue concentrations of bromophycolides were determined in these samples.
Since the development of devices that could first measure the mass of molecules, the technology of MS has continued to enjoy an exponential growth. In the last 5 years much of this growth has been in the development of new ionization methods, primarily serving the needs of forensic, proteomic, and metabolomic investigations. MALDI or ESI are currently the most common methods by which to ionize biomolecules. Because MALDI and ESI are soft ionization methods they allow for routine MS on biologically-relevant molecules and have become the standard in the life sciences. It was therefore no surprise that the development of soft desorption ionization methods was recognized with the Nobel Prize in 2002 (4, 5). However, since the development of MALDI and ESI, other modes of ionization and improvements to existing techniques are providing a rich suite of complementary methods. These new methods enable MS to be used in a variety of new and innovative applications. Many of these newer methods are well suited to analyzing surfaces, are tolerant to impurities, and do not require extensive sample preparations. Examples include low-temperature plasma ionization (LTP), direct analysis in real time (DART), electrospray desorption/ionization (ELDI), DESI, and many others (6–9). Collectively, these approaches are called ambient ionization methods, a term first introduced by Graham Cooks' laboratory in 2004 with the introduction of DESI (8).
DESI ionizes molecules by spraying a physical surface with charged droplets of solvent containing various ionic species. On impact, molecules present on the surface are ionized and then introduced into a long (≈30 cm) transfer line connected to the atmospheric inlet of a mass spectrometer. In the initial report on DESI (8) it was demonstrated that the technique could observe chemical warfare agents, explosives, and over-the-counter drugs from different surfaces such as plastics, leather, and human skin. In addition, the report by Cooks and colleagues (8) demonstrated that DESI could measure different quantities of a natural product metabolite through a cross-section of a plant stem, thus enabling DESI imaging as well. DESI imaging is accomplished by moving a sample in an x–y direction under the DESI ionization source in a predefined fashion. Throughout the raster grid, different mass spectra are acquired and the ions of interest are displayed in color and superimposed onto a picture of the sample. The greater abundance the molecular ion at a given position of the raster, the higher the intensity of color displayed, allowing for visualization of the distribution of ions (10–13). The DESI methodology used in Lane et al.'s study (1) has been described in greater detail in a parallel technical report by Nyadong et al. (14).
Members of the Kubanek laboratory (15–17) reported a series of novel, bioactive diterpene natural products from the Fijian seaweed Callophycus serratus. In the new study (1), they explore the possible natural roles of these molecules, termed the bromophycolides, callophycoic acids, and callophycols. Using 18S rRNA phylogenetic and liquid chromatography (LC)MS analysis of 10 distinct collections of the seaweed they reveal 2 probable clades of C. serratus, with the “callo” compounds associated with one group and the bromophycolides associated with the other. Moreover, in a standard growth inhibition assay, both of these families of compounds strongly inhibit the marine pathogenic fungus Lindra thalassia but show little to no activity toward the marine pathogenic bacterium Pseudoalteromonas bacteriolytica.
Next, the antifungal activities of whole algal extracts to L. thalassia were measured at naturally-occurring whole-cell concentrations. Only 2 of the callophycoic acids, C and G, had IC50 values at or below the naturally-occurring concentrations of 100–200 μM, whereas all of the bromophycolides encountered had significant antifungal activity at these concentrations and were generally more potent. Thus, in principle both sets of compounds could act as effective antifungal deterrents against L. thalassia.
Having established the chemical identity, whole algal concentrations, and biological properties of the putative antifungal compounds, it was necessary to demonstrate their physical locations and tissue concentrations. To accomplish this, Lane et al. (1) used DESI MS, which, as noted above, is superbly suited to surface chemical analysis. The work focused on bromophycolides A and B because they were the most potent antifungals and most abundant metabolites. Using pure bromophycolide A and a synthetic surface, they determined the limit of detection (LOD) in this analysis and found it to be highly sensitive with an LOD of 0.9 pmol/mm2. Then, DESI was used to probe the surfaces of 6 algal samples and resulted in the finding that bromophycolides A and B are exclusively concentrated on distinct, but randomly scattered, light-colored patches of tissue that cover ≈5% of the algal surface. By rastering the DESI probe along the surface of whole samples, DESI images of the surface distribution of bromophycolides A and B were obtained and confirmed that these antifungal agents are found only in the light-colored patches. In 3 tested patches, the combined bromophycolide concentrations were more than sufficient to display an effective antifungal activity. Light microscopy of algal surfaces before and after DESI imaging revealed no physical damage to subtending cells from this analysis, and thus the bromophycolides analyzed were located in the outermost portions of the tissue.
MS imaging techniques are enabling a precise view of the location of natural products.
Next, the distribution of bromophycolides in cells of light-colored patches and nonpatch regions was determined. The patch and nonpatch locations were first mechanically damaged, and then examined by DESI. Bromophycolides A and B were observed by DESI in both regions of the thallus, which was confirmed by LC-MS, suggesting that their detection in undamaged light-colored patch regions is caused by a specific release mechanism. Lane et al. (1) propose 2 scenarios consistent with these findings: first, the surface presence of the bromophycolides exclusively in the patch regions may indicate a response to microbial infections in these areas. However, light and epifluorescence microscopy in combination with DAPI staining for microbial DNA failed to show conclusive evidence for the presence of microbes in the patches. A second speculation proposed that the patches are sites of damage and enhanced vulnerability to pathogen attack. In response, C. serratus secretes the antifungal bromophycolides into these damaged areas as a defense mechanism.
As illustrated by Lane et al. (1), a highly-creative interface is formed between the confluence of natural products chemistry, biology, and ecology and new advances in MS. These MS imaging techniques are enabling a precise view of the location of natural products in biological tissues, as if at long last we had “molecular eyes” with which to see these unique substances. The degree of innovation occurring with developing various direct soft and ambient ionizations is spectacular, including LTP, DESI, MALDI, ESI, secondary ionization MS, DART, surface-enhanced laser desorption/ionization, nanotechnology-assisted laser desorption/ionization, and others, and they are creating a new definition for the “chemistry–biology interface” concept. Overall, this collaborative effort provides a superb example of how a MS laboratory and a biology laboratory have crossed paths to use a new MS tool that provides insight into the antifungal roles of natural products in seaweeds. Indeed, Lane et al.'s article (1) is one of the very first applications of MS imaging to provide a functional and ecological understanding of natural products.
Acknowledgments.
E.E. is supported by National Institutes of Health Training Grant GM 067550. The MS in P.C.D.'s laboratory is supported by the Beckman Foundation, the V Foundation, and National Institutes of Health Grants GM086283 and GM085128. Work on this topic in W.H.G.'s laboratory was supported by National Institutes of Health Grants CA52955, CA100851, and NS053398.
Footnotes
The authors declare no conflict of interest.
See companion article on page 7314.
References
- 1.Lane A, et al. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc Natl Acad Sci USA. 2009;106:7314–7319. doi: 10.1073/pnas.0812020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simmons TL, et al. Biosynthetic origin of natural products isolated from marine microorganism-invertebrate assemblages. Proc Natl Acad Sci USA. 2008;105:4587–4594. doi: 10.1073/pnas.0709851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esquenazi E, et al. Visualizing the spatial distribution of secondary metabolites produced by marine cyanobacteria and sponges via MALDI-TOF imaging. Mol BioSyst. 2008;4:562–570. doi: 10.1039/b720018h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenn JB. Electrospray wings for molecular elephants (Nobel lecture) Angew Chem Int Ed. 2003;42:3871–3894. doi: 10.1002/anie.200300605. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka K. The origin of macromolecule ionization by laser irradiation (Nobel lecture) Angew Chem Int Ed. 2003;42:3861–3870. doi: 10.1002/anie.200300585. [DOI] [PubMed] [Google Scholar]
- 6.Peng IX, Ogorzalek Loo RR, Shiea J, Loo JA. Reactive-electrospray-assisted laser desorption/ioniza-tion for characterization of peptides and proteins. Anal Chem. 2008;80:6995–7003. doi: 10.1021/ac800870c. [DOI] [PubMed] [Google Scholar]
- 7.Cody RB, Larame JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 8.Taka′ts Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 9.Ifa DR, Jackson AU, Paglia, Cooks RG. Forensic applications of ambient ionization mass spectrometry. Anal Bioanal Chem. 2009 doi: 10.1007/s00216-009-2659-2. [DOI] [PubMed] [Google Scholar]
- 10.Wiseman JM, Ifa DR, Venter A, Cooks RG. Ambient molecular imaging by desorption electrospray ionization mass spectrometry. Nat Protocols. 2008;3:517–524. doi: 10.1038/nprot.2008.11. [DOI] [PubMed] [Google Scholar]
- 11.Kertesz V, Van Berkel GJ. Improved imaging resolution in desorption electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:2639–2644. doi: 10.1002/rcm.3662. [DOI] [PubMed] [Google Scholar]
- 12.Sampson JS, Hawkridge AM, Muddiman DC. Construction of a versatile high-precision ambient ionization source for direct analysis and imaging. J Am Soc Mass Spectrom. 2008;19:1527–1534. doi: 10.1016/j.jasms.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiseman JM, et al. Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. Proc Natl Acad Sci USA. 2008;105:18120–18125. doi: 10.1073/pnas.0801066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyadong L, et al. Reactive desorption electrospray ionization mass spectrometry (DESI MS) of natural products of a marine alga. Anal Bioanal Chem. 2009 doi: 10.1007/s00216-009-2674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane AL, et al. Callophycoic acids and callophycols from the Fijian red alga Callophycus serratus. J Org Chem. 2007;72:7343–7351. doi: 10.1021/jo071210y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubanek J, et al. Bromophycolides C-I from the Fijian red alga Callophycus serratus. J Nat Prod. 2006;69:731–735. doi: 10.1021/np050463o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubanek J, et al. Antineoplastic diterpene-benzoate macrolides from the Fijian red alga Callophycus serratus. Org Lett. 2005;7:5261–5264. doi: 10.1021/ol052121f. [DOI] [PMC free article] [PubMed] [Google Scholar]