Abstract
IL-15 has potential as an immunotherapeutic agent for cancer treatment because it is a critical factor for the proliferation and activation of natural killer (NK) and CD8+ T cells. Administration of anti-CD40 antibodies has shown anti-tumor effects in vivo through a variety of mechanisms. Furthermore, activation of CD40 led to increased expression of IL-15 receptor-α by dendritic cells, an action that is critical for trans-presentation of IL-15 to NK and CD8+ T cells. In this study, we investigated the therapeutic efficacy of the combination regimen of murine IL-15 (mIL-15) with an agonistic anti-CD40 antibody (FGK4.5) in murine lung metastasis models involving CT26 and MC38, which are murine colon cancer cell lines syngeneic to BALB/c and C57BL/6 mice, respectively. Treatment with mIL-15 or the anti-CD40 antibody alone significantly prolonged survival of both CT26 and MC38 tumor-bearing mice compared with the mice in the PBS solution control group (P < 0.01). Furthermore, combination therapy with both mIL-15 and the anti-CD40 antibody provided greater therapeutic efficacy as demonstrated by prolonged survival of the mice compared with either mIL-15 or the anti-CD40 antibody-alone groups (P < 0.001). We found that NK cells isolated from the mice that received the combination regimen expressed increased levels of intracellular granzyme B and showed stronger cytotoxic activity on the target cells. The findings from this study provide the scientific basis for clinical trials using the combination regimen of IL-15 with an anti-CD40 antibody for the treatment of patients with cancer.
Keywords: animal model, cancer immunotherapy, IL-15 receptor-α
IL-2 has been approved by the US Food and Drug Administration for use in the treatment of patients with metastatic renal cell carcinoma and malignant melanoma (1, 2). However IL-2 is not optimal for inhibiting tumor growth, because in the presence of IL-2, the cytotoxic lymphocytes generated might recognize the tumor as self and undergo activation-induced cell death (AICD), or alternatively, the immune response may be inhibited by IL-2-dependent regulatory T (T reg) cells. By contrast, IL-15, with its ability to activate T and natural killer (NK) cells, its inhibition of AICD, and its role in the persistence of CD8-memory T cells might be a better choice than IL-2 for the treatment of cancer. Attempts to prevent tumor growth in various mouse models by IL-15 have proved effective (3–5). IL-15 and its specific high-affinity receptor, IL-15Rα, support NK cell homeostasis in resting animals via a novel trans-presentation mechanism (6) and mediate NK cell survival and differentiation into fully functional NK cells capable of killing virally infected cells and tumor cells (7). Mice with genetic deletions of IL-15 or its private receptor, IL-15Rα, are characterized by decreased numbers of NK, NKT, CD8+/CD44high T, TCRγ+/δ+ T, and intestinal intraepithelial CD8α+/β− T cells, suggesting that physiologically relevant IL-15 signals require IL-15Rα and that these molecules function in close cooperation (8, 9). Furthermore, pre-association of IL-15 with soluble IL-15Rα-IgG1-Fc enhanced its activity in inducing proliferation of NK and CD8+/CD44high T cells as well as its antitumor action (10, 11). Soluble IL-15Rα generated by alternative splicing yielding an IL-15 binding “sushi” domain had agonistic activity whereas the full-length IL-15Rα ecto-domain generated by proteolytic cleavage is antagonistic to IL-15 action (12).
Although IL-15 administration may ultimately show efficacy in the treatment of metastatic malignancy in clinical trials, it is not optimal as a single agent. As noted earlier, for its long-term persistence and its optimal transaction on NK and CD8+ T-cells, IL-15 must be bound to IL-15Rα on the surface of dendritic cells (DCs) or monocytes, where it is trans-presented to the effector NK and CD8+ T-cells (6, 8, 9, 13, 14). However, there is only a low level of expression of IL-15Rα on unactivated DCs, and therefore IL-15Rα would be very limiting in therapeutic trials involving IL-15 alone. In the present study we examined the use of agents that induce IL-15Rα that could be given in conjunction with IL-15 to circumvent the problems with IL-15 as a single agent.
CD40 is a member of the tumor necrosis factor receptor superfamily that plays a critical role in both humoral and cellular immune responses (15). CD40 has a broad pattern of expression, including B cells, epithelial and endothelial cells, as well as a variety of antigen- presenting cells (APCs) (15). CD40 ligation triggers a range of cellular functions including the activation of APCs (16, 17). Agonistic anti-CD40 antibodies have been shown to promote T cell-mediated immunity in treatment of neoplastic diseases in animals (18, 19). In other tumor models, the effector cells were shown to be CD8+ T-cells and macrophages. It was reported that a stimulatory anti-CD40 antibody indirectly activated NK cells by IL-12 secreted by CD40+ DCs and monocytes, which produced significant antitumor effects (20). In another potential contribution to elimination of tumors, we demonstrated that activation of CD40 was associated with an increased expression of IL-15Rα by DCs (21). In this context, IL-15Rα retains IL-15 via a high-affinity interaction on the cell surface and trans-presents it to neighboring NK and CD8+/CD44high T cells that express IL-2/IL-15Rβ (CD122) and γc but not IL-15Rα, thus inducing the proliferation of responsive cells. The induction of IL-15Rα expression by CD40 activation (21) provided the scientific basis for this study, in which we investigated the therapeutic efficacy mediated by the combination regimen of mIL-15 with an agonistic anti-CD40 antibody in 2 murine lung metastasis models of colon cancer. The findings from this study suggest that the combination regimen is very promising for the treatment of patients with cancer.
Results
An Agonistic Anti-CD40 Antibody Induced the Expression of IL-15Rα.
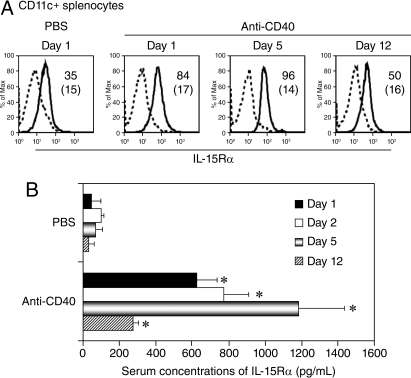
IL-15 and its private receptor, IL-15Rα, are both essential for the support of NK and CD8+ T cell homeostasis (13, 22, 23). Expression of IL-15Rα specifically on DCs is critical for the trans-presentation of IL-15 and activation of NK cells (6). Previously, we and others reported that IFN-γ, toll-like receptor (TLR) stimulation, or CD40 ligation with CD40 ligand induced IL-15Rα expression by DCs and monocytes (21, 22, 24). We examined the cell surface levels of IL-15Rα by flow cytometric analysis and the serum concentrations of soluble IL-15Rα by ELISA. Treatment with the anti-CD40 antibody increased the expression of IL-15Rα on the CD11c+ population of splenocytes compared with the PBS solution group (Fig. 1A). In addition, the serum concentrations of IL-15Rα were significantly higher in the anti-CD40 antibody treated mice than in the PBS solution-treated mice (Fig. 1B; P < 0.0001).
Fig. 1.
Expression of Il-15Rα was increased by the anti-CD40 antibody. (A) Histograms of flow cytometric analysis of IL-15Rα expression on DCs. C57BL/6 mice were treated with PBS solution or with the anti-CD40 antibody at a dose of 200 μg on day 0, followed by 100 μg on days 3, 7, and 10. At different time points, the splenocytes were separated from the mice for flow cytometric analysis. Treatment with the anti-CD40 antibody increased the expression of IL-15Rα on the CD11c+ population of splenocytes compared with that observed in the mice treated with PBS solution. The dashed lines represent isotype controls. Numbers refer to mean fluorescence intensity and the numbers in parentheses represent isotype controls. The data are representative of 3 separate experiments. (B) ELISA detection of IL-15Rα in serum. C57BL/6 mice were treated as described earlier. The blood samples were taken at different time points. The serum concentrations of IL-15Rα were significantly higher in the anti-CD40 antibody-treated mice than those in the PBS solution-treated mice (*P < 0.0001). The data represent the mean ± SD (n = 4–6).
Therapeutic Studies in the CT26 Model.
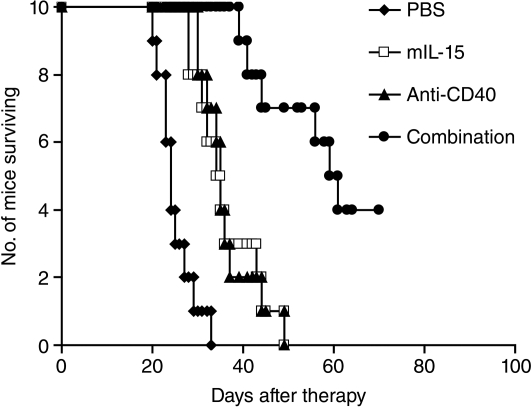
Therapy was started on day 0, 2 days following the i.v. injection of the CT26 cells. The experimental protocol used in the therapeutic study performed in the CT26 model is shown in Fig. S1. Treatment with either mIL-15 at a dose of 2.5 μg, 5 days a week for 3 weeks, or with the anti-CD40 antibody at a dose of 200 μg on day 0, then 100 μg on days 3, 7, and 10, significantly prolonged the survival of the CT26 tumor-bearing mice compared with the mice in the PBS solution control group (Fig. 2; P < 0.001). Furthermore, combination therapy with both mIL-15 and the anti-CD40 antibody provided greater therapeutic efficacy as seen by prolonged survival of the mice compared with monotherapy with either mIL-15 or the anti-CD40 antibody (Fig. 2; P < 0.001). All the mice in the PBS control, mIL-15 alone, and anti-CD40 antibody-alone groups died from tumor progression by day 50. In contrast, 7 of 10 mice in the combination group were alive at that time (Fig. 2).
Fig. 2.
The combination of mIL-15 with the anti-CD40 antibody provided enhanced therapeutic efficacy in the CT26 model. Therapy started at 48 h after 2 × 105 CT26 cells were injected i.v. into BALB/c mice, and we referred to the therapy-starting day as day 0. Treatment with mIL-15 alone at a dose of 2.5 μg every day 5 days a week for 3 weeks or with the anti-CD40 antibody (200 μg on day 0, then 100 μg on days 3, 7, and 10) significantly prolonged survival of the CT26 tumor-bearing mice compared with the mice in the PBS solution control group (P < 0.001). Furthermore, combination therapy with mIL-15 and the anti-CD40 antibody provided a greater therapeutic efficacy, as seen by prolonged survival of the mice compared with the mIL-15 or anti-CD40 antibody-alone groups (P < 0.001).
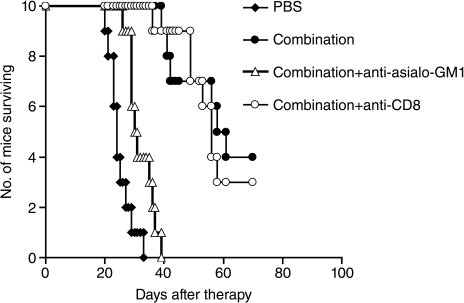
To investigate possible involvement of NK or CD8+ T-cells as effectors in the combination regimen-mediated antitumor activity in the CT26 model, we treated CT26 tumor-bearing mice with rabbit anti-asialo-GM1 or with purified rat anti-mouse CD8 antibody to eliminate NK or CD8+ cells, respectively, together with the combination regimen. Compared with the group receiving the combination therapy alone, depletion of NK cells with rabbit anti-asialo-GM1 nearly abrogated the antitumor efficacy (Fig. 3; P < 0.01), whereas depletion of CD8+ T cells did not show any effect on the antitumor efficacy mediated by the combination therapy (Fig. 3).
Fig. 3.
NK cells are involved in the anti-tumor action mediated by the combination regimen in the CT26 model. To investigate possible involvement of NK or CD8+ T cells as effectors in the combination regimen-mediated antitumor activity, CT26 tumor-bearing mice were treated with rabbit anti-asialo-GM1 or rat anti-mouse CD8 monoclonal antibody, together with the combination regimen of mIL-15 and the anti-CD40 antibody as described in Materials and Methods. Compared with the group receiving the combination therapy alone, depletion of NK cells with rabbit anti-asialo-GM1 nearly abrogated the antitumor efficacy (P < 0.01), whereas depletion of CD8+ T cells did not show any effect on the antitumor efficacy mediated by the combination therapy.
Therapeutic Study in the MC38 Model.
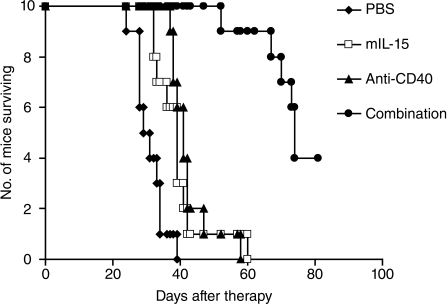
The therapeutic study was also performed in the MC38 model with mIL-15 alone, anti-CD40 antibody alone, or the combination regimen at the same doses and dosing schedule used in the CT26 model, with the exception that mIL-15 was used for 2 weeks instead of 3 weeks. Treatment with mIL-15 or the anti-CD40 antibody alone significantly prolonged survival of the MC38 tumor-bearing mice compared with the mice in the PBS solution control group (Fig. 4; P < 0.01). Combination therapy with both mIL-15 and the anti-CD40 antibody provided greater therapeutic efficacy as seen by prolonged survival of the mice compared with monotherapy with mIL-15 or the anti-CD40 antibody (Fig. 4; P < 0.001). All the mice in the PBS solution control, mIL-15 alone, and anti-CD40 antibody-alone groups died from tumor progression by day 60. In contrast, 9 of 10 mice in the combination group were alive at that time (Fig. 4).
Fig. 4.
The combination of mIL-15 with the anti-CD40 antibody provided improved therapeutic efficacy in the MC38 model. Therapy started at 48 h after 2 × 106 MC38 cells were injected i.v. into female C57BL/6 mice, and we referred to the therapy-starting day as day 0. Treatment with mIL-15 at a dose of 2.5 μg every day 5 days a week for 2 weeks or with the anti-CD40 antibody (200 μg on day 0, then 100 μg on days 3, 7, and 10) significantly prolonged the survival of the MC38 tumor-bearing mice compared with the mice in the PBS solution control group (P < 0.01). Combination therapy with mIL-15 and the anti-CD40 antibody provided greater therapeutic efficacy, as seen by prolonged survival of the mice compared with the mIL-15 or anti-CD40 antibody-alone groups (P < 0.001).
Combination of mIL-15 with the Anti-CD40 Antibody Enhanced the Cytotoxic Activity of NK Cells.
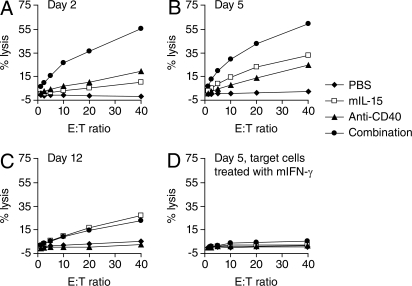
The in vivo therapeutic studies showed that treatment with either mIL-15 or the anti-CD40 antibody inhibited both CT26 and MC38 tumor growth in mice and the combination regimen showed greater therapeutic efficacy compared with monotherapy with either mIL-15 or the anti-CD40 antibody (Figs. 2 and 4). The in vivo cell depletion experiment showed that NK cells are involved in the antitumor action mediated by the combination therapy in the CT26 model (Fig. 3). Furthermore, we had previously shown that the effect of IL-15 in inhibiting the MC38 tumor growth depended on NK cells (4). To determine whether the increased antitumor efficacy mediated by the combination regimen in vivo was associated with increased cytotoxic activity of NK cells, the lysis activity of NK and CD8+ T-cells toward the CT26 cells was examined ex vivo. 111In-labeled CT26 cells were co-incubated at various effector to target ratios with NK cells overnight or with CD8+ T-cells for 24 h. Both NK and CD8+ T-cells were freshly isolated from the CT26 tumor-bearing mice that had received the same treatments as those in the therapeutic study. When compared with the NK cells isolated from the mice in the PBS solution control group, NK cells from the mIL-15-treated mice efficiently lysed the CT26 cells at all time points tested (Fig. 5 A–C) and NK cells from the anti-CD40 antibody-treated mice showed increased lysis activity at days 2 and 5 (Fig. 5 A and B), but did not show any cytolytic activity at day 12 (Fig. 5C), although the treatment with the anti-CD40 antibody continued until day 10. Furthermore, the combination therapy with mIL-15 and the anti-CD40 antibody induced the greatest NK cell-mediated lysis activity toward the CT26 cells at days 2 and 5 compared with mIL-15 or anti-CD40 antibody treatment alone (Fig. 5 A and B). At day 12, the lysis activity of NK cells isolated from the mice in both mIL-15 alone and the combination groups were comparable (Fig. 5C). Pretreatment of the CT26 cells with murine IFN-γ for 24 h increased the cell surface expression of MHC-I (data not shown), resulting in a loss of NK cell lysis activity (Fig. 5D). CD8+ T-cells isolated from all of the treated mice at day 12 were unable to lyse the CT26 cells regardless of the pretreatment with IFN-γ (data not shown).
Fig. 5.
The combination of mIL-15 with the anti-CD40 antibody enhanced the cytotoxic activity of NK cells. Lysis activity of NK cells mediated by mIL-15 or the anti-CD40 antibody or their combination was examined ex vivo. BALB/c mice were treated as indicated in Materials and Methods, and the experimental protocol is shown in Fig. S2. The splenocytes were separated at day 2 (A), day 5 (B), and day 12 (C). 111In-labeled CT26 cells were co-incubated with freshly isolated NK cells at various effector-to-target ratios overnight. Lysis activity was assessed by the amount of radioactivity in the supernatant. The data represent the mean of triplicates. NK cells isolated from the mice that received mIL-15 showed cytotoxic activity toward CT26 cells compared with those isolated from the mice that received PBS solution at all time points tested. Treatment with the anti-CD40 antibody increased lysis activity of NK cells at days 2 and 5, but not at day 12, compared with that in the PBS solution group. Furthermore, the combination therapy with both mIL-15 and the anti-CD40 antibody induced the greatest NK cell-mediated lysis activity toward CT26 cells compared with mIL-15 or anti-CD40 antibody treatment alone at days 2 and 5. (D) Pretreatment of CT26 cells with murine IFN-γ for 24 h resulted in a loss of lysis activity mediated by NK cells, which were isolated at day 5.
Induction of NK Cell Activation.
NK cells isolated from the mice treated with the combination regimen showed stronger cytotoxic activity to the target cells ex vivo compared with those isolated from mIL-15-treated or anti-CD40 antibody-treated mice (Fig. 5 A and B). We further examined the NK cell surface activation marker, CD69, and intracellular granzyme B expression by flow cytometric analysis ex vivo. Treatment with mIL-15 (2.5 μg) or the anti-CD40 antibody (200 μg) or their combination up-regulated the expression of CD69 (Fig. S3 Upper) on NK1.1+ splenocytes compared with the PBS solution control group. Furthermore, treatment with the combination regimen induced the highest level of intracellular granzyme B in the NK1.1+ splenocytes compared with those from all other groups (Fig. S3 Lower), consistent with the observation that these cells showed the strongest cytotoxic activity to the target cells (Fig. 5 A and B). In addition, we also checked the total numbers of NK cells in spleens at day 5 after therapy. Treatment with mIL-15 at a dose of 2.5 μg every day for 5 days markedly increased the total NK cell numbers (approximately 6 times that of the PBS solution group; data not shown), and treatment with the anti-CD40 antibody at a dose of 200 μg on day 0 followed by 100 μg on day 3 modestly increased the total NK cell numbers (approximately 2 times that of the PBS solution group; data not shown). The total number of NK cells in the spleens from the combination regimen-treated mice was comparable to that of mIL-15-treated mice (data not shown).
Discussion
The immune system is dedicated to the generation of rapid innate and adaptive immune responses to invading pathogens, the elimination of auto-reactive T cells to generate tolerance to self, and the maintenance of specific memory responses to pathogens. Such immune responses are normally regulated by cytokines, including IL-2 and IL-15. The receptors for IL-2 and IL-15 are heterotrimeric and both contain the common γ (γc) subunit (25) as well as IL-2/IL-15Rβ (CD122) (26–29). In addition, the high-affinity forms of IL-2R and IL-15R contain a unique cytokine-specific α subunit, IL-2Rα or IL-15Rα, respectively (30). In light of the common receptor components, the γc and IL-2/IL-15Rβ, the 2 cytokines share several functions, including the induction of the proliferation of activated CD4−CD8−, CD4+CD8+, CD4+, and CD8+ T cells (14, 27, 28, 31, 32). IL-2 and IL-15 also stimulate the generation, proliferation, and activation of NK cells (27, 28, 32). In addition to these similarities, there are distinct differences between the actions of IL-2 and IL-15 (14, 31, 33–35). IL-2 is involved in checkpoints on the immune system in that it is required to maintain the competitive fitness of forkhead box P3 expressing T reg cells (36–38). IL-2 also has a critical role in AICD, a process that leads to the elimination of self-reactive T cells (34). In contrast, IL-15 has no marked effect on T reg cells and is an anti-apoptotic factor that inhibits IL-2-induced AICD in select systems (34). In accordance with these ex vivo functional studies with IL-15, mice that are deficient in IL-15 or IL-15Rα have a marked reduction in the number of NK cells, NKT cells, and intestinal intraepithelial lymphocytes (8, 9). Furthermore, IL-15Rα-deficient mice show a marked reduction in CD8+CD44hi memory phenotype T cells (9). Koka and coworkers demonstrated that murine IL-15Rα deficient DCs fail to support NK cell cytolytic activity and elaboration of IFN-γ, despite the fact that these DCs express normal levels of costimulatory molecules and IL-12 (39). A critical factor in the functional differences between IL-2 and IL-15 involves the distinct mode of action of these 2 cytokines. IL-2 is a secreted molecule that binds to preformed high-affinity heterotrimeric receptors at the surfaces of activated T cells (40, 41). In contrast to IL-2, IL-15 is secreted only in small quantities and is predominantly membrane-bound and induces signaling in the context of an immunological synapse (6). Monocytes and DCs stimulated with type I or type II IFNs, together with activation of NF-κB through TLR stimulation or ligation of CD40 with CD40 ligand, boosts the coordinate expression of IL-15 and IL-15Rα (6, 21, 22, 24). IL-15Rα and IL-15 expressed by these monocytes and DCs then become associated on the cell surface and recycle through endosomic vesicles (6). In animals in which high expression of IL-15Rα is induced on APCs, there is a very long temporal persistence of externally administered IL-15 following a bolus administration into mice, which may persist for more than 3 or 4 days on lymph nodes and more than 10 days in the lungs (42). In contrast, IL-15Rα-deficient mice show only a very short survival of exogenously administered IL-15 (42). Furthermore, IL-15Rα on activated DCs presents IL-15 in trans to cells that express IL-2/IL-15Rβ and γc but not IL-15Rα (6).
IL-2 has been approved by the Food and Drug Administration for use in the treatment of patients with metastatic renal cell carcinoma and malignant melanoma (1, 2). However, as noted earlier, IL-15 might be superior to IL-2 for the treatment of cancer because of the distinct functions of IL-2 and IL-15 (14, 27). Efforts to inhibit tumor growth in mice by the administration of IL-15 have been relatively effective (5, 43). The importance of IL-15 in the elimination of syngenic MC38 colon carcinoma cells has been evaluated using IL-15 transgenic mice (4). Whereas WT mice died with pulmonary metastases within 6 weeks after an i.v. infusion of MC38 cells, IL-15 transgenic mice did not develop such metastases and survived more than 8 months (4). In addition, IL-15 alone or in conjunction with IL-21 was effective in the treatment of mice bearing B16 melanoma (44). The importance of IL-15Rα-mediated IL-15 trans-presentation was evaluated by determining the survival of the mice that were injected with either WT or IL-15Rα-transfected MC38 cells (4). The administration of unmodified MC38 cells to mice led to death within 6 weeks. By contrast, the majority of the mice injected with IL-15Rα-transfected MC38 cells that bind endogenous IL-15 did not show tumor development in the period of observation (4). On the basis of the studies of IL-15 in tumor models, our research group, in conjunction with the Biopharmaceutical Development Program of the National Cancer Institute (NCI), has produced recombinant IL-15 under current Good Manufacturing Practice conditions for use in lieu of IL-2 in human clinical trials that involve patients with metastatic malignancy.
Although IL-15 administration may show efficacy in the treatment of metastatic malignancy in human clinical trials, it is not optimal as a single agent. As noted earlier, IL-15Rα expressed on monocytes and DCs is critical for trans-presentation of IL-15 to the effector NK and CD8+ T-cells (6, 8, 9, 14). However, there is only a low level of expression of IL-15Rα on non-activated DCs and, therefore, IL-15Rα would be very limiting in therapeutic trials involving IL-15 alone. In the present study, we are examining the use of agents that induce IL-15Rα that could be given in conjunction with IL-15. The stimulation of monocytes or DCs with IFNs or through the activation of TLR4 with lipopolysaccharide or through the ligation of CD40 with an agonistic agent induces the expression of IL-15Rα (21, 22, 24). Although the IFNs induce the expression of IL-15Rα, they also induce the expression of MHC-I on the tumor cells, an action that abrogates the cytotoxic effector function of NK cells in the CT26 (Fig. 5D) and MC38 tumor models (4). As we wish to use an IL-15Rα-inducing agent that is now being evaluated in human clinical trials involving patients with cancer, we have focused on an agonistic antibody to CD40 in the present study.
CD40 is a member of the tumor necrosis factor receptor superfamily that plays a critical role in both humoral and cellular immune responses (15). Activation with agonistic agents directed toward CD40 has a wide range of anti-tumor actions (18, 19, 45, 46). Anti-CD40 antibody administration evoked cytotoxic T cell responses that eradicated a lymphoma and bypassed T cell help (47). In an orthotopic metastatic renal cell carcinoma model (45), an agonistic antibody to CD40 and IL-2 gave synergistic anti-tumor responses that depended on CD8+ T cells as well as IFN-γ and IL-12 p40. In other tumor models, synergistic activation of macrophages via CD40 and TLR9 resulted in T cell-independent anti-tumor effects (48). In yet another system (20), the administration of anti-CD40 antibody resulted in production of IL-12 and IFN-γ followed by an increase in NK cell cytolytic activity that was associated with substantial anti-tumor and anti-metastatic effects in multiple tumor models. In addition, in some cases, the tumor cells themselves express CD40 and are a target of the anti-CD40 antibody. However, the CT26 and MC38 tumor cells in the present study did not express CD40 (data not shown). We did demonstrate that the administration of the anti-CD40 antibody was associated with augmented expression of IL-15Rα on DCs and an increased concentration of released IL-15Rα in the serum. On the basis of our previous studies, the induced expression of IL-15Rα is associated with a marked prolongation of the action of externally administered IL-15 (42). In addition, it is associated with more effective trans-presentation of IL-15 to effector cells, including NK and CD8+CD44hi T cells (6). Furthermore, we found that NK cells isolated from the mice receiving the combination of mIL-15 and the anti-CD40 antibody expressed increased levels of intracellular granzyme B (Fig. S3) and manifested stronger cytotoxic activity toward target cells (Fig. 5).
Thus, in summary, given the multiple mechanisms of action of agonistic anti-CD40 antibodies, we cannot conclude that their primary mode of action in the present study was the observed augmentation of IL-15Rα expression on DCs. Nevertheless, in addition to its other anti-tumor actions, the administration of an agonistic antibody to CD40 was associated with an increased expression of IL-15Rα on DCs, which might facilitate a long-term survival of administered IL-15 and augment the action of IL-15 on target effector NK cells. The combination therapy was also associated with increased NK cell cytotoxic activity. These findings in the present study provide the scientific basis for human clinical trials to determine if there is similar synergistic anti-tumor activity observed by the administration of agonistic antibodies to CD40 in combination with IL-15 for the treatment of patients with metastatic malignant melanoma or renal cell cancer.
Materials and Methods
Reagents.
mIL-15 was purchased from PeproTech. Rat anti-mouse CD40 monoclonal antibody (FGK4.5) was obtained from BioExpress Cell Culture Services. Rat anti-asialo-GM1 was purchased from Wako Chemicals and rat anti-mouse CD8 (clone 2.43) was obtained from the Frederick Cancer Research and Development Center.
Flow Cytometry Analysis.
IL-15Rα expression on DCs (CD11c+ population of splenocytes) was detected by flow cytometry using biotinylated anti-mIL-15Rα antibody or biotinylated normal goat IgG as an isotype control (R&D Systems), followed by PE-labeled streptavidin (eBioscience). Granzyme B expression in NK cells (NK1.1+ population of splenocytes) was determined by intracellular cytokine staining using a BD fixation/permeabilization kit (BD Biosciences). Cell surface expression of NK1.1, CD69, and CD11c was performed using commercial antibodies from BD Biosciences and eBioscience.
Measurement of the Serum Concentration of IL-15Rα.
C57BL/6 mice were injected i.p. with PBS solution or with the anti-CD40 antibody at a dose of 200 μg on day 0, followed by 100 μg on days 3, 7, and 10. The blood samples were taken on days 1, 2, 5, and 12. Measurement of the serum concentrations of IL-15Rα was performed by using the Due-set ELISA kit (R&D Systems), and the ELISA was performed as indicated in the manufacturer's instructions.
Tumor Cell Lines and Mouse Models.
CT26 is a N-nitro-N-methylurethane-induced BALB/c murine colon carcinoma cell line and MC38 is a metastatic murine colon cancer cell line syngeneic to C57BL/6 mice. Female BALB/c and C57BL/6 mice were purchased from NCI-Frederick. The CT26 model was established by i.v. injection of 2 × 105 CT26 cells into BALB/c mice and the MC38 model was established by i.v. injection of 2 × 106 MC38 cells into C57BL/6 mice. All mice were maintained in a pathogen-free animal facility and were used at 7 to 10 weeks of age. All animal experiments were approved by the NCI Animal Care and Use Committee and were performed in accordance with its guidelines. NCI-Frederick is accredited by American Association for the Accreditation of Laboratory Animal Care International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the Guide for Care and Use of Laboratory Animals (National Research Council, 1996).
Therapeutic Studies in Animal Models.
The therapeutic studies were performed in both CT26 and MC38 models. The treatment schema of the therapeutic study in the CT26 model is shown in Fig. S1. Female BALB/c mice were injected with the CT26 cells i.v. Therapy started 2 days later, and we referred the treatment-starting day as day 0. Groups of 10 mice each received mIL-15 i.p., 2.5 μg per mouse daily, 5 days a week for 3 weeks; the anti-CD40 antibody, 200 μg on day 0, then 100 μg on days 3, 7, and 10; or a combination of mIL-15 with the anti-CD40 antibody at the same doses and dosing schedule as those in mIL-15 and the anti-CD40 antibody groups. The initial 200-μg loading dose of anti-CD40 antibody was twice that used in subsequent doses because there is persistent antibody remaining from the initial dose at later dosing time points because of the several-day survival half-life of the antibody in vivo. An additional group of mice that received PBS solution injections served as a control. In the MC38 model, therapy started after the C57BL/6 mice had received the injection of the MC38 cells for 2 days. The treatment schema was the same as that used in the CT26 model (Fig. S1), with the exception that mIL-15 was used for 2 weeks instead of 3 weeks.
In Vivo Cell Depletion Experiment.
Female BALB/c mice were injected with CT26 cells i.v. Two days later, the mice received the combination therapy of mIL-15 with the anti-CD40 antibody, at the same doses and dosing schedules described earlier. An additional group of mice that received PBS solution injections served as a control. NK and CD8+ T cells were depleted in vivo by i.p. injections of anti-asialo-GM1 (50 μL) and purified rat anti-mouse CD8 antibody (200 μg), respectively, together with the combination regimen. One dose of the anti-asialo-GM1 or the anti-mouse CD8 antibody was administered 1 day before the combination therapy started, and subsequent doses were administered 3 times weekly for 3 weeks.
In Vitro Lysis Assay.
The protocol for this experiment is shown in Fig. S2. Briefly, female BALB/c mice were injected with the CT26 cells i.v. Two days later, groups of the tumor-bearing mice received mIL-15 or the anti-CD40 antibody alone or together at the same doses and dosing schedules used in the therapeutic studies described earlier. An additional group of mice was given PBS solution as a control. The mice were killed and the spleens were taken at days 2, 5, or 12 after therapy. NK and CD8+ T cells were isolated from the spleens using negative isolation micro-beads (Miltenyi Biotec). The CT26 cells were used as target cells. The target cells (2 × 106) were labeled with 0.1 mCi of 111In-Oxine (GE Healthcare) for 30 min at 37 °C in FBS. The washed labeled target cells were incubated with isolated NK cells overnight or with CD8+ T cells for 24 h at various effector-to-target ratios. Radioactivity in the liquid phase was measured in a γ counter. Specific lysis was determined by using the following formula: % lysis = 100× [(experiment cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm)]. The maximum release value was determined from target cells treated with 1% (vol/vol) Triton X-100 (Sigma).
Statistical Analysis.
Comparison of mean values of serum concentration of IL-15Rα between PBS solution and anti-CD40 antibody groups was analyzed by using an unpaired Student t test. The statistical significance of differences in survival of the mice in different groups was determined by the log-rank test using GraphPad Prism software (GraphPad).
Supplementary Material
Acknowledgments.
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), NCI, Center for Cancer Research. This project has been funded in whole or in part with federal funds from the NCI, NIH, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902637106/DCSupplemental.
References
- 1.Heemskerk B, et al. Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Hum Gene Ther. 2008;19:496–510. doi: 10.1089/hum.2007.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klapper JA, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diab A, Cohen AD, Alpdogan O, Perales MA. IL-15: targeting CD8+ T cells for immunotherapy. Cytotherapy. 2005;7:23–35. doi: 10.1080/14653240510018037. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi H, et al. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105:721–727. doi: 10.1182/blood-2003-12-4187. [DOI] [PubMed] [Google Scholar]
- 5.Evans R, Fuller JA, Christianson G, Krupke DM, Troutt AB. IL-15 mediates anti-tumor effects after cyclophosphamide injection of tumor-bearing mice and enhances adoptive immunotherapy: the potential role of NK cell subpopulations. Cell Immunol. 1997;179:66–73. doi: 10.1006/cimm.1997.1132. [DOI] [PubMed] [Google Scholar]
- 6.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 7.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 10.Dubois S, Patel HJ, Zhang M, Waldmann TA, Müller JR. Preassociation of IL-15 with IL-15Rα-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein MP, et al. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc Natl Acad Sci USA. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulanova E, et al. Soluble interleukin (IL)-15Rα is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J Biol Chem. 2007;282:13167–13179. doi: 10.1074/jbc.M610036200. [DOI] [PubMed] [Google Scholar]
- 13.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 14.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 15.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 17.van Kooten C, Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr Opin Immunol. 1997;9:330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 18.Honeychurch J, Glennie MJ, Johnson PW, Illidge TM. Anti-CD40 monoclonal antibody therapy in combination with irradiation results in a CD8 T-cell-dependent immunity to B-cell lymphoma. Blood. 2003;102:1449–1457. doi: 10.1182/blood-2002-12-3717. [DOI] [PubMed] [Google Scholar]
- 19.French RR, et al. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109:4810–4815. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 20.Turner JG, et al. Anti-CD40 antibody induces antitumor and antimetastatic effects: the role of NK cells. J Immunol. 2001;166:89–94. doi: 10.4049/jimmunol.166.1.89. [DOI] [PubMed] [Google Scholar]
- 21.Dubois SP, Waldmann TA, Muller JR. Survival adjustment of mature dendritic cells by IL-15. Proc Natl Acad Sci USA. 2005;102:8662–8667. doi: 10.1073/pnas.0503360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Rα signals are required for bystander proliferation. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferlazzo G, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noguchi M, et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 26.Bamford RN, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- 28.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 29.Grabstein KH, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 30.Giri JG, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 32.Carson WE, et al. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J Clin Invest. 1997;99:937–943. doi: 10.1172/JCI119258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 34.Marks-Konczalik J, et al. IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 36.D'Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 37.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 38.Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- 39.Koka R, et al. Cutting edge: murine dendritic cells require IL-15Rα to prime NK cells. J Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- 40.Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73:5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- 41.Waldmann TA. The interleukin-2 receptor. J Biol Chem. 1991;266:2681–2684. [PubMed] [Google Scholar]
- 42.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Rα on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci USA. 2007;104:588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munger W, et al. Studies evaluating the antitumor activity and toxicity of interleukin-15, a new T cell growth factor: comparison with interleukin-2. Cell Immunol. 1995;165:289–293. doi: 10.1006/cimm.1995.1216. [DOI] [PubMed] [Google Scholar]
- 44.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy WJ, et al. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J Immunol. 2003;170:2727–2733. doi: 10.4049/jimmunol.170.5.2727. [DOI] [PubMed] [Google Scholar]
- 46.Uno T, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med. 2006;12:693–698. doi: 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 47.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 48.Buhtoiarov IN, et al. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol. 2006;176:309–318. doi: 10.4049/jimmunol.176.1.309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.