Abstract
The sensorimotor striatum is important for procedural learning, including skill learning. Our previous findings indicate that this part of the striatum mediates the acquisition of a motor skill in a running-wheel task and that this skill learning is dependent on striatal D1 dopamine receptors. Here, we investigated whether the sensorimotor striatum is also involved in the consolidation of this skill memory and whether this consolidation is modified by the indirect dopamine receptor agonist cocaine. Rats were trained on a running wheel for two days (40 min/day) to learn a new motor skill, that is, the ability to control the movement of the wheel. Before each training session, the animals received an injection of vehicle or cocaine (25 mg/kg; i.p.). Immediately following the training session, an intrastriatal infusion of 2% lidocaine (1 μl) or a sham infusion were administered. Wheel-skill performance was tested before and repeatedly after the training. Our results show that post-trial intrastriatal infusion of lidocaine disrupted late-stage long-term skill memory (post-training days 6-26), but spared early long-term memory (1 day after the training). Skill consolidation was more susceptible to such disruption in animals that practiced less during the training. Cocaine given pre-trial prevented this post-trial disruption of skill consolidation. These findings indicate that the sensorimotor striatum is critical for consolidation of late but not early long-term skill memory. Furthermore, cocaine appeared to stabilize motor memory formation by protecting consolidation processes after the training.
Keywords: striatum, cocaine, lidocaine, motor-memory consolidation, skill learning, rat
1. Introduction
The sensorimotor striatum is implicated in various forms of procedural learning (e.g., [25]). Best established perhaps is a role for the striatum in instrumental learning, as demonstrated in maze and lever-press tasks (e.g., [11,16,26,47]). However, evidence indicates that motor-skill learning, another form of procedural learning [38], is also dependent on normal function of the sensorimotor striatum [25]. For example, moderate loss of dopamine [1,24] or deletion of NMDA receptors [7] in the striatum impaired skill learning in a rotarod task. Also, our previous findings show that blockade of striatal D1 dopamine receptors during training prevents acquisition of a motor skill in a running-wheel paradigm [45].
Motor-skill learning is an attractive model for procedural learning because it offers various advantages over other models, including relatively fast acquisition of a learned motor response within a few training sessions and stable long-term memory that can last for weeks to months [19]. Such advantages facilitate investigation of the cellular mechanisms underlying procedural learning.
Similar to declarative memory, a critical step in the formation of long-term procedural memory is the consolidation of the memory trace, which involves neuronal processing after the training (“off-line” processing) (for reviews, see [18,34]). This memory stabilization converts the memory from a labile state that can easily be disrupted by various manipulations to a more stabile state that is resistant to interference [18,34]. Human and animal studies demonstrated that such memory stabilization is also necessary for the formation of enduring motor skills (e.g., [5,20,36]). Little is known regarding the neuronal substrates of motor-skill consolidation [37]. For example, a recent study reported critical involvement of the motor cortex in the consolidation of a reaching skill [20]. The motor cortex is a major source of inputs to the sensorimotor striatum (cf. [46]), and simultaneous changes in activity patterns have been found in both brain regions during motor-skill acquisition [6,32,41]. However, it is currently unknown whether the sensorimotor striatum also participates in the consolidation (off-line processing) of motor-skill memories.
We investigated the role of the sensorimotor striatum in consolidation of wheel-skill memory. Moreover, given the role of dopamine in skill learning (see above) and the finding that the indirect dopamine receptor agonist cocaine modified acquisition of motor-skill memory [45], we also assessed possible effects of cocaine on consolidation of this skill memory. Our findings show that post-trial interference with striatal function disrupted motor-skill memory consolidation. Administration of cocaine before the training prevented this disruption of skill-memory formation.
2. Materials and methods
2.1. Subjects
Male Sprague-Dawley rats (220-250 g; Harlan, Madison, WI) were individually housed under standard laboratory conditions (12:12 h light/dark cycle, lights on at 0700 h) with food and water available ad libitum. The experiments were performed between 1300 and 1600 h. All procedures met the NIH guidelines for the care and use of laboratory animals and were approved by the Rosalind Franklin University Animal Care and Use Committee.
2.2. Implantation of guide cannulas
Rats were anesthetized with ketamine/xylazine (60/20 mg/kg) and given a dose of the analgesic banamine (1.5 mg/kg, s.c.). Guide cannulas (26 gauge; Plastics One, Roanoke, VA), occluded by “dummy cannulas” of equal length, were implanted into the dorsal striatum bilaterally (A, -0.1 mm; L, +/-3.0 mm; V, -4.0 mm [29]), aiming at the striatal region that showed wheel learning-associated changes in gene regulation [44]. One week later, one day before the first infusion, the dummy cannula was replaced with a longer one that protruded 2.5 mm beyond the guide cannula, to reduce the probability of acute damage caused by the infusion cannula (33 gauge, 1 mm longer than the guide cannula) [39]. The infusion site in the striatum was confirmed in Nisslstained coronal sections after completion of the experiments.
2.3. Running-wheel training and drug administration
Rats were trained on a running wheel on two consecutive days (40 min/day) (see [45], for details). The running wheels (Wahmann Company, Baltimore, MD) consisted of a rotating metal chamber with a wire mesh floor (diameter, 35 cm; width, 11 cm), attached to a stationary metal wall with an access opening that could be closed. A mechanical counter recorded full wheel revolutions. Training and testing were conducted in the same room. In order to minimize the effects of surrounding noise, constant “white noise” was provided during both training and testing sessions.
Before each training session (pre-trial), rats received an injection of vehicle (experiment 1) or cocaine (cocaine hydrochloride; Sigma, St. Louis, MO; 25 mg/kg, in 0.02% ascorbic acid, 1 ml/kg, i.p.) (experiment 2). Immediately after each training session (post-trial), the animals received bilateral intrastriatal infusions of lidocaine (Sigma; 2%, in saline, pH 7.4; 1 μl each side; 0.2 μl/min) [17,27] or a sham infusion (insertion of infusion cannulas without infusion) (n=8-9 each). After the infusion, the cannulas were left in place for 3 min to allow for diffusion of the drug.
2.4. Wheel-skill testing
In the beginning of the running-wheel training, rats learn to control/balance the wheel in order to run at the bottom of the wheel and avoid swinging (wheel skill) [44]. The wheel-skill test assesses this skill by measuring the rat’s ability to stop the wheel from swinging [45]. To start the test, the wheel with the rat is gently rotated by 90 degrees (rat’s head up; see [45]). Upon release, the wheel swings back and forth until the rat stops the swinging by counterbalancing. Behavior on the wheel is videotaped for analysis. Wheel-skill performance is measured by counting the number of interruptions by the rat’s body (minus tail) of a horizontal plane at one third of the wheel diameter above the wheel bottom (marked on the video monitor), until the animal fails to interrupt for 3 sec.
The skill performance was tested on the day before the first training session (“pre”) and 1, 6, 18, and 26 days after the last training session (“post”). One day before the pre-test, the animal was habituated to testing apparatus/room by placing it on a locked wheel for 1 h. On test days, the rat was again first placed inside the locked wheel for a 2-min habituation period. Then, two test trials were performed (inter-trial interval, 1 min), and the two scores were averaged for a measure of performance error. The treatment groups were balanced with respect to pre-test scores.
2.5. Statistical analysis
Training effects over time within a group were determined by nonparametric analysis of variance (Friedman test), and post hoc Wilcoxon paired-sample tests were used to describe differences between pre- and post-training test scores (Statistica, Statsoft, Tulsa, OK). Between-group comparisons were performed with Mann-Whitney U tests. In order to facilitate comparisons between experiments, the test scores are expressed as percentage of mean pre-test scores.
3. Results
3.1. Post-trial lidocaine infusion into the striatum disrupts wheel-skill consolidation
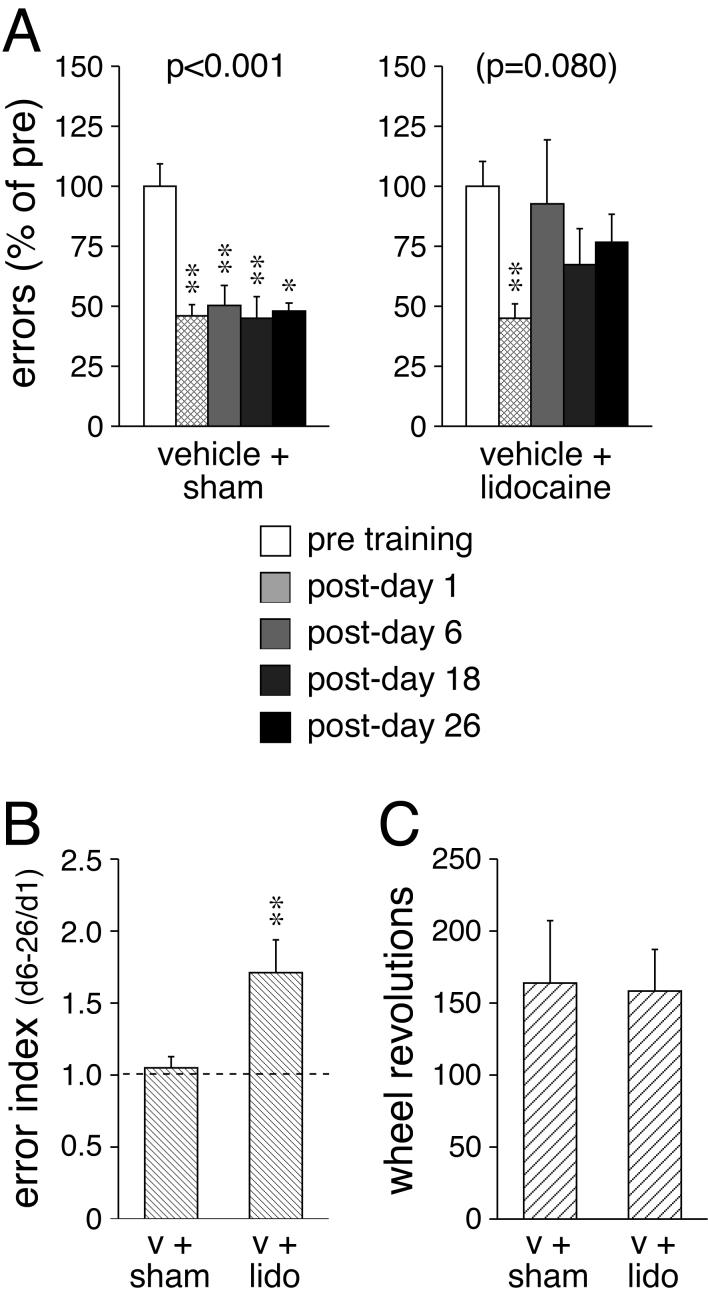
The effects of post-trial intrastriatal infusions of lidocaine or sham infusions on wheel-skill performance were assessed 1, 6, 18 and 26 days after the training (Fig. 1). Statistical analysis of the test scores over time in individual groups revealed that rats that received post-trial sham infusions significantly improved in their wheel-skill performance (p<0.001, Friedman test). Post hoc tests comparing post- with pre-training scores showed that these rats committed significantly fewer performance errors at all time points after the training (p<0.05, Wilcoxon test) (Fig. 1A). In contrast, animals that received post-trial infusions of lidocaine into the striatum displayed no significant overall training effect (p>0.05). However, they did show a tendency for an improved skill performance (p=0.080) (Fig. 1A), which was mainly due to the performance on post-day 1 when they displayed fewer errors (p<0.01, vs. pre-test), comparable to the sham controls (Figs. 1, 2). Thus, in lidocaine-treated animals, enhanced skill performance did not endure past post-day 1. To compare skill stability over time between the two treatment groups, we used an error index (averaged errors on days 6-26 divided by errors on day 1). Results revealed a significantly higher error index in lidocaine-treated animals compared with sham-treated controls (p<0.01, Mann-Whitney U test) (Fig. 1B), indicating decreasing skill stability over time after lidocaine infusion. There was no significant difference in the total number of wheel revolutions during the training between the two groups (p>0.05) (Fig. 1C), indicating that they did not differ in the amount of practicing.
Fig. 1.
Effects of post-trial interference by lidocaine infusion into the striatum on motor-skill learning. A The number (mean±SEM) of performance errors (in percent of pre-test values) committed before (pre) and 1, 6, 18 and 26 days after (post) the 2-day running-wheel training is given for rats that received a bilateral intrastriatal infusion of lidocaine (2%, 1 μl each side) or a sham infusion after each training session. All rats received a systemic injection of vehicle (0.02% ascorbic acid) before each training session. The p value for the overall training effect is also shown. B The error index (averaged errors on post-days 6-26 divided by errors on post-day 1; mean±SEM) is shown for sham (v+sham)- and lidocaine (v+lido)-treated groups. C The total number (mean±SEM) of wheel revolutions during the training for these groups is depicted. ** p<0.01, * p<0.05 vs. pre (A) or v+sham (B).
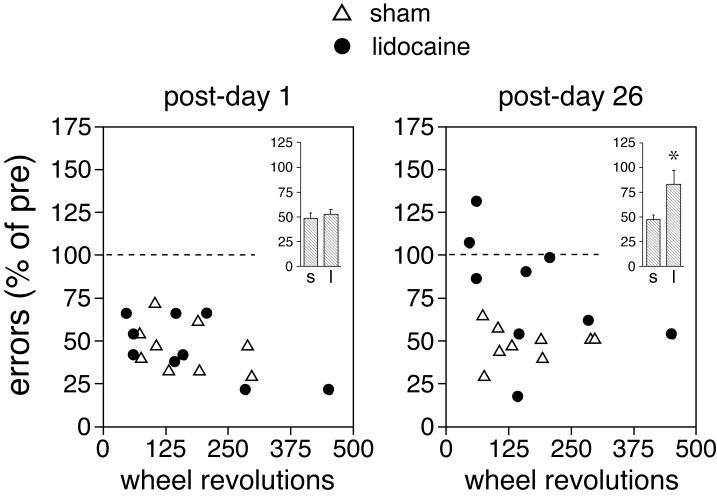
Fig. 2.
Relationship between running (practicing) during the training and post-trial interference by lidocaine infusion. Scatter plots show the total number of wheel revolutions during the 2-day training and the number of performance errors (in percent of pre-test values) committed in the skill tests 1 day (left) and 26 days (right) after the training for individual animals that received intrastriatal infusions of lidocaine (full circles) or sham infusions (open triangles) after each training session plus a pre-trial injection of vehicle. Insets depict, for each time point, error scores (mean±SEM) for sham (s)- and lidocaine (l)-treated animals that showed less than 250 wheel revolutions during the training. * p<0.05.
Further analysis showed that there was an inverse relationship between the amount of practicing (running) during the training and the effects of post-trial interference by lidocaine infusion. This interference was more effective in animals that ran less during the training (Fig. 2). This effect emerged over time and was most robust on post-training day 26. On that day, the lidocaine-treated group showed significantly more performance errors than the sham controls (p<0.05). However, this difference was predominantly produced by animals whose number of wheel revolutions during the training was in the lower range of revolutions and not by animals that practiced more (Fig. 2). Thus, the mean error score for lidocaine-treated rats that ran less than 250 revolutions was approximately twice as high as that in controls (p<0.05; Fig. 2).
3.2. Cocaine prevents disruption of wheel-skill consolidation by post-trial interference in the striatum
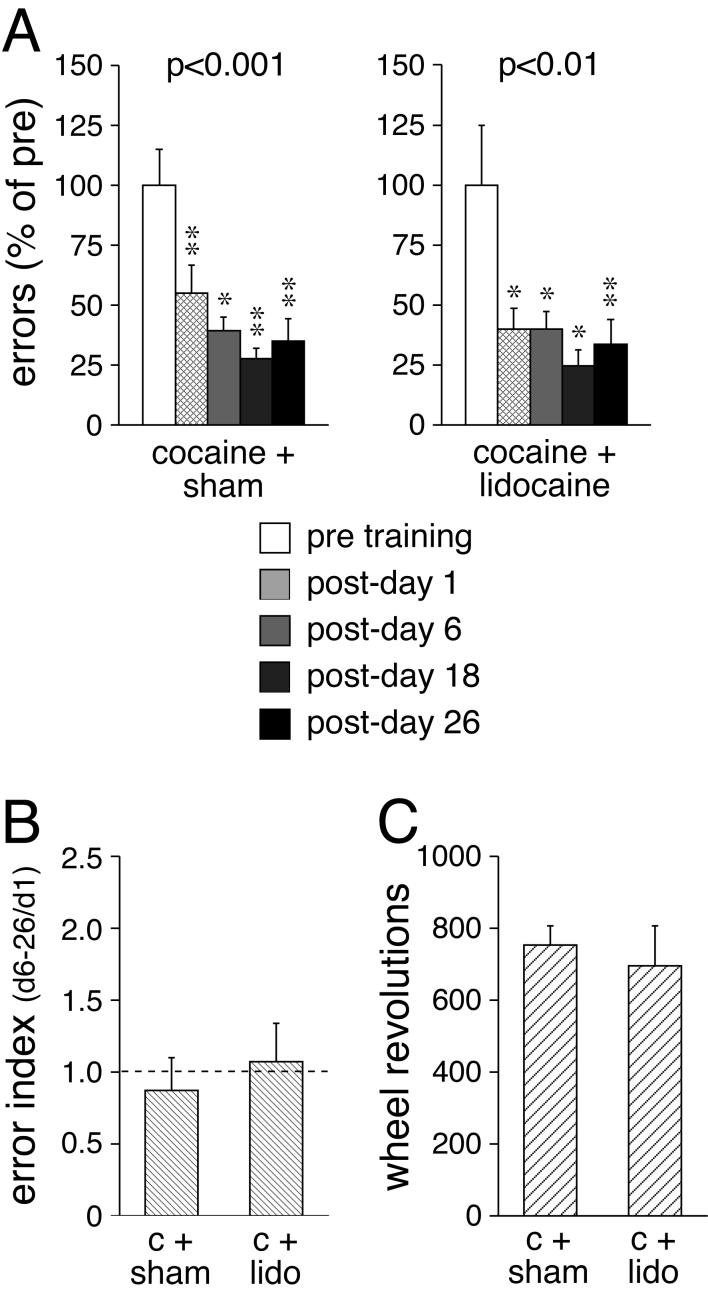
We also investigated whether cocaine administered immediately before each training session altered the effects of post-trial interference on wheel-skill consolidation (Fig. 3). Statistical analysis revealed that the controls that received pre-trial cocaine injections and post-trial sham infusions improved significantly in their skill performance (p<0.001) (Fig. 3A); they committed significantly fewer performance errors up to 26 days after training (p<0.05). A similar effect was found with post-trial lidocaine treatment. Thus, animals that received pre-trial cocaine plus post-trial intrastriatal infusions of lidocaine displayed significant skill improvement after the training (p<0.01) and retained this skill for at least 4 weeks (p<0.05) (Fig. 3A). In contrast to the vehicle-treated groups (experiment 1; Fig. 1B), these two cocaine-treated groups did not differ in the error index (skill stability over time) (p>0.05) (Fig. 3B). Moreover, the group that received pre-trial cocaine plus post-trial lidocaine showed a significantly lower error index than the vehicle plus lidocaine-treated group (p<0.05). Similar to the 2 vehicle-treated groups, the 2 cocaine-treated groups did not differ in the number of wheel revolutions performed during the training (p>0.05) (Fig. 3C).
Fig. 3.
Effects of post-trial interference by lidocaine infusion into the striatum on motor-skill learning under the influence of cocaine. A The number (mean±SEM) of errors (in percent of pre-test values) committed before (pre) and 1, 6, 18 and 26 days after (post) the 2-day training is given for rats that received a bilateral intrastriatal infusion of lidocaine (2%, 1 μl each side) or a sham infusion after each training session. All rats received an injection of cocaine (25 mg/kg, i.p.) before each training session. The p value for the overall training effect is also shown. B The error index (averaged errors on post-days 6-26 divided by errors on post-day 1; mean±SEM) is shown for sham (c+sham)- and lidocaine (c+lido)-treated groups. C The total number (mean±SEM) of wheel revolutions during the training for these groups is depicted. ** p<0.01, * p<0.05 vs. pre.
4. Discussion
We investigated the role of the sensorimotor striatum in skill-memory consolidation in a running-wheel paradigm. Our results show that post-trial interference with neuronal activity by intrastriatal infusion of lidocaine following the training session disrupted consolidation of late-stage (post-training days 6-26), but not early (post-day 1), long-term skill memory. Cocaine administered before the training session prevented this post-trial disruption of skill-memory consolidation. These results indicate that the sensorimotor striatum plays a critical role in the consolidation of long-term motor-skill memory. Furthermore, our findings suggest that cocaine stabilizes motor-memory formation.
4.1. Post-trial infusion of lidocaine into the striatum disrupts skill-memory consolidation
We have previously shown that formation of stable motor-skill memory in the running-wheel paradigm is dependent on normal function of the sensorimotor striatum, as blockade of striatal D1 receptors attenuated this skill learning [45]. However, in that study, the acquisition of this motor skill was prevented by intrastriatal infusion of a D1 receptor antagonist before the training sessions (pre-trial). In order to determine whether the striatum is also involved in the consolidation of this skill, we here employed transient interference with striatal function immediately after the training (post-trial), by intrastriatal lidocaine infusions. These infusions targeted parts of the dorsal striatum that displayed wheel-skill learning-associated molecular changes as described in our previous studies [44,46]. Our findings demonstrate that these post-trial infusions disrupted skill-memory consolidation. Moreover, consolidation was more susceptible to interference in animals that practiced less during the training.
Lidocaine infusions have been found before to interfere with memory consolidation. For example, similar lidocaine doses as used here blocked procedural-memory formation in maze and avoidance tasks when infused into the dorsal striatum (e.g., [17,27,33]). Based on previous findings [21,35,40], the effective spread of the present lidocaine dose was estimated to be less than a millimeter, and was thus most likely limited to sensorimotor parts of the dorsal striatum. Our findings show that these infusions impaired long-term skill stability. These animals displayed normal skill learning one day after the training, but lost their improved wheel skill by day 6 after the training (see below).
Regarding the neuronal mechanisms affected, a caveat of lidocaine inactivation is the possibility of effects on fibers of passage, in addition to activity-dependent processes in neurons at the infusion site. While the relative contributions of striatal processes vs. passing fibers to the present lidocaine interference remain undecided, it should be noted that our previous study with intrastriatal dopamine receptor antagonism demonstrated similar effects on skill-memory formation. Thus, infusion of the D1 receptor antagonist SCH-23390 into the same striatal regions before the training sessions also attenuated wheel-skill learning [45]. Moreover, this D1 receptor effect had similar temporal characteristics, as late long-term memory was preferentially attenuated [45]. These findings demonstrate a role for D1 receptor-mediated striatal processes in long-term skill-memory formation.
Our present results demonstrate that post-trial interference in the striatum readily interrupted motor-skill memory consolidation, suggesting a critical role for striatal processing after the training session for such long-term memory. These findings are consistent with previous studies showing that normal function of the striatum is necessary for consolidation of other forms of learning. For example, post-training inactivation of the dorsal striatum or disruption of striatal glutamate signaling blocked consolidation of fear memory [28,33] or spatial and non-spatial memories in a water-maze task [9,28]. The present study is the first, to our knowledge, to indicate that the functional integrity of the sensorimotor striatum is critical for the consolidation of motor-skill memory.
4.2. Late-stage, but not early, long-term skill memory is disrupted by post-trial interference with striatal function
One of the most important findings of the present study is that post-trial intrastriatal interference affected the consolidation of late-stage (days 6-26) long-term skill memory, but had no effect on skill improvement one day after the training (which reflects early-stage long-term memory; e.g., [14]). Thus, these rats displayed near-normal skill-test performance on post-day 1, but subsequently lost this skill. A similar dissociation in the mechanisms supporting early vs. late-stage skill memory was observed in our previous study on wheel-skill learning [45]. In that study, acquisition of late-stage, but not early, wheel-skill memory was critically dependent on D1 receptors. The present findings extend this dissociation to mechanisms of skill consolidation.
While our present and previous [45] results demonstrate that striatal mechanisms are critical for late long-term skill memory, it is presently unclear which mechanisms mediate early long-term skill memory. Previous studies showed changes in neuronal processing in both cortical and striatal nodes of corticostriatal circuits during procedural learning. For example, parallel modifications in neuronal activity patterns in motor cortex and sensorimotor striatum have been found during the acquisition of motor skills on a rotarod [6]. Moreover, a role for the motor cortex in consolidation of a skilled reaching response has also been demonstrated [20]. It remains to be seen whether the motor cortex (or other structures [37]) or striatal mechanisms not affected by the present post-trial manipulation are important for consolidation of early long-term skill memory. Regardless, our present results demonstrate that consolidation of early- and late-stage long-term skill memory is mediated by, at least in part, differential mechanisms.
4.3. Stabilization of skill-memory consolidation by cocaine
Our previous findings showed that the indirect dopamine receptor agonist cocaine modified wheel-skill learning [45]. In the present study, we thus also investigated whether the same cocaine treatment (pre-trial) affected skill-memory consolidation. While it has previously been shown that cocaine or amphetamine can enhance some forms of procedural learning (e.g., [12,13,15]), our results demonstrate that administration of cocaine before the training session prevented post-trial interference with skill-memory consolidation by lidocaine infusions into the striatum. Our findings thus suggest that one action of psychostimulants/dopamine agonists is to “stabilize” neuronal processes of consolidation, thus rendering them resistant to interference.
Several mechanisms by which cocaine could produce such an effect are conceivable. First, considering the direct relationship between practicing and skill learning [45], it could be argued that cocaine preserved memory formation simply by increasing the amount of running/practicing during the training. While such an effect of increased training is possible, it seems unlikely to be the principal mechanism. For one, our results indicate that cocaine did not enhance skill learning in sham-infused animals as compared with sham-infused vehicle controls, despite enhancing running about four-fold. This was hardly due to a ceiling effect; rats can improve more, for example, with more or longer training sessions [45]. Also, in our previous study, depending on the training conditions, cocaine tended to impair, rather than improve, skill learning despite increasing the amount of running [45].
Second, cocaine may have helped overcome the effects of transient local interference by increasing neuronal activity in the sensorimotor striatum [30,42]. High doses of systemic cocaine raise extracellular levels of dopamine in the striatum for at least 90 minutes (e.g., [23,31]), indicating that some of the present cocaine effects likely outlasted training session and subsequent transient lidocaine action [4,40]. Such effects may have allowed consolidation to proceed.
Third, cocaine may have facilitated memory consolidation by affecting molecular mechanisms of synapse modification necessary for long-term memory. Our previous findings demonstrated that cocaine enhanced skill learning-associated changes in gene regulation in the sensorimotor striatum. Specifically, animals trained under the influence of cocaine in the running-wheel task showed transiently enhanced inducibility of genes encoding transcription factors such as c-fos and zif 268 and molecules that directly regulate synaptic plasticity (Homer) in the striatum selectively during the learning phase ([43,44]; Willuhn and Steiner, in preparation). The induction of such genes during learning is thought to initiate cellular changes that lead to altered structure/efficacy of synapses, mediating long-term memory consolidation [2,8,14,22]. Consistent with this notion, our most recent results show that blockade of striatal D1 receptors prevented both the above described learning-associated molecular changes in the striatum (Willuhn and Steiner, in preparation) as well as wheel-skill learning [45]. Thus, it is conceivable that cocaine stabilized motor-memory consolidation by enhancing D1 receptor-mediated molecular processes in the striatum, thus reducing their susceptibility to interference.
It has been suggested that psychostimulants such as cocaine and amphetamine alter procedural learning mediated by the dorsal striatum and that these changes play a role in drug addiction [3,10]. Abnormal stabilization of motor memory consolidation by cocaine may contribute to such aberrant motor learning. Future studies will have to determine the mechanism(s) by which cocaine stabilizes motor memory and whether this effect is involved in drug addiction.
Acknowledgements
This work was supported by Grant DA011261 from the National Institute on Drug Abuse. We thank Joel Beverley for excellent technical assistance and Dr. Kuei-Yuan Tseng for helpful discussions.
References
- [1].Akita H, Ogata M, Jitsuki S, Ogura T, Oh-Nishi A, Hoka S, Saji M. Nigral injection of antisense oligonucleotides to synaptotagmin I using HVJ-liposome vectors causes disruption of dopamine release in the striatum and impaired skill learning. Brain Res. 2006;1095:178–189. doi: 10.1016/j.brainres.2006.04.039. [DOI] [PubMed] [Google Scholar]
- [2].Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- [4].Boehnke SE, Rasmusson DD. Time course and effective spread of lidocaine and tetrodotoxin delivered via microdialysis: an electrophysiological study in cerebral cortex. J Neurosci Meth. 2001;105:133–141. doi: 10.1016/s0165-0270(00)00348-4. [DOI] [PubMed] [Google Scholar]
- [5].Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- [6].Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- [7].Dang MT, Yokoi F, Yin HH, Lovinger DM, Wang Y, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci USA. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- [9].De Leonibus E, Oliverio A, Mele A. A study on the role of the dorsal striatum and the nucleus accumbens in allocentric and egocentric spatial memory consolidation. Learn Mem. 2005;12:491–503. doi: 10.1101/lm.94805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- [11].Featherstone RE, McDonald RJ. Lesions of the dorsolateral striatum impair the acquisition of a simplified stimulus-response dependent conditional discrimination task. Neuroscience. 2005;136:387–395. doi: 10.1016/j.neuroscience.2005.08.021. [DOI] [PubMed] [Google Scholar]
- [12].Fulginiti S, Cancela LM. Effect of naloxone and amphetamine on acquisition and memory consolidation of active avoidance responses in rats. Psychopharmacology. 1983;79:45–48. doi: 10.1007/BF00433015. [DOI] [PubMed] [Google Scholar]
- [13].Introini-Collison IB, McGaugh JL. Cocaine enhances memory storage in mice. Psychopharmacology. 1989;99:537–541. doi: 10.1007/BF00589905. [DOI] [PubMed] [Google Scholar]
- [14].Izquierdo I, Bevilaqua LR, Rossato JI, Bonini JS, Medina JH, Cammarota M. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [15].Janak PH, Keppel G, Martinez JL. Cocaine enhances retention of avoidance conditioning in rats. Psychopharmacology. 1992;106:383–387. doi: 10.1007/BF02245422. [DOI] [PubMed] [Google Scholar]
- [16].Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- [17].Kantak KM, Green-Jordan K, Valencia E, Kremin T, Eichenbaum HB. Cognitive task performance after lidocaine-induced inactivation of different sites within the basolateral amygdala and dorsal striatum. Behav Neurosci. 2001;115:589–601. doi: 10.1037//0735-7044.115.3.589. [DOI] [PubMed] [Google Scholar]
- [18].Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29:58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Luft AR, Buitrago MM. Stages of motor skill learning. Mol Neurobiol. 2005;32:205–216. doi: 10.1385/MN:32:3:205. [DOI] [PubMed] [Google Scholar]
- [20].Luft AR, Buitrago MM, Ringer T, Dichgans J, Schulz JB. Motor skill learning depends on protein synthesis in motor cortex after training. J Neurosci. 2004;24:6515–6520. doi: 10.1523/JNEUROSCI.1034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Malpeli JG, Schiller PH. A method of reversible inactivation of small regions of brain tissue. J Neurosci Meth. 1979;1:143–151. doi: 10.1016/0165-0270(79)90011-6. [DOI] [PubMed] [Google Scholar]
- [22].Moser MB. Making more synapses: a way to store information? Cell Mol Life Sci. 1999;55:593–600. doi: 10.1007/s000180050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nicolaysen LC, Pan HT, Justice JBJ. Extracellular cocaine and dopamine concentrations are linearly related in rat striatum. Brain Res. 1988;456:317–323. doi: 10.1016/0006-8993(88)90234-x. [DOI] [PubMed] [Google Scholar]
- [24].Ogura T, Ogata M, Akita H, Jitsuki S, Akiba L, Noda K, Hoka S, Saji M. Impaired acquisition of skilled behavior in rotarod task by moderate depletion of striatal dopamine in a pre-symptomatic stage model of Parkinson’s disease. Neurosci Res. 2005;51:299–308. doi: 10.1016/j.neures.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [25].Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- [26].Packard MG, McGaugh JL. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci. 1992;106:439–446. doi: 10.1037//0735-7044.106.3.439. [DOI] [PubMed] [Google Scholar]
- [27].Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- [28].Packard MG, Vecchioli SF, Schroeder JP, Gasbarri A. Task-dependent role for dorsal striatum metabotropic glutamate receptors in memory. Learn Mem. 2001;8:96–103. doi: 10.1101/lm.37401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- [30].Pederson CL, Wolske M, Peoples LL, West MO. Firing rate dependent effect of cocaine on single neurons of the rat lateral striatum. Brain Res. 1997;760:261–265. doi: 10.1016/s0006-8993(97)00395-8. [DOI] [PubMed] [Google Scholar]
- [31].Pettit HO, Pan HT, Parsons LH, Justice JBJ. Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem. 1990;55:798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- [32].Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ. The neural correlates of motor skill automaticity. J Neurosci. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pérez-Ruiz C, Prado-Alcalá RA. Retrograde amnesia induced by lidocaine injection into the striatum: protective effect of the negative reinforcer. Brain Res Bull. 1989;22:599–603. doi: 10.1016/0361-9230(89)90076-2. [DOI] [PubMed] [Google Scholar]
- [34].Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nat Rev Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- [35].Sandkühler J, Maisch B, Zimmermann M. The use of local anaesthetic microinjections to identify central pathways: a quantitative evaluation of the time course and extent of the neuronal block. Exp Brain Res. 1987;68:168–178. doi: 10.1007/BF00255242. [DOI] [PubMed] [Google Scholar]
- [36].Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memor. J Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- [38].Squire LR. Memory and Brain. Oxford University Press; Oxford: 1987. [Google Scholar]
- [39].Steiner H, Gerfen CR. Dynorphin opioid inhibition of cocaine-induced, D1 dopamine receptor-mediated immediate-early gene expression in the striatum. J Comp Neurol. 1995;353:200–212. doi: 10.1002/cne.903530204. [DOI] [PubMed] [Google Scholar]
- [40].Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Meth. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- [41].Toni I, Rowe J, Stephan KE, Passingham RE. Changes of cortico-striatal effective connectivity during visuomotor learning. Cereb Cortex. 2002;12:1040–1047. doi: 10.1093/cercor/12.10.1040. [DOI] [PubMed] [Google Scholar]
- [42].White IM, Doubles L, Rebec GV. Cocaine-induced activation of striatal neurons during focused stereotypy in rats. Brain Res. 1998;810:146–152. doi: 10.1016/s0006-8993(98)00905-6. [DOI] [PubMed] [Google Scholar]
- [43].Willuhn I, Steiner H. Motor learning-related gene regulation in the striatum: Effects of cocaine. In: Bolam JP, Ingham CA, Magill PJ, editors. The Basal Ganglia VIII. Plenum Press; New York: 2005. pp. 197–207. [Google Scholar]
- [44].Willuhn I, Steiner H. Motor-skill learning-associated gene regulation in the striatum: Effects of cocaine. Neuropsychopharmacology. 2006;31:2669–2682. doi: 10.1038/sj.npp.1300995. [DOI] [PubMed] [Google Scholar]
- [45].Willuhn I, Steiner H. Motor-skill learning in a novel running-wheel task is dependent on D1 dopamine receptors in the striatum. Neuroscience. 2008;153:249–258. doi: 10.1016/j.neuroscience.2008.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: Relationship to cortical inputs and role of behavioural context. Eur J Neurosci. 2003;17:1053–1066. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- [47].Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–9. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]