Abstract
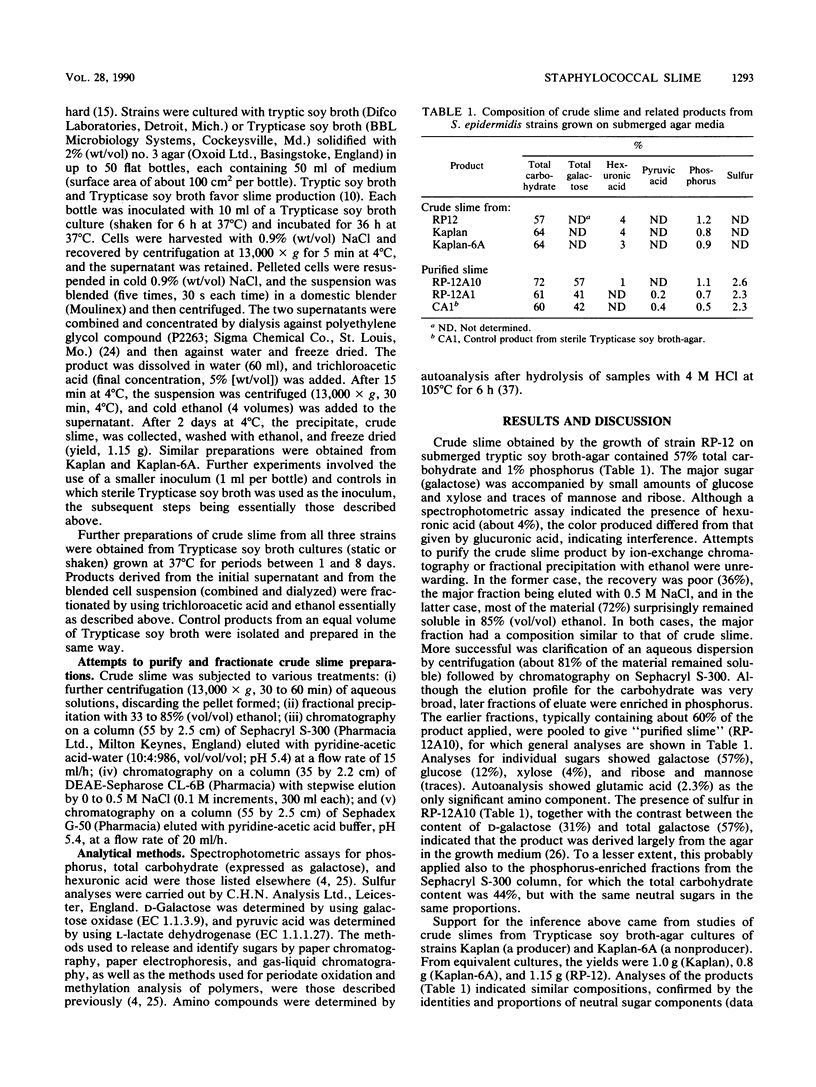
Slime production by Staphylococcus epidermidis may be important in the adherence to and colonization of biomedical devices, and slime has been proposed to have various effects on the immune system. Attempts were made to isolate, purify, and chemically characterize slime from S. epidermidis cultivated under fluid on tryptic soy broth-agar medium. "Crude slime" from slime-producing strain RP-12 was characterized by a high galactose content. Similar materials in similar yields were isolated from slime-producing strain Kaplan, a non-slime-producing mutant, Kaplan-6A, and sterile medium controls, suggesting that crude slime was derived mainly from the medium. The occurrence of D- and L-galactose and pyruvate and sulfate residues and methylation analysis of these crude slime preparations, monitored by gas-liquid chromatography and mass spectrometry, showed that the agar was the main source of crude slime, suggesting that the preparation was largely an artifact of the growth and isolation procedures. Similar high-galactose-content preparations from both S. epidermidis and Staphylococcus aureus, assumed to be bacterial products and with a variety of biological activities, have been described by other investigators. Growth attached to a solid surface appears to be important for slime production. An accumulation of turned-over cell surface molecules and released macromolecules such as DNA may contribute to slime production. Avoidance of agar and development of a chemically defined medium for slime production are recommended for further studies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit R. D., Karakawa W. W., Vann W. F., Robbins J. B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984 Apr;2(2):85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- BAIRD-PARKER A. C. THE CLASSIFICATION OF STAPHYLOCOCCI AND MICROCOCCI FROM WORLD-WIDE SOURCES. J Gen Microbiol. 1965 Mar;38:363–387. doi: 10.1099/00221287-38-3-363. [DOI] [PubMed] [Google Scholar]
- Bayston R., Penny S. R. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol Suppl. 1972;27:25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- Brock J. H., Reiter B. Chemical and biological properties of extracellular slime produced by Staphylococcus aureus grown in high-carbohydrate, high-salt medium. Infect Immun. 1976 Mar;13(3):653–660. doi: 10.1128/iai.13.3.653-660.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykowska K., Ludwicka A., Wegrzynowicz Z., Lopaciuk S., Kopeć M. Anticoagulant properties of extracellular slime substance produced by Staphylococcus epidermidis. Thromb Haemost. 1985 Dec 17;54(4):853–856. [PubMed] [Google Scholar]
- CATLIN B. W., CUNNINGHAM L. S. Studies of extracellular and intracellular bacterial deoxyribonucleic acids. J Gen Microbiol. 1958 Dec;19(3):522–539. doi: 10.1099/00221287-19-3-522. [DOI] [PubMed] [Google Scholar]
- Caputy G. G., Costerton J. W. Morphological examination of the glycocalyces of Staphylococcus aureus strains Wiley and Smith. Infect Immun. 1982 May;36(2):759–767. doi: 10.1128/iai.36.2.759-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L., Fischetti V. A. Variation in the expression of cell wall proteins of Staphylococcus aureus grown on solid and liquid media. Infect Immun. 1988 May;56(5):1061–1065. doi: 10.1128/iai.56.5.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Cripps R. E., Work E. The accumulation of extracellular macromolecules by Staphylococcus aureus grown in the presence of sodium chloride and glucose. J Gen Microbiol. 1967 Oct;49(1):127–137. doi: 10.1099/00221287-49-1-127. [DOI] [PubMed] [Google Scholar]
- Davenport D. S., Massanari R. M., Pfaller M. A., Bale M. J., Streed S. A., Hierholzer W. J., Jr Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J Infect Dis. 1986 Feb;153(2):332–339. doi: 10.1093/infdis/153.2.332. [DOI] [PubMed] [Google Scholar]
- Ekstedt R. D., Bernhard J. M. Preparation and characterization of a slime layer material produced by Staphylococcus aureus. Proc Soc Exp Biol Med. 1973 Jan;142(1):86–91. doi: 10.3181/00379727-142-36964. [DOI] [PubMed] [Google Scholar]
- Gray E. D., Peters G., Verstegen M., Regelmann W. E. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet. 1984 Feb 18;1(8373):365–367. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- JONES D., DEIBEL R. H., NIVEN C. F., Jr Identity of Staphylococcus epidermidis. J Bacteriol. 1963 Jan;85:62–67. doi: 10.1128/jb.85.1.62-67.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. M., Lee D. A., Regelmann W. E., Gray E. D., Peters G., Quie P. G. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986 Oct;54(1):13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsson K. G., Hastings J. G., Spencer R. C. The role of extracellular slime in opsonophagocytosis of Staphylococcus epidermidis. J Med Microbiol. 1988 Nov;27(3):207–213. doi: 10.1099/00222615-27-3-207. [DOI] [PubMed] [Google Scholar]
- Lorian V. In vitro simulation of in vivo conditions: physical state of the culture medium. J Clin Microbiol. 1989 Nov;27(11):2403–2406. doi: 10.1128/jcm.27.11.2403-2406.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwicka A., Uhlenbruck G., Peters G., Seng P. N., Gray E. D., Jeljaszewicz J., Pulverer G. Investigation on extracellular slime substance produced by Staphylococcus epidermidis. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Dec;258(2-3):256–267. doi: 10.1016/s0176-6724(84)80043-7. [DOI] [PubMed] [Google Scholar]
- Madiraju M. V., Brunner D. P., Wilkinson B. J. Effects of temperature, NaCl, and methicillin on penicillin-binding proteins, growth, peptidoglycan synthesis, and autolysis in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Nov;31(11):1727–1733. doi: 10.1128/aac.31.11.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald H. C., Karakawa W. W. Immunochemical analysis of a uronic acid polymer of Staphylococcus epidermidis, strain 53. J Immunol. 1970 Aug;105(2):389–395. [PubMed] [Google Scholar]
- Oxley D., Wilkinson S. G. Structure of the O-specific polysaccharide from the lipopolysaccharide of Serratia marcescens O8. Eur J Biochem. 1986 May 2;156(3):597–601. doi: 10.1111/j.1432-1033.1986.tb09619.x. [DOI] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Reifsteck F., Wee S., Wilkinson B. J. Hydrophobicity-hydrophilicity of staphylococci. J Med Microbiol. 1987 Aug;24(1):65–73. doi: 10.1099/00222615-24-1-65. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Samra Z., Karakawa W. W., Vann W. F., Schneerson R., Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985 Nov;22(5):828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Yamashita N., Goldmann D. A., Pier G. B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988 Apr;157(4):713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- Tsai C. L., Schurman D. J., Smith R. L. Quantitation of glycocalyx production in coagulase-negative Staphylococcus. J Orthop Res. 1988;6(5):666–670. doi: 10.1002/jor.1100060507. [DOI] [PubMed] [Google Scholar]
- WILKINSON J. F. The extracellualr polysaccharides of bacteria. Bacteriol Rev. 1958 Mar;22(1):46–73. doi: 10.1128/br.22.1.46-73.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbrath L. Studies of lipopolysaccharides from Pseudomonas aeruginosa. Eur J Biochem. 1975 Mar 17;52(2):331–343. doi: 10.1111/j.1432-1033.1975.tb04001.x. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Ekstedt R. D. Relation of mucoid growth of Staphylococcus aureus to clumping factor reaction, morphology in serum-soft agar, and virulence. J Bacteriol. 1968 Oct;96(4):902–908. doi: 10.1128/jb.96.4.902-908.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Umeda A., Ichiman Y., Yamada T. Cross protection between a strain of Staphylococcus epidermidis and eight other species of coagulase-negative staphylococci. Can J Microbiol. 1988 Jul;34(7):913–915. doi: 10.1139/m88-160. [DOI] [PubMed] [Google Scholar]


