Abstract
Mitochondria are the powerhouse of the cell. Their primary physiological function is to generate adenosine triphosphate through oxidative phosphorylation via the electron transport chain. Reactive oxygen species generated from mitochondria have been implicated in acute brain injuries such as stroke and neurodegeneration. Recent studies have shown that mitochondrially-formed oxidants are mediators of molecular signaling, which is implicated in the mitochondria-dependent apoptotic pathway that involves pro- and antiapoptotic protein binding, the release of cytochrome c, and transcription-independent p53 signaling, leading to neuronal death. Oxidative stress and the redox state of ischemic neurons are also implicated in the signaling pathway that involves phosphatidylinositol 3-kinase/Akt and downstream signaling, which lead to neuronal survival. Genetically modified mice or rats that overexpress or are deficient in superoxide dismutase have provided strong evidence in support of the role of mitochondrial dysfunction and oxidative stress as determinants of neuronal death/survival after stroke and neurodegeneration.
Keywords: apoptosis, ischemia, mitochondria, oxidative stress, p53, reactive oxygen species
Introduction
Mitochondria are central integrators for and transducers of apoptotic signals in neurons. Mitochondria physiologically generate adenosine triphosphate through oxidative phosphorylation via the electron transport chain. Reactive oxygen species (ROS) generated from mitochondria mediate molecular signaling, such as p53. In mitochondria-dependent apoptosis, molecular signaling returns to mitochondria, then triggers the release of critical apoptotic activators and effectors of cell death, such as cytochrome c or apoptosis-inducing factor, from the mitochondrial intermembrane space.
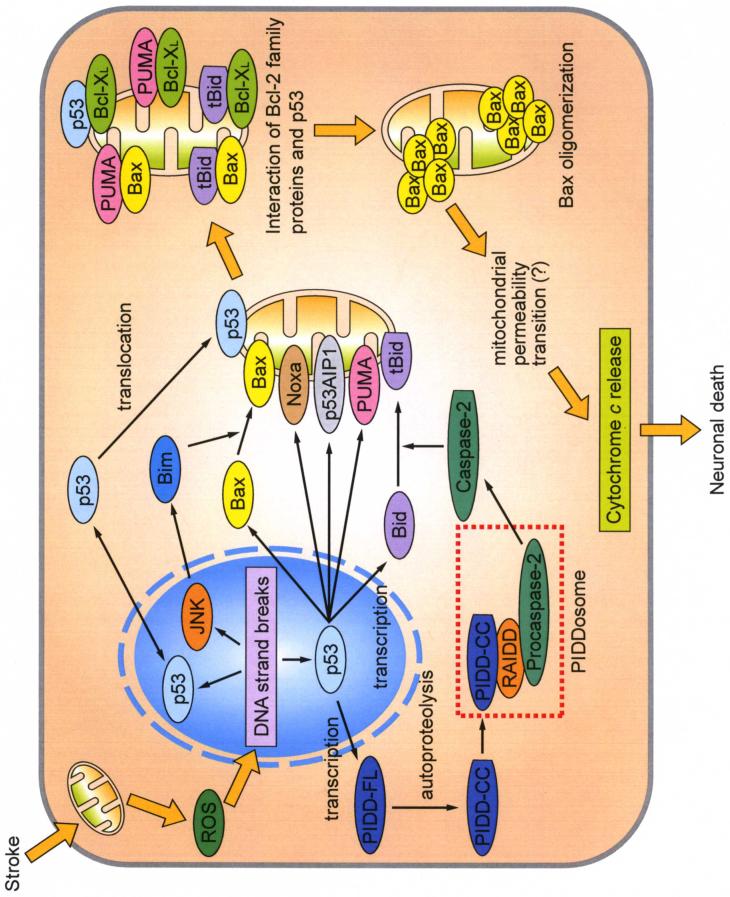
p53 is the master regulator of cell death by inducing apoptosis (Schmitt et al. 2002). p53 encodes a sequence-specific transcription factor that controls apoptosis-related gene expression. Bcl-2-associated X protein (Bax) (Miyashita and Reed 1995), BH3 interacting domain death agonist (Bid) (Wang et al. 1996; Sax et al. 2002), NADPH oxidase activator 1 (Noxa) (Oda et al. 2000b), p53 acetate-induced protein 1 (p53AIP1) (Oda et al. 2000a), and p53-upregulated modulator of apoptosis (PUMA) (Nakano and Vousden 2001; Yu et al. 2001), all of which are products of p53, act directly on mitochondria and induce apoptosis. p53-induced protein with a death domain (PIDD), which is also a product of p53, activates caspase-2, resulting in the activation of mitochondria-dependent apoptosis (Tinel and Tschopp 2004; Berube et al. 2005; Ren et al. 2005; Seth et al. 2005). Moreover, p53 mediates apoptosis in a transcription-independent manner (Chipuk et al. 2004) (Fig. 1).
Fig. 1.
Involvement of p53 signaling after ROS generation. After ROS generation from mitochondria, p53 transcriptionally generates pro-apoptotic proteins such as Bax, Noxa, p53AIP1, PUMA, and Bid. These products act directly on mitochondria. Mitochondrial translocation of Bax is promoted by JNK through transcriptional activation of Bim. Full-length PIDD (PIDD-FL) is also transcriptionally upregulated by p53. PIDD-CC, a fragment of PIDD-FL cleaved by autoproteolysis, activates caspase-2 through the formation of the PIDDosome, which precedes Bid truncation and translocation to mitochondria. Moreover, p53 translocates to the mitochondrial membrane and activates the mitochondria-dependent apoptotic pathway in a transcription-independent manner. BH3-only proteins and p53 interact with both pro-apoptotic Bax and anti-apoptotic Bcl-XL on the mitochondrial membrane. This interaction causes Bax oligomerization and activation, which triggers cytochrome c release, leading to neuronal death. tBid, truncated Bid.
Recent findings demonstrate that p53 is involved in neuronal death that occurs with stroke and neurodegeneration (Crumrine et al. 1994; Li et al. 1994; Tomasevic et al. 1999; Saito et al. 2005; Endo et al. 2006a). Overexpression of copper/zinc-superoxide dismutase (SOD1) downregulated PUMA (Niizuma et al. 2009), suggesting a functional relationship between oxidative stress and the p53 signaling pathway. Here, we discuss the role of mitochondrial dysfunction and oxidative stress as determinants of neuronal death after stroke and neurodegeneration, focusing on Bax, PUMA, PIDD, transcription-independent p53 translocation, and SOD1 overexpression.
Bax signaling pathway
Bax has an extensive amino acid homology with Bcl-2. Bax homodimerizes and forms heterodimers with Bcl-2 (Oltvai et al. 1993). Cell fractionation and confocal microscopy showed that Bax localized in the cytosol of most cells, although it has the C-terminal putative transmembrane domain, similar to that of Bcl-2 (Hsu et al. 1997). With apoptotic stimuli, Bax is post-transcriptionally activated, then it oligomerizes and translocates to mitochondria. Mitochondrial Bax triggers cytochrome c release from mitochondria (Gross et al. 1998; Fiskum et al. 1999).
Bax is known to have roles in neuronal death. Bax mRNA was upregulated after transient global cerebral ischemia (tGCI) (Honkaniemi et al. 1996). Bax protein levels increased after tGCI (Krajewski et al. 1995) and focal cerebral ischemia (FCI) (Gillardon et al. 1996). Recent studies indicate that Bax transcription was regulated by p53, and translocation was mediated by c-Jun N-terminal kinase (JNK) in focal ischemia and experimental Parkinson’s disease (Okuno et al. 2004; Perier et al. 2007). Bax interacts with truncated Bid, Bim, or PUMA, which triggers cytochrome c release in neurons (Desagher et al. 1999; Okuno et al. 2004; Niizuma et al. 2009).
In summary, apoptotic stimuli cause Bax to increase and translocate to mitochondria. Mitochondrial Bax interacts with other Bcl-2 family proteins, which triggers cytochrome c release (Fig. 1).
PUMA signaling pathway
PUMA was originally identified as a direct target of p53 with two putative p53 binding sites (Nakano and Vousden 2001; Yu et al. 2001). PUMA has been reported to be a strong inducer of apoptosis. In an in vitro study, PUMA expression caused rapid apoptosis (Yu et al. 2001) and PUMA inhibition by antisense oligonucleotide reduced apoptosis (Nakano and Vousden 2001). PUMA induced apoptosis through a mitochondria-dependent pathway (Nakano and Vousden 2001; Yu et al. 2001).
Recent reports have demonstrated that PUMA induces apoptosis by interacting with anti- or pro-apoptotic proteins (Nakano and Vousden 2001; Yu et al. 2001; Chen et al. 2005; Kim et al. 2006; Steckley et al. 2007). PUMA can interact with multiple Bcl-2 family members through the BH3 domain (Chen et al. 2005). It localized to mitochondria and interacted with both pro-apoptotic Bax (Kim et al. 2006; Steckley et al. 2007) and anti-apoptotic Bcl-2 or Bcl-XL (Nakano and Vousden 2001; Yu et al. 2001) through a BH3 domain, followed by cytochrome c release and caspase activation.
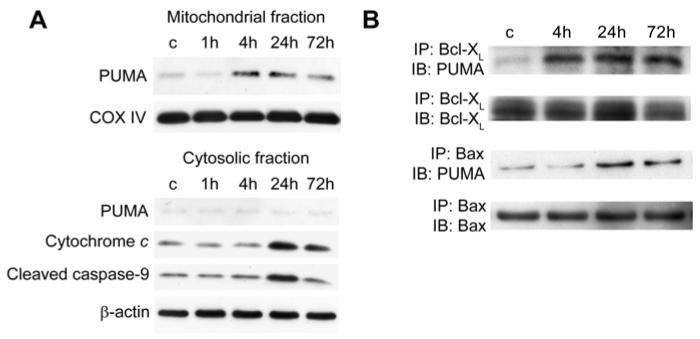
PUMA is also known to have important roles in neuronal apoptosis. Its overexpression induced apoptosis in primary neurons (Cregan et al. 2004), and PUMA nullizygous neurons are resistant to araC-induced apoptosis (Wyttenbach and Tolkovsky 2006). PUMA mediated oxidative stress-induced neuronal apoptosis through cytochrome c release and caspase activation in a primary culture of mouse neurons (Steckley et al. 2007). It also mediated camptothecin-induced neuronal death in a primary mouse neuron culture (Uo et al. 2007). PUMA regulated neuronal death after tGCI (Reimertz et al. 2003; Niizuma et al. 2009) (Fig. 2).
Fig. 2.
Mitochondrial PUMA upregulation after tGCI. (A) Western blot analysis shows that mitochondrial PUMA increased 4 and 24 h after tGCI, followed by cytosolic upregulation of cleaved caspase-9 and cytochrome c release. β-actin and cytochrome oxidase subunit IV (COX IV) analyses are shown as internal controls. c, control. (B) Coimmunoprecipitation analyses show that PUMA immunoreactivity precipitated by Bcl-XL or Bax increased after tGCI. Bcl-XL precipitated by Bcl-XL, and Bax precipitated by Bax were used to show equal precipitation. IP, immunoprecipitation; IB, immunoblotting. (Data modified from Niizuma et al. 2009.)
In summary, PUMA is induced by p53, and interacts with pro-apoptotic and anti-apoptotic Bcl-2 family proteins, resulting in cytochrome c release (Fig. 1).
PIDD signaling pathway
PIDD was also identified as a target of p53 (Lin et al. 2000). Since PIDD overexpression in p53-deficient human cell lines induces cell-cycle arrest and apoptosis, PIDD is considered to act downstream of p53. Full length PIDD is constitutively cleaved into an N-terminal fragment and a C-terminal fragment (PIDD-C) by autoproteolysis. PIDD-C is further cleaved into PIDD-CC by autoproteolysis (Tinel and Tschopp 2004; Tinel et al. 2007).
Evidence for the role of PIDD-CC in the activation of caspase-2 has been accumulating (Tinel and Tschopp 2004; Berube et al. 2005; Ren et al. 2005; Seth et al. 2005). PIDD-CC, receptor-interacting protein-associated ICH-1/CED-3 homologous protein with a death domain (RAIDD), and procaspase-2 form a large protein complex, which is referred to the PIDDosome, similar to the caspase-9—activating apoptosome complex (Tinel and Tschopp 2004). PIDD interacts with RAIDD through the death domain, and RAIDD interacts with caspase-2 through the caspase recruitment domain, resulting in the crystal structure of the PIDDosome (Park et al. 2007). Procaspase-2 was schematically dimerized and activated by the PIDDosome (Park et al. 2007). Similar to the caspase-9—activating apoptosome complex, the PIDDosome regulates stress-induced apoptosis (Tinel and Tschopp 2004).
In contrast to PIDD-CC, PIDD-C is thought to have an anti-apoptotic role. In a recent study, PIDD-C formed a protein complex with a nuclear factor-κB essential modulator and receptor-interacting protein 1. This activated the transcription factor nuclear factor-κB pathway in response to genotoxic stress (Janssens et al. 2005).
In neuronal death, PIDD-CC increased after tGCI, followed by caspase-2 activation and Bid cleavage (Niizuma et al. 2008). Truncated Bid interacts with Bax, exposing the N-terminus of Bax and inducing its oligomerization followed by release of pro-apoptotic proteins from mitochondria (Desagher et al. 1999).
In summary, PIDD is transcriptionally induced by p53, then cleaved by autoproteolysis. PIDD-CC forms the PIDDosome, which activates caspase-2. Caspase-2 cleaved Bid, followed by the interaction of Bax and truncated Bid, resulting in cytochrome c release (Fig. 1).
Transcription-independent p53 translocation
Most of the effects of p53 are ascribed to its function as a transcription factor. However, reports have suggested that p53 can also induce apoptosis independently of its transcriptional activity (Caelles et al. 1994; Bennett et al. 1998; Mihara et al. 2003). In response to certain death stimuli, a fraction of stabilized p53 rapidly translocates to mitochondria in some cell types (Marchenko et al. 2000; Mihara et al. 2003; Erster et al. 2004). In p53 null cancer cells, exogenous p53 targeted to mitochondria induced apoptosis and suppressed colony formation in a transcription-independent manner (Mihara et al. 2003). Furthermore, endogenous mitochondrial p53 forms inhibitory complexes with anti-apoptotic Bcl-XL and Bcl-2 proteins, which cause cytochrome c release and caspase activation (Mihara et al. 2003). Mitochondrial translocation of p53 launches a rapid pro-apoptotic response in a transcription-independent manner that jump-starts and amplifies the slower transcription-dependent response (Erster et al. 2004). This translocation may be regulated by the Akt-Mdm2 pathway through monoubiquitylation of p53 (Marchenko et al. 2007).
In neuronal cell death, p53 translocated to mitochondria and interacted with anti-apoptotic Bcl-XL, followed by cytochrome c release after tGCI (Endo et al. 2006a). Inhibition of p53 translocation caused by a specific dosage of the p53 inhibitor, pifithrin-α, resulted in neuroprotection of the hippocampal CA1 subregion against cerebral ischemia and reperfusion (Endo et al. 2006a).
In summary, p53 can induce apoptosis in a transcription-independent manner by interacting with Bcl-XL after ischemia (Fig. 1).
SOD1 overexpression
Evidence is accumulating in support of the idea that activation of p53 signaling pathways, which precedes release of pro-apoptotic proteins from mitochondria, can cause apoptosis in ischemic neurons. However, the upstream events that lead to p53 signaling and neuronal death are unclear. ROS formation during reperfusion after cerebral ischemia in the mitochondria appears to be one such event. This is supported by the finding that p53 target genes upregulated in response to elevated oxidative stress in liver samples (Han et al. 2008). ROS cause DNA damage, which activates DNA-dependent kinase and ataxia telangiecta protein, resulting in phosphorylation of p53 at specific serine residues (Nakagawa et al. 1999; Shangary et al. 2000).
Specific scavengers of ROS, such as SOD1, may play a major role in modulating death signaling. SOD1 is an antioxidant isoenzyme mainly localized in the cytosol that dismutates superoxide anions to hydrogen peroxide (Fridovich 1975). SOD1 is constitutively present in all cells (Huang et al. 1999). In animals that overexpress SOD1, cytochrome c release and neuronal death were highly inhibited after FCI (Kinouchi et al. 1991; Chan 1996; Fujimura et al. 2000), tGCI (Murakami et al. 1997; Chan et al. 1998; Endo et al. 2006b), and subarachnoid hemorrhage (Endo et al. 2007). The phospho-Akt survival pathway was significantly upregulated in SOD1-overexpressing animals compared with wild-type animals (Noshita et al. 2003; Endo et al. 2006b, 2007). In contrast to the survival pathway, p53 upregulation was inhibited by SOD1 overexpression after FCI (Saito et al. 2005). Moreover, PUMA upregulation was inhibited in SOD1-overexpressing animals after tGCI (Niizuma et al. 2009), suggesting that oxidative stress may modulate pro-survival Akt signaling and pro-death p53 signaling that determine death or survival of ischemic neurons.
In contrast to SOD1 overexpression, SOD1 homozygous mutants (SOD1-/-) and heterozygous mutants (SOD1-/+) showed high mortality, increased infarct volume, and greater apoptotic neuronal cell death after FCI (Kondo et al. 1997). SOD1 deficiency also showed increased neuronal death after tGCI (Kawase et al. 1999).
These results cumulatively suggest that oxidative stress, known to be generated during reperfusion following an ischemic event, is associated with cell survival signaling such as Akt, cell death pathways such as p53, and the determination of subsequent neuronal survival or death.
Conclusion
Oxidative stress may regulate p53-dependent transcription, p53 translocation, and pro-survival Akt signaling through phosphorylation, at least in part. Decreasing oxidative stress by SOD1 overexpression results in neuroprotection. Mitochondrial dysfunction and oxidative stress may determine neuronal death/survival after stroke and neurodegeneration.
Acknowledgements
We thank Liza Reola and Bernard Calagui for technical assistance, Cheryl Christensen for editorial assistance, and Elizabeth Hoyte for assistance with figure preparation. Supported by National Institutes of Health grants P50 NS014543, R01 NS025372, R01 NS036147, and R01 NS038653.
Abbreviations used
- ROS
reactive oxygen species
- Bax
Bcl-2-associated X protein
- Bid
BH3 interacting domain death agonist
- Noxa
NADPH oxidase activator 1
- p53AIP1
p53 acetate-induced protein 1
- PUMA
p53-upregulated modulator of apoptosis
- PIDD
p53-induced protein with a death domain
- SOD1
copper/zinc-superoxide dismutase
- tGCI
transient global cerebral ischemia
- FCI
focal cerebral ischemia
- JNK
c-Jun N-terminal kinase
- RAIDD
receptor-interacting protein-associated ICH-1/CED-3 homologous protein with a death domain
References
- Bennett M, Macdonald K, Chan S-W, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290–293. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- Berube C, Boucher L-M, Ma W, Wakeham A, Salmena L, Hakem R, Yeh W-C, Mak TW, Benchimol S. Apoptosis caused by p53-induced protein with death domain (PIDD) depends on the death adapter protein RAIDD. Proc. Natl. Acad. Sci. USA. 2005;102:14314–14319. doi: 10.1073/pnas.0506475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caelles C, Helmberg A, Karin M. p53-Dependent apoptosis in the absence of transcriptional activation of p53-target genes [Letter] Nature. 1994;370:220–223. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, Reola L, Carlson E, Epstein CJ. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J. Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Cregan SP, Arbour NA, MacLaurin JG, Callaghan SM, Fortin A, Cheung ECC, Guberman DS, Park DS, Slack RS. p53 activation domain 1 is essential for PUMA upregulation and p53-mediated neuronal cell death. J. Neurosci. 2004;24:10003–10012. doi: 10.1523/JNEUROSCI.2114-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumrine RC, Thomas AL, Morgan PF. Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J. Cereb. Blood Flow Metab. 1994;14:887–891. doi: 10.1038/jcbfm.1994.119. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou J-C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Kamada H, Nito C, Nishi T, Chan PH. Mitochondrial translocation of p53 mediates release of cytochrome c and hippocampal CA1 neuronal death after transient global cerebral ischemia in rats. J. Neurosci. 2006a;26:7974–7983. doi: 10.1523/JNEUROSCI.0897-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Nito C, Kamada H, Nishi T, Chan PH. Activation of the Akt/GSK3β signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2006b;26:1479–1489. doi: 10.1038/sj.jcbfm.9600303. [DOI] [PubMed] [Google Scholar]
- Endo H, Nito C, Kamada H, Yu F, Chan PH. Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3β survival signaling. J. Cereb. Blood Flow Metab. 2007;27:975–982. doi: 10.1038/sj.jcbfm.9600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erster S, Mihara M, Kim RH, Petrenko O, Moll UM. In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol. Cell. Biol. 2004;24:6728–6741. doi: 10.1128/MCB.24.15.6728-6741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J. Cereb. Blood Flow Metab. 1999;19:351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu. Rev. Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Morita-Fujimura Y, Noshita N, Sugawara T, Kawase M, Chan PH. The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J. Neurosci. 2000;20:2817–2824. doi: 10.1523/JNEUROSCI.20-08-02817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillardon F, Lenz C, Waschke KF, Krajewski S, Reed JC, Zimmermann M, Kuschinsky W. Altered expression of Bcl-2, Bcl-X, Bax, and c-Fos colocalizes with DNA fragmentation and ischemic cell damage following middle cerebral artery occlusion in rats. Mol. Brain Res. 1996;40:254–260. doi: 10.1016/0169-328x(96)00059-9. [DOI] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han E-S, Muller FL, Pérez VI, et al. The in vivo gene expression signature of oxidative stress. Physiol. Genomics. 2008;34:112–126. doi: 10.1152/physiolgenomics.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkaniemi J, Massa SM, Breckinridge M, Sharp FR. Global ischemia induces apoptosis-associated genes in hippocampus. Mol. Brain Res. 1996;42:79–88. doi: 10.1016/s0169-328x(96)00121-0. [DOI] [PubMed] [Google Scholar]
- Hsu Y-T, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T-T, Carlson EJ, Raineri I, Gillespie AM, Kozy H, Epstein CJ. The use of transgenic and mutant mice to study oxygen free radical metabolism. Ann. N. Y. Acad. Sci. 1999;893:95–112. doi: 10.1111/j.1749-6632.1999.tb07820.x. [DOI] [PubMed] [Google Scholar]
- Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-κB activation in response to DNA damage. Cell. 2005;123:1079–1092. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Kawase M, Murakami K, Fujimura M, Morita-Fujimura Y, Gasche Y, Kondo T, Scott RW, Chan PH. Exacerbation of delayed cell injury after transient global ischemia in mutant mice with CuZn superoxide dismutase deficiency. Stroke. 1999;30:1962–1968. doi: 10.1161/01.str.30.9.1962. [DOI] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu H-C, Jeffers JR, Zambetti GP, Hsieh JJ-D, Cheng EH-Y. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc. Natl. Acad. Sci. USA. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Reaume AG, Huang T-T, et al. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J. Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski S, Mai JK, Krajewska M, Sikorska M, Mossakowski MJ, Reed JC. Upregulation of Bax protein levels in neurons following cerebral ischemia. J. Neurosci. 1995;15:6364–6376. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chopp M, Zhang ZG, Zaloga C, Niewenhuis L, Gautam S. p53-immunoreactive protein and p53 mRNA expression after transient middle cerebral artery occlusion in rats. Stroke. 1994;25:849–855. doi: 10.1161/01.str.25.4.849. [DOI] [PubMed] [Google Scholar]
- Lin Y, Ma W, Benchimol S. Pidd, a new death-domain–containing protein, is induced by p53 and promotes apoptosis [Letter] Nat. Genet. 2000;26:124–127. doi: 10.1038/79102. [DOI] [PubMed] [Google Scholar]
- Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J. Biol. Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- Marchenko ND, Wolff S, Erster S, Becker K, Moll UM. Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J. 2007;26:923–934. doi: 10.1038/sj.emboj.7601560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Epstein CJ, Chan PH. Overexpression of CuZn-superoxide dismutase reduces hippocampal injury after global ischemia in transgenic mice [Erratum: Stroke 28:2573, 1997] Stroke. 1997;28:1797–1804. doi: 10.1161/01.str.28.9.1797. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Taya Y, Tamai K, Yamaizumi M. Requirement of ATM in phosphorylation of the human p53 protein at serine 15 following DNA double-strand breaks. Mol. Cell. Biol. 1999;19:2828–2834. doi: 10.1128/mcb.19.4.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Nito C, Myer DJ, Chan PH. The potential role of PUMA in delayed death of hippocampal CA1 neurons after transient global cerebral ischemia. Stroke. 2009 doi: 10.1161/STROKEAHA.108.524447. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Nito C, Myer DJ, Kim GS, Chan PH. The PIDDosome mediates delayed death of hippocampal CA1 neurons after transient global cerebral ischemia in rats. Proc. Natl. Acad. Sci. USA. 2008;105:16369–16374. doi: 10.1073/pnas.0806222105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshita N, Sugawara T, Lewén A, Hayashi T, Chan PH. Copper-zinc superoxide dismutase affects Akt activation after transient focal cerebral ischemia in mice. Stroke. 2003;34:1513–1518. doi: 10.1161/01.STR.0000072986.46924.F4. [DOI] [PubMed] [Google Scholar]
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000a;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Oda K, Arakawa H, Tanaka T, et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell. 2000b;102:849–862. doi: 10.1016/s0092-8674(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J. Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Park HH, Logette E, Raunser S, Cuenin S, Walz T, Tschopp J, Wu H. Death domain assembly mechanism revealed by crystal structure of the oligomeric PIDDosome core complex. Cell. 2007;128:533–546. doi: 10.1016/j.cell.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perier C, Bové J, Wu D-C, et al. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2007;104:8161–8166. doi: 10.1073/pnas.0609874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimertz C, Kögel D, Rami A, Chittenden T, Prehn JHM. Gene expression during ER stress—induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J. Cell Biol. 2003;162:587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Shi M, Liu R, Yang Q-H, Johnson T, Skarnes WC, Du C. The Birc6 (Bruce) gene regulates p53 and the mitochondrial pathway of apoptosis and is essential for mouse embryonic development. Proc. Natl. Acad. Sci. USA. 2005;102:565–570. doi: 10.1073/pnas.0408744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Hayashi T, Okuno S, Nishi T, Chan PH. Modulation of p53 degradation via MDM2-mediated ubiquitylation and the ubiquitin-proteasome system during reperfusion after stroke: role of oxidative stress. J. Cereb. Blood Flow Metab. 2005;25:267–280. doi: 10.1038/sj.jcbfm.9600028. [DOI] [PubMed] [Google Scholar]
- Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS. BID regulation by p53 contributes to chemosensitivity. Nat. Cell Biol. 2002;4:842–849. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP. p53-dependent caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in renal tubular epithelial cell injury. J. Biol. Chem. 2005;280:31230–31239. doi: 10.1074/jbc.M503305200. [DOI] [PubMed] [Google Scholar]
- Schmitt CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–298. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Shangary S, Brown KD, Adamson AW, Edmonson S, Ng B, Pandita TK, Yalowich J, Taccioli GE, Baskaran R. Regulation of DNA-dependent protein kinase activity by ionizing radiation-activated Abl kinase is an ATM-dependent process. J. Biol. Chem. 2000;275:30163–30168. doi: 10.1074/jbc.M004302200. [DOI] [PubMed] [Google Scholar]
- Steckley D, Karajgikar M, Dale LB, et al. Puma is a dominant regulator of oxidative stress induced Bax activation and neuronal apoptosis. J. Neurosci. 2007;27:12989–12999. doi: 10.1523/JNEUROSCI.3400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- Tinel A, Janssens S, Lippens S, Cuenin S, Logette E, Jaccard B, Quadroni M, Tschopp J. Autoproteolysis of PIDD marks the bifurcation between pro-death caspase-2 and pro-survival NF-κB pathway. EMBO J. 2007;26:197–208. doi: 10.1038/sj.emboj.7601473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasevic G, Shamloo M, Israeli D, Wieloch T. Activation of p53 and its target genes p21WAF1/Cip1 and PAG608/Wig-1 in ischemic preconditioning. Mol. Brain Res. 1999;70:304–313. doi: 10.1016/s0169-328x(99)00146-1. [DOI] [PubMed] [Google Scholar]
- Uo T, Kinoshita Y, Morrison RS. Apoptotic actions of p53 require transcriptional activation of PUMA and do not involve a direct mitochondrial/cytoplasmic site of action in postnatal cortical neurons. J. Neurosci. 2007;27:12198–12210. doi: 10.1523/JNEUROSCI.3222-05.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Yin X-M, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Wyttenbach A, Tolkovsky AM. The BH3-only protein Puma is both necessary and sufficient for neuronal apoptosis induced by DNA damage in sympathetic neurons. J. Neurochem. 2006;96:1213–1226. doi: 10.1111/j.1471-4159.2005.03676.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]