Abstract
Tumor associated microtubule associated protein (TMAP), also known as cytoskeleton associated protein 2 (CKAP2) is a mitotic spindle-associated protein whose expression is cell cycle-regulated and also frequently deregulated in cancer cells. Two monoclonal antibodies (mAbs) against TMAP/CKAP2 were produced: B-1-13 and D-12-3. Interestingly, the reactivity of mAb D-12-3 to TMAP/CKAP2 was markedly decreased specifically in mitotic cell lysate. The epitope mapping study showed that mAb D-12-3 recognizes the amino acid sequence between 569 and 625 and that phosphorylation at T596 completely abolishes the reactivity of the antibody, suggesting that the differential reactivity originates from the phosphorylation status at T596. Immunofluorescence staining showed that mAb D-12-3 fails to detect TMAP/CKAP2 in mitotic cells between prophase and metaphase, but the staining becomes evident again in anaphase, suggesting that phosphorylation at T596 occurs transiently during early phases of mitosis. These results suggest that the cellular functions of TMAP/CKAP2 might be regulated by timely phosphorylation and dephosphorylation during the course of mitosis.
Keywords: antibodies, monoclonal; cell cycle; CKAP2 protein, human; fluorescent antibody technique, direct; phosphorylation
Introduction
Tumor associated microtubule associated protein (TMAP), also known as cytoskeleton associated protein 2 (CKAP2) is frequently upregulated in various malignancies, including gastric adenocarcinoma, diffuse B-cell lymphoma and cutaneous T-cell lymphoma (Maouche-Chretien et al., 1998; Eichmuller et al., 2001; Bae et al., 2003), and also detected in various cancer cell lines (Bae et al., 2003; Jin et al., 2004). Knockdown of TMAP/CKAP2 reduces cell proliferation, whereas constitutive overexpression at a moderate level enhances proliferation of human foreskin fibroblasts (HFFs) and NIH 3T3 cells (Jeon et al., 2006), indicating that TMAP/CKAP2 is essential for normal cell growth. However, the exact cellular functions of TMAP/CKAP2 remain unknown. TMAP/CKAP2 is primarily localized to microtubules and centrosomes during interphase and to mitotic spindles and spindle poles during mitosis (Maouche-Chretien et al., 1998; Bae et al., 2003; Jin et al., 2004; Hong et al., 2007). During late stages of mitosis, however, TMAP/CKAP2 localizes near the chromatin region and to the midbody microtubules (Hong et al., 2007).
While the level of TMAP/CKAP2 expression is low or undetectable in growth-arrested primary HFFs and NIH 3T3 cells, its expression starts to incline as cells enter the cell cycle and peaks at G2/M phases (Jeon et al., 2006). Moreover, its cell cycl-edependent expression pattern coincides with those of various known mitotic regulators (Iyer et al., 1999; Whitfield et al., 2002), which suggests a possibility that the cellular functions of TMAP/CKAP2 may pertain to mitotic processes. In support of this, recent reports have shown that TMAP/CKAP2 has microtubule-stabilizing properties and may contribute to the assembly and maintenance of mitotic spindles by regulating microtubule dynamics during mitosis (Jin et al., 2004; Hong et al., 2007). In addition, it has been recently reported that TMAP/CKAP2 is a novel substrate of the anaphase promoting complex (APC) (Hong et al., 2007). TMAP/CKAP2 is degraded during mitotic exit by the APC-Cdh1 in a KEN box-dependent manner. Cells expressing a non-degradable mutant of TMAP/CKAP2 exhibit delayed mitosis or defects in spindle formation in the following mitosis and often fail to complete cytokinesis. These results suggest that TMAP/CKAP2 is a potential regulator of mitotic spindle functions and that proper regulation of its protein level is functionally important for completion of cytokinesis and for proper maintenance of spindle bipolarity. Thus, the increased level of TMAP/CKAP2 protein in cancers and subsequent deregulation of the spindle function may contribute to abnormal cell divisions and chromosomal instability frequently observed in cancers.
It is well known that various aspects of the cell cycle are regulated by a number of kinases via inducing serine/threonine phosphorylation of specific substrates (Marumoto et al., 2005). Among these, Cdk1, Aurora kinases, Nek2, and Plk1 are some of the major mitotic kinases which regulate mitotic processes (Carmena and Earnshaw, 2003; Murray, 2004; Marumoto et al., 2005; Rapley et al., 2005). To date, phosphorylation kinetics of TMAP/CKAP2 during mitosis has never been reported. In the present study, cell cycle phase-specific phosphorylation of TMAP/CKAP2 at T596 was demonstrated using monoclonal antibodies. The level of phosphorylation at T596 increased as cells entered prophase and remained elevated up to metaphase, and the de-phosphorylated form became dominant again starting at anaphase.
Materials and Methods
Production of monoclonal antibodies
Open reading frame of mouse TMAP/CKAP2 (mTMAP/CKAP2) was cloned into pET-28(a) vector. Over-expressed protein was purified by His-tag column chromatography, and was used for immunization. Balb/c mice were immunized three times at a 2-week interval, each time with 100 µg of purified recombinant mouse TMAP/CKAP2. On the fifth day after the final injection, cell fusion was performed with spleen cells and sp2/O-Ag14 myeloma cells as described by Lim et al. (2006). Positive clones were selected by enzyme immunoassay.
Expression constructs
Generation of a green fluorescent protein (GFP) fusion TMAP/CKAP2 construct (pEGFP-C2 CKAP2) has been described previously (Bae et al., 2003). A myc-tagged TMAP/CKAP2 expression construct (pCMV6-myc-TMAP/CKAP2) or pCAGGS-mTMAP/CKAP2 (for mTMAP/CKAP2) was generated by subcloning the human TMAP/CKAP2 or mTMAP/CKAP2 into the pCMV6-myc vector (kindly provided by Dr. M. J. Hahn, Sungkyunkwan University) or pCAGGS vector (kindly provided by Dr. H.-W. Lee, Yonsei University). Deletion mutant constructs or point mutants for human TMAP/CKAP2 used in the present study were generated by PCR and subcloned into pCMV6-myc vector or pEGFP-C2 (Clontech) vector. Characterization of human TMAP/CKAP2-specific siRNA has been previously described (Jeon et al., 2006). For siRNA-mediated silencing of mTMAP/CKAP2, the following target sequence was used: 5'-AAGATACTGACCAGCGCAGAT-3'. The mTMAP/CKAP2 siRNA was synthesized at Dharmacon.
Cell culture and transfection
HeLa, HEK 293, and C2C12 (mouse myoblast) cells were cultured in DMEM containing 10% FBS. For transfection of plasmid DNAs and siRNAs, Lipofectamine Plus (Invitrogen) and DharmaFECT1 (Dharmacon) were used, respectively, according to manufacturer's instructions.
Western and dot blot analysis
Western blot analyses of cells transfected with siRNAs, or various full-length or deletion mutant constructs of TMAP/CKAP2 described in the text were done in the following manner. Transfected or treated cells were harvested by boiling in Laemmli buffer. The protein concentration was determined by BCA protein assay. Protein samples (25 or 50 µg) were resolved by SDS-PAGE and transferred to a PVDF membrane (Millipore). Following 30 min of incubation in the blocking solution (5% skim milk in TBST) at room temperature, the blot was incubated with an appropriate dilution of the primary antibody (in the blocking solution) for 1 h at room temperature or overnight at 4℃. The blot was washed twice in TBST, and incubated with an appropriate HRP-conjugated secondary antibody for 1 h at room temperature. The antibody-antigen complex was then detected using SuperSignal West Pico solution (Pierce). For dot blot analysis, whole cell lysate (5 µg) obtained from HighFive insect cells uninfected or infected with hTMAP/CKAP2-expressing baculovirus was spotted onto a nitrocellulose membrane (Schleicher & Schuell). The dot blot was then processed through the same procedures described above.
Flow cytometry
Cells were fixed in cold 75% ethanol overnight or longer. They were spun down, washed in PBS containing 1% BSA, and incubated in the propidium iodide (PI) solution containing 50 µg/ml PI, 0.1% sodium citrate, 0.3% NP-40, RNase A (50 µg/ml) and 1× PBS for 1 h at 37℃. DNA profiles of PI-stained cells were obtained using a FACSCalibur system (BectonDickinson) and analyzed using CELLQuest software, ver. 3.3 (BectonDickinson) as previously reported (Sihn et al., 2005).
Amino acid sequence alignment
Amino acid sequence corresponding to 569-625 of human TMAP/CKAP2 was aligned for comparison with the homologous regions of TMAP/CKAP2 from the indicated species using ClustalW program.
Synthetic peptides
Both nonphosphorylated and phosphorylated peptides corresponding to the region surrounding each potential phosphorylation residue (i.e., T578 and T596) were synthesized at Peptron (Daejeon, Korea). The following peptides were synthesized: CDPTHDVKTPNTETRT (T578) and CIKYNVSTTPYLQSWK (T596) (phosphorylated residues are underlined). For dot blot analysis, each peptide (1 µg) was spotted onto a nitrocellulose membrane.
Nocodazole treatment
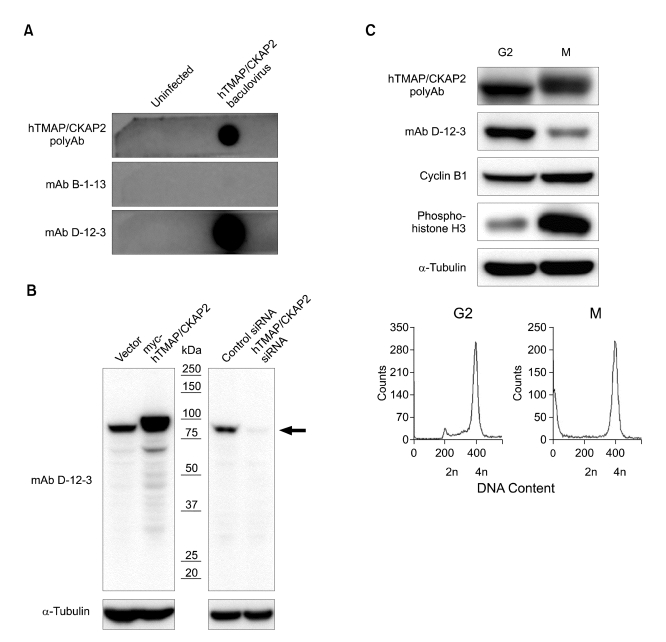
HeLa cells were treated with 1 µM nocodazole (Sigma) for 16-20 h to arrest the cells at G2 or M phase. Cells at M phase were collected by gentle pipetting, and the remaining adherent cells at G2 were harvested separately. Cells were then washed in PBS, and either lysed in Laemmli buffer or fixed in cold 75% ethanol. The cell cycle phase of each cell population was confirmed by Western blot analysis (for cyclin B1, a G2/M marker and phospho-histone H3, an M phase-specific marker) and flow cytometry (for DNA content) (see Figure 2C).
Figure 2.
Reactivity of mAb D-12-3 to human TMAP/CKAP2. (A) Lysate of insect cells uninfected or infected with baculovirus expressing human TMAP/CKAP2 was used for dot blot analysis. While mAb B-1-13 was unreactive to human TMAP/CKAP2, mAb D-12-3 strongly cross-reacted with human TMAP/CKAP2. Rabbit polyclonal antibody against human TMAP/CKAP2 (hTMAP/CKAP2 PolyAb) was used as a positive control. (B) Western blot analysis was done on HEK 293A cells transfected with pCMV6-myc vector (Vector), pCMV6-myc-hTMAP/CKAP2, control luciferase siRNA (Control siRNA), or hTMAP/CKAP2 siRNA. mAb D-12-3 specifically detected an 80-kDa band (endogenous human TMAP/CKAP2 protein) in the vector control lane, as well as a protein band of a slightly larger molecular weight for the exogenously introduced myc-tagged hTMAP/CKAP2 in the neighboring lane. The intensity of the 80-kDa band was decreased by the treatment of human TMAP/CKAP2 siRNA (arrow), confirming the specificity of the antibody. (C) Nocodazole arrested HeLa cells at G2 or M phase were analyzed by Western blot and flow cytometry (FACS). The relative reactivity of mAb D-12-3 to TMAP/CKAP2 was significantly reduced in HeLa cells at the mitotic phase (M) when compared to cells in G2 phase. The levels of cyclin B1 (a G2/M marker) and phospho-histone H3 (an M phase-specific marker) and the analysis of DNA content show that each cell population is enriched at the indicated cell cycle phase.
Antibodies
Generation of rabbit polyclonal antiserum to human TMAP/CKAP2 has been previously described (Bae et al., 2003). Rabbit polyclonal anti-α-tubulin antibody was purchased from Abcam (ab18251). Mouse monoclonal antibodies against α-tubulin (clone B-5-1-2) and cyclin B1 (clone GNS1) were purchased from Sigma and Santa Cruz, respectively. Rabbit polyclonal anti-phospho-histone H3 (Ser10) was purchased from Upstate. Alexa488-conjugated anti-rabbit IgG was purchased from Molecular Probes. Cy3-conjugated anti-mouse IgG was purchased from Rockland.
Immunofluorescence staining and image acquisition
C2C12 (mouse myoblast) cells grown on glass coverslips were fixed in 3.7% formaldehyde in PBS and permeabilized in 0.25% Triton X-100 in PBS. Following incubation in the blocking solution (5% BSA in PBS), the cells were incubated in primary antibody solution (diluted in the blocking solution). They were then incubated in a solution of secondary antibodies conjugated to fluorochromes (diluted in the blocking solution). They were finally counterstained with 4',6-diamidino-2-phenylindole (DAPI). Fluorescence images were viewed and acquired using a LSM 510 confocal microscope (Carl Zeiss). AxioVision 4.3 software (Carl Zeiss) was used for processing images.
Results
Production of monoclonal antibodies against TMAP/CKAP2
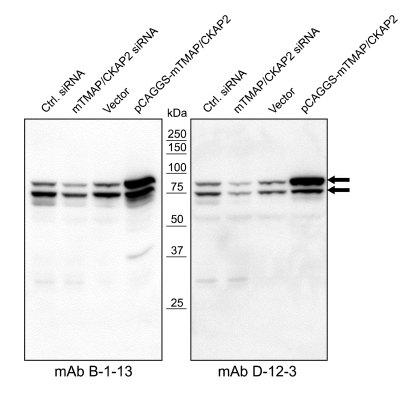
By immunizing recombinant mouse TMAP/CKAP2 (mTMAP/CKAP2) protein from bacterial over-expression, two monoclonal antibodies against mTMAP/CKAP2, B-1-13 and D-12-3, were produced. The specificity was checked by Western blot on protein extracts from C2C12 (mouse myoblast) cells. The antibodies detected two distinct bands of mTMAP/CKAP2 protein with approximate molecular weights of 75 and 85 kDa (Figure 1). Of note, it has been previously reported that mTMAP/CKAP2 protein always appears as double bands regardless of the cell cycle phase, whereas the human counterpart appears as a single band (Bae et al., 2003; Hong et al., 2007), and the cause of such difference is currently unknown. The detection signals for both protein bands were decreased by the treatment with mTMAP/CKAP2 siRNA and increased by the over-expression of mTMAP/CKAP2 (Figure 1), further confirming the specificity of the antibodies. The reactivity of the monoclonal antibodies to human TMAP/CKAP2 was then examined. In dot blot analysis with human TMAP/CKAP2 expressed in insect cells, only mAb D-12-3 cross-reacted to human TMAP/CKAP2 (Figure 2A). Western blot analysis using human HEK 293 cell lysates confirmed that mAb D-12-3 detected one specific band (Figure 2B), and the specificity was confirmed by the increase in the signal intensity following overexpression of myc-tagged human TMAP/CKAP2 and the decrease in the intensity following the treatment with human TMAP/CKAP2-specific siRNA (Figure 2B).
Figure 1.
Production of monoclonal antibodies (B-1-13 and D-12-3) against mouse TMAP/CKAP2. Control luciferase siRNA (Ctrl. siRNA), TMAP/CKAP2 siRNA (mTMAP/CKAP2 siRNA), pCAGGS vector (Vector) or TMAP/CKAP2 overexpression vector (pCAGGS-mTMAP/CKAP2) was transfected into C2C12 (mouse myoblast) cells and analyzed by Western blot using mAb B-1-13 or D-12-3. Both antibodies detected two specific bands with molecular weights of 75 and 85 kDa (arrows). The intensities of both bands were decreased by TMAP/CKAP2 siRNA treatment and increased by overexpression of TMAP/CKAP2, which shows the specificity of the antibodies.
Cell cycle-dependent changes in the reactivity of mAb D-12-3
While studying the cell cycle-dependent changes in the TMAP/CKAP2 protein level using mAb D-12-3, we observed a dramatic reduction in the reactivity of the antibody to TMAP/CKAP2 as cells began to enter mitosis (data not shown). In order to further verify this observation, HeLa cells were treated with nocodazole overnight to arrest them at G2/M. The round cells arrested at M phase were collected by gentle pipetting, and the remaining adherent cells mostly at G2 were harvested separately. The cell cycle stage of each cell population was confirmed by both Western blot (for cyclin B1, a G2/M marker, and phospho-histone H3, a M phase-specific marker) and flow cytometry (for the DNA content) (Figure 2C). G2 and M phase cell lysates were then analyzed by Western blot using rabbit polyclonal human TMAP/CKAP2 antibody or mAb D-12-3. Interestingly, while the total TMAP/CKAP2 protein levels were not significantly different between G2 and M phase cell lysates when checked by the polyclonal antibody, there was a marked decrease in the reactivity of mAb D-12-3 to TMAP/CKAP2 specifically in the M phase cell lysate (Figure 2C). Based on this observation, we postulated that the decrease in antibody reactivity to M phase TMAP/CKAP2 could be due to an M phase-specific post-translational modification (e.g., phosphorylation).
Epitope mapping of mAb D-12-3
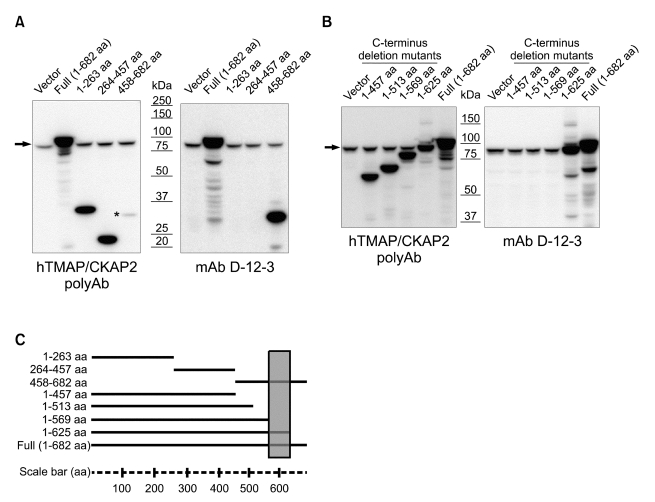
To further study the mechanism of the cell cycle-dependent changes in the reactivity of mAb D-12-3, an epitope mapping was carried out. For the epitope mapping, myc-tagged constructs of full-length or three different deletion mutants (containing amino acid sequences of 1-263, 264-457, or 458-682) of TMAP/CKAP2 were transfected into HeLa cells, and analyzed by Western blot. mAb D-12-3 specifically detected the C-terminal portion (corresponding to amino acids 458 and 682) of TMAP/CKAP2 protein (Figure 3A). In order to further narrow down the epitope region, additional myc-tagged C-terminus deletion mutants (containing amino acid sequences of 1-457, 1-513, 1-569, or 1-625) were tested against the antibody. On Western blot analysis, mAb D-12-3 specifically reacted with the deletion mutant containing 1-625 aa and the full-length protein, while remaining unreactive to the rest (Figure 3B). This result indicated that the epitope region resided within the amino acid sequence between 569 and 625 (Figure 3C).
Figure 3.
Epitope mapping of mAb D-12-3. (A) HeLa cells were transfected with pCMV6-myc vector (Vector), myc-tagged full-length (Full) or indicated deletion mutant construct of human TMAP/CKAP2 and lysates were analyzed by Western blot. Rabbit polyclonal antibody against human TMAP/CKAP2 (hTMAP/CKAP2 PolyAb) was used as a positive control. Of note, hTMAP/CKAP2 PolyAb does not detect the C-terminal portion efficiently (asterisk). mAb D-12-3 specifically detected the C-terminal portion of human TMAP/CKAP2 (corresponding to amino acids 458-682). The endogenous hTMAP/CKAP2 protein band is detected by both hTMAP/CKAP2 PolyAb and mAb D-12-3 (arrow). (B) Additional C-terminus deletion mutants of indicated lengths were expressed in HeLa cells and analyzed by Western blot. mAb D-12-3 showed strong reactivity towards the full-length protein and the mutant containing 1-625 aa, while remaining unreactive to the rest. The arrow indicates the endogenous hTMAP/CKAP2 protein band. (C) A representation of the deletion mutants tested in panels A and B. The epitope region for mAb D-12-3 is indicated by the shaded box.
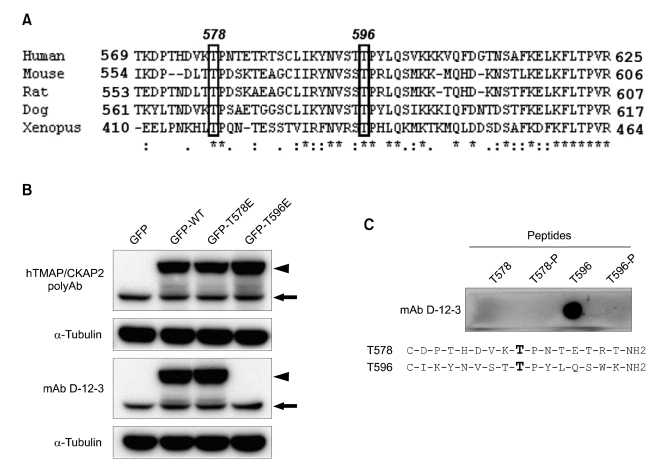
As mentioned above, the differential reactivity of the antibody to M phase TMAP/CKAP2 may originate from M phase-specific post-translational modification of TMAP/CKAP2. Previously, we have observed an up-shift of the TMAP/CKAP2 protein band specifically in M phase cell lysate by SDS-PAGE (Hong et al., 2007), and the mitosis-specific band shift was also reproduced in Figure 2C. This suggested a possibility that TMAP/CKAP2 is phosphorylated specifically during mitosis. Based on this observation, we speculated that M phase-specific phosphorylation of TMAP/CKAP2 might result in the loss of the antibody reactivity. Thus, we searched for potential phosphorylation sites between 569 and 625 amino acids by identifying evolutionarily conserved residues within the sequence. This was based on the rationale that evolutionarily conserved residues are often likely candidates of functionally important sites. Among the conserved residues, two threonine residues, T578 and T596, both of which were followed by a conserved proline, were chosen for further analysis (Figure 4A). The GFP-fusion wildtype or mutant constructs for T578 and T596, in which the threonine residue was replaced by a glutamate (T578E and T596E), were transfected into HEK 293 cells and analyzed by Western blot. While the binding of mAb D-12-3 to TMAP/CKAP2 was unaffected by T578E mutation, the reactivity was completely abolished by T596E mutation, suggesting that the threonine residue at 596 is important for the binding of mAb D-12-3 to TMAP/CKAP2 (Figure 4B). To test if actual phosphorylation at T596 can indeed cause the loss of the antibody reactivity, peptides corresponding to the sequences surrounding T578 or T596 and their phosphorylated counterparts were synthesized. Dot blot analysis with the synthetic peptides showed that the T596-phosphorylated peptide was completely un-reactive to the antibody while the nonphosphorylated T596 peptide retained the reactivity (Figure 4C). These results suggest that mAb D-12-3 recognizes an epitope surrounding T596 and that phosphorylation at T596 abolishes the reactivity of the antibody to the epitope. Taken together, the results indicate that TMAP/CKAP2 is phosphorylated at T596 specifically during mitosis, which results in the loss of the reactivity of mAb D-12-3 to M phase TMAP/CKAP2. This also implies that mAb D-12-3 can be used to survey the phosphorylation status of TMAP/CKAP2 at T596.
Figure 4.
Phosphorylation at T596 abolishes the reactivity of mAb D-12-3 to TMAP/CKAP2. (A) Partial amino acid sequences of TMAP/CKAP2 from indicated species were aligned using ClustalW. Conserved residues (asterisk) and two conserved potential phosphorylation residues, T578 and T596 (boxes) are indicated. (B) HEK 293 cells transfected with GFP-fusion wildtype (WT), T578E, or T596E mutant construct of human TMAP/CKAP2 were analyzed by Western blot. mAb D-12-3 completely failed to react with the T596E mutant, while remaining reactive to the WT and T578E mutant. Endogenous TMAP/CKAP2 protein and the GFP-fusion protein are indicated by the arrow and arrowhead, respectively. (C) Dot blot analysis of synthetic peptides containing nonphosphorylated (T578 or T596) or phosphorylated (T578-P or T596-P) threonine residues using mAb D-12-3. The antibody reacted only with the nonphosphorylated T596 peptide, and the phosphorylation at T596 (T596-P) completely abolished the reactivity.
Transient phosphorylation of T596 during early phases of mitosis
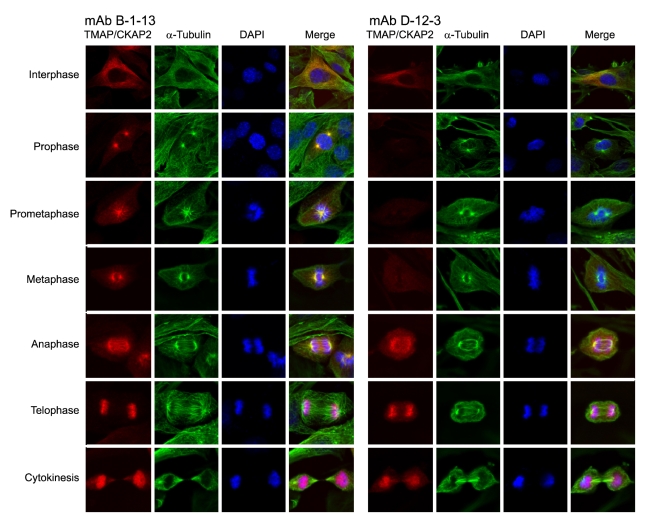
To further characterize the M phase-specific phosphorylation of T596 in vivo, we performed immunofluorescence staining of C2C12 cells using the two monoclonal antibodies. mAb B-1-13 detected TMAP/CKAP2 at microtubules during interphase; at microtubules and separating centrosomes in prophase; at mitotic spindles and spindle poles during prometaphase and metaphase; at mitotic chromosomes during telophase; and in newly formed nuclei and midbody microtubules during cytokinesis (Figure 5, left), which was consistent with the previous observations made using rabbit polyclonal antibodies (Bae et al., 2003; Jin et al., 2004; Hong et al., 2007). Although mAb D-12-3 also successfully detected microtubule-associated TMAP/CKAP2 in interphase cells, the immunoreactivity was abruptly lost in mitotic cells at prophase, prometaphase and metaphase (Figure 5, right). Starting from anaphase, however, TMAP/ CKAP2 staining returned and was indistinguishable from those observed with mAb B-1-13 (Figure 5). This observation suggests that TMAP/CKAP2 phosphorylation at T596 is regulated during mitosis and that the timing of T596 phosphorylation is restricted to early phases of mitosis (i.e., from prophase to metaphase).
Figure 5.
Immunofluorescence staining showing the status of T596 phosphorylation at different stages of mitosis. C2C12 (mouse myoblast) cells were fixed and immunostained for TMAP/CKAP2 (Cy3; red) and α-tubulin (AlexaFluor 488; green) using mAb B-1-13 (left panel) and mAb D-12-3 (right panel). DAPI (blue) staining shows nuclei and chromosomes. mAb B-1-13 produced the staining patterns which were consistent with previous reports (left panels). Although mAb D-12-3 also successfully detected microtubule-associated TMAP/CKAP2 in interphase cells, it failed to detect TMAP/CKAP2 in mitotic cells at prophase, prometaphase, and metaphase (right panels). However, the expected staining patterns returned again starting from the anaphase and were indistinguishable to those produced by mAb B-1-13.
Discussion
The expression of TMAP/CKAP2 is dependent upon the state of cell division. Confluent or serum-starved fibroblasts arrested at the G0/G1 phase, do not express TMAP/CKAP2, whereas subconfluent or dividing fibroblasts do (Jeon et al., 2006). The expression level of TMAP/CKAP2 is also up-regulated in several cancers (Maouche-Chretien et al., 1998; Eichmuller et al., 2001; Bae et al., 2003). Overexpression of TMAP/CKAP2 causes mitotic delay and spindle formation defects resulting in prometaphase/metaphase arrest, abnormal cell divisions and increased chromosomal instability frequently observed in cancers (Tsuchihara et al., 2005; Hong et al., 2007). For further characterization of TMAP/CKAP2 functions, two clones of specific monoclonal antibodies were produced (see Figure 1). Interestingly, the reactivity of mAb D-12-3 to TMAP/CKAP2 was cell cycle-dependent; there was a dramatic decrease in its reactivity to M phase TMAP/CKAP2 (see Figure 2C). We hypothesized that TMAP/CKAP2 undergoes a post-translational modification such as phosphorylation during mitosis. We have previously observed a mitosis-specific shift/retardation of the TMAP/CKAP2 protein band on a SDS-PAGE gel (Hong et al., 2007), which suggests that TMAP/CKAP2 is modified by phosphorylation at the M phase. To test this possibility, an epitope mapping study was performed. Using deletion mutants of TMAP/CKAP2, it was found that mAb D-12-3 binds to the sequence between 569 and 625 amino acids. Among evolutionarily conserved residues within this region, two potential phosphorylation sites (T578 and T596) were tested further. When T to E mutants were tested, the T596E mutation alone resulted in the complete loss of the reactivity of mAb D-12-3, suggesting that the integrity of T596 is important for the binding of the antibody to TMAP/CKAP2. Moreover, analysis of synthetic peptides containing phosphorylated threonine residues revealed that modification of T596 by phosphorylation completely abolished the reactivity of mAb D-12-3 to TMAP/CKAP2. These data indicate that the change in the antibody reactivity to TMAP/CKAP2 shown in Figure 2C is the result of M phase-specific phosphorylation at T596. We have also independently confirmed that T596 is indeed phosphorylated during mitosis through in vivo 32P-orthophosphate labeling study, whereas a neighboring residue, T595, is not (unpublished observations, K. U. Hong, C. D. Bae, and J. Park). The present finding is also supported by a recent report by Nousiainen et al. (2006), which showed that T596 phosphorylation is one of the phosphorylation sites of TMAP/CKAP2 in a phosphoproteome analysis of the human mitotic spindle.
The exact timing and kinetics of T596 phosphorylation during mitosis was then investigated by immunofluorescence staining using mAb D-12-3. During interphase, the antibody stained microtubule-associated TMAP/CKAP2. However, as cells began to separate the duplicated centrosomes and enter into prophase, the antibody was no longer able to detect TMAP/CKAP2 located either at centrosomes or spindle microtubules (see Figure 5). Starting at anaphase, the expected TMAP/CKAP2 staining patterns returned. This result indicates that the window of T596 phosphorylation is restricted to early phases of mitosis (i.e., prophase, prometaphase, and metaphase), and its de-phosphorylation is initiated at some point during metaphase to anaphase transition. It is likely that such pattern of phosphorylation reflects the timing of its kinase activity. Based on the fact that T596 is immediately followed by an evolutionarily conserved proline (see Figure 4A), it is tempting to speculate that the responsible kinase is one of the proline-directed kinases, such as Cdk's and MAPK's (Roux and Blenis, 2004; Malumbres, 2005). Of note, the timing of T596 phosphorylation also coincides with the window of activation of Cdk1-cyclin B complex (Murray et al., 2004). However, additional studies are in need to identify the kinase responsible for T596 phosphorylation during early mitosis.
It is well known that the serine/threonine phosphorylation of a number of molecules plays a critical role for essentially all events occurring during mitosis (Carmena and Earnshaw, 2003; Murray, 2004; Marumoto et al., 2005). However, the functional significance of cell cycle-specific phosphorylation of TMAP/CKAP2 is currently unclear. One possibility is that degradation of TMAP/CKAP2 during mitotic exit (Hong et al., 2007) is regulated by de-phosphorylation, similar to the degradation of Plk1 which requires de-phosphorylation of Plk1 for its efficient destruction by the APC-Cdh1 during mitotic exit (Lindon and Pines, 2004). Another possibility is that the ability of TMAP/CKAP2 to bind and stabilize microtubules may be regulated by phosphorylation. Properties of microtubule-associated proteins (MAPs) are often regulated by phosphorylation. For instance, phosphorylation of MAP2C, MAP1B, and Tau by GSK-3 results in a decrease in their ability to bind and stabilize microtubules (Lovestone et al., 1996; Wagner et al., 1996; Utton et al., 1997). Similarly, Cdk1-mediated phosphorylation of another MAP, XMAP215 at the onset of mitosis has been reported to reduce its microtubule-stabilizing activity (Vasquez et al., 1999). Thus, it is possible that the microtubule-stabilizing properties of TMAP/CKAP2 may be similarly regulated by timely phosphorylation and de-phosphorylation in a cell cycle phase-specific manner.
In summary, TMAP/CKAP2 is differentially phosphorylated during the cell cycle. TMAP/CKAP2 is phosphorylated at T596 specifically during prophase, prometaphase, and metaphase, and its de-phosphorylation becomes evident starting at anaphase. Functional significance of phosphorylation of TMAP/CKAP2 at T596 is not clear yet, but the possibilities discussed above are currently being explored using phosphorylation-deficient and phosphorylation-mimic mutants of TMAP/CKAP2. The present study and future studies on the mechanism and functional significance of phosphorylation of TMAP/CKAP2 will further enhance our understanding of the cellular functions of TMAP/CKAP2 during mitosis.
Acknowledgements
This work was supported by a research grant from National Cancer Center, Korea (grant 0510370 and 0810240), from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (grant 0720370), and from the Molecular and Cellular Bio-Discovery Project, KISTEP (grant 2004-01789).
Abbreviations
- CKAP2
cytoskeleton associated protein 2
- TMAP
tumor associated microtubule associated protein
References
- 1.Bae CD, Sung YS, Jeon SM, Suh Y, Yang HK, Kim YI, Park KH, Choi J, Ahn G, Park J. Up-regulation of cytoskeletal-associated protein 2 in primary human gastric adenocarcinomas. J Cancer Res Clin Oncol. 2003;129:621–630. doi: 10.1007/s00432-003-0484-0. [DOI] [PubMed] [Google Scholar]
- 2.Carmena W, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 3.Eichmuller S, Usener D, Dummer R, Stein A, Thiel D, Schadendorf D. Serological detection of cutaneous T-cell lymphoma-associated antigens. Proc Natl Acad Sci USA. 2001;98:629–634. doi: 10.1073/pnas.021386498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong KU, Park YS, Seong YS, Kang D, Bae CD, Park J. Functional importance of the anaphase-promoting complex-Cdh1-mediated degradation of TMAP/CKAP2 in regulation of spindle function and cytokinesis. Mol Cell Biol. 2007;27:3667–3681. doi: 10.1128/MCB.01386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J, Jr, Boguski MS, Lashkari D, Shalon D, Botstein D, Brown PO. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 6.Jeon SM, Choi B, Hong KU, Kim E, Seong YS, Bae CD, Park J. A cytoskeleton-associated protein, TMAP/CKAP2, is involved in the proliferation of human foreskin fibroblasts. Biochem Biophys Res Commun. 2006;348:222–228. doi: 10.1016/j.bbrc.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 7.Jin Y, Murakumo Y, Ueno K, Hashimoto M, Watanabe T, Shimoyama Y, Ichihara M, Takahashi M. Identification of a mouse cytoskeleton-associated protein, CKAP2, with microtubule-stabilizing properties. Cancer Sci. 2004;95:815–821. doi: 10.1111/j.1349-7006.2004.tb02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim BH, Cho BI, Kim YN, Kim JW, Park ST, Lee CW. Overexpression of nicotinamide N-methyltransferase in gastric cancer tissues and its potential post-translational modification. Exp Mol Med. 2006;38:455–465. doi: 10.1038/emm.2006.54. [DOI] [PubMed] [Google Scholar]
- 9.Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J Cell Biol. 2004;164:233–241. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lovestone S, Hartley CL, Pearce J, Anderton BH. Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience. 1996;73:1145–1157. doi: 10.1016/0306-4522(96)00126-1. [DOI] [PubMed] [Google Scholar]
- 11.Malumbres M. Revisiting the "Cdk-centric" view of the mammalian cell cycle. Cell Cycle. 2005;4:206–210. doi: 10.4161/cc.4.2.1406. [DOI] [PubMed] [Google Scholar]
- 12.Maouche-Chretien L, Deleu N, Badoual C, Fraissignes P, Berger R, Gaulard P, Romeo PH, Leroy-Viard K. Identification of a novel cDNA, encoding a cytoskeletal associated protein, differentially expressed in diffuse large B cell lymphomas. Oncogene. 1998;17:1245–1251. doi: 10.1038/sj.onc.1202048. [DOI] [PubMed] [Google Scholar]
- 13.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 14.Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/s0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- 15.Nousiainen M, Silljé HH, Sauer G, Nigg EA, Köner R. Phosphoproteome analysis of the human mitotic spindle. Proc Natl Acad Sci USA. 2006;103:5391–5396. doi: 10.1073/pnas.0507066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapley J, Baxter JE, Blot J, Wattam SL, Casenghi M, Meraldi P, Nigg EA, Fry AM. Coordinate regulation of the mother centriole component nlp by nek2 and plk1 protein kinases. Mol Cell Biol. 2005;25:1309–1324. doi: 10.1128/MCB.25.4.1309-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sihn CR, Suh EJ, Lee KH, Kim SH. Sec13 induces genomic instability in U2OS cells. Exp Mol Med. 2005;37:255–260. doi: 10.1038/emm.2005.34. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchihara K, Lapin V, Bakal C, Okada H, Brown L, Hirota-Tsuchihara M, Zaugg K, Ho A, Itie-Youten A, Harris-Brandts M, Rottapel R, Richardson CD, Benchimol S, Mak TW. Ckap2 regulates aneuploidy, cell cycling, and cell death in a p53-dependent manner. Cancer Res. 2005;65:6685–6691. doi: 10.1158/0008-5472.CAN-04-4223. [DOI] [PubMed] [Google Scholar]
- 20.Utton MA, Vandecandelaere A, Wagner U, Reynolds CH, Gibb GM, Miller CC, Bayley PM, Anderton BH. Phosphorylation of tau by glycogen synthase kinase 3beta affects the ability of tau to promote microtubule self-assembly. Biochem J. 1997;323:741–747. doi: 10.1042/bj3230741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner U, Utton M, Gallo JM, Miller CC. Cellular phosphorylation of tau by GSK-3 beta influences tau binding to microtubules and microtubule organization. J Cell Sci. 1996;109:1537–1543. doi: 10.1242/jcs.109.6.1537. [DOI] [PubMed] [Google Scholar]
- 22.Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE, Matese JC, Perou CM, Hurt MM, Brown PO, Botstein D. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell. 2002;13:1977–2000. doi: 10.1091/mbc.02-02-0030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasquez RJ, Gard DL, Cassimeris L. Phosphorylation by CDK1 regulates XMAP215 function in vitro. Cell Motil Cytoskeleton. 1999;43:310–321. doi: 10.1002/(SICI)1097-0169(1999)43:4<310::AID-CM4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]