Abstract
The novel class of protein kinase C (nPKC) isoform η is expressed in platelets, but not much is known about its activation and function. In this study, we investigated the mechanism of activation and functional implications of nPKCη using pharmacological and gene knock-out approaches. nPKCη was phosphorylated (at Thr-512) in a time- and concentration-dependent manner by 2MeSADP. Pretreatment of platelets with MRS-2179, a P2Y1 receptor antagonist, or YM-254890, a Gq blocker, abolished 2MeSADP-induced phosphorylation of nPKCη. Similarly, ADP failed to activate nPKCη in platelets isolated from P2Y1 and Gq knock-out mice. However, pretreatment of platelets with P2Y12 receptor antagonist, AR-C69331MX did not interfere with ADP-induced nPKCη phosphorylation. In addition, when platelets were activated with 2MeSADP under stirring conditions, although nPKCη was phosphorylated within 30 s by ADP receptors, it was also dephosphorylated by activated integrin αIIbβ3 mediated outside-in signaling. Moreover, in the presence of SC-57101, a αIIbβ3 receptor antagonist, nPKCη dephosphorylation was inhibited. Furthermore, in murine platelets lacking PP1cγ, a catalytic subunit of serine/threonine phosphatase, αIIbβ3 failed to dephosphorylate nPKCη. Thus, we conclude that ADP activates nPKCη via P2Y1 receptor and is subsequently dephosphorylated by PP1γ phosphatase activated by αIIbβ3 integrin. In addition, pretreatment of platelets with η-RACK antagonistic peptides, a specific inhibitor of nPKCη, inhibited ADP-induced thromboxane generation. However, these peptides had no affect on ADP-induced aggregation when thromboxane generation was blocked. In summary, nPKCη positively regulates agonist-induced thromboxane generation with no effects on platelet aggregation.
Platelets are the key cellular components in maintaining hemostasis (1). Vascular injury exposes subendothelial collagen that activates platelets to change shape, secrete contents of granules, generate thromboxane, and finally aggregate via activated αIIbβ3 integrin, to prevent further bleeding (2, 3). ADP is a physiological agonist of platelets secreted from dense granules and is involved in feedback activation of platelets and hemostatic plug stabilization (4). It activates two distinct G-protein-coupled receptors (GPCRs) on platelets, P2Y1 and P2Y12, which couple to Gq and Gi, respectively (5–8). Gq activates phospholipase Cβ (PLCβ), which leads to diacyl glycerol (DAG)2 generation and calcium mobilization (9, 10). On the other hand, Gi is involved in inhibition of cAMP levels and PI 3-kinase activation (4, 6). Synergistic activation of Gq and Gi proteins leads to the activation of the fibrinogen receptor integrin αIIbβ3. Fibrinogen bound to activated integrin αIIbβ3 further initiates feed back signaling (outside-in signaling) in platelets that contributes to the formation of a stable platelet plug (11).
Protein kinase Cs (PKCs) are serine/threonine kinases known to regulate various platelet functional responses such as dense granule secretion and integrin αIIbβ3 activation (12, 13). Based on their structure and cofactor requirements, PKCs are divided in to three classes: classical (cofactors: DAG, Ca2+), novel (cofactors: DAG) and atypical (cofactors: PIP3) PKC isoforms (14). All the members of the novel class of PKC isoforms (nPKC), viz. nPKC isoforms δ, θ, η, and ε, are expressed in platelets (15), and they require DAG for activation. Among all the nPKCs, PKCδ (15, 16) and PKCθ (17–19) are fairly studied in platelets. Whereas nPKCδ is reported to regulate protease-activated receptor (PAR)-mediated dense granule secretion (15, 20), nPKCθ is activated by outside-in signaling and contributes to platelet spreading on fibrinogen (18). On the other hand, the mechanism of activation and functional role of nPKCη is not addressed as yet.
PKCs are cytoplasmic enzymes. The enzyme activity of PKCs is modulated via three mechanisms (14, 21): 1) cofactor binding: upon cell stimulus, cytoplasmic PKCs mobilize to membrane, bind cofactors such as DAG, Ca2+, or PIP3, release autoinhibition, and attain an active conformation exposing catalytic domain of the enzyme. 2) phosphorylations: 3-phosphoinositide-dependent kinase 1 (PDK1) on the membrane phosphorylates conserved threonine residues on activation loop of catalytic domain; this is followed by autophosphorylations of serine/threonine residues on turn motif and hydrophobic region. These series of phosphorylations maintain an active conformation of the enzyme. 3) RACK binding: PKCs in active conformation bind receptors for activated C kinases (RACKs) and are lead to various subcellular locations to access the substrates (22, 23). Although various leading laboratories have elucidated the activation of PKCs, the mechanism of down-regulation of PKCs is not completely understood.
The premise of dynamic cell signaling, which involves protein phosphorylations by kinases and dephosphorylations by phosphatases has gained immense attention over recent years. PP1, PP2A, PP2B, PHLPP are a few of the serine/threonine phosphatases reported to date. Among them PP1 and PP2 phosphatases are known to regulate various platelet functional responses (24, 25). Furthermore, PP1c, is the catalytic unit of PP1 known to constitutively associate with αIIb and is activated upon integrin engagement with fibrinogen and subsequent outside-in signaling (26). Among various PP1 isoforms, recently PP1γ is shown to positively regulate platelet functional responses (27). Thus, in this study we investigated if the above-mentioned phosphatases are involved in down-regulation of nPKCη. Furthermore, reports from other cell systems suggest that nPKCη regulates ERK/JNK pathways (28). In platelets ERK is known to regulate agonist induced thromboxane generation (29, 30). Thus, we also investigated if nPKCη regulates ERK phosphorylation and thereby agonist-induced platelet functional responses.
In this study, we evaluated the activation of nPKCη downstream of ADP receptors and its inactivation by an integrin-associated phosphatase PP1γ. We also studied if nPKCη regulates functional responses in platelets and found that this isoform regulates ADP-induced thromboxane generation, but not fibrinogen receptor activation in platelets.
EXPERIMENTAL PROCEDURES
Approval for this study was obtained from the Institutional Review Board of Temple University (Philadelphia, PA).
Materials—Apyrase (type VII), bovine serum albumin (fraction V), thrombin, 2MeSADP, MRS-2179 (N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate) (tetra sodium salt), fibrinogen (type I), and acetylsalicylic acid were obtained from Sigma. Phospho-ERK antibodies against threonine 202 and tyrosine 204 residues and β-actin antibodies were obtained from Cell Signaling Technologies (Beverly, MA). Alkaline phosphatase-labeled secondary antibody was from Kierkegaard & Perry Laboratories (Gaithersburg, MD). AR-C69931MX (N6-(2-methyl-tioethyl)-2-(3,3,3-trifluoro-propylthio)-β,γ-dichloromethylene ATP) (tetrasodium salt) was a kind gift from AstraZeneca (Loughborough, UK). YM-254890 was a generous gift from Yamanouchi Pharmaceutical (Ibaraki, Japan). SC-57101 was a gift from Searle and Co (Greenwich, CT). Phospho-PKCη antibodies against p-Thr512 were custom made from 21st Century Biochemicals. Inc (Morlboro, MA). η-RACK antagonistic peptide and control peptide were a kind gift from Dr. Daria Mochly Rosen (Stanford University).
Animals—129/Sv mice carrying Gαq-null mutation were obtained from Dr. T. Kent Gartner (31), with permission from Dr. Stefan Offermanns (University of Heidelberg, Heidelberg, Germany). CD-1 mice carrying PP1cγ-null mutation were generated in the laboratory of Susannah Varmuza (University of Toranto) (32). C57/BL6 mice carrying P2Y1-null mutation were generated by subcontract with Lexicon Genetics Inc. (Woodlands, TX) through knock-out constructs as described previously (33). These P2Y1 receptor-deficient mice were described previously by our group (29, 34–36).
Isolation of Human Platelets—All experiments using human subjects were performed in accordance with the Declaration of Helsinki. Whole blood was drawn from healthy, consenting human volunteers into tubes containing one-sixth volume of ACD (2.5 g of sodium citrate, 1.5 g of citric acid, and 2 g of glucose in 100 ml of deionized water). Blood was centrifuged (Eppendorf 5810R centrifuge, Hamburg, Germany) at 230 × g for 20 min at room temperature to obtain platelet-rich plasma (PRP). If indicated, PRP was incubated with 1 mm acetylsalicylic acid (aspirin) for 30 min at 37 °C. The PRP was then centrifuged for 10 min at 980 × g at room temperature to pellet the platelets. Platelets were resuspended in Tyrode's buffer (138 mm NaCl, 2.7 mm KCl, 1 mm MgCl2, 3 mm NaH2PO4, 5 mm glucose, 10 mm HEPES, pH 7.4, 0.2% bovine serum albumin) containing 0.1 units/ml apyrase. Cells were counted using the Coulter Z1 Particle Counter (Miami, FL), and concentration of cells was adjusted to 2 × 108 platelets/ml. All experiments using washed platelets were performed in the absence of extracellular calcium unless otherwise mentioned.
Isolation of Mouse Platelets—Blood was collected from the vena cava of anesthetized mice into syringes containing 1:10th blood volume of 3.8% sodium citrate as anticoagulant. Red blood cells were removed by centrifugation at 100 × g for 10 min. Platelet-rich plasma was recovered, and platelets were pelleted at 400 × g for 10 min. The platelet pellet was resuspended in Tyrode's buffer (pH 7.4) containing 0.01 units/ml apyrase. The washed platelets were subsequently used for experiments.
Platelet Cell Lysates Preparation—Platelets were stimulated with agonists for the appropriate time under non-stirring or stirring conditions at 37 °C. The reaction was stopped by the addition of 3× SDS-Laemmli's buffer. Platelet lysates were boiled for 10 min and stored for Western blotting analysis.
Aggregometry—Aggregation of 0.5 ml of washed platelets was analyzed using a P.I.C.A. lumiaggregometer (Chrono-log Corp. Havertown, PA). Aggregation was measured using light transmission under stirring conditions (900 rpm) at 37 °C. Each sample was allowed to aggregate for at least 3 min. The chart recorder (Kipp and Zonen, Bohemia, NY) was set for 0.2 mm/s.
Measurement of Thromboxane A2 Generation—Washed human platelets without aspirin treatment were prepared as noted and brought to a concentration of 4 × 108 platelets/ml. Stimulations were performed in a platelet aggregometer under stirring conditions (900 rpm) at 37 °C. The η-RACK antagonistic peptide and control peptide were added for 10 min before addition of the agonist. Stimulations were performed for 3.5 min, and the reaction was stopped by snap-freezing. Samples were stored at –80 °C until TXB2 analysis was performed. Levels of TXB2 were determined in duplicates using a Correlate-EIA Thromboxane B2 Enzyme Immunoassay kit (Assay Designs, Ann Arbor, MI), according to the manufacturer's instructions. The mean ± S.E. was derived from experiments performed in duplicate using platelets obtained from three independent donors.
Western Blotting Analysis—Lysates prepared from platelets were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membrane. Nonspecific binding sites were blocked by incubation in Tris-buffered saline and Tween (TBST; 20 mm Tris, 140 mm NaCl, 0.1% (v/v) Tween 20) containing 0.5% (w/v) milk protein and 3% (w/v) bovine serum albumin for 30 min at room temperature, and membranes were incubated overnight at 4 °C with the primary antibody (1:10,000 dilution in TBST with 2% bovine serum albumin) with gentle agitation. After three washes for 5 min each with TBST, the membranes were probed with an alkaline phosphatase-labeled secondary antibody (1:5000 dilutions in TBST with 2% bovine serum albumin) for 1 h at room temperature. After additional washing steps, membranes were then incubated with CDP-Star chemiluminescent substrate (Tropix, Bedford, MA) for 10 min at room temperature, and immunoreactivity was detected using a Fuji Film Luminescent Image Analyzer (LAS-1000 CH, Japan).
Statistical Analysis—We have analyzed statistical significance of our data using paired Student's t-test or analysis of variance. Statistically significant data bearing p value < 0.05 are annotated by an asterisk symbol. Data are expressed as mean ± S.E.
RESULTS
Activation of nPKCη Isoform by ADP—Platelets express all the four nPKC isoforms, viz. δ, θ, ε, and η (15). We have previously shown that ADP activates ERK and p38 MAP kinase in platelets (29, 37). It is also known that nPKCη activates MAP kinases in keratinocytes (28) and other cell systems (38). Hence, we initiated our studies with evaluation of nPKCη activation in platelets by ADP.
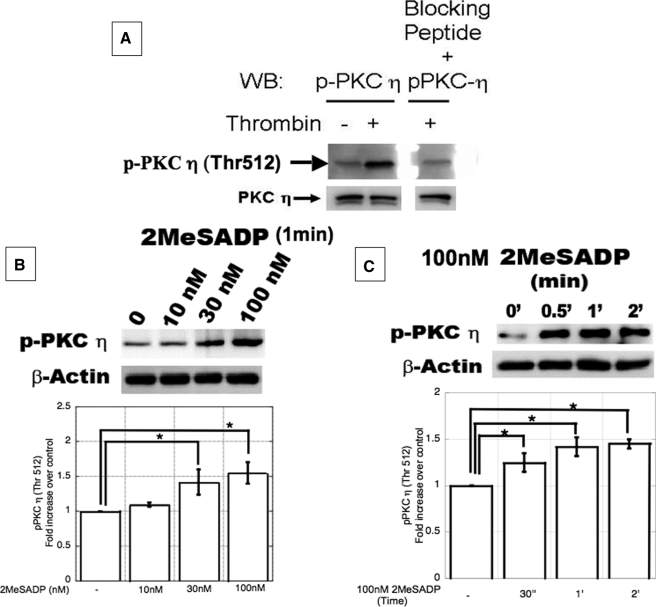
DAG binding to C1 domain followed by phosphorylation on conserved threonine residues in the activation loop primes activation of nPKC isoforms (14). As activation of nPKCη is dependent on phosphorylation at Thr-512 (the consensus threonine residue (39)) in the activation loop, we first custom-synthesized and characterized the anti-phospho-Thr-512 nPKCη antibody using the peptide GVTTA(pT)FCGTPD. Upon stimulation of aspirin-treated and washed platelets with thrombin (0.1 units/ml), nPKCη was activated as detected with the phosphospecific antibody (Fig. 1A). However, preincubation of the antibody with the peptide (Immunogen) used to generate antibodies in rabbits, the signal was blocked. These results confirm the specificity of the phosphospecific antibody against the Thr-512 of nPKCη. The same antibody was used to study the activation of nPKCη by ADP. When platelets were stimulated with different concentrations of 2MeSADP, an ADP analog, under non-stirring conditions for 1 min, nPKCη was activated in a concentration-dependent manner (Fig. 1B). nPKCη was also activated by 2MeSADP (100 nm) under non-stirring conditions in a time-dependent manner, with activation occurring as early as 30 s (Fig. 1C). Similar results were obtained with ADP as the agonist (data not shown).
FIGURE 1.
A, characterization of antiphospho-Thr-512 nPKCη antibody. Washed and aspirin-treated human platelets were stimulated with 0.1 units/ml of thrombin for 30 s. The samples were subjected to SDS-PAGE in duplicates. Proteins were transferred on polyvinylidene difluoride membrane, and each membrane is subjected to immunoblotting with antiphospho-Thr-512 nPKCη antibody or the same antibody was preincubated with the blocking peptide for 2 h. β-Actin was used to ensure equal protein concentrations in all lanes. B and C, ADP activates PKCη in a concentration- and time-dependent manner. Washed and aspirin-treated human platelets were stimulated with increasing concentrations (B) of 2MeSADP for different time periods (C) under non-stirring conditions at 37 °C. The reaction was stopped by adding the Laemmli's buffer. The cell lysates were analyzed for Thr-512 phosphorylations on nPKCη by Western blotting using phosphospecific antibodies as indicated. β-Actin was used to monitor protein concentrations in all lanes. The blot shown is representative of experiments performed using platelets from three different donors. Furthermore, data obtained from three different sets of experiments were quantified and expressed as mean ± S.E.; * indicates p < 0.05.
Role of P2Y1 and P2Y12 Receptors in Activation of nPKCη by ADP—ADP activates platelets via Gq-coupled P2Y1 receptor and Gi-coupled P2Y12 receptor (5, 6). The role of each of these receptors in activation of nPKCη is evaluated using pharmacological and gene knock-out approaches. To evaluate the role of P2Y1 and P2Y12 receptor in activation of nPKCη, we activated platelets with 2MeSADP in the presence of MRS-2179, a P2Y1 receptor antagonist (40) and AR-C69331MX, a P2Y12 antagonist (4) under non-stirring conditions. As shown in Fig. 2, pretreatment of platelets with MRS-2179 abolished 2MeSADP-induced nPKCη activation. On the other hand, pretreatment of platelets with ARC-69931MX, had minimal effect on ADP-induced nPKCη activation (Fig. 2). Furthermore, P2Y1 receptor couples to Gq, which leads to PLC activation, DAG generation, and calcium mobilization (6). To evaluate whether Gq pathway, downstream of P2Y1 receptor, causes nPKCη activation, we pretreated platelets with YM-254890, which prevents Gq coupling to GPCRs (41) and activated with 2MeSADP under non-stirring conditions. YM-254890 has been successfully used in platelets to block Gq signaling pathways (42). As shown in Fig. 2, YM-254890 abolished ADP-induced nPKCη activation. These results suggest that ADP activates PKCη via P2Y1 receptor coupled to Gq.
FIGURE 2.
ADP activates nPKCη via P2Y1receptor, which is coupled to Gαq-pharmacological approach. Washed and aspirin-treated human platelets were pretreated with 100 nm AR-C69931MX, a P2Y12 antagonist, 100 μm MRS-2179, a P2Y1 antagonist or 100 nm YM-245890, a Gq blocker for 5 min (as indicated) and activated by 100 nm 2MeSADP under non-stirring at 37 °C. The reaction was stopped after 1 min by adding the Laemmli's buffer. The cell lysates were analyzed for Thr-512 phosphorylations on nPKCη by Western blotting using phosphospecific antibodies as indicated. Total β-actin antibody was used to ensure equal protein concentrations in all lanes. The blot shown is representative of experiments performed using platelets from three different donors. Furthermore, data obtained from three different sets of experiments were quantified and expressed as mean ± S.E.; * indicates p < 0.05.
Complementary to the pharmacological approach, we also evaluated the role of P2Y1-coupled Gq pathway in activation of nPKCη using P2Y1 and Gq knock-out mice. Platelets isolated from P2Y1, Gq knock-out mice and wild type littermates were activated with 2MeSADP under non-stirring conditions for different time periods and phosphorylation of nPKCη was studied using anti-phospho PKCη antibody. As shown in Fig. 3A, while 2MeSADP activated nPKCη in wild type murine platelets in a time-dependent manner, it failed to activate nPKCη in P2Y1 knock-out murine platelets. Furthermore, as shown in Fig. 3B, 2MeSADP also failed to activate nPKCη in Gq knock-out murine platelets, in comparison with wild type murine platelets (Fig. 3B). These data further confirm that ADP activates nPKCη via P2Y1 receptor coupled to Gq.
FIGURE 3.
ADP activates nPKCη via P2Y1 receptor coupled to Gαq protein-gene knock-out approach. Washed platelets from P2Y1 (Fig. 3A) or Gαq (Fig. 3B)-deficient mice (dark bars) and wild type littermates (white bars) were treated with 100 nm 2MeSADP under non-stirring conditions at 37 °C for different time periods as indicated. The reaction was stopped by adding the Laemmli's buffer. The cell lysates were analyzed for Thr-512 phosphorylations on nPKCη by Western blotting using phosphospecific antibodies as indicated. Total β-actin antibody was used to ensure equal protein concentrations in all lanes. The blot shown is representative of experiments performed using platelets from three separate set of pooled blood from knock-out and wild type animals. Furthermore, data obtained from three different sets of experiments were quantified and expressed as mean ± S.E.; * indicates p < 0.05.
Role of Activated αIIbβ3 Integrin Signaling in Activation of nPKCη—Signaling cascades initiated from P2Y1 and P2Y12 receptors lead to integrin αIIbβ3 activation (5, 6). Once activated, αIIbβ3 binds its ligand, fibrinogen, and initiates outside-in signaling cascade, leading to PLCγ activation, DAG generation, and Ca2+ mobilization (11). To evaluate the role of outside-in signaling in activation of nPKCη, platelets were activated by ADP with fibrinogen under stirring conditions. Under stirring conditions, activated integrin αIIbβ3 binds fibrinogen and initiates outside-in signaling. As shown in Fig. 4, 2MeSADP not only phosphorylated nPKCη within 30 s, it also dephosphorylated nPKCη by 2 min. These data indicate that nPKCη is activated and also inactivated downstream of ADP receptors. To confirm whether the dephosphorylation of nPKCη is a consequence of activated integrin αIIbβ3, we used SC-57101, an integrin αIIbβ3 (fibrinogen receptor) antagonist. Pretreatment of platelets with SC-57101 inhibited ADP-induced dephosphorylation of nPKCη at 2 min (Fig. 4) under stirring conditions. These data indicate a temporal phosphorylation pattern for nPKCη, wherein, the initial phosphorylation of nPKCη caused by ADP receptors is followed by a dephosphorylation that is mediated by integrin αIIbβ3 mediated outside-in signals.
FIGURE 4.
nPKCη is dephosphorylated byαIIbβ3 mediated outside-in signaling. Washed and aspirin-treated human platelets were treated with 100 nm 2MeSADP under stirring conditions, in the presence (dark bars) or absence (white bars) of 10 μm SC-57101, anαIIbβ3 antagonist at 37 °C for different time periods as indicated. The reaction was stopped by adding the Laemmli's buffer. The cell lysates were analyzed for Thr-512 phosphorylations on nPKCη by Western blotting using phosphospecific antibodies as indicated. Total nPKCη antibody was used to ensure equal protein concentrations in all lanes. The blot shown is representative of experiments performed using platelets from three different donors. Furthermore, data obtained from three different sets of experiments were quantified and expressed as mean ± S.E.; * indicates p < 0.05.
Role of PP1γ, a Serine/Threonine Phosphatase in Dephosphorylation of nPKCη—Serine/threonine phosphatases are reported to regulate PKCs in other cell systems. For example, PKCα is regulated by PP2B in endothelial cells (43), PP2A is reported to regulate atypical PKC isoforms in epithelial cells (44). PP1c is a catalytic subunit of PP1, a serine/threonine phosphatase that constitutively associates with αIIb tail (26) in integrin αIIbβ3 complex. Upon activation and fibrinogen (ligand) binding to integrin αIIbβ3, PP1c dissociates from αIIbβ3 and becomes catalytically active (26). Among different PP1 isoforms (PP1α, PP1β, PP1γ), PP1γ is known to regulate platelet functional responses (27). Because of the unavailability of specific pharmacological inhibitors for the above-mentioned phosphatases, we adapted gene knock-out approach to study the role of phosphatases in regulation of nPKCη activation. We investigated whether integrin αIIbβ3 engagement during stirring conditions, dephosphorylates nPKCη via PP1cγ phosphatase using PP1cγ knock-out mice. Platelets isolated from PP1cγ knock-out mice and wild type littermates were activated by 2MeSADP under stirring conditions. As shown in Fig. 5, ADP caused transient phosphorylation of nPKCη in wild type murine platelets. However, in murine platelets lacking PP1cγ, ADP caused sustained nPKCη phosphorylation. These results suggest that binding of fibrinogen to integrin αIIbβ3 dephosphorylates nPKCη via PP1cγ. Furthermore, it should also be noted that activated αIIbβ3 integrin-induced dephosphorylation was only partially rescued in PP1cγ knock-out mice. These data indicate that other isoforms of PP1 phosphatase may also be involved in dephosphorylation of nPKCη. However, as PP1α and PP1β knockouts are embryonic lethal, we could not evaluate the role of these phosphatases in dephosphorylation of nPKCη.
FIGURE 5.
αIIbβ3-mediated integrin outside-in signaling dephosphorylates nPKCη via PP1cγ phosphatase. Washed platelets from PP1cγ-deficient mice (dark bars) and wild type littermates (white bars) were treated with 100 nm 2MeSADP under stirring conditions at 37 °C for different time periods as indicated. The reaction was stopped by adding the Laemmli's buffer. The cell lysates were analyzed for Thr-512 phosphorylations on nPKCη by Western blotting using phosphospecific antibodies as indicated. β-actin was used to ensure equal protein concentrations in all lanes. The blot shown is representative of experiments performed using platelets from three separate set of pooled blood from knock-out animals. Furthermore, data obtained from three different sets of experiments were quantified and expressed as mean ± S.E.; * indicates p < .05.
Role of nPKCη in Agonist-induced Platelet Functional Responses—Previous reports from other cell systems suggest that nPKCη regulates MAP kinases such as ERK (28). We have previously demonstrated that ERK is involved in agonist-induced thromboxane generation (29). Thus, we evaluated the role of nPKCη in ADP-induced thromboxane generation. We utilized η-RACK antagonistic peptides in our studies. These peptides are designed to bind intracellular RACKs, the proteins involved in transportation of activated PKC from the membrane to the intracellular substrate. Therefore, although the enzyme (nPKCη) is activated (phosphorylated), it cannot render its catalytic activity due to unavailability of its substrate and as a consequence corresponding signaling pathway is inhibited (45, 46). The peptides designed with similar strategy have been successfully used in various studies in other cell systems (46, 47). We evaluated the functional role of nPKCη in agonist-induced platelet functional responses by pretreating non-aspirin-treated platelets with η-RACK antagonistic peptides and activating with 2MeSADP. As shown in Fig. 6, 2MeSADP-induced aggregation (Fig. 6A) and thromboxane generation (Fig. 6B) were inhibited in platelets pretreated with η-RACK antagonistic peptide compared with the platelets pretreated with equimolar control peptide. Furthermore, inhibition of thromboxane generation is also evident by the fact that ADP-induced dense granule secretion (measured as ATP release in Fig. 6A) in non-aspirin-treated platelets, which is solely dependent on thromboxane generation (48) is also inhibited. In addition, the peptides by itself had no effect on platelet aggregation and thromboxane generation. These data suggest that nPKCη positively regulates ADP-induced thromboxane generation.
FIGURE 6.
nPKCη positively regulates ADP-induced thromboxane generation. Washed and non-aspirin-treated platelets were pretreated with 1 μm η-RACK antagonistic peptide or control peptide for 10 min at 37 °C and were activated by 100 nm 2MeSADP. A, A1, representative aggregation (measured as deflections in light transmission using aggregometry), and secretion (measured as ATP release using lumichrome assay) tracings of non-aspirin-treated platelets activated by ADP. The % change in aggregation and secretion up on treatment with η-RACK antagonist compared with control is represented in A2 and A3, respectively. B, graphical representation of thromboxane generated in non-aspirin-treated platelets activated by ADP, as measured using ELISA. The graphs are representative of data drawn from three separate experiments conducted using blood from three different donors. Data are expressed as mean ± S.E.; * indicates p value <0.05.
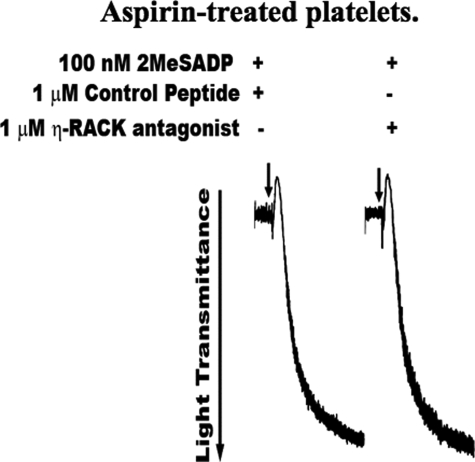
We further evaluated whether nPKCη directly regulates ADP-induced platelet aggregation, independent of feedback effects from inhibited thromboxane, using aspirin-treated platelets. Aspirin is a well established cyclooxygenase (COX) inhibitor, which abolishes thromboxane generation, when used under the working conditions described under “Experimental Procedures” (37, 49). Aspirin-treated platelets were pretreated with η-RACK antagonistic peptides or control peptide and activated by 2MeSADP. As shown in Fig. 7, ADP-induced platelet aggregation was same in both platelets pretreated with η-RACK antagonistic peptides and control peptides. Thromboxane generation was completely inhibited in aspirin-treated platelets upon stimulation with 2MeSADP (data not shown). These results suggest that nPKCη has no direct effect on ADP-induced aggregation. In addition, these data also confirm that the decrease in platelet aggregation observed in non-aspirin-treated platelets pretreated with η-RACK antagonist (Fig. 6A) is only due to decrease in thromboxane generation, which in turn results in decreased thromboxane-induced aggregation.
FIGURE 7.
nPKCη has no effect on ADP-induced platelet aggregation and dense granule secretion. Washed and aspirin-treated platelets were pretreated with 1 μm η-RACK antagonistic peptide or control peptide for 10 min at 37 °C were activated by 100 nm 2MeSADP for 3 min. Shown are the representative aggregation tracings of three separate experiments conducted using blood drawn from three different donors.
Molecular Mechanism By Which nPKCη Regulates Agonist-induced Functional Responses—Previous reports suggest that agonist-induced thromboxane generation is regulated by ERK (29). Furthermore, results from Fig. 6A, suggest that nPKCη positively regulates thromboxane generation. Thus, we evaluated if nPKCη regulates thromboxane generation in platelets by regulating ERK. Aspirin-treated platelets pretreated with η-RACK antagonistic and control peptides were activated by 2MeSADP. The extent of activation of ERK was measured by Western blotting analysis using phospho-ERK antibody. As shown in Fig. 8, phosphorylation of ERK was not affected in platelets pretreated with η-RACK antagonist or control peptides. These data suggest that nPKCη does not regulate thromboxane generation through ERK.
FIGURE 8.
nPKCη does not regulate agonist-induced thromboxane generation via ERK pathway. Washed and aspirin-treated platelets were pretreated with 1 μm η-RACK antagonistic peptide or control peptide for 10 min at 37 °C were activated by 100 nm 2MeSADP for 1 min, and the reaction was stopped by adding the Laemmli's buffer. The cell lysates were subjected to Western blot analysis and ERK activation was analyzed using phospho-ERK antibody. Total ERK antibody was used to ensure equal protein concentrations in all lanes. The blot shown is representative of experiments performed using platelets from three separate donors.
DISCUSSION
The mechanism of activation of PKCs has been extensively studied in various cell systems including platelets. However, the mechanism by which they are inactivated is not completely understood. In this study, we demonstrate a novel mechanism of inactivation of nPKCη isoform by integrin-associated serine/threonine phosphatase. Furthermore, although the role of some PKC isoforms in agonist-induced platelet functional responses have been previously studied, the role of nPKCη in platelets has not been studied. In this study, we demonstrated that ADP activates nPKCη via P2Y1 receptor coupled to Gq. As expected, Gi pathway, which does not generate DAG or mobilize calcium, has no role in regulation of nPKCη. Furthermore, nPKCη positively regulates ADP-induced thromboxane generation without directly affecting ADP-induced aggregation. Finally, we show that upon activation of platelets, αIIbβ3 mediated outside-in signaling dephosphorylates nPKCη through PP1γ phosphatase.
Recent reports suggest that following activation, PKCs are subjected to lysosomal or proteasomal degradation involving ubiquitination (50–52). However, such proteosomal degradation is possibly not occurring in platelets, as ubiquitinated nPKCη bands, which typically appear as a ladder, were not observed. (Figs. 4 and 5). Furthermore, total nPKCη levels remains constant upon integrin signaling (as studied using anti-PKCη antibody, Fig. 4), Hence, PKCη is not inactivated by ubiquitin-mediated degradation in platelets. We have previously demonstrated that although Syk is ubiquitinated upon stimulation by collagen, it does not lead to its degradation in platelets (53).
We have also evaluated the role of nPKCη using η-RACK antagonistic peptides that interfere with enzyme-substrate interaction. Similar antagonistic peptides have been successfully used in various cell systems such as cardiomyocytes (46) and neuronal cells (54). Using η-RACK antagonists we have demonstrated that nPKCη positively regulates agonist-induced thromboxane generation (Fig. 6) with no effect on agonist-induced platelet aggregation (Fig. 7). The peptides were targeted in to the cell using TAT carrier protein, which is also used as a negative control for these experiments. The specificity of η-RACK antagonistic peptides is further elucidated by the fact that they do not affect the platelet aggregation (Fig. 7). Downstream of ADP receptors platelet aggregation is regulated by calcium and other PKCs such as cPKCα. Thus, as the antagonistic peptides did not affect platelet aggregation, its effect on other molecular events could be ruled out. Furthermore the nPKCδ and θ are neither activated by ADP nor regulate ADP-induced functional responses (19, 20). Thus, the effects observed upon pretreatment with nPKCη RACK antagonist could primarily be because of its interaction with nPKCη.
In platelets, ADP-induced thromboxane generation is regulated by P2Y1, P2Y12, and αIIbβ3 receptor-mediated signaling (55). Furthermore, in our previous studies we have shown that ERK is a positive regulator of agonist-induced thromboxane generation in platelets, and it requires signaling from both P2Y1 and P2Y12 receptor-mediated pathways for its activation (29, 30). As nPKCη regulates ADP-induced thromboxane generation, we further investigated if the mechanism is by regulating P2Y1 or P2Y12 signaling. However, nPKCη does not regulate thromboxane generation via ERK (Fig. 8). Furthermore, to evaluate if P2Y12 receptor-mediated, Gi pathway alone is regulated by nPKCη, we studied Akt activation and measured decrease in cAMP levels. We chose to conduct these studies, as Akt activation and decrease in cAMP production are solely dependent on Gi signaling (35). However, inhibition of nPKCη by η-RACK antagonistic peptides did not affect cAMP levels or Akt activation in aspirin-treated platelets activated by ADP (data not shown). Thus nPKCη appears to regulate thromboxane generation via an unknown mechanism possibly mediated by outside-in signaling, since the P2Y1 and P2Y12 receptor-mediated signaling is not affected.
In platelets, PP1c positively regulates agonist-induced platelet functional responses (27). Our data show that nPKCη also positively regulates ADP-induced thromboxane generation. In addition, PP1c dephosphorylates nPKCη. We believe that PP1c regulation of platelet function is not through nPKCη. We demonstrated that nPKCη is activated within 30 s (Fig. 1C) of agonist-induced platelet stimulation. In addition, nPKCη is dephosphorylated by PP1cγ phosphatase, activated by αIIbβ3 integrin by 2 min after agonist-induced platelet activation. Thus, as nPKCη phosphorylation precedes PP1cγ activation and, hence, we predict that the molecular mechanism by which PP1c regulates platelet functional responses is not via nPKCη. Rather PP1γ-mediated dephosphorylation of nPKCη is probably the mechanism by which catalytic activity of nPKCη is regulated in platelets.
In addition, deletion of PP1cγ does not completely rescue activated αIIbβ3 integrin-induced dephosphorylation of nPKCη (Fig. 5). These data leave us with the possibility that the other isoforms of PP1c such as PP1cα and PP1cβ might also be involved in dephosphorylation of nPKCη.
In summary, nPKCη is activated by ADP via P2Y1 receptor. Once activated it is also dephosphorylated by integrin αIIbβ3 via PP1γ phosphatase. Furthermore, activated nPKCη positively regulates ADP-induced thromboxane generation with no effect on aggregation. In addition, nPKCη possibly regulates thromboxane generation via an unknown pathway downstream of integrin αIIbβ3 (Fig. 9).
FIGURE 9.
Model depicting pathways involved in activation and inactivation of nPKCη. nPKCη is activated by ADP via P2Y1 receptor coupled to the Gq pathway. Furthermore, nPKCη is down-regulated by activated integrin αIIbβ3 induced outside-in signaling via PP1cγ phosphatase. Upon activation, nPKCη positively regulates ADP-induced thromboxane generation without affecting platelet aggregation and dense granule secretion.
Supplementary Material
Acknowledgments
We thank Dr. Daria Mochly-Rosen, Stanford University School Of Medicine, for providing us η-RACK antagonistic peptides. We also thank Dr. Grant R. Budas for his suggestions regarding the usage of these peptides.
This work was supported, in whole or in part, by National Institutes of Health Grants HL81322, HL93231, HL80444, and HL60683.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
Footnotes
The abbreviations used are: DAG, diacylglycerol; PI, phosphatidylinositol; PKC, protein kinase C; ERK, extracellular signal-regulated kinase.
References
- 1.Shattil, S. J., Kashiwagi, H., and Pampori, N. (1998) Blood 91 2645–2657 [PubMed] [Google Scholar]
- 2.Brass, L. F. (1999) J. Clin. Investig. 104 1663–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brass, L. F., Hoxie, J. A., Kieber-Emmons, T., Manning, D. R., Poncz, M., and Woolkalis, M. (1993) Adv. Exp. Med. Biol. 344 17–36 [DOI] [PubMed] [Google Scholar]
- 4.Dorsam, R. T., and Kunapuli, S. P. (2004) J. Clin. Investig. 113 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel, J. L., Dangelmaier, C., Jin, J., Ashby, B., Smith, J. B., and Kunapuli, S. P. (1998) J. Biol. Chem. 273 2024–2029 [DOI] [PubMed] [Google Scholar]
- 6.Jin, J., Daniel, J. L., and Kunapuli, S. P. (1998) J. Biol. Chem. 273 2030–2034 [DOI] [PubMed] [Google Scholar]
- 7.Jin, J., and Kunapuli, S. P. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 8070–8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Offermanns, S. (2000) Biol. Chem. 381 389–396 [DOI] [PubMed] [Google Scholar]
- 9.Kunapuli, S. P. (2002) Scientific World J. 2 424–433 [Google Scholar]
- 10.Leon, C., Hechler, B., Freund, M., Eckly, A., Vial, C., Ohlmann, P., Dierich, A., LeMeur, M., Cazenave, J. P., and Gachet, C. (1999) J. Clin. Investig. 104 1731–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shattil, S. J., and Newman, P. J. (2004) Blood 104 1606–1615 [DOI] [PubMed] [Google Scholar]
- 12.Quinton, T. M., Kim, S., Dangelmaier, C., Dorsam, R. T., Jin, J., Daniel, J. L., and Kunapuli, S. P. (2002) Biochem. J. 368 535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang, X., Sun, L., Gabbeta, J., and Rao, A. K. (1997) Thromb. Res. 88 317–328 [DOI] [PubMed] [Google Scholar]
- 14.Newton, A. C. (1997) Curr. Opin. Cell Biol. 9 161–167 [DOI] [PubMed] [Google Scholar]
- 15.Murugappan, S., Tuluc, F., Dorsam, R. T., Shankar, H., and Kunapuli, S. P. (2004) J. Biol. Chem. 279 2360–2367 [DOI] [PubMed] [Google Scholar]
- 16.Crosby, D., and Poole, A. W. (2003) J. Biol. Chem. 278 24533–24541 [DOI] [PubMed] [Google Scholar]
- 17.Crosby, D., and Poole, A. W. (2002) J. Biol. Chem. 277 9958–9965 [DOI] [PubMed] [Google Scholar]
- 18.Soriani, A., Moran, B., de Virgilio, M., Kawakami, T., Altman, A., Lowell, C., Eto, K., and Shattil, S. J. (2006) J. Thromb. Haemost. 4 648–655 [DOI] [PubMed] [Google Scholar]
- 19.Nagy, B., Jr., Bhavaraju, K., Getz, T., Bynagari, Y. S., Kim, S., and Kunapuli, S. P. (2009) Blood 113 2557–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chari, R., Getz, T., Nagy, B., Jr., Bhavaraju, K., Mao, Y., Bynagari, Y. S., Murugappan, S., Nakayama, K., and Kunapuli, S. P. (2009) Arterioscler. Thromb. Vasc. Biol., in press [DOI] [PMC free article] [PubMed]
- 21.Newton, A. C. (2003) Biochem. J. 370 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochly-Rosen, D., Khaner, H., Lopez, J., and Smith, B. L. (1991) J. Biol. Chem. 266 14866–14868 [PubMed] [Google Scholar]
- 23.Ron, D., Chen, C. H., Caldwell, J., Jamieson, L., Orr, E., and Mochly-Rosen, D. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 839–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gushiken, F. C., Patel, V., Liu, Y., Pradhan, S., Bergeron, A. L., Peng, Y., and Vijayan, K. V. (2008) J. Biol. Chem. 283 12862–12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vijayan, K. V., Liu, Y., Sun, W., Ito, M., and Bray, P. F. (2005) J. Biol. Chem. 280 21756–21762 [DOI] [PubMed] [Google Scholar]
- 26.Vijayan, K. V., Liu, Y., Li, T. T., and Bray, P. F. (2004) J. Biol. Chem. 279 33039–33042 [DOI] [PubMed] [Google Scholar]
- 27.Gushiken, F. C., Patel, V., Rumbaut, R., Varmuza, S., and Vijayan, K. V. (2007) Blood 110 135a [Google Scholar]
- 28.Brandlin, I., Hubner, S., Eiseler, T., Martinez-Moya, M., Horschinek, A., Hausser, A., Link, G., Rupp, S., Storz, P., Pfizenmaier, K., and Johannes, F. J. (2002) J. Biol. Chem. 277 6490–6496 [DOI] [PubMed] [Google Scholar]
- 29.Garcia, A., Shankar, H., Murugappan, S., Kim, S., and Kunapuli, S. P. (2007) Biochem. J. 404 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankar, H., Garcia, A., Prabhakar, J., Kim, S., and Kunapuli, S. P. (2006) J. Thromb. Haemost. 4 638–647 [DOI] [PubMed] [Google Scholar]
- 31.Offermanns, S., Hashimoto, K., Watanabe, M., Sun, W., Kurihara, H., Thompson, R. F., Inoue, Y., Kano, M., and Simon, M. I. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 14089–14094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varmuza, S., Jurisicova, A., Okano, K., Hudson, J., Boekelheide, K., and Shipp, E. B. (1999) Dev. Biol. 205 98–110 [DOI] [PubMed] [Google Scholar]
- 33.Fabre, J. E., Nguyen, M., Latour, A., Keifer, J. A., Audoly, L. P., Coffman, T. M., and Koller, B. H. (1999) Nat. Med. 5 1199–1202 [DOI] [PubMed] [Google Scholar]
- 34.Dorsam, R. T., Kim, S., Murugappan, S., Rachoor, S., Shankar, H., Jin, J., and Kunapuli, S. P. (2005) Blood 105 2749–2756 [DOI] [PubMed] [Google Scholar]
- 35.Kim, S., Jin, J., and Kunapuli, S. P. (2004) J. Biol. Chem. 279 4186–4195 [DOI] [PubMed] [Google Scholar]
- 36.Kim, S., Jin, J., and Kunapuli, S. P. (2006) Blood 107 947–954 [DOI] [PubMed] [Google Scholar]
- 37.Dangelmaier, C., Jin, J., Daniel, J. L., Smith, J. B., and Kunapuli, S. P. (2000) Eur. J. Biochem. 267 2283–2289 [DOI] [PubMed] [Google Scholar]
- 38.Uht, R. M., Amos, S., Martin, P. M., Riggan, A. E., and Hussaini, I. M. (2007) Oncogene 26 2885–2893 [DOI] [PubMed] [Google Scholar]
- 39.Newton, A. C. (2001) Chem. Rev. 101 2353–2364 [DOI] [PubMed] [Google Scholar]
- 40.Baurand, A., Raboisson, P., Freund, M., Leon, C., Cazenave, J. P., Bourguignon, J. J., and Gachet, C. (2001) Eur. J. Pharmacol. 412 213–221 [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi, M., Suzumura, K., Nagai, K., Kawasaki, T., Takasaki, J., Sekiguchi, M., Moritani, Y., Saito, T., Hayashi, K., Fujita, S., Tsukamoto, S., and Suzuki, K. (2004) Bioorg. Med. Chem. 12 3125–3133 [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki, T., Taniguchi, M., Moritani, Y., Hayashi, K., Saito, T., Takasaki, J., Nagai, K., Inagaki, O., and Shikama, H. (2003) Thromb. Haemost. 90 406–413 [DOI] [PubMed] [Google Scholar]
- 43.Lum, H., Podolski, J. L., Gurnack, M. E., Schulz, I. T., Huang, F., and Holian, O. (2001) Am. J. Physiol. Lung Cell Mol. Physiol. 281 L546–L555 [DOI] [PubMed] [Google Scholar]
- 44.Liedtke, C. M., Wang, X., and Smallwood, N. D. (2005) J. Biol. Chem. 280 25491–25498 [DOI] [PubMed] [Google Scholar]
- 45.Csukai, M., Chen, C. H., De Matteis, M. A., and Mochly-Rosen, D. (1997) J. Biol. Chem. 272 29200–29206 [DOI] [PubMed] [Google Scholar]
- 46.Ron, D., Luo, J., and Mochly-Rosen, D. (1995) J. Biol. Chem. 270 24180–24187 [DOI] [PubMed] [Google Scholar]
- 47.Inagaki, K., Hahn, H. S., Dorn, G. W., 2nd, and Mochly-Rosen, D. (2003) Circulation 108 869–875 [DOI] [PubMed] [Google Scholar]
- 48.Mills, D. C. (1996) Thromb. Haemost. 76 835–856 [PubMed] [Google Scholar]
- 49.Schror, K. (1997) Semin Thromb. Hemost. 23 349–356 [DOI] [PubMed] [Google Scholar]
- 50.Parker, P. J., Bosca, L., Dekker, L., Goode, N. T., Hajibagheri, N., and Hansra, G. (1995) Biochem. Soc. Trans. 23 153–155 [DOI] [PubMed] [Google Scholar]
- 51.Goode, N. T., Hajibagheri, M. A., and Parker, P. J. (1995) J. Biol. Chem. 270 2669–2673 [DOI] [PubMed] [Google Scholar]
- 52.Lee, H. W., Smith, L., Pettit, G. R., and Smith, J. B. (1997) Mol. Pharmacol. 51 439–447 [PubMed] [Google Scholar]
- 53.Dangelmaier, C. A., Quinter, P. G., Jin, J., Tsygankov, A. Y., Kunapuli, S. P., and Daniel, J. L. (2005) Blood 105 3918–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joseph, E. K., Bogen, O., Alessandri-Haber, N., and Levine, J. D. (2007) Pain 132 67–73 [DOI] [PubMed] [Google Scholar]
- 55.Jin, J., Quinton, T. M., Zhang, J., Rittenhouse, S. E., and Kunapuli, S. P. (2002) Blood 99 193–198 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.