Abstract
A retrospective analysis of adenovirus serotypes associated with gastroenteritis involved the examination of 143 stool specimens collected between 1983 and 1986 from symptomatic patients whose stools were positive for adenovirus by electron microscopy. The virus isolates obtained from 140 of the specimens were typed according to the SmaI cleavage pattern of the viral DNA and by neutralization with specific antisera. The predominant types were adenovirus type 31 (Ad31) (18%), Ad40 (16.9%), and Ad41 (38%), which together accounted for more than 70% of the isolates. The remaining virus isolates were typed as Ad1, 2, 3, 5, 7, and 12. DNA restriction analysis proved to be better than serum neutralization for identification of the enteric adenovirus serotypes in stool specimens. HindIII cleavage identified four Ad41 variants, none of which had a HindIII restriction pattern identical to that of the prototype strain Tak. Over the time period of the study, the incidence of Ad40 showed an overall decrease accompanied by an increased incidence of Ad41, while the incidence of Ad31 was relatively stable.
Full text
PDF

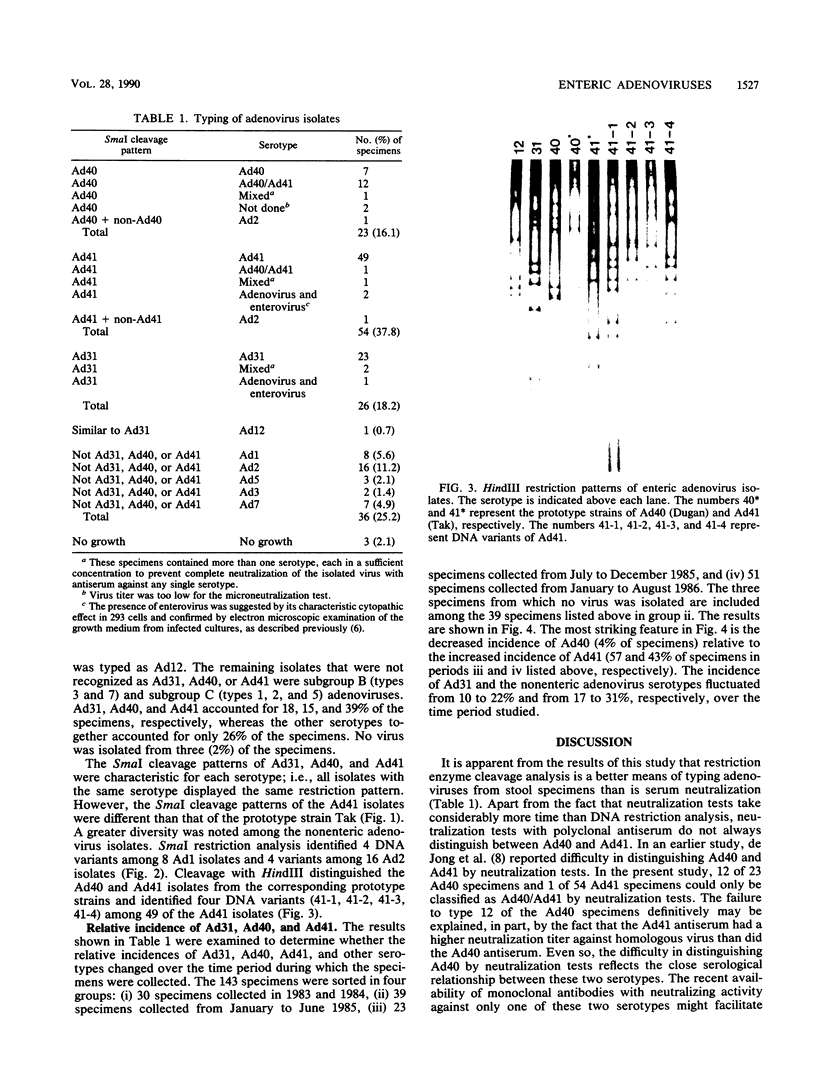


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T., Wigand R., Richter J. Gastroenteritis in infants, associated with a genome type of adenovirus 31 and with combined rotavirus and adenovirus 31 infection. Eur J Pediatr. 1987 Jan;146(1):38–40. doi: 10.1007/BF00647280. [DOI] [PubMed] [Google Scholar]
- August M. J., Warford A. L. Evaluation of a commercial monoclonal antibody for detection of adenovirus antigen. J Clin Microbiol. 1987 Nov;25(11):2233–2235. doi: 10.1128/jcm.25.11.2233-2235.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidler J. L., Hilliard P. R., Rill R. L. Ultrasensitive staining of nucleic acids with silver. Anal Biochem. 1982 Nov 1;126(2):374–380. doi: 10.1016/0003-2697(82)90530-9. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Arrobio J. O., Jeffries B. C., Stallings E. P., Lewis C., Miles A. J., Gardner M. K., Parrott R. H. Adenoviruses and pediatric gastroenteritis. J Infect Dis. 1985 Mar;151(3):437–443. doi: 10.1093/infdis/151.3.437. [DOI] [PubMed] [Google Scholar]
- Brown M., Petric M. Evaluation of cell line 293 for virus isolation in routine viral diagnosis. J Clin Microbiol. 1986 Apr;23(4):704–708. doi: 10.1128/jcm.23.4.704-708.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Petric M., Middleton P. J. Diagnosis of fastidious enteric adenoviruses 40 and 41 in stool specimens. J Clin Microbiol. 1984 Sep;20(3):334–338. doi: 10.1128/jcm.20.3.334-338.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. Selection of nonfastidious adenovirus species in 293 cells inoculated with stool specimens containing adenovirus 40. J Clin Microbiol. 1985 Aug;22(2):205–209. doi: 10.1128/jcm.22.2.205-209.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Shami Y., Zywulko M., Singh-Naz N., Middleton P. J. Time-resolved fluoroimmunoassay for enteric adenoviruses using the europium chelator 4,7-bis(chlorosulfophenyl)-1,10-phenanthroline-2,9-dicarboxylic acid. J Clin Microbiol. 1990 Jun;28(6):1398–1402. doi: 10.1128/jcm.28.6.1398-1402.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. P., Brandt C. D., Wassermann F. E., Hall C. E., Spigland I., Kogon A., Elveback L. R. The virus watch program: a continuing surveillance of viral infections in metropolitan New York families. VI. Observations of adenovirus infections: virus excretion patterns, antibody response, efficiency of surveillance, patterns of infections, and relation to illness. Am J Epidemiol. 1969 Jan;89(1):25–50. doi: 10.1093/oxfordjournals.aje.a120913. [DOI] [PubMed] [Google Scholar]
- Fox J. P., Hall C. E., Cooney M. K. The Seattle Virus Watch. VII. Observations of adenovirus infections. Am J Epidemiol. 1977 Apr;105(4):362–386. doi: 10.1093/oxfordjournals.aje.a112394. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Grandien M., Pettersson C. A., Svensson L., Uhnoo I. Latex agglutination test for adenovirus diagnosis in diarrheal disease. J Med Virol. 1987 Dec;23(4):311–316. doi: 10.1002/jmv.1890230402. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Mauthe G., Joshua J., Hannan C. K. Examination of uncommon clinical isolates of human adenoviruses by restriction endonuclease analysis. J Clin Microbiol. 1985 Apr;21(4):611–616. doi: 10.1128/jcm.21.4.611-616.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Perron-Henry D. M., Blacklow N. R. Antigen detection with monoclonal antibodies for the diagnosis of adenovirus gastroenteritis. J Infect Dis. 1987 Jun;155(6):1167–1171. doi: 10.1093/infdis/155.6.1167. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Perron-Henry D. M., Stobbs-Walro D., Blacklow N. R. Preparation and characterization of monoclonal antibodies to enteric adenovirus types 40 and 41. Arch Virol. 1987;94(3-4):259–265. doi: 10.1007/BF01310718. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Johansson K. H., Anderson L. J., Tsou C. J., Halonen P. E. Comparison of monoclonal time-resolved fluoroimmunoassay with monoclonal capture-biotinylated detector enzyme immunoassay for adenovirus antigen detection. J Clin Microbiol. 1987 Sep;25(9):1662–1667. doi: 10.1128/jcm.25.9.1662-1667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Cosgrove B. P., Brown R. A., Madeley C. R. Faecal adenoviruses from Glasgow babies. Studies on culture and identity. J Hyg (Lond) 1982 Jun;88(3):463–474. doi: 10.1017/s0022172400070327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd A. H. Genome variants of adenovirus 41 (subgroup G) from children with diarrhoea in South Africa. J Med Virol. 1984;14(1):49–59. doi: 10.1002/jmv.1890140108. [DOI] [PubMed] [Google Scholar]
- Madeley C. R. The emerging role of adenoviruses as inducers of gastroenteritis. Pediatr Infect Dis. 1986 Jan-Feb;5(1 Suppl):S63–S74. doi: 10.1097/00006454-198601001-00012. [DOI] [PubMed] [Google Scholar]
- Mortensson-Egnund K., Kjeldsberg E. Improved ELISA for the detection of adenovirus antigen in faeces extracts by the biotin/streptavidin interaction. J Virol Methods. 1986 Aug;14(1):57–63. doi: 10.1016/0166-0934(86)90007-8. [DOI] [PubMed] [Google Scholar]
- Schmitz H., Wigand R., Heinrich W. Worldwide epidemiology of human adenovirus infections. Am J Epidemiol. 1983 Apr;117(4):455–466. doi: 10.1093/oxfordjournals.aje.a113563. [DOI] [PubMed] [Google Scholar]
- Singh-Naz N., Rodriguez W. J., Kidd A. H., Brandt C. D. Monoclonal antibody enzyme-linked immunosorbent assay for specific identification and typing of subgroup F adenoviruses. J Clin Microbiol. 1988 Feb;26(2):297–300. doi: 10.1128/jcm.26.2.297-300.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984 Sep;20(3):365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigand R. Fast identification of adenovirus 40/41 in infants with enteritis. Eur J Clin Microbiol. 1987 Oct;6(5):606–607. doi: 10.1007/BF02014267. [DOI] [PubMed] [Google Scholar]
- Wood D. J., Bijlsma K., de Jong J. C., Tonkin C. Evaluation of a commercial monoclonal antibody-based enzyme immunoassay for detection of adenovirus types 40 and 41 in stool specimens. J Clin Microbiol. 1989 Jun;27(6):1155–1158. doi: 10.1128/jcm.27.6.1155-1158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. J., de Jong J. C., Bijlsma K., van der Avoort H. G. Development and evaluation of monoclonal antibody-based immune electron microscopy for diagnosis of adenovirus types 40 and 41. J Virol Methods. 1989 Sep;25(3):241–250. doi: 10.1016/0166-0934(89)90051-7. [DOI] [PubMed] [Google Scholar]
- de Jong J. C., Wigand R., Kidd A. H., Wadell G., Kapsenberg J. G., Muzerie C. J., Wermenbol A. G., Firtzlaff R. G. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11(3):215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]
- van der Avoort H. G., Wermenbol A. G., Zomerdijk T. P., Kleijne J. A., van Asten J. A., Jensma P., Osterhaus A. D., Kidd A. H., de Jong J. C. Characterization of fastidious adenovirus types 40 and 41 by DNA restriction enzyme analysis and by neutralizing monoclonal antibodies. Virus Res. 1989 Feb;12(2):139–157. doi: 10.1016/0168-1702(89)90060-9. [DOI] [PubMed] [Google Scholar]