Abstract
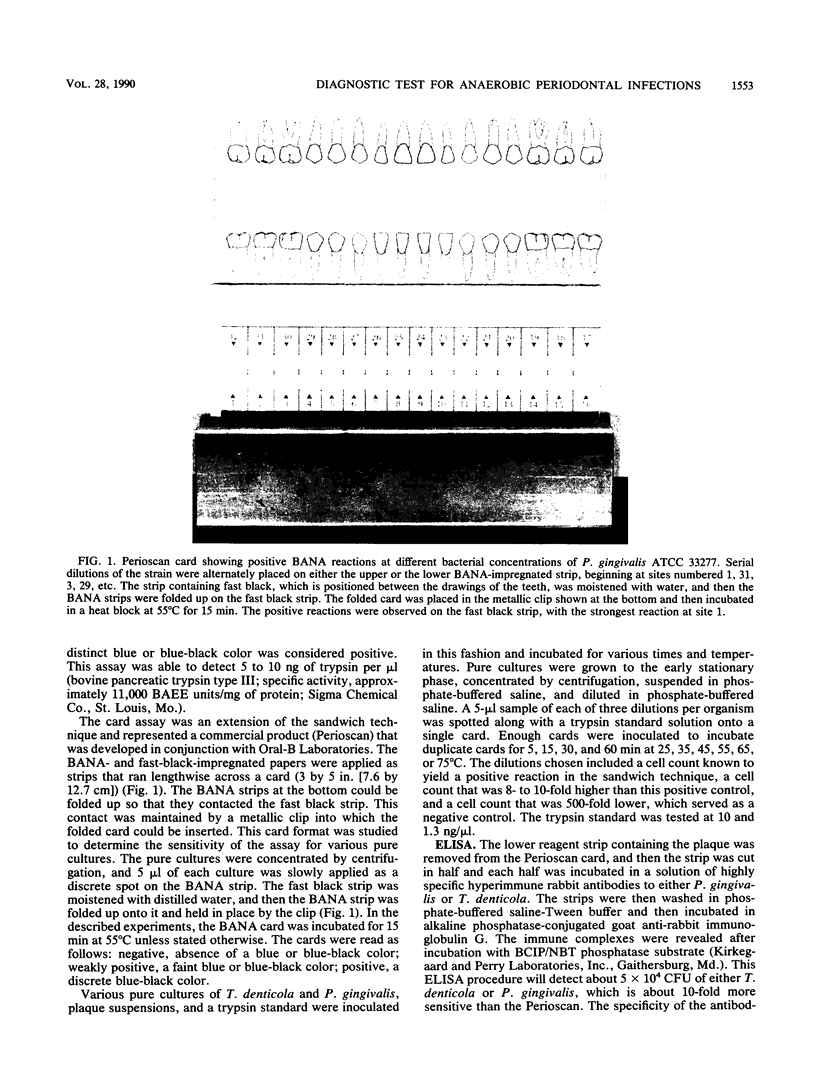
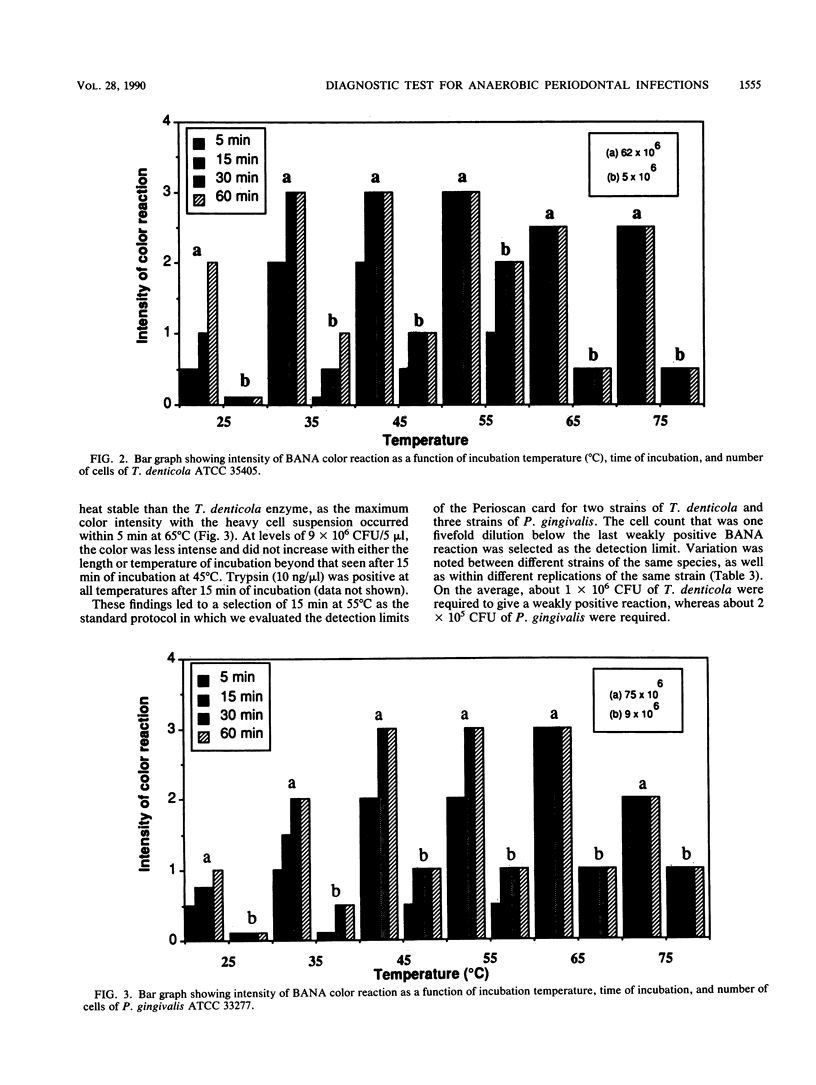
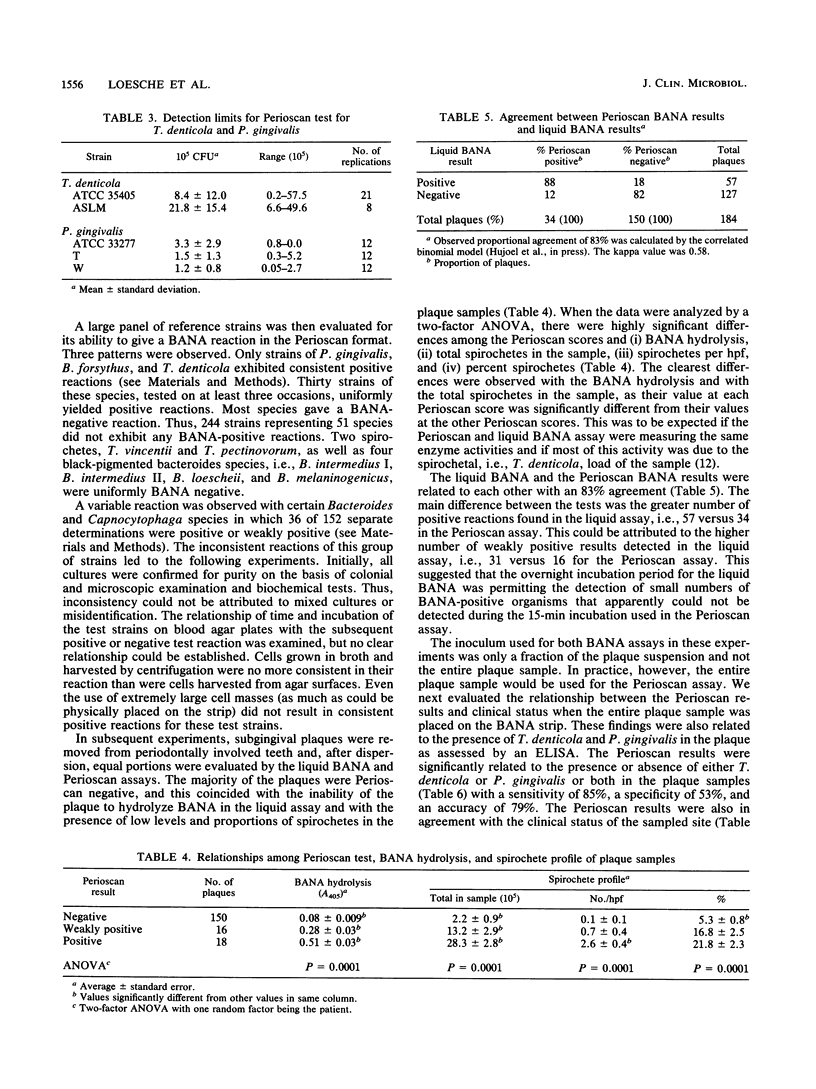
Treponema denticola, Porphyromonas (Bacteroides) gingivalis, and Bacteroides forsythus are among the anaerobic species frequently associated with adult forms of periodontal disease. These organisms hydrolyze the synthetic peptide benzoyl-DL-arginine-naphthylamide (BANA), and such enzyme activity can be detected in the plaque and related to clinical disease and the presence of spirochetes. In this investigation, the liquid BANA assay was compared with a commercially developed BANA assay which employed a paper format and which could be read after a 15-min incubation. In the paper format, strips of a Whatman filter paper were impregnated with BANA and strips of nitrocellulose paper were impregnated with fast black K salt. Both strips were applied lengthwise across a paper card (3 by 5 in. [7.6 by 12.7 cm]). The BANA strip at the bottom was inoculated with the test sample (pure culture, plaque), folded back so that it contacted the fast black strip, and then incubated for 15 min at 55 degrees C. T. denticola, P. gingivalis, and B. forsythus always gave a positive reaction, whereas 51 other plaque species were always negative. Six Bacteroides and Capnocytophaga species on occasion had weak reactions. The proportional agreement between BANA positiveness and clinical disease was similar for both the liquid and the paper assays. The sensitivity, specificity, and accuracy relative to the clinical standard of the liquid assay were 74, 76, and 77%, respectively, while those of the paper assay were 81, 78, and 80%, respectively. The paper assay was significantly associated with the presence of either T. denticola or P. gingivalis or both in the plaque samples, with a sensitivity of 85%, a specificity of 53%, and an accuracy of 79%. These findings indicate that a rapid paper assay for BANA hydrolysis gives data comparable to those obtained with the liquid BANA assay.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretz W. A., Loesche W. J. Characteristics of trypsin-like activity in subgingival plaque samples. J Dent Res. 1987 Nov;66(11):1668–1672. doi: 10.1177/00220345870660111301. [DOI] [PubMed] [Google Scholar]
- Dzink J. L., Smith C., Socransky S. S. Semiautomated technique for identification of subgingival isolates. J Clin Microbiol. 1984 May;19(5):599–605. doi: 10.1128/jcm.19.5.599-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzink J. L., Socransky S. S., Haffajee A. D. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988 May;15(5):316–323. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Genco R. J. Highlights of the conference and perspectives for the future. J Periodontal Res. 1987 May;22(3):164–171. doi: 10.1111/j.1600-0765.1987.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Gmür R., Strub J. R., Guggenheim B. Prevalence of Bacteroides forsythus and Bacteroides gingivalis in subgingival plaque of prosthodontically treated patients on short recall. J Periodontal Res. 1989 Mar;24(2):113–120. doi: 10.1111/j.1600-0765.1989.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Hockett R. N., Syed S. A. The predominant cultivable flora of tooth surface plaque removed from institutionalized subjects. Arch Oral Biol. 1972 Sep;17(9):1311–1325. doi: 10.1016/0003-9969(72)90164-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Morrison E. C., Kerry G. A., Higgins T., Stoll J. Metronidazole in periodontitis. I. Clinical and bacteriological results after 15 to 30 weeks. J Periodontol. 1984 Jun;55(6):325–335. doi: 10.1902/jop.1984.55.6.325. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Stoll J. Trypsin-like activity in subgingival plaque. A diagnostic marker for spirochetes and periodontal disease? J Periodontol. 1987 Apr;58(4):266–273. doi: 10.1902/jop.1987.58.4.266. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. The identification of bacteria associated with periodontal disease and dental caries by enzymatic methods. Oral Microbiol Immunol. 1986 Nov;1(1):65–72. doi: 10.1111/j.1399-302x.1986.tb00322.x. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. The role of spirochetes in periodontal disease. Adv Dent Res. 1988 Nov;2(2):275–283. doi: 10.1177/08959374880020021201. [DOI] [PubMed] [Google Scholar]
- Moore W. E. Microbiology of periodontal disease. J Periodontal Res. 1987 Sep;22(5):335–341. doi: 10.1111/j.1600-0765.1987.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt E. D., Strzempko M. N., Vaccaro K. K., Peros W. J., French C. K. Comparison of cultural methods and DNA probe analyses for the detection of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis, and Bacteroides intermedius in subgingival plaque samples. J Periodontol. 1988 Jul;59(7):431–438. doi: 10.1902/jop.1988.59.7.431. [DOI] [PubMed] [Google Scholar]
- Schmidt E. F., Bretz W. A., Hutchinson R. A., Loesche W. J. Correlation of the hydrolysis of benzoyl-arginine naphthylamide (BANA) by plaque with clinical parameters and subgingival levels of spirochetes in periodontal patients. J Dent Res. 1988 Dec;67(12):1505–1509. doi: 10.1177/00220345880670121201. [DOI] [PubMed] [Google Scholar]
- Simonson L. G., Goodman C. H., Bial J. J., Morton H. E. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988 Apr;56(4):726–728. doi: 10.1128/iai.56.4.726-728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Bragd L., Wikström M., Dahlén G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986 Jul;13(6):570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Strzempko M. N., Belsky C. A., McKinley G. A. API ZYM and API An-Ident reactions of fastidious oral gram-negative species. J Clin Microbiol. 1985 Sep;22(3):333–335. doi: 10.1128/jcm.22.3.333-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J., Reynolds H. S., Chen P., Genco R. J. Rapid identification of periodontal pathogens in subgingival dental plaque. Comparison of indirect immunofluorescence microscopy with bacterial culture for detection of Bacteroides gingivalis. J Periodontol. 1985 Nov;56(11 Suppl):32–40. doi: 10.1902/jop.1985.56.11s.32. [DOI] [PubMed] [Google Scholar]



