Abstract
Myocardial G protein-coupled receptor kinase 2 (GRK2) is a critical regulator of cardiac β-adrenergic receptor (βAR) signaling and cardiac function. Its upregulation in heart failure (HF) may further depress cardiac function and contribute to mortality in this syndrome. Preventing GRK2 translocation to activated βAR with a GRK2-derived peptide that binds Gβγ (βARKct) has benefited some models of HF, but the precise mechanism is uncertain, as GRK2 is still present and βARKct has other potential effects.
We generated mice in which cardiac myocyte GRK2 expression was normal during embryonic development, but was ablated after birth (αMHC-Cre × GRK2 fl/fl), or only after administration of tamoxifen (αMHC-MerCreMer×GRK2 fl/fl) and examined the consequences of GRK2 ablation before and after surgical coronary artery ligation on cardiac adaptation after myocardial infarction. Absence of GRK2 prior to coronary artery ligation prevented maladaptive post-infarction remodeling and preserved βAR responsiveness. Strikingly, GRK2 ablation initiated 10 days after infarction increased survival, enhanced cardiac contractile performance, and halted ventricular remodeling.
These results demonstrate a specific causal role for GRK2 in post-infarction cardiac remodeling and HF and support therapeutic approaches of targeting GRK2 or restoring βAR signaling by other means to improve outcomes in HF.
Keywords: heart failure, myocardial infarction, conditional gene targeting, GRK2
INTRODUCTION
The role of myocardial β-adrenergic receptor (βAR) signaling in heart failure (HF) is controversial, with abundant data supporting dominant effects that are either pathological, or beneficial. Pathological effects are strongly suggested by the cardiomyopathies that develop under conditions of chronic βAR activation after long-term catecholamine administration 1 or as a consequence of high-levels of overexpressed cardiac β1 or β2-ARs 2, 3. Likewise, enhanced signaling from overexpression of the βAR signal transducer Gαs causes HF 4. Most importantly, however, are the human patient data indicating that βAR signaling is deleterious in HF: Genetic polymorphisms that enhance myocardial sympathetic tone are independent risk factors for HF 5, and pharmacological βAR antagonism has been known for over two decades to strikingly prolong life when used as primary therapy in HF 6.
Despite the strength of data in experimental systems and the human condition that excessive βAR signaling can contribute to HF, there continue to be those who advocate modulating βARs in HF as a form of therapy 7. This viewpoint is based upon observations that two transgenic mouse lines overexpressing low levels of β2-AR in the heart, which were developed in two independent laboratories, demonstrated enhanced contractile function without myocardial disease 8, 9, and β2-ARs can improve function of cardiomyopathy models 10, 11. Another group has suggested that transgenic or adenoviral-mediated overexpression of the downstream βAR signaling effector, adenylyl cyclase, has similar effects 12. Also consistent with this idea are studies pioneered in our laboratory where prevention of typical HF-associated G protein-coupled receptor kinase 2 (GRK2)-mediated desensitization and downregulation of myocardial βARs has rescued numerous genetic 13, 14 and physiological 15, 16 models of HF.
The reasons for the apparently contradictory data sets relating to βAR signaling in failing myocardium are unknown, but understanding them will be essential to fully optimizing HF treatment. Regarding GRK2 inhibition specifically, concerns have been expressed about the specificity and true mechanism of action of the βARKct GRK2 inhibitor used in many of these studies 17. It is also not entirely clear that GRK2 is the best target in HF, as GRK3, GRK5, and GRK6 are also expressed in the heart, and forced expression of GRK5 had similar desensitization effects on myocardial βARs as does forced expression of GRK2 18. Indeed, cardiac-specific ablation of GRK2 in the early embryo actually sensitized adult mice to catecholamine cardiomyopathy, rather than conferring protection, which was interpreted as evidence for pathological effects of βAR signaling 19. These results raise additional questions about whether GRK2 inhibition may have different effects in different experimental HF models, or at different time points relative to the induction of HF.
To address these issues we created mice in which the GRK2 gene could be selectively ablated in cardiac myocytes in a temporally-defined manner, and compared the consequences of heart-specific GRK2 ablation before and after induction of HF by myocardial infarction.
MATERIALS AND METHODS
Experimental animals
Conditional mice bearing floxed GRK2 alleles (GRK2 fl/fl) have previously been described 19. Transgenic mice overexpressing Cre-recombinase protein fused to two mutant estrogen receptor ligand-binding domains under the control of the αMHC promoter (αMHC-MerCreMer) 20 were received from The Jackson Laboratory (JAX Mice and Services, Βar Harbor, Maine, USA). Homozygous mice with the floxed GRK2 alleles were crossed with αMHC-MerCreMer mice and resulting αMHC-MerCreMer × GRK2 fl/fl mice and GRK2 fl/fl, as well as αMHC-MerCreMer, were studied. To induce Cre recombination and subsequent deletion of GRK2, adult αMHC-MerCreMer × GRK2 fl/fl transgenic mice were treated with tamoxifen (Tmx) (Sigma-Aldrich, St. Louis, Missouri, USA) as described previously 20.
In addition αMHC-Cre mice 21 were bred on to the GRK2 fl/fl background to generate cardiac GRK2-deleted mice, initiated by the activation of the αMHC-promoter. αMHC-Cre × GRK2 fl/fl mice and GRK2 fl/fl were included in the study. All animal procedures and experiments were performed in accordance with the guidelines of the IACUC of Thomas Jefferson University. All animals were bred and maintained on a C57Bl/6 background.
Model of LV myocardial infarction
MI was induced by high ligation of the left anterior descending coronary artery (LAD) as described previously 22 and more detailed in the online expanded methods supplement.
Echocardiographic and hemodynamic analysis of cardiac function
LV ventricular function was assessed by transthoracic echocardiography and LV hemodynamics in anesthetized mice as described previously 23 and more detailed in the online expanded methods supplement. For measurements of LV hemodynamics all recordings were performed with a closed chest approach at baseline and after increasing doses of isoproterenol (Sigma-Aldrich, St. Louis, Missouri, USA) as described previously 23.
Isolation of cardiac myocytes
Adult mouse cardiac myocytes were isolated from sham and infarcted GRK2 fl/fl and αMHC-Cre × GRK2 fl/fl mice as previously described 24 and more detailed in the online expanded methods supplement.
Single myocyte contractility studies
Isolated cardiac myocytes were stimulated in an electrical field, and continuously flushed with tyrodes containing 1mM CaCl2 without (baseline) and with 10−8M isoproterenol (isoproterenol-stimulation). Single-cell contractions were measured by video edge detection (Fluorescence and Contractility System, IonOptix, Milton, Massachusetts, USA) .
RNA isolation, reverse transcription and quantitative real-time RT-PCR
Isolated LV cardiac myocytes (rescue-study) or myocardial tissue from the remote zone (prevention-study) was snap-frozen. Analysis was carried out 35 days post MI/ 21 days after the last dose of Tmx (rescue-study) or 28 days post MI (prevention study). Quantitative real-time polymerase chain reaction was performed as described previously 22 and more detailed in the online expanded methods supplement.
Western blot analysis
Western blotting was performed as described previously 22 and more detailed in the online expanded methods supplement. Cardiac protein levels of GRK2 (sc-562, C-15, Santa Cruz Biotechnology, 1:5,000), α-actin (A7811, Sigma-Aldrich, 1:5,000) and calsequestrin (CSQ) (208915; Calbiochem; 1:10,000), were assessed in cardiac myocyte cellular preparations.
Statistical analysis
Data are generally expressed as mean ± SEM. An unpaired two tailed t-test, a one-way ANOVA and a two-way repeated measurement ANOVA was performed for between-group comparisons. Survival analysis was performed by the Kaplan-Meier method and between-group differences in survival were tested by the log-rank test. For all tests, a P value < 0.05 was considered significant.
RESULTS
Defined loss of GRK2 in cardiac myocytes after MI rescues the HF phenotype
Survival of HF is improved after loss of GRK2
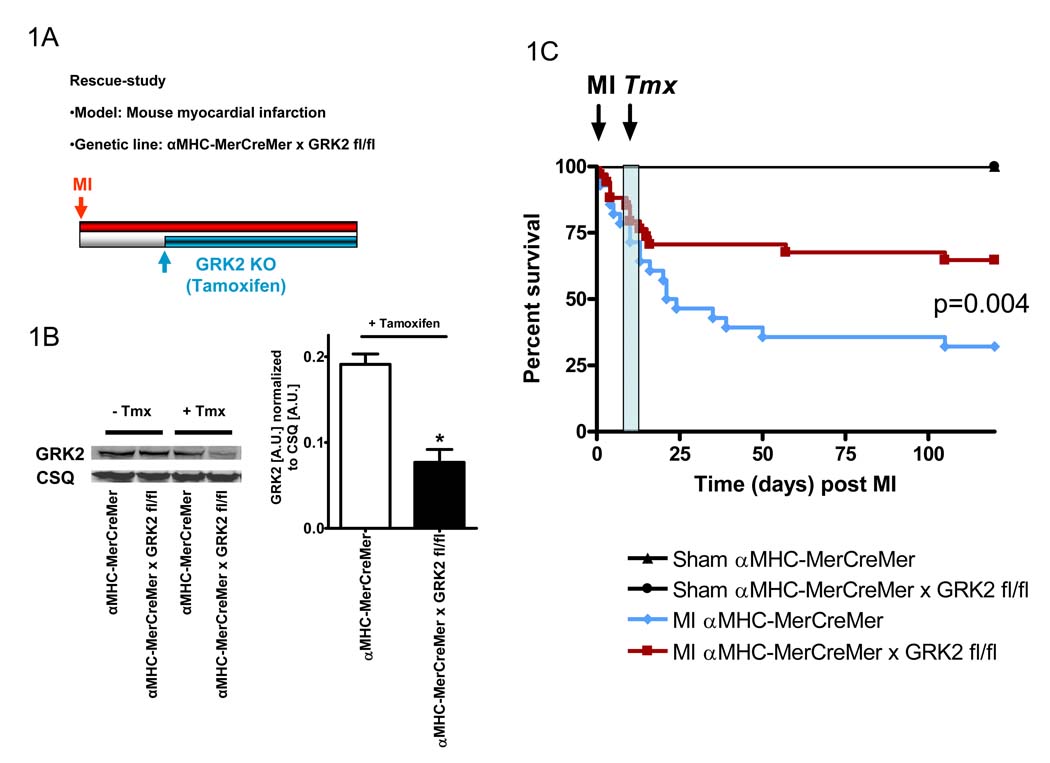
To gain insight into whether GRK2 ablation could be therapeutic in hearts already in HF, we used Tmx-inducible GRK2 KO (αMHC-MerCreMer × GRK2 fl/fl) mice. These mice along with αMHC-MerCreMer mice as controls were subjected to coronary artery ligation and loss of GRK2 was induced by 5 consecutive days of Tmx injections (on days 10–14 post-MI) (Fig. 1A). GRK2 levels in these two groups of mice were similar until after Tmx induction (Fig. 1B). We found similar mortality in both mice until ~12–13 days post-MI (Fig. 1C). Interestingly, following the Tmx-injections survival in Tmx-treated αMHC-MerCreMer × GRK2 fl/fl mice was significantly preserved compared to the continued death seen in Tmx-treated control mice (Fig. 1C). Thus, the loss of cardiac GRK2 after MI prevents the significant mortality seen chronically in HF mice with normal levels of GRK2 in their hearts.
Figure 1.
Survival of HF is improved after post-MI induced loss of GRK2 in cardiac myocytes. A, Experimental protocol showing the study-design of the rescue-study using Tmx-inducible GRK2KO mice (αMHC-MerCreMer × GRK2 fl/fl, GRK2 fl/fl and αMHC-MerCreMer mice). B, Time-defined controlled loss of GRK2 in cardiac myocytes from Tmx-inducible αMHC-MerCreMer × GRK2 fl/fl and αMHC-MerCreMer mice. Impact of Tmx-treatment on cardiac myocyte GRK2 protein levels determined via Western Blotting. Representative Western Blot of GRK2 and CSQ (as a loading control) and quantification of the results, n=6 animals/group, *P<0.05 αMHC-MerCreMer × GRK2 fl/fl vs. αMHC-MerCreMer, unpaired two-tailed t-test. C, Survival of Tmx treated sham and infarcted αMHC-MerCreMer and αMHC-MerCreMer×GRK2 fl/fl mice throughout the study period. n=8–11 animals/group for Sham mice, n=30–34 animals/group for infarcted mice, survival was analyzed by the Kaplan-Meier method and between-group differences in survival were tested by the log-rank test.
Loss of cardiac GRK2 in already established HF rescues cardiac function
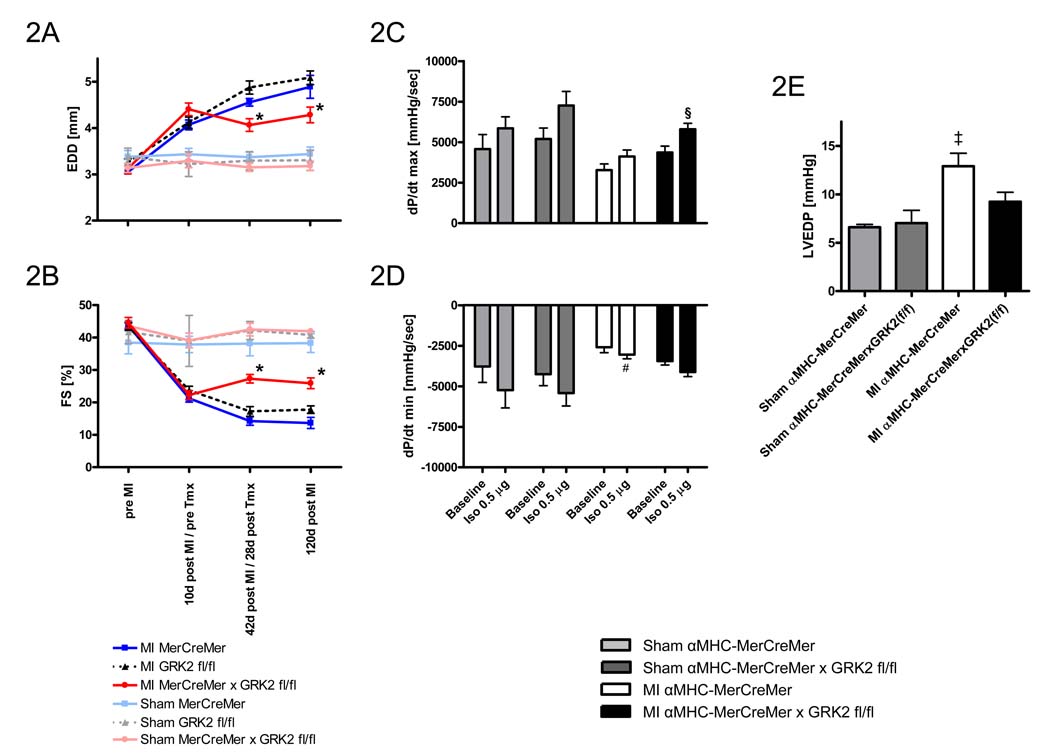
We next examined whether loss of cardiac GRK2 after MI could reverse the cardiac dysfunction, remodeling and reduced contractility associated with HF. In these studies αMHC-MerCreMer × GRK2 fl/fl, αMHC-MerCreMer, and GRK2 fl/fl mice were subjected to coronary artery ligation and cardiac function and dimensions were measured by serial echocardiography for up to 4 months after MI (Fig. 2). Importantly, all groups of mice 10 days post-MI had similar levels of chamber dilatation and cardiac dysfunction compared to pre-MI measurements (Fig. 2A and B). The 3 sham groups showed no alterations in cardiac function or dimensions at any time-point. Following loss of GRK2 in αMHC-MerCreMer × GRK2 fl/fl mice, echocardiography at 42 and 120 days post-MI showed stabilization of LV remodeling (Fig. 2A). Further, significant enhancement of cardiac function (as measured by FS [%]) was observed after the loss of cardiac GRK2 compared to both groups of Tmx-treated control mice (αMHC-MerCreMer and GRK2 fl/fl) (Fig. 2B). These salutary effects, which were clearly associated with the loss of GRK2, could be observed throughout the time span studied, showing beneficial long-term therapeutic effects.
Figure 2.
Induced loss of GRK2 rescues adverse LV remodeling and cardiac function post-MI. A, Echocardiographic assessment of EDD pre-MI, 10d post-MI (prior to Tmx treatment), 42d post-MI (28d post-Tmx treatment) and 120d post-MI. B, Echocardiographic assessment of FS pre-MI, 10d post-MI (prior to Tmx treatment), 42d post-MI (28d post-Tmx treatment) and 120d post-MI. n=6–8 animals/group for sham-operated mice, n=12–16 animals/group for MI mice, *P<0.05 vs. MI αMHC-MerCreMer and MI GRK2 fl/fl. Hemodynamic measurements of LV contractility (as measured by LV dP/dtmax) (C), and relaxation (as measured by LV dP/dtmin) (D), and LV end diastolic pressure (LVEDP) (E), 120 d post-MI (106 d post-Tmx). n=5 animals/group for sham-operated mice, n=9–11 animals/group for MI mice, §P<0.05 MI αMHC-MerCreMer × GRK2 fl/fl vs. MI αMHC-MerCreMer under respective condition, #P<0.05 MI αMHC-MerCreMer vs. Sham groups under respective condition, ‡P<0.05 MI αMHC-MerCreMer vs. MI αMHC-MerCreMer × GRK2 fl/fl and Sham groups, two-way ANOVA for A, B, C, and D, one-way ANOVA for E.
Measurements of LV hemodynamics 120 days post MI showed preserved responsiveness to β-AR stimulation in infarcted Tmx-treated αMHC-MerCreMer × GRK2 fl/fl mice, whereas infarcted Tmx-treated αMHC-MerCreMer mice revealed an impaired β-AR reserve compared to sham mice (Fig. 2C and D). LVEDP was significantly lower in infarcted αMHC-MerCreMer × GRK2 fl/fl mice as compared to infarcted αMHC-MerCreMer mice (Fig. 2E).
Loss of GRK2 in post MI failing cardiac myocytes reduces activation of the fetal gene program
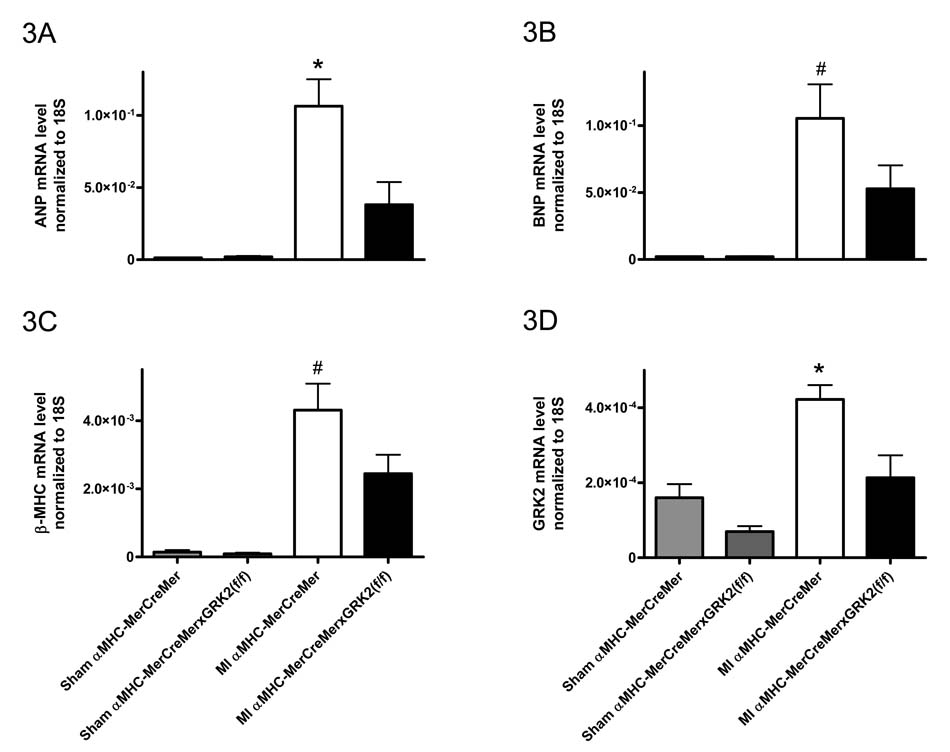
RT-PCR analysis was performed 5 weeks post MI (3 weeks after the last dose of Tmx) on cardiac myocytes isolated from the LV. Interestingly, less induction of selected fetal genes (atrial natriuretic peptide/ANP, brain natriuretic peptide/BNP, and β myosin heavy chain/β-MHC) was found in infarcted Tmx-treated αMHC-MerCreMer × GRK2 fl/fl mice as compared to post-MI Tmx-treated αMHC-MerCreMer cardiac myocytes, which showed significant up-regulation of these genes compared to sham-controls (Fig. 3).
Figure 3.
GRK2 abolishment in the post-MI failing heart alters gene expression. Quantitative RT-PCR analysis on cardiac myocytes isolated from the LV 5 weeks post MI (3 weeks after the last dose of Tmx). A, ANP; B, BNP; C, β-MHC; and D, GRK2. All values normalized to 18S levels. n=5–6 animals/group for Sham mice, n=7–11 animals/group for MI mice, *P<0.05 MI αMHC-MerCreMer vs. MI αMHC-MerCreMer × GRK2 fl/fl and Sham groups, #P<0.05 MI αMHC-MerCreMer vs. Sham groups, one-way ANOVA.
We also examined expression of GRK mRNA post-MI in cardiac myocytes isolated from the LV. As expected in control Tmx-treated αMHC-MerCreMer mice, MI produced a significant increase in GRK2 expression over sham levels, whereas infarcted Tmx-treated αMHC-MerCreMer × GRK2 fl/fl mice showed significantly lower GRK2 levels (Fig. 3D). Interestingly, GRK5 mRNA was selectively upregulated in infarcted Tmx-treated αMHC-MerCreMer mice (supplemental Fig. 5).
Loss of GRK2 before myocardial infarction prevents the development of HF
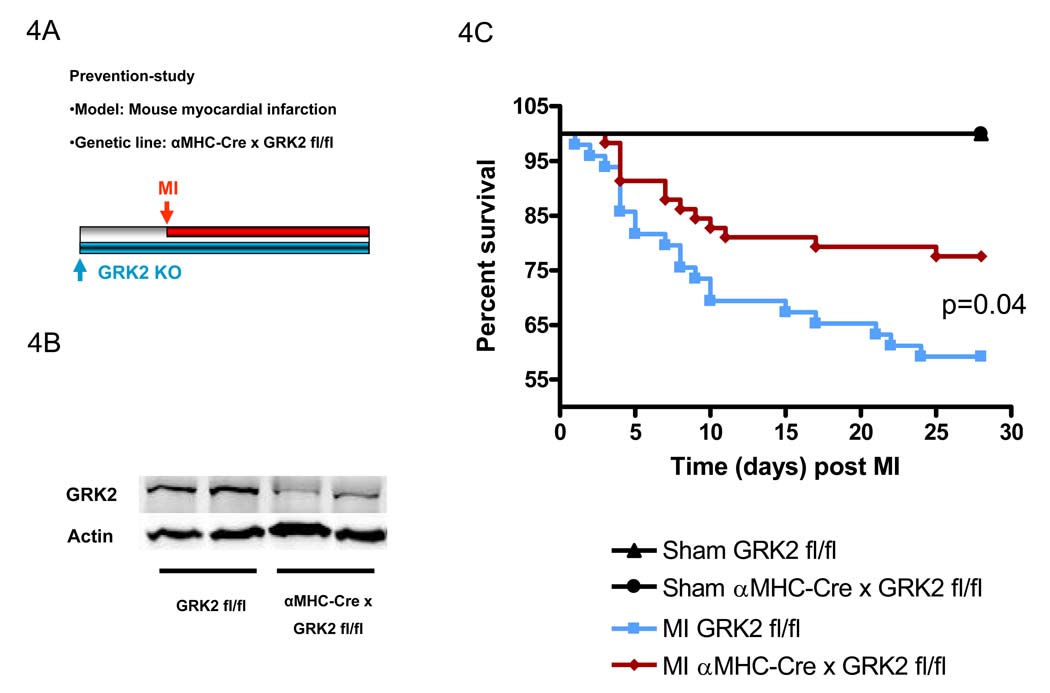
Loss of cardiac myocyte GRK2 before MI reduces infarct-related mortality
To study the consequence of GRK2 loss on the development of HF, adult (8 week old) αMHC-Cre × GRK2 fl/fl mice and their corresponding littermate controls (GRK2 fl/fl) were subjected to coronary artery ligation and subsequent MI (Fig. 4A). αMHC-Cre × GRK2 fl/fl mice have an approximate 80% loss of cardiac myocyte GRK2 (Fig. 4B). Of note, infarct size was not different 24 hours or 28 days after MI between mice with normal levels of GRK2 and the GRK2 KO mice (supplemental Fig. 2). Interestingly, survival was significantly improved in male αMHC-Cre × GRK2 fl/fl mice subjected to MI compared to control GRK2 fl/fl mice (see Fig. 4C). There was a trend towards improved survival in infarcted αMHC-Cre × GRK2 fl/fl mice of both sexes (p=0.06) (supplemental Fig. 3). The differences in survival were present, despite only the αMHC-Cre × GRK2 fl/fl mice and not the control mice in this study (GRK2 fl/fl) possessed Cre recombinase, which has previously been proven to contribute to cardiac pathology 25.
Figure 4.
Infarct-related mortality is reduced with loss of GRK2 in cardiac myocytes prior to MI. A, Experimental protocol showing the study-design of the prevention-study using conditional GRK2KO mice (αMHC-Cre × GRK2 fl/fl and GRK2 fl/fl mice). B, Conventional conditional loss of GRK2 in cardiac myocytes in αMHC-Cre × GRK2 fl/fl and GRK2 fl/fl mice. Western blot analysis for GRK2 protein and actin (as a loading control) in isolated cardiac myocytes. C, Survival of male GRK2 fl/fl and αMHC-MHC × GRK2 fl/fl mice throughout the study period. n=8 animals/group for Sham mice, n=49–58 animals/group for infarcted mice, survival was analyzed by the Kaplan-Meier method and between-group differences in survival were tested by the log-rank test.
Loss of GRK2 in cardiac myocytes before MI reduces the extent and progression of cardiac dysfunction
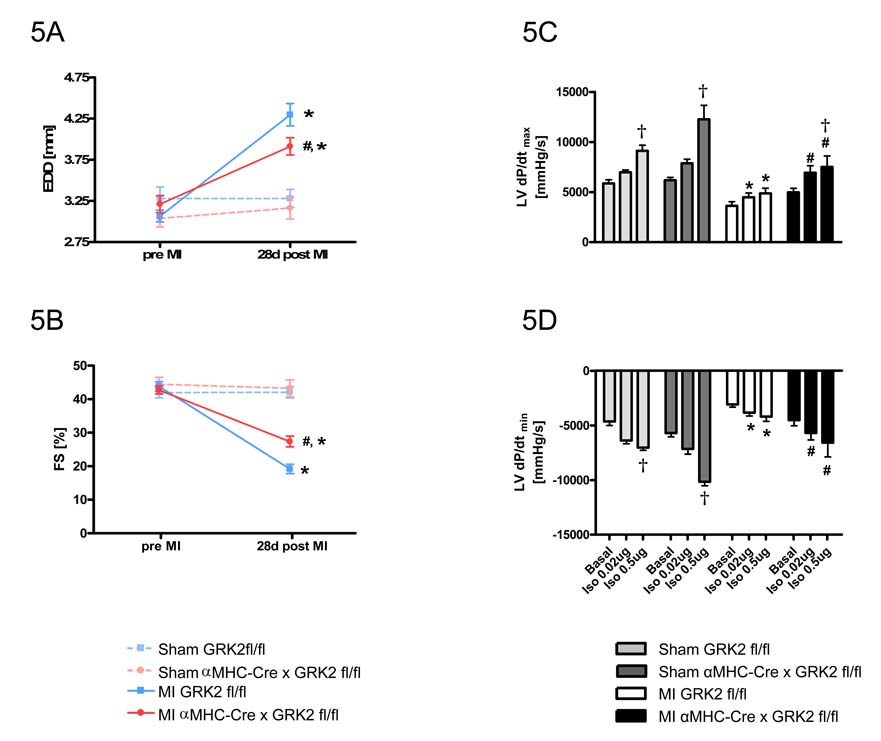
In vivo cardiac function was assessed by echocardiography prior to and 28 days after MI (or sham). 28 days after MI, control GRK2 fl/fl mice displayed significantly depressed systolic function and enlarged cardiac chambers as compared to pre-MI values (Fig. 5A and 5B). In striking contrast, αMHC-Cre × GRK2 fl/fl mice subjected to MI did not display the same degree of cardiac deterioration (Fig. 5A and B).
Figure 5.
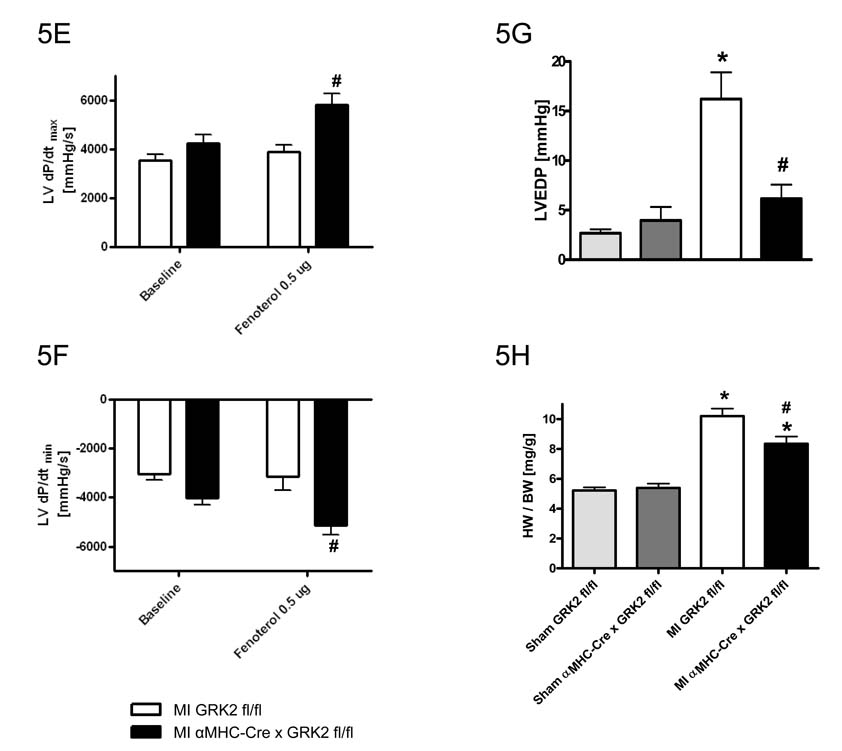
Constitutive loss of GRK2 in cardiac myocytes prior to MI reduces the extent of cardiac dysfunction. Echocardiographic assessment 28d post-MI of EDD (A) and FS (B), n=8–10 animals/group for Sham mice, n=21–30 animals/group for MI mice, *P<0.01 MI vs. Sham for respective group, #P<0.05 MI αMHC-Cre × GRK2 fl/fl vs. MI GRK2 fl/fl, one-way ANOVA. Hemodynamic measurements of LV contractility (as measured by LV dP/dtmax) (C), and relaxation (as measured by LV dP/dtmin) (D) at baseline and in response to increasing intraperitoneal injected doses of isoproterenol. E, left ventricular enddiastolic pressure (LVEDP) n=5 animals/group for sham-operated mice, n=9–10 animals/group for MI mice; †P<0.05 Iso 0.5µg vs. basal for each group, *P<0.05 MI GRK2 fl/fl vs. Sham GRK2 fl/fl for each condition, #P<0.05 MI αMHC-Cre × GRK2 fl/fl vs. MI GRK2 fl/fl for each condition, two-way ANOVA for C, and D, one-way ANOVA for E. F, Heart-to-body weight ratio at day 28 post MI. *P<0.01 MI vs. Sham, #P<0.05 MI αMHC-Cre × GRK2 fl/fl vs. MI GRK2 fl/fl, one-way ANOVA.
Loss of myocyte GRK2 before MI improves LV hemodynamics and preserves βAR responsiveness
Marked desensitization of cardiac βARs is considered a hallmark of the failing heart 26–28. To determine whether the loss of GRK2 in cardiac myocytes can reverse dysfunctional βAR signaling, we measured in vivo LV hemodynamics 28 days after MI at baseline and after isoproterenol. LV contractility (as measured by dP/dtmax) and LV relaxation (as measured by LV dP/dtmin) after isoproterenol were significantly impaired in control GRK2 fl/fl mice compared to sham control mice demonstrating a loss of inotropic reserve consistent with HF (Fig. 5C and 5D). Similarly, hemodynamic studies on αMHC-Cre × GRK2 fl/fl mice revealed signs of cardiac dysfunction at baseline; however, responses to isoproterenol were significantly improved compared to post-MI control mice showing improved contractile reserve and preserved βAR signaling in hearts without GRK2 (Fig. 5C and D). Finally, elevated LV end diastolic pressure (LVEDP) in control post-MI GRK2 fl/fl mice was significantly lowered in mice with GRK2 expression lost (Fig. 5E).
Loss of GRK2 is associated with a lower extent of cardiac hypertrophy and normalized gene expression after MI
Heart-to-body weight ratio (HW/BW-ratio) was significantly increased 28 days post-MI in control GRK2 fl/fl mice compared to corresponding sham-operated animals (Fig. 5F). Although αMHC-Cre × GRK2 fl/fl mice subjected to MI displayed LV hypertrophy compared to sham-operated controls, the extent of hypertrophy was significantly less than that observed in GRK2 fl/fl mice subjected to MI (Fig. 5F).
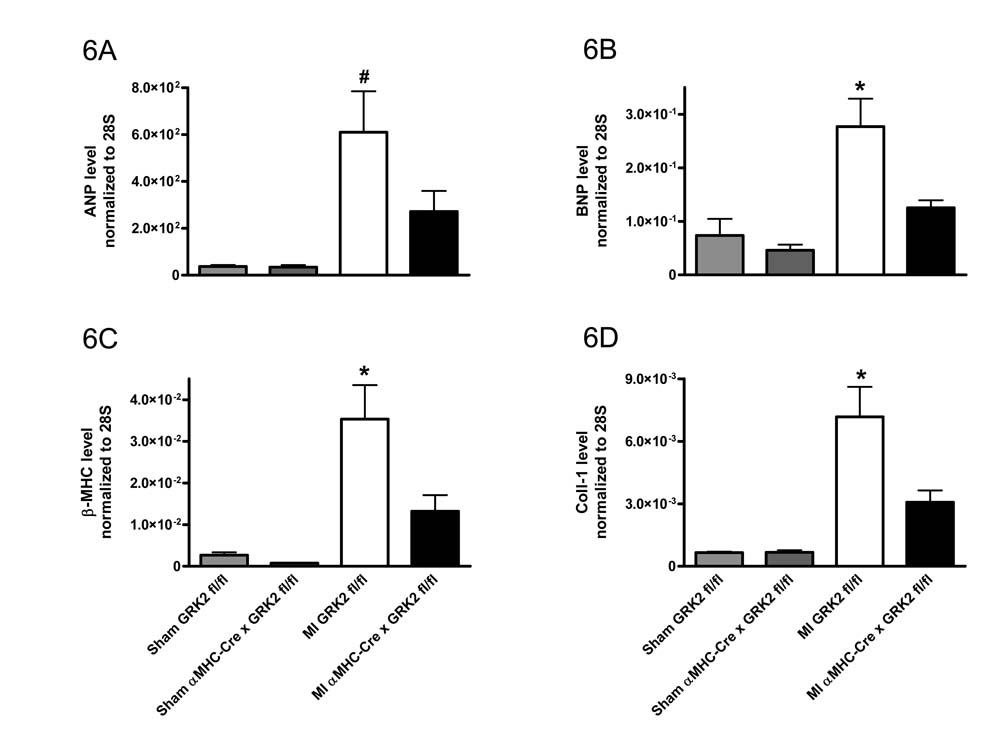
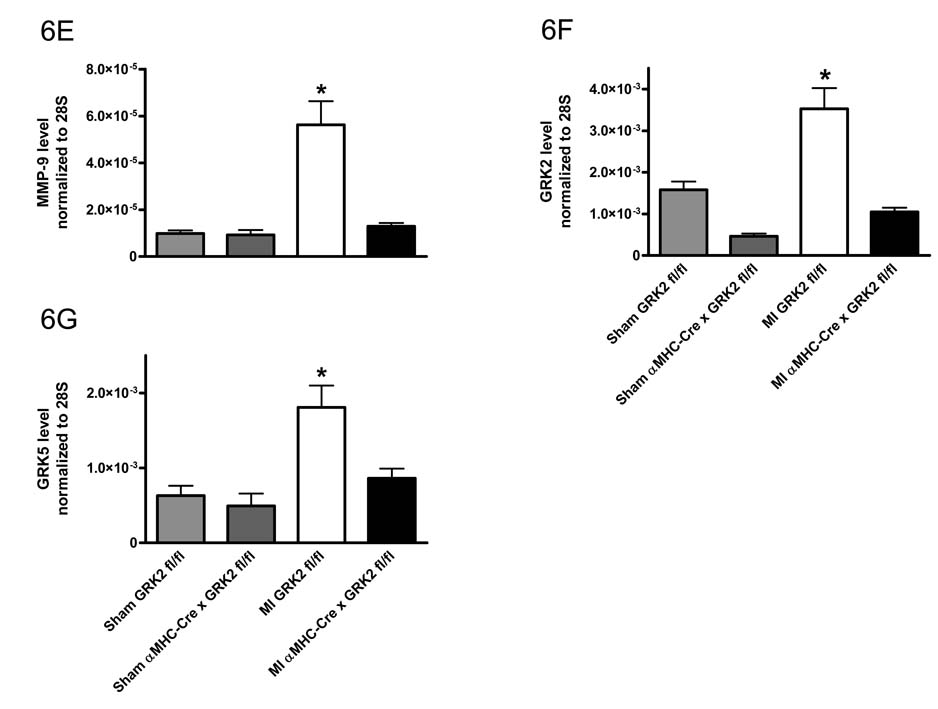
Consistent with decreased hypertrophy post-MI in GRK2 KO mice, RT-PCR analysis of the remote (non-ischemic) portion of the LV showed significantly less induction of selected fetal genes (atrial natriuretic peptide/ANP, brain natriuretic peptide/BNP, and β myosin heavy chain/β-MHC) as seen in post-MI control hearts, which showed significant up-regulation of these genes compared to sham-controls (Fig. 6A–C). Up-regulation of the mRNAs encoding matrix metalloproteinase 9 (MMP-9) and Collagen-1 (Coll-1) in the non-ischemic LV zone were also seen in post-MI GRK2 fl/fl mice indicating adverse remodeling, however in GRK2 KO mice, these changes in MMP-9 and Coll-1 were not seen (Fig. 6D and 6E). These observations in combination with the attenuation of LV chamber dilation (see above) argue in favor of significant repression of adverse LV remodeling by the loss of GRK2 in myocytes.
Figure 6.
Loss of GRK2 in cardiac myocytes alters gene expression in the failing heart. Quantitative RT-PCR analysis 28 days post MI in the remote non-infarcted region of the LV. A, ANP; B, BNP; C, β-MHC; D, Coll-1; E, MMP-9; F, GRK2; and G, GRK5. All values normalized to 28S levels. n=6–8 animals/group, *P<0.05 MI GRK2 fl/fl vs. MI αMHC-Cre × GRK2 fl/fl and Sham GRK2 fl/fl and Sham αMHC-Cre × GRK2 fl/fl, #P<0.05 MI GRK2 fl/fl vs. Sham GRK2 fl/fl, one-way ANOVA.
We also examined myocardial expression of GRK mRNA post-MI in remote (non-ischemic) sections of the LV. In agreement with the data obtained from the rescue-study, MI led to a significant increase in GRK2 expression over sham levels, which was absent in GRK2 KO mice (Fig. 6F). Interestingly, GRK5, another major GRK found in the heart 29, which was up-regulated in post-MI control (GRK2 fl/fl) hearts compared to sham was not significantly changed in the hearts of post-MI αMHC-Cre × GRK2 fl/fl mice (Fig. 6G). The results in wild-type mice were somewhat expected as GRK5 has been shown to be up-regulated in HF as well as GRK2, however, the fact that the GRK2 KO mice had no increase in GRK5 argues for a lack of compensation by this GRK. Of note, GRK3 a close homologue of GRK2 was not altered in any of our groups post MI or sham (data not shown).
Loss of GRK2 normalizes βAR responsiveness of single cardiac myocytes post-MI
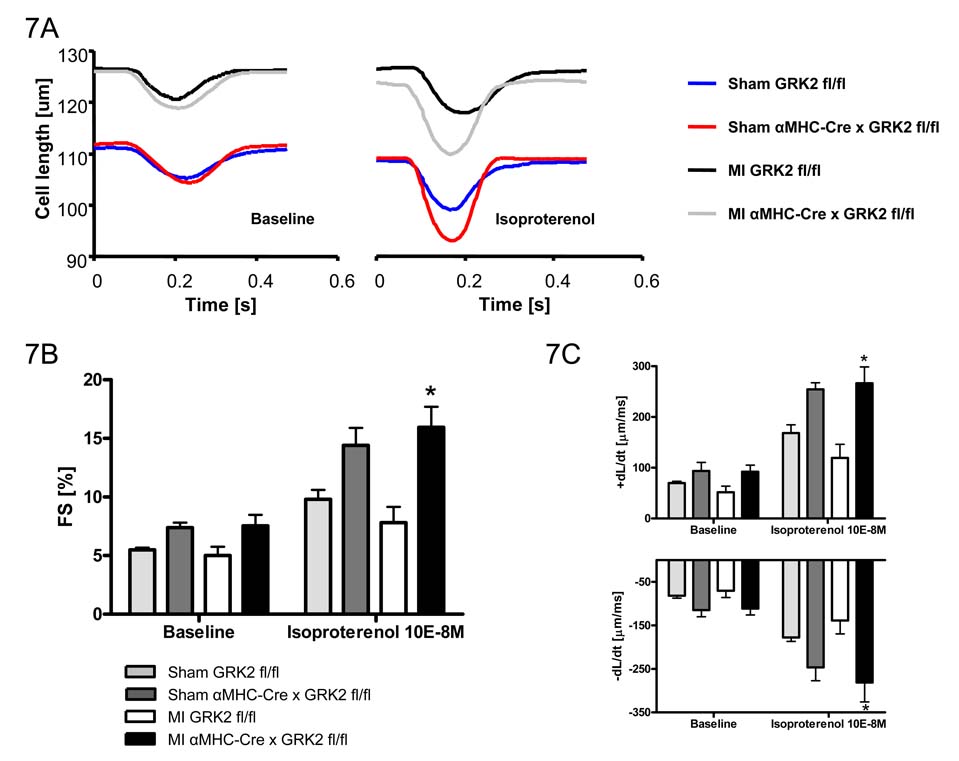
Since the loss of GRK2 in cardiac myocytes resulted in preserved in vivo LV contractility globally post-MI without affecting infarct size, we explored whether the beneficial effect was due to improved β-AR signaling and contractile function in single cardiac myocytes. Functional recordings of cardiac myocyte twitches were obtained at 2 Hz (field stimulation), 20–22°C, and 1 mM extracellular Ca2+ ([Ca2+]e). Fig. 7A shows representative steady state twitches from cardiac myocytes (from sham and infarcted GRK2 fl/fl and αMHC-Cre × GRK2 fl/fl mice) under baseline conditions (left panel) and following isoproterenol stimulation (10−8M) (right panel). Isolated cardiac myocytes from post-MI αMHC-Cre × GRK2 fl/fl mice demonstrated significantly enhanced FS, improved rate of cell shortening (+dL/dt), and improved rate of re-lengthening (−dL/dt) under isoproterenol stimulation compared to control, post-MI GRK2(fl/fl) myocytes (Fig. 7B and 7C). These results indicate that β-AR responsiveness post-MI is preserved by the loss of GRK2.
Figure 7.
Loss of GRK2 improves post-MI βAR responsiveness of single cardiac myocytes. A, Representative tracings of single myocyte contractility under baseline conditions and stimulation with isoproterenol. B, Quantitative data of FS. C, Maximum rate of cardiac myocyte contractility and relengthening. Cardiac myocytes were measured at baseline conditions and after stimulation with isoproterenol. n=3 animals/group for Sham mice, n= 6 animals/group for MI mice, *P<0.05 MI αMHC-Cre/GRK2(fl/fl) vs. MI GRK2(fl/fl) for respective condition, two-way ANOVA.
DISCUSSION
The incidence of HF is increasing and its long-term prognosis has remained at 50% survival after five years despite the broad application of life-saving anti-neurohormonal pharmacological therapies with ACE inhibition and β-blockade over two decades ago 6, 30. Thus, more efficient HF therapeutics are needed, which may be achieved by identifying non-traditional molecular targets downstream of the neurohormonal ligand-receptor interaction. For a number of years we have proposed that GRK2, a kinase that regulates the signaling activity and number of βAR in the heart, and that itself is regulated in HF, might represent such a non-traditional target 26. Indeed, transgenic and adenoviral expression of a peptide inhibitor of GRK2 translocation, βARKct, has benefited a number of experimental HF models 13, 14, 31. Mechanistically however, questions have arisen about the specific effects of βARKct, and about the specific pathophysiological role of GRK2 in heart disease. In the current studies we attempted to unambiguously define the role of GRK2 in post-ischemic HF and to assess the efficacy of its genetic ablation in a highly relevant model of HF, myocardial infarction, and at clinically relevant times in the course of the disease, i.e. both before and after the inciting ischemic event. Finally, we did this in a manner such that, for the first time, any major effects of GRK2 gene ablation on embryonic cardiac development 19, 32 would not confound our results, as cardiac myocyte-specific GRK2 gene ablation was induced around the second postnatal day with the activation of the α-MHC promoter or the application of Tmx in adult animals. In short, our data contains definitive evidence that GRK2 contributes to ischemic HF, and demonstrate a critical role for this kinase in the disease. Moreover our data show the striking efficacy of restoring cardiac βAR signaling homeostasis by GRK2 ablation either before or after myocardial infarction.
GRK2 is the critical GRK in the failing heart
Using constitutive (starting post-natally) αMHC-Cre or inducible αMHC-MerCreMer mice we were able to test the role of GRK2 expression in adult cardiac myocytes in both the development of, and rescue of ischemic cardiomyopathy. The use of these mice is necessary since global GRK2 KO mice die in utero 19, 32. Interestingly, mortality post-MI was similar between groups until the Tmx treatment when the induced loss of GRK2 prevented further death up to 120 days post-MI. Thus, the loss of GRK2 expression in myocytes after MI, when HF was already established, clearly led to significantly improved survival chronically after MI. Furthermore, loss of GRK2 before MI significantly reduced mortality in male GRK2KO mice. In addition, GRK2 abolishment prevented LV dilatation and deterioration of cardiac function that is seen in control mice post-MI. This is the first study showing that controlled loss of GRK2 in failing cardiac myocytes rescues a model of established HF and directly demonstrates that minimizing GRK2 activity in the failing heart is beneficial. Although the loss of GRK2 was not complete, this loss of HF-associated upregulation of cardiac myocyte GRK2 essentially restored in vivo cardiac systolic and diastolic function. Our data strongly suggest GRK2 as the primary GRK critically involved in the functional regulation of the failing heart.
Restoration of βAR-signaling in the failing heart by GRK2 ablation similar to effects of the GRK2 inhibitor peptide βARKct
Both early and late after the acute inciting event, our current data provide further support for the benefits of restoration or normalization of βAR-signaling in HF. The increased LV hemodynamic response and the improved contractility of isolated single LV cardiac myocytes to βAR stimulation in mice lacking cardiac myocyte GRK2 shows that signaling through this inotropic system is preserved despite the presence of a large MI. Moreover, this data is consistent with previous rescue studies using the βARKct and demonstrates and supports the primary mechanism of action of this Gβγ-sequestering peptide in HF therapeutics to be GRK2 inhibition. Further, our data strongly support that the beneficial effect of βARKct expression observed in several HF models 13, 14, 31 is indeed due to inhibition of membranous GRK2 activity (primarily targeting βAR desensitization) causing a restoration towards normalized signaling. In addition, previous studies with βARKct and our present study with conditional GRK2 ablation provide strong evidence that GRK2 is the critical GRK regulating inotropic responsiveness and adverse βAR desensitization after MI.
An apparent narrow therapeutic window for altering βAR-signaling in HF
GRK2 is a major regulator of myocardial βAR signaling and dysfunctional βAR signaling is a consequence of HF and might at least in part contribute to the progression of cardiac dysfunction as failing human myocardium is characterized by diminished βAR responsiveness, loss of overall cardiac and single myocyte contractility, and disruption of intracellular Ca2+-cycling 26, 33. Importantly, βAR derangements include the up-regulation of GRK2 34, 35. βAR stimulation in myocardium modulates intracellular Ca2+-fluxes and Ca2+-responsiveness of the sarcomere, thereby linking βAR signaling to cardiac myocyte contractility. Of note, chronic upstream activation of the βAR signaling cascade by cardiac overexpression of the β1-AR 2 or the adenylyl cyclase stimulating G protein α-subunit (Gαs) 4 led to the development of dilated cardiomyopathy in mice. Moreover, overexpression of PKA, a down-stream effector of βAR-Gs signaling also resulted in fatal dilated cardiomyopathy 36. Despite the evidence showing detrimental effects of chronic βAR and down-stream activation in the heart, other studies manipulating βAR signaling have led to positive outcomes. This includes studies by us showing the prevention and rescue of HF using the βARKct 13, 14, 31 as well as overexpression of adenylyl cyclase VI that has been shown to increase contractile function in the heart as well as inhibiting the development of HF post-MI 37, 38. Recently, β1-AR transactivation of the epidermal growth factor (EGF) receptor mediated by β-arrestin was shown to confer cardioprotection in stressed hearts 39. Taken together, these genetic manipulations at different levels of βAR signaling demonstrate that the point of intervention is important to the function of the heart and suggest that it is more desirable to circumvent βAR desensitization than to simply facilitate βAR activation. Studies using the βARKct are consistent with this notion 13, 14, 31. Overall, the data discussed and our data suggest a narrow therapeutic window for βAR-signaling in HF, and indicate that normalization of βAR-signaling, which can be best achieved by preventing their uncoupling and downregulation through GRK2 inhibition/ablation, might be the optimal approach, rather than forced overexpression of receptors or their downstream signaling effectors.
Conclusions
Protein kinases are rapidly emerging as a new pharmacological approach that may complement the existing drug classes targeting G protein-coupled receptors. Taken together, our current results show that targeted deletion and lowering of cardiac myocyte GRK2 activity leads to a novel protective and inotropic phenotype, which prevents post-ischemic HF and rescues a phenotype of established HF. Our results herein combined with our previous βARKct data add to the significance of the βAR system in the failing heart as it is clear that resensitization can positively affect cardiac function. The data would appear to indicate that βAR desensitization and down-regulation is indeed maladaptive. We have not ruled out that other G protein-coupled receptors signals, no doubt altered by the loss of GRK2, are also contributing to the above phenotypes, however the above described changes in βAR inotropic reserve are significant. Overall, our data demonstrate that GRK2 activity is pathogenic in HF and targeted inhibition or lowering its expression lead to beneficial effects for contractile function of the heart and this lone molecular change in the post-ischemic heart can prevent and rescue structural HF and offer novel benefits over existing HF therapies.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft (Ra 1668/1-1, to Philip W. Raake). Walter J. Koch is the W.W. Smith Professor of Medicine and this research was supported by his NIH grants R01 HL61690, R01 HL56205 and P01 HL075443 (Project 2). The effort of Gerald W. Dorn II on this project was supported by NIH R01 HL87871.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Kudej RK, Iwase M, Uechi M, Vatner DE, Oka N, Ishikawa Y, Shannon RP, Bishop SP, Vatner SF. Effects of chronic beta-adrenergic receptor stimulation in mice. Journal of molecular and cellular cardiology. 1997;29(10):2735–2746. doi: 10.1006/jmcc.1997.0508. [DOI] [PubMed] [Google Scholar]
- 2.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(12):7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, Yatani A, Dorn GW., 2nd Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101(14):1707–1714. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 4.Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, Wight DC, Wagner TE, Ishikawa Y, Homcy CJ, Vatner SF. Adverse effects of chronic endogenous sympathetic drive induced by cardiac GS alpha overexpression. Circulation research. 1996;78(4):517–524. doi: 10.1161/01.res.78.4.517. [DOI] [PubMed] [Google Scholar]
- 5.Mialet Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, Schwartz A, Dorn GW, Liggett SB. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nature medicine. 2003;9(10):1300–1305. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein S. Benefits of beta-blocker therapy for heart failure: weighing the evidence. Archives of internal medicine. 2002;162(6):641–648. doi: 10.1001/archinte.162.6.641. [DOI] [PubMed] [Google Scholar]
- 7.Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A, Yacoub MH. Left ventricular assist device and drug therapy for the reversal of heart failure. The New England journal of medicine. 2006;355(18):1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 8.Cross HR, Steenbergen C, Lefkowitz RJ, Koch WJ, Murphy E. Overexpression of the cardiac beta(2)-adrenergic receptor and expression of a beta-adrenergic receptor kinase-1 (betaARK1) inhibitor both increase myocardial contractility but have differential effects on susceptibility to ischemic injury. Circulation research. 1999;85(11):1077–1084. doi: 10.1161/01.res.85.11.1077. [DOI] [PubMed] [Google Scholar]
- 9.Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ. Science. 5158. Vol. 264. New York, N.Y.: 1994. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor; pp. 582–586. [DOI] [PubMed] [Google Scholar]
- 10.Dorn GW, 2nd, Tepe NM, Lorenz JN, Koch WJ, Liggett SB. Low- and high-level transgenic expression of beta2-adrenergic receptors differentially affect cardiac hypertrophy and function in Galphaq-overexpressing mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(11):6400–6405. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah AS, Lilly RE, Kypson AP, Tai O, Hata JA, Pippen A, Silvestry SC, Lefkowitz RJ, Glower DD, Koch WJ. Intracoronary adenovirus-mediated delivery and overexpression of the beta(2)-adrenergic receptor in the heart : prospects for molecular ventricular assistance. Circulation. 2000;101(4):408–414. doi: 10.1161/01.cir.101.4.408. [DOI] [PubMed] [Google Scholar]
- 12.Lai NC, Roth DM, Gao MH, Tang T, Dalton N, Lai YY, Spellman M, Clopton P, Hammond HK. Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation. 2004;110(3):330–336. doi: 10.1161/01.CIR.0000136033.21777.4D. [DOI] [PubMed] [Google Scholar]
- 13.Harding VB, Jones LR, Lefkowitz RJ, Koch WJ, Rockman HA. Cardiac beta ARK1 inhibition prolongs survival and augments beta blocker therapy in a mouse model of severe heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(10):5809–5814. doi: 10.1073/pnas.091102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr., Lefkowitz RJ, Koch WJ. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(12):7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tachibana H, Naga Prasad SV, Lefkowitz RJ, Koch WJ, Rockman HA. Level of beta-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure overload-induced heart failure. Circulation. 2005;111(5):591–597. doi: 10.1161/01.CIR.0000142291.70954.DF. [DOI] [PubMed] [Google Scholar]
- 16.Shah AS, White DC, Emani S, Kypson AP, Lilly RE, Wilson K, Glower DD, Lefkowitz RJ, Koch WJ. In vivo ventricular gene delivery of a beta-adrenergic receptor kinase inhibitor to the failing heart reverses cardiac dysfunction. Circulation. 2001;103(9):1311–1316. doi: 10.1161/01.cir.103.9.1311. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Laugwitz KL, Pinkernell K, Pragst I, Baumgartner C, Hoffmann E, Rosport K, Munch G, Moretti A, Humrich J, Lohse MJ, Ungerer M. Effects of two Gbetagamma-binding proteins--N-terminally truncated phosducin and beta-adrenergic receptor kinase C terminus (betaARKct)--in heart failure. Gene therapy. 2003;10(16):1354–1361. doi: 10.1038/sj.gt.3301995. [DOI] [PubMed] [Google Scholar]
- 18.Chen EP, Bittner HB, Akhter SA, Koch WJ, Davis RD. Myocardial function in hearts with transgenic overexpression of the G protein-coupled receptor kinase 5. The Annals of thoracic surgery. 2001;71(4):1320–1324. doi: 10.1016/s0003-4975(00)01754-9. [DOI] [PubMed] [Google Scholar]
- 19.Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW., 2nd Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circulation research. 2006;99(9):996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 20.Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circulation research. 2001;89(1):20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 21.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. The Journal of clinical investigation. 1997;100(1):169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Most P, Seifert H, Gao E, Funakoshi H, Volkers M, Heierhorst J, Remppis A, Pleger ST, DeGeorge BR, Jr., Eckhart AD, Feldman AM, Koch WJ. Cardiac S100A1 protein levels determine contractile performance and propensity toward heart failure after myocardial infarction. Circulation. 2006;114(12):1258–1268. doi: 10.1161/CIRCULATIONAHA.106.622415. [DOI] [PubMed] [Google Scholar]
- 23.Funakoshi H, Kubota T, Kawamura N, Machida Y, Feldman AM, Tsutsui H, Shimokawa H, Takeshita A. Disruption of inducible nitric oxide synthase improves beta-adrenergic inotropic responsiveness but not the survival of mice with cytokine-induced cardiomyopathy. Circulation research. 2002;90(9):959–965. doi: 10.1161/01.res.0000017632.83720.68. [DOI] [PubMed] [Google Scholar]
- 24.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279(1):H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 25.Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, Pu WT, Izumo S, Jay PY. Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. Journal of cardiac failure. 2006;12(5):392–398. doi: 10.1016/j.cardfail.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415(6868):206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 27.Bristow MR. Why does the myocardium fail? Insights from basic science. Lancet. 1998;352 Suppl 1:SI8–SI14. doi: 10.1016/s0140-6736(98)90311-7. [DOI] [PubMed] [Google Scholar]
- 28.Keys JR, Koch WJ. The adrenergic pathway and heart failure. Recent progress in hormone research. 2004;59:13–30. doi: 10.1210/rp.59.1.13. [DOI] [PubMed] [Google Scholar]
- 29.Vinge LE, Oie E, Andersson Y, Grogaard HK, Andersen G, Attramadal H. Myocardial distribution and regulation of GRK and beta-arrestin isoforms in congestive heart failure in rats. American journal of physiology. 2001;281(6):H2490–H2499. doi: 10.1152/ajpheart.2001.281.6.H2490. [DOI] [PubMed] [Google Scholar]
- 30.Dunlap ME. New studies influencing treatment of heart failure: 2006 update. Pharmacotherapy. 2007;27(4 Pt 2):3S–11S. doi: 10.1592/phco.27.4part2.3S. [DOI] [PubMed] [Google Scholar]
- 31.Williams ML, Hata JA, Schroder J, Rampersaud E, Petrofski J, Jakoi A, Milano CA, Koch WJ. Targeted beta-adrenergic receptor kinase (betaARK1) inhibition by gene transfer in failing human hearts. Circulation. 2004;109(13):1590–1593. doi: 10.1161/01.CIR.0000125521.40985.28. [DOI] [PubMed] [Google Scholar]
- 32.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr., Lefkowitz RJ, Caron MG, Giros B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(23):12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piacentino V, 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circulation research. 2003;92(6):651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 34.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation. 1993;87(2):454–463. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 35.Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. European heart journal. 2005;26(17):1752–1758. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- 36.Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, Marks AR, Olson EN. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circulation research. 2001;89(11):997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 37.Gao MH, Lai NC, Roth DM, Zhou J, Zhu J, Anzai T, Dalton N, Hammond HK. Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation. 1999;99(12):1618–1622. doi: 10.1161/01.cir.99.12.1618. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi T, Tang T, Lai NC, Roth DM, Rebolledo B, Saito M, Lew WY, Clopton P, Hammond HK. Increased cardiac adenylyl cyclase expression is associated with increased survival after myocardial infarction. Circulation. 2006;114(5):388–396. doi: 10.1161/CIRCULATIONAHA.106.632513. [DOI] [PubMed] [Google Scholar]
- 39.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. The Journal of clinical investigation. 2007;117(9):2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.