Summary
The fusion of cells to generate syncytial tissues is a crucial event in the development of many organisms. In the lens of the vertebrate eye, proteins and other macromolecules diffuse from cell to cell via the large molecule diffusion pathway (LMDP). We used the tamoxifen-induced expression of GFP to investigate the nature and role of the LMDP in living, intact lenses. Our data indicate that the LMPD preferentially connects cells lying within a stratum of the lens cortex and that formation of the LMPD depends on the expression of Lim2, a claudin-like molecule. The conduits for intercellular protein exchange are most likely regions of partial cellular fusion, which are commonly observed in wild-type lenses but rare or absent in Lim2-deficient lenses. The observation that lens tissue constitutes a stratified syncytium has implications for the transparency, refractive function and pathophysiology of the tissue.
Keywords: Lens, Green fluorescent protein, Fluorescence correlation spectroscopy, Lim2, Syncytium, Confocal microscopy
Introduction
The generation of syncytia by cell fusion is an important stage in the development of many eukaryotic organisms and generally involves the fusion of cells of a single lineage at a well-defined stage of differentiation. In Caenorhabditis elegans, nearly one third of cells fuse, in reactions that ultimately generate the syncytial hypodermis, vulva, excretory gland, male tail, anchor cell, uterus and pharynx (Gattegno et al., 2007). Muscle development in species ranging from Drosophila melanogaster to humans, involves the fusion of mononucleated myoblasts to form multinucleated muscle fibers (Chen et al., 2007). Epithelial cell fusion occurs in the production of the syncytiotrophoblast – the placental layer separating maternal and fetal blood supplies (Potgens et al., 2002). Finally, the fusion of macrophages produces multinucleated osteoclasts, which function in bone resorption, and giant cells, which are active at chronic inflammatory sites (Chen et al., 2007).
Fusion of cells is believed to be a multi-step process involving recognition, adhesion, hemifusion formation (in which the outer membrane leaflets of apposed cells merge) and, finally, formation of an aqueous pore (Oren-Suissa and Podbilewicz, 2007). Although the fundamental steps in membrane fusion may be common to all systems, the molecular mechanisms by which fusion is achieved are surprisingly diverse and are understood in detail in only a few cases. In C. elegans, for example, the fusion failure (FF) genes EFF-1 and AFF-1 are necessary and sufficient to drive cell fusion during development (Sapir et al., 2008). Similarly, in humans, syncytin 1 [also known as ENW1 (HERV-W_7q21.2 provirus ancestral Env polyprotein)] appears to function as an authentic fusogen during placental syncytiotrophoblast formation (Mi et al., 2000).
Recent evidence suggests that cell fusion might occur during terminal differentiation of fiber cells in the vertebrate lens (Shestopalov and Bassnett, 2000a; Shestopalov and Bassnett, 2003). Regions of limited fusion were noted in electron microscopic studies of the lens (Kuszak et al., 1989; Kuszak et al., 1985) and identified subsequently in volumetric reconstructions of tissue from the lens core (Shestopalov and Bassnett, 2000a). Functional evidence for cell fusion was provided by experiments in which GFP-tagged membrane proteins expressed in one fiber cell were found to diffuse into the membranes of neighboring cells, suggesting the presence of a continuous (i.e. fused) plasma membrane system (Shestopalov and Bassnett, 2000a). Similarly, cytoplasmic proteins or high molecular weight tracers introduced into the cytoplasm of one cell were able to diffuse intercellularly through the large molecule diffusion pathway (LMDP).
An unusual, perhaps unique, feature of the lens syncytium is that the fusion of constituent cells is incomplete. Thus, once fusion pores are formed, they do not expand indefinitely. Consequently, the cytoplasms of fused fiber cells remain partitioned by an intervening membrane pair over much of their length (Shestopalov and Bassnett, 2000a). The physiological significance of the lens syncytium is obscure, but it has been proposed to connect metabolically active cells near the lens surface, with metabolically quiescent cells in the center of the lens (Shestopalov and Bassnett, 2003). In this manner, the pool of aged proteins in cells of the lens core might be refreshed. In addition to uncertainty over the physiological role, it is not known whether fusogenic proteins, analogous to EFF-1 or syncytin 1, are expressed during lens syncytial formation.
In the present paper, we used induced expression of GFP to probe the nature and role of the lens syncytium. Our results indicated that the formation of the syncytium depends on the presence of Lim2 (also known as lens fiber membrane intrinsic protein LMIP or MP20), a claudin-like protein found at high concentration in lens fiber cell plasma membranes. Long-term GFP labeling revealed that the LMDP preferentially couples cells located in the same stratum of the lens cortex, and it is therefore unlikely to facilitate centripetal diffusion of proteins. The results are discussed in relation to the role of the lens in image formation in the eye. Together, the data indicate that the lens is comprised of not one, but several overlapping syncytia, each facilitating intercellular diffusion predominantly within a stratum of the tissue. To our knowledge, this arrangement is unique and hence we suggest the use of the term `stratified syncytium' to describe the syncytial organization of lens tissue.
Results
Structure of the mouse lens
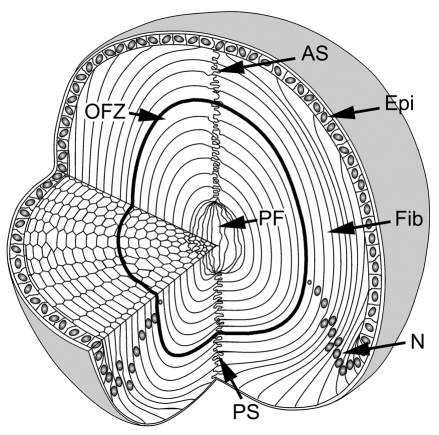
The cellular organization of the mouse lens is shown in Fig. 1. The lens grows by the deposition of newly differentiated fiber cells at the surface of the tissue. There is no cell turnover in the lens, therefore the age of a fiber cell can be deduced from its radial position: cells in the lens core are the oldest and those nearest the surface are the youngest.
Fig. 1.
Cellular organization of the mouse lens. The lens consists of two cell types: epithelial cells (Epi) located at the anterior surface, and fiber cells (Fib), which comprise the remainder and majority of the tissue. At the lens equator, epithelial cells differentiate into fiber cells. As they differentiate, fibers become highly elongated. The tips of the elongating fibers converge at the anterior and posterior lens sutures (AS and PS, respectively). In cross section, the fiber cells have a flattened hexagonal profile with two broad faces (oriented parallel to the lens surface) and four narrow faces. Initially, all fiber cells are nucleated but, during differentiation, nuclei (N) and other organelles are degraded. As a result, the central region of the lens constitutes an organelle-free zone (OFZ). The innermost cells, termed primary fiber cells (PF), are formed during embryonic development and are less regular in shape and arrangement than the other fiber cells.
Induced expression of GFP
GFP expression was induced in 3-week-old Cre-ER™;Z/EG mice by intraperitoneal injection of tamoxifen. Tamoxifen injection resulted in GFP expression by 1-5% of lens epithelial cells and superficial fiber cells. The relatively low level of induction has been attributed to mosaic expression of the Z/EG reporter transgene in the lens (Shi and Bassnett, 2007). For the present purposes, the low induction efficiency was advantageous, because it enabled individual GFP-expressing cells to be visualized in the intact tissue. Mature fiber cells located in the lens core did not express GFP, presumably because those cells were incapable of protein synthesis, as a result of the disintegration of cytoplasmic organelles that occurs in the course of embryonic development (Faulkner-Jones et al., 2003; Vrensen et al., 1991). To confirm that GFP was freely diffusible in the cytoplasm of lens fiber cells, we measured its diffusion coefficient (D) using fluorescence correlation spectroscopy (FCS) (supplementary material Fig. S1). In the outermost fiber cells, D=3.9±0.4×10–11 m2/second (mean ± s.d.; n=3), a value similar to that reported for GFP in the cytoplasm of CHO and HeLa cells (Brock et al., 1999; Chen et al., 2002) and >97% of GFP molecules exhibited brownian motion (i.e. only a small fraction of the GFP was bound to cytoplasmic components). There was a modest reduction in D (to 2.1±0.3×10–11 m2/second; n=3) in cells located deeper in the tissue.
The LMDP is established early in fiber cell differentiation and persists in mature fibers
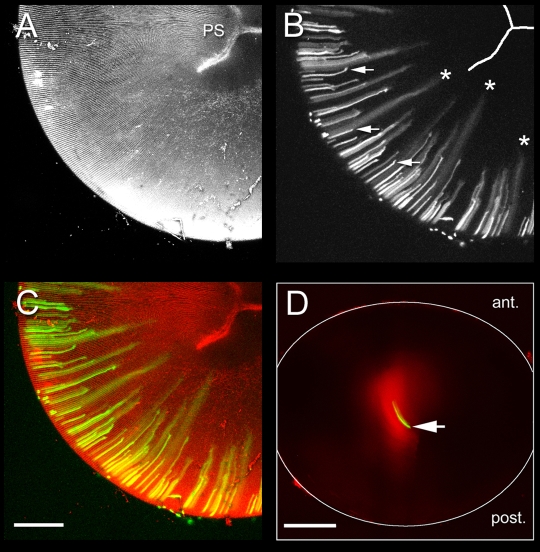
In tamoxifen-treated animals, GFP was restricted initially to the cytoplasm of expressing fiber cells located near the lens periphery. However, as differentiation proceeded, and elongating fiber cells were internalized, GFP began to diffuse from expressing cells into the cytoplasm of neighboring, non-expressing cells, signifying the formation of the LMDP (Fig. 2A-C). The LMDP was established relatively early in fiber differentiation while the fiber cells were actively elongating (i.e. before the fiber tips had reached the lens suture) (see Fig. 1). To test whether the LMDP remained patent in mature fiber cells, we microinjected Alexa Fluor 488-dextran (10 kDa) into anucleated cells located in the center of organ-cultured lenses. A comparison of images collected 10 minutes or 2 days after injection, indicated that the dextran diffused throughout the lens core in the intervening period (Fig. 2D). Thus, the LMDP is established early in fiber cell differentiation and remains patent in mature fiber cells.
Fig. 2.
The large molecule diffusion pathway (LMDP) is established in elongating fiber cells and persists in mature fiber cells. A-C. Maximum intensity projections of the posterior lens hemisphere from a 33-day-old Cre-ER™;Z/EG mouse, 10 days after tamoxifen treatment. The lens was incubated in FM4-64 to visualize the membrane architecture. (A) FM4-64 staining reveals the regular arrangement of the fiber cells and the position of the Y-shaped posterior suture (PS). (B) In short, newly differentiated cells near the periphery, GFP is restricted to the cytoplasm of expressing cells (arrows). As fiber elongation proceeds, GFP diffuses from expressing cells into the cytoplasm of neighboring cells (*). Note that intercellular diffusion commences before cells reach the suture (i.e. in actively elongating fiber cells) (see Fig. 1). (C) Co-visualization of GFP (green) and FM4-64 fluorescence (red). (D) Wild-type mouse lens 10 minutes (green) or 2 days (red) after injection of Alexa Fluor 488-dextran (10 kDa) into a fiber cell located in the center of the lens (arrow). Note the extensive intercellular spread of tracer over the 2 day incubation period. Scale bars: 250 μm.
The LMDP preferentially couples cells of the same age
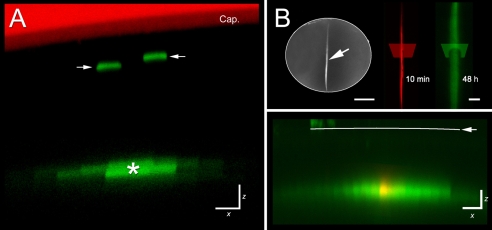
In cross section, fiber cells in the lens cortex have a flattened hexagonal appearance and are closely surrounded by six neighboring fibers (see Fig. 1). We examined the initial diffusion pattern of GFP to determine whether cells were equally well coupled to each of their six neighbors. X-Z images of GFP-expressing cells (Fig. 3A) or lens tissue injected with Alexa Fluor 488-dextran (Fig. 3B) revealed that, in both cases, tracer spread more readily between cells located in the same stratum (i.e. between cells of the same age) than between cells located in different strata (i.e. between cells of differing ages).
Fig. 3.
The LMDP preferentially links cells of the same age. (A) X-Z image of the equatorial region of a tamoxifen-treated Cre-ER™;Z/EG lens. In this orientation, fiber cells are seen in transverse section. Near the surface, individual GFP-expressing cells are evident (arrows). At greater depths (>50 μm), GFP fluorescence is detected in a cluster of cells, suggesting that a GFP-expressing cell (*) has become coupled to its lateral neighbors by the LMDP. (B) Lateral spread of large molecules is also observed following injection of fluorescent dextran. The upper left panel shows a lens in which an individual fiber cell (arrow) was injected with Alexa Fluor 488-Dextran and, at higher magnification (upper right panel), the appearance of the injected fiber cell 10 minutes (red) and 48 hours (green) after injection. The transects indicate the orientation of the optical sections shown in the lower panel. The X-Z section (lower panel) is oriented such that cells are seen in cross section. The distribution of Alexa Fluor 488-Dextran is shown 10 minutes (red) and 2 days (green) after injection. Ten minutes after injection the dextran is confined to the cytoplasm of the injected cell but, after 2 days in organ culture the dextran has spread laterally, into the cytoplasm of a layer of neighboring cells. Cap, capsule. Scale bars: 10 μm (A); 500 μm (B, upper left); 100 μm (B, upper right); 20 μm (B, lower panel).
Preliminary experiments (Fig. 2) indicated that the LMDP was first established in cells located approximately 50 μm below the equatorial surface of the lens. We used the confocal microscope in line-scan mode to obtain X-Z sections of cells located in this region of the lens. The X-Z plane was oriented such that fiber cells were imaged in cross section. Images were collected at regular intervals over a period of 14 hours and assembled into time-lapse movies (supplementary material Movie 1). Analysis of the time-lapse sequences revealed instances in which transfer of GFP was initiated between GFP-expressing and non-expressing cells during the culture period, signifying the formation of the LMDP between the cell pair.
The fiber cell plasma membrane is polarized into distinct apical, basal and lateral membrane domains. The lens sutures are regions of exclusive apical to apical membrane contact (anterior suture) and basal to basal (posterior suture) membrane contact (see Fig. 1). Although GFP diffused readily across the lateral fiber membranes, there was little or no transfer across the anterior or posterior lens sutures (supplementary material Fig. S2). Thus, the LMDP is a specific property of the lateral domain.
Long-term GFP labeling reveals the lamellar substructure of the lens
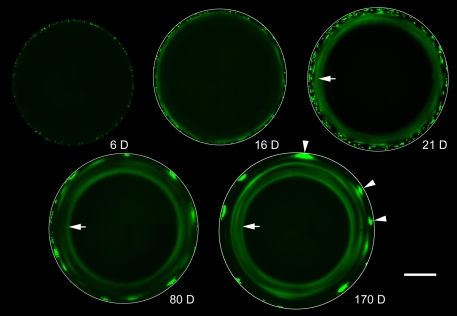
Nucleated, metabolically active cells constitute a thin layer near the lens surface (Fig. 1). The remainder of the lens is composed of mature fiber cells that are metabolically quiescent and incapable of de novo protein synthesis (Faulkner-Jones et al., 2003). It has been proposed that the LMDP might facilitate the centripetal flow of newly synthesized protein from cells in the active surface layers into the quiescent cells of the interior (Shestopalov and Bassnett, 2000a; Shestopalov and Bassnett, 2003). To test this hypothesis directly, we induced GFP expression in 3-week-old mice and followed the distribution of GFP within the lens at intervals thereafter (Fig. 4). Six days after tamoxifen treatment, scattered GFP-positive fiber cells were present near the periphery of the lens. By 16 days, the initial cohort of GFP-expressing cells was internalized and GFP had begun to diffuse from the innermost GFP-positive cells. There was little evidence of centripetal flow, however. Instead, cell-to-cell diffusion occurred predominantly between cells in the same stratum of the lens cortex, resulting, by 21 days, in the formation of a fluorescent annulus (Fig. 4, arrows). The inner diameter of the annulus was not smaller at 170 days than at 21 days, indicating that little or no centripetal diffusion of GFP occurred in the intervening period. Lenses from older animals (80 days and 170 days) were characterized by the presence of a series of complete and incomplete secondary fluorescent rings.
Fig. 4.
Long-term GFP labeling reveals the lamellar substructure of the lens. Lenses from Cre-ER™;Z/EG mice were examined at intervals (6-170 days) after tamoxifen injection. Optical sections were obtained through the lens equator. In this orientation, fiber cells are seen in cross section. Six days after tamoxifen treatment (6 D), scattered GFP-expressing fiber cells are observed near the lens surface. By 16 days (16 D), the fiber cells have become internalized and the innermost GFP-expressing cells are connected by the LMDP. Consequently, GFP is beginning to diffuse from the cytoplasm of expressing cells into the cytoplasm of non-expressing neighbors. The spread of fluorescence is largely between cells located in the same lens stratum, resulting in the formation of a fluorescent annulus which becomes better defined at later ages (arrowed in 21 D, 80 D, 170 D). At later ages (80 D and 170 D), secondary fluorescent rings (complete and incomplete) are present. These result from the periodic incorporation of clusters of highly fluorescent fiber cells (arrowheads in 170 D) into the lens syncytium. Scale bar: 500 μm.
Age-dependent changes in clonal expansion of fiber cell progenitors account for the generation of multiple fluorescent rings
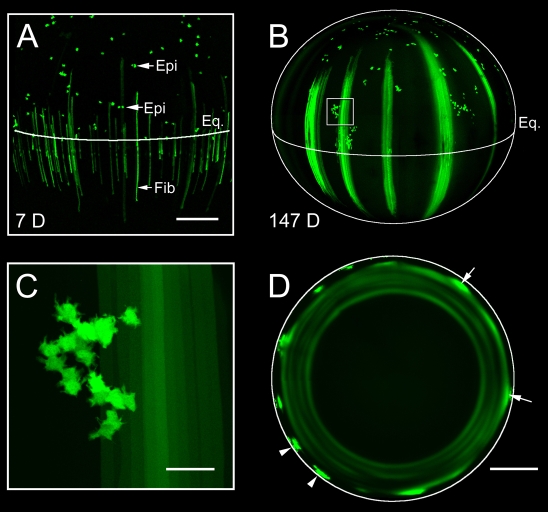
The pattern of GFP expression in elongating fiber cells was qualitatively different at early and late time points. In lenses examined 7 days after tamoxifen treatment, scattered, GFP-positive fiber cells were evenly dispersed in the peripheral lens tissue (Fig. 5A). By contrast, at later time points, bundles of GFP-expressing fibers were present near the periphery. Fluorescent cell bundles were separated from each other by broad strips of non-fluorescent fiber cells (Fig. 5B). The difference in labeling patterns appeared to result from age-dependent changes in the behavior of epithelial progenitor cells. In young lenses, GFP-labeled epithelial cells located in the proliferative zone of the epithelium were usually present as cell pairs (Fig. 5A). The subsequent differentiation of these cells generated an evenly dispersed pattern of fluorescent fiber cells in the lens cortex. By contrast, in older lenses, several mitoses occurred between the initial cell division and fiber differentiation. As a result of this clonal expansion, a cluster of GFP-positive epithelial cells (numbering 20-30) accumulated adjacent to the lens equator (Fig. 5C). The synchronous differentiation of these cells gave rise to the formation of the broad, fluorescent bundles of fiber cells evident in Fig. 5B. When GFP-positive fiber cells within the bundles became coupled to non-expressing cells by the LMDP, the lateral diffusion of a bolus of GFP resulted in the formation of a fluorescent annulus (Fig. 5D). Dark rings in equatorial sections of older lenses presumably correspond to periods when no GFP-labeled cell clusters differentiated.
Fig. 5.
Age-dependent changes in clonal expansion of fiber cell progenitors accounts for the presence of secondary rings. (A) Maximum intensity projection of the lens equatorial region from a 29-day-old Cre-ER™;Z/EG mouse 7 days (7 D) after tamoxifen treatment. The position of the lens equator (Eq.) and individual GFP-expressing epithelial cells (Epi) is indicated. Note that GFP-positive epithelial cells are apparently randomly distributed in the lens epithelium and many, especially those located near the equator, are present as cell pairs. GFP-expressing fiber cells (Fib) are evenly distributed in the cortical lens tissue beneath the equator. (B) Maximum intensity projection of the lateral aspect of a lens from a Cre-ER™;Z/EG mouse 147 days (147 D) after tamoxifen treatment. Immediately anterior to the lens equator, clusters of GFP-positive epithelial cells are present (one cluster is boxed in B and shown at higher magnification in C). Diffusely labeled bundles of fiber cells are present interspersed with unlabeled fibers, resulting in a characteristic striped fluorescence pattern. (C) Clusters of GFP-positive epithelial cells contain 20-30 cells. (D) Equatorial optical sections of the lens at 147 days reveal the spatial relationship between the fluorescent fiber bundles and the pattern of secondary fluorescent rings. Near the periphery, discretely labeled bundles of GFP-positive cells (typically containing 20-30 fluorescent fiber cells) are present (arrowheads). As the fiber bundles are overlaid with more recently differentiated cells, GFP begins to diffuse into the cytoplasm of neighboring cells located in the same stratum of the lens as the GFP bundles (arrows). Diffusion of GFP throughout an entire stratum results in the formation of secondary fluorescent rings. Scale bars: 250 μm (A); 500 μm (B,D); 50 μm (C).
Formation of the LMDP requires Lim2
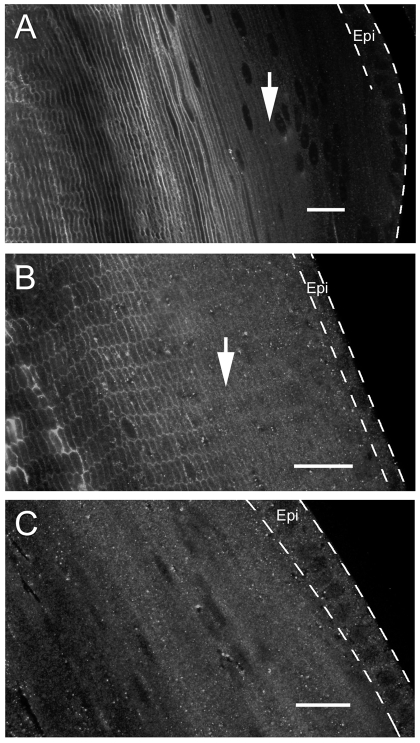
Lim2 is an abundant integral protein of lens fiber cell plasma membranes (Alcala et al., 1975). Using immunofluorescence techniques, Lim2 was first detected in the membranes of fiber cells located approximately 50 μm beneath the equatorial lens surface (Fig. 6). Thus, the onset of Lim2 expression coincided spatially and temporally with the formation of the LMDP (see Figs 2, 3, 4, 5), suggesting a role for Lim2 in the establishment of intercellular communication. To test this hypothesis directly, we examined intercellular protein diffusion in lenses deficient in Lim2. We disrupted the Lim2 locus using the genetrap technique (Shiels et al., 2007). Lim2Gt/Gt mice, lacking Lim2, were crossed with either Cre-ER™;Z/EG mice or TgN(GFPU)5Nagy mice, a strain in which a GFP transgene is expressed spontaneously in the lens in a mosaic pattern (Shestopalov and Bassnett, 2003).
Fig. 6.
Expression of Lim2 in the cortical region of 1-month-old mouse lenses. Lenses were sectioned in the mid-sagittal (A) or equatorial (B,C) plane. The position of the lens epithelium (Epi) is indicated by dashed lines. In wild-type mouse lenses (A,B), Lim2 fluorescence is first detected in the membranes of fiber cells located approximately 50 μm beneath the lens surface (arrows) and increases in intensity in the older, central cells. No membrane fluorescence was observed in Lim2-null mouse lenses, although granular background staining was present in the cytoplasm of epithelial cells (Epi) and superficial fibers. Scale bars: 20 μm.
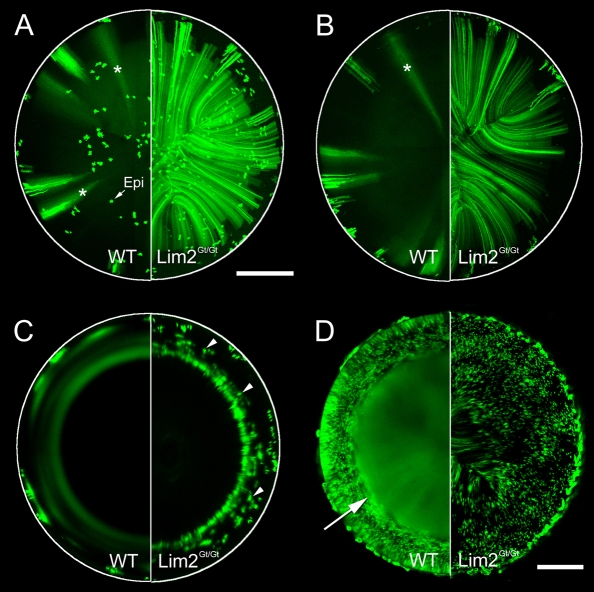
When GFP expression was induced in Lim2+/+;Cre-ER™;Z/EG mice, the characteristic pattern of diffuse fluorescent stripes was evident in projections of the lens anterior (Fig. 7A) or posterior (Fig. 7B) hemispheres and a series of fluorescent annuli was observed in equatorial sections (Fig. 7C). By contrast, on a Lim2Gt/Gt background, there was no evidence of intercellular diffusion of GFP. GFP was localized to discretely labeled fiber cells scattered throughout the tissue volume (Fig. 7A,B). In equatorial sections (Fig. 7C), fluorescent annuli were absent and, instead, labeled single cells or small cell clusters were observed. The presence of discretely labeled GFP-expressing cells was confirmed by examining cryosections of Lim2Gt/Gt;Cre-ER™;Z/EG lenses (supplementary material Fig. S3). To examine the fate of GFP expressed by the innermost fiber cells, we crossed Lim2Gt/Gt mice with TgN(GFPU)5Nagy mice (Fig. 7D). In Lim2+/+;TgN(GFPU)5Nagy mice, the cortical tissue contained GFP-expressing and non-expressing fiber cells but the central cells were uniformly fluorescent, indicating the establishment, in these cells, of the LMDP (Shestopalov and Bassnett, 2003). In Lim2Gt/Gt;TgN(GFPU)5Nagy mice, the pattern differed markedly from the wild type. No intercellular diffusion of GFP was noticeable. Rather, a mixture of GFP-expressing and non-expressing fiber cells was present in the lens core. GFP diffusion in the lenses of Lim2+/Gt animals was indistinguishable from the wild type (data not shown). Together, these data indicate that, in the absence of Lim2, the LMDP is not established, and the lens syncytium does not form.
Fig. 7.
Formation of the LMDP requires Lim2. Lim2Gt/Gt (right semicircles) or wild-type (WT) mice (left semicircles) were crossed with the inducible reporter strain Cre-ER™;Z/EG (A-C) or TgN(GFPU)5Nagy (D), a strain in which GFP is spontaneously expressed by scattered lens fiber cells. In Cre-ER™;Z/EG mice, fluorescence was examined 3 months after tamoxifen treatment (A-C). As noted elsewhere (see Figs 4 and 5), on a wild-type background, bundles of GFP-expressing fibers are present in the lens cortex. As these become incorporated into the lens syncytium, GFP diffuses into neighboring cells, resulting initially in the formation of broad diffuse stripes of fluorescence (*) in anterior (A) and posterior projections (B) and, at later time points, the formation of characteristic fluorescence rings in equatorial optical sections of the lens (C). In Lim2-deficient mice, intercellular diffusion in the lens was not observed. Numerous discretely labeled fibers were evident throughout the anterior (A) and posterior (B) hemispheres of lenses in Lim2-null mice. In equatorial sections (C), where fibers are seen in cross section, individual labeled fibers were noted (arrowheads), extending to a depth of approximately 300 μm below the lens surface (corresponding to the tissue stratum in which GFP expression was first induced). In TgN(GFPU)5Nagy mice, GFP is expressed in the lens throughout development, enabling the formation of the LMDP to be visualized in the center of young (5-day-old) lenses (D). In wild-type mice, the LMDP is established in fibers located 100-200 μm below the lens surface (arrow). However, in Lim2-deficient animals, the LMDP does not form and the central region of the lens has a checkerboard appearance as a result of the presence of uncoupled GFP-expressing and non-expressing cells. Epi, epithelial cells. Scale bars: 500 μm (A-C); 250 μm (D).
We also examined whether Lim2 was necessary for gap-junction-mediated intercellular communication in the lens. The small fluorescent tracer neurobiotin (367 Da) was injected into central fiber cells in 2-day-old lenses from wild-type or Lim2Gt/Gt mice. In both cases, the tracer diffused widely within the tissue (supplementary material Fig. S4) indicating that neither Lim2 nor the LMDP were necessary for the gap-junction-mediated diffusion of small molecules.
Lim2-dependent cell fusions may be conduits for intercellular diffusion of macromolecules
We sought to determine the structural basis for the LMDP by comparing the lenses of wild-type mice (in which intercellular diffusion of protein occurs) with those of Lim2Gt/Gt mice (in which intercellular diffusion of proteins does not occur). We focused our attention on the cells immediately adjacent to the anterior suture. The suture is readily identified in both intact and sectioned lens preparations and serves as a useful landmark when making objective comparisons between lenses. Intact living lenses were stained with the lipophilic dye, FM4-64, and examined by confocal microscopy. In wild-type lenses, the lens fiber plasma membranes were strongly stained by the dye and the suture was clearly visible (Fig. 8, upper panel). The ordered appearance of the fiber membranes was interrupted by the presence of numerous cellular dilations, short regions in which the membrane separating adjacent cells appeared to be absent. Volumetric analysis indicated that the dilations were dispersed throughout the tissue in this region, to depths of at least 200 μm below the lens surface (the working distance of the objective lens). Dilations were not observed in comparable regions of Lim2Gt/Gt lenses (Fig. 8, lower panel), suggesting that they might represent regions of cellular fusion.
Fig. 8.
Conduits for intercellular diffusion of macromolecules might be Lim2-dependent cell fusions. Living, intact lenses were imaged by confocal microscopy following incubation in the lipophilic fluorescent dye, FM4-64. The ordered arrangement of the fiber cells in the vicinity of the anterior suture is evident but, in wild-type lenses, numerous cellular dilations are also present (arrows). Dilations are distributed throughout the tissue volume in this region, as shown by three representative sections from a z-stack collected beneath the anterior pole of the lens. A similar region (note the presence of the Y-shaped suture in each case) from a Lim2Gt/Gt mouse lens is shown in the lower panels. Cellular dilations are not present in the Lim2Gt/Gt lens. Scale bar: 50 μm.
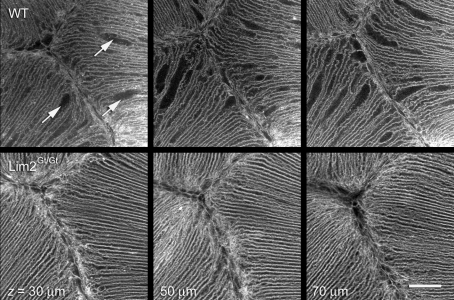
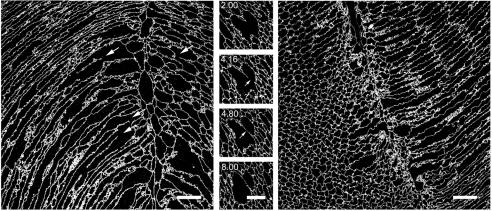
Reconstructions of the cellular dilations imaged in intact lenses lacked the axial resolution to unambiguously identify fiber cell fusions. Therefore, we used the array tomography technique (which offers about a tenfold increase in z-axis resolution over conventional confocal microscopy) to examine the membrane architecture in the anterior polar region of the lens. Ultrathin (80 nm) sections of wild-type lenses revealed numerous examples of partially fused cells in longitudinal (Fig. 9, left panel) or transverse section (center panel). The fused regions of the fiber cells were generally of greater caliber than the unfused regions, supporting the notion that the dilations observed in intact preparations (Fig. 8, upper panel) correspond to regions of cellular fusion. Fused cells were not observed in equivalent regions of lenses from Lim2Gt/Gt mice (Fig. 9, right panel). Six randomly selected sections from each of three Lim2Gt/Gt lenses were carefully examined for the presence of fiber cell fusions in the anterior and posterior suture regions. Fusions were never observed, suggesting that fusions are rare or absent in the lenses of Lim2Gt/Gt animals.
Fig. 9.
Regions of cellular dilation correspond to zones of limited cell fusion. The anterior polar regions of lenses from 3-week-old wild-type mice were processed for array tomography. Ultrathin (80 nm) sections were incubated with an antibody against Aqp0, an abundant integral protein of lens fiber membranes. In longitudinal sections (left panel) numerous examples of cellular fusions were observed (arrows) adjacent to the anterior suture (visible as a vertical discontinuity in the cell packing arrangement). In transverse sections (center panel), large caliber cells were identified which, on volumetric reconstruction, were found to correspond to zones of fusion between neighboring fibers. The four images in the center panel are selected from a z-series of 150 serial ultrathin sections. The numbers represent the position (in μm) of sections in the image stack. Note that the membrane septum dividing the cells at position 8.00 μm has disappeared by position 2.00 μm and, as a result, the cells are fused in this region. The right panel shows an equivalent region from a Lim2Gt/Gt mouse lens. Note the more regular arrangement of the cells (compare with wild-type lens in the left panel), and the absence of dilated or fused regions. Scale bars: 20 μm.
Discussion
The stratified syncytium of the lens cortex
Syncytia are found throughout the plant and animal kingdoms; however, in mammals, there are generally considered to be only three syncytial tissues: muscle fibers, syncytiotrophoblasts and osteoclasts or giant cells. In each case, the syncytium is the result of fusion of cells of a single lineage at a defined point in terminal differentiation. Results of the present study, in conjunction with earlier findings (Shestopalov and Bassnett, 2003) suggest that the LMDP forms relatively early in the fiber differentiation process. Intercellular diffusion is first triggered in actively elongating cells located approximately 50 μm (∼25 cell layers) below the equatorial lens surface (see Figs 2 and 3), although this distance might be somewhat greater in the lenses of very young mice (Shestopalov and Bassnett, 2003). Thus, LMDP formation occurs after the onset of fiber differentiation, but before fibers reach the lens sutures or undergo organelle breakdown.
In other systems, the aqueous pore that joins the cytoplasm of fusing cells is a transient structure, which expands quickly, and thus clears away the intervening membrane. The result is a multinucleated cell bounded by a single plasma membrane. By contrast, the fusion pore between lens cells appears to be a fixed structure, and most of the membrane that originally separated the cells remains intact. The persistence of a differentiated plasma membrane system within the lens syncytium ensures that the movement of proteins between cells is not isotropic throughout much of the tissue volume. Cortical fiber cells are hexagonal in cross section with two broad faces (oriented parallel to the lens surface) and four narrow faces (oriented approximately radially). The broad and narrow faces are functionally differentiated. Gap junctions, for example, are concentrated at the broad faces (Donaldson et al., 2001), whereas adhesive proteins, such as N-cadherin, are enriched at the narrow faces (Beebe et al., 2001). Electron microscopic studies have detected fusions at both the broad and the narrow faces (Kuszak et al., 1985). This implies that intercellular diffusion should occur both within and between strata. However, the current observation that proteins diffuse predominantly within a stratum of the lens cortex suggests that fusions are likely to be more numerous at the narrow faces than the broad faces. Detailed mapping studies will be required to test this hypothesis directly.
It has been suggested that the role of the LMDP is to facilitate the centripetal flow of newly synthesized protein from metabolically active cells near the lens periphery into the aged cells of the lens core (Shestopalov and Bassnett, 2003). However, the present data indicated that little or no centripetal diffusion of proteins occurs in the lens. This is consistent with the results of a recent isotopic study on human lenses in which the 14C content of proteins extracted from the lens nucleus was determined (Lynnerup et al., 2008). The concentration of atmospheric 14C in the atmosphere peaked in the 1950s as a result of nuclear weapons testing. Mass spectrometric analysis showed that the 14C content of proteins extracted from the lens nucleus reflected the level of atmospheric 14C in the birth year rather than lower, more contemporary values. Thus, there is no evidence that the proteins of core fiber cells are refreshed over time by newly synthesized components from the lens periphery.
Although intercellular diffusion of protein in the lens cortex occurs predominantly within a stratum of the tissue, this appears to be less true in the center of the lens. The innermost cells, formed early in embryonic development, are not packed together in the hexagonal arrangement that characterizes the majority of the tissue volume. Broad and narrow faces are not well defined (Shestopalov and Bassnett, 2000b). Perhaps for this reason, diffusion in the lens core appears to be more isotropic (Fig. 2D) (Shestopalov and Bassnett, 2000a) than in the cortex. On the basis of these observations, we propose a tripartite model for the lens. In a thin (<50 μm) layer of cells near the lens surface, proteins are restricted to the cytoplasm of the cell in which they were synthesized. In the lens cortex, which comprises the majority of the tissue volume, proteins diffuse from cell to cell, predominantly within a stratum of tissue. In the innermost fiber cells, the intercellular spread of protein is isotropic.
Role of Lim2
The current data indicate that the lens membrane protein Lim2 is required for both cell fusion and syncytium formation. Lim2 is a glycophosphoprotein (Ervin et al., 2005) that binds galectin 3 (Gonen et al., 2001) and calmodulin (Galvan et al., 1989). Lim2 is a member of the large PMP22/EMP/MP20/claudin, or pfam00822, superfamily of membrane proteins. Members of this family share a tetraspan topology and a W-GLW-C-C signature motif in the first extracellular loop (Van Itallie and Anderson, 2006) and have diverse roles in intercellular adhesion and the control of paracellular permeability. Mutations in LIM2 underlie certain types of inherited cataract in humans (Ponnam et al., 2008; Pras et al., 2002). Although it is the second most abundant membrane protein in the vertebrate lens (Alcala et al., 1975), no definitive physiological role for Lim2 has emerged. Studies in rats have indicated that Lim2 might act to minimize extracellular space (Grey et al., 2003) and an adhesive function is also suggested by the phenotype of Lim2-deficient mice, in which lens fiber cells are readily separated from each other (Shiels et al., 2007).
Although we have not tested the ability of Lim2 to induce fusion in heterologous systems, it seems unlikely that Lim2 acts directly as a fusogenic protein in a role analogous to, for example, syncytin 1 in syncytiotrophoblasts (Mi et al., 2000). Previous studies on lenses from newborn mice indicated that Lim2 is uniformly distributed in the plasma membrane and not concentrated at the narrow faces, the region that we argue is likely to be rich in cell fusion. In the current study, the expression of Lim2 on the broad and narrow faces (supplementary material Fig. S5), and along the length of the fiber cells (Fig. 6A), was found to be similarly uniform in adult lenses. In other cell types, fusion is a multi-step process, beginning with cell-cell recognition and adhesion, followed by formation of a hemifusion and, finally, an aqueous pore (Oren-Suissa and Podbilewicz, 2007). Interfering with any of these steps prevents fusion from occurring. For example, the Drosophila proteins dumbfounded, roughest and sticks-and-stones, are required for myoblast fusion, but probably do not function as fusogens per se. Rather, each is a member of the immunoglobulin superfamily of adhesion proteins and their absence presumably affects the ability of myoblasts to adhere to each other, an early, obligate step in the fusion process. Lim2 might act in a similar manner, facilitating fusion of lens cells by promoting the adhesion of lens fibers.
Implications for transparency, refraction and cataract
The role of the lens in the optical train of the eye is to focus light sharply on the retina. The stratified syncytial organization described here might contribute to this in several ways. First, by allowing the cytoplasmic contents of neighboring cells to mix, small differences in cytosolic protein content are avoided. Since light is scattered at the interface between media of differing refractive index, equalizing the protein content of neighboring cells will ensure refractive index matching and contribute directly to lens transparency. Second, the stratified nature of the lens syncytium might contribute to the correction of spherical aberration in the lens. It is well established that a standing gradient of protein concentration is maintained in the lens and that, as a result, the concentration of protein in the central lens cells can be two- or three-fold higher than at the periphery (De Korte et al., 1994). The graded distribution of protein produces a refractive index gradient within the lens tissue. In the rat lens, for example, the refractive index varies parabolically from 1.37 (near the surface) to 1.51 (in the center) (Campbell, 1984). The precisely shaped refractive index gradient serves to correct longitudinal spherical aberration and is essential, therefore, for forming a sharp image on the retina. The stratified syncytium might contribute to this, by ensuring a uniform refractive index within any one stratum of the lens while permitting a difference in refractive index to be maintained between strata. In our tripartite model of the lens, the centermost fiber cells constitute a spherical volume of tissue in which proteins intermingle. This would imply that the protein content and refractive index are uniform within this region. Studies have demonstrated that the refractive index gradient is relatively flat in this region (Fernald and Wright, 1983), supporting the hypothesis. Finally, by ensuring that cells of a given age have a common refractive index, the stratified syncytium effectively centers the index gradient about the optical axis.
The stratified syncytium does not form in Lim2Gt/Gt mice. The lenses of such animals are of normal size and shape and are relatively transparent. However, laser analysis has shown that the internal refractive properties of Lim2Gt/Gt lenses are profoundly disturbed (Shiels et al., 2007). This observation is the strongest evidence to date that the stratified syncytium contributes to optical quality. It is also possible, however, that Lim2 has functions in the lens beyond its role in syncytial formation, and that deficits in such functions contribute to the lens phenotype in Lim2Gt/Gt mice.
Co-existence of two pathways for intercellular diffusion
Gap-junction plaques are most abundant on the broad faces of the lens fiber cells (Jacobs et al., 2003). As a consequence, the flow of ions and small metabolites in the lens cortex is predominantly radial in direction. However, the intercellular diffusion of large molecules is predominantly circumferential (i.e. within a tissue stratum). Thus, the two intercellular pathways are oriented orthogonal to each other. The functional roles of the two cell-cell pathways in the lens are also distinct and separable. In mice, knockout of connexins Cx46 and Cx50 (the major components of lens fiber gap junctions) results in cataracts, elevated intracellular calcium and, in the case of Cx50, small lenses (White, 2002). Thus, connexin-mediated intercellular communication is essential for ionic homeostasis and lens growth. By contrast, the absence of the LMDP from the lenses of Lim2Gt/Gt mice is associated with profoundly disturbed refractive properties, suggesting a specific role for the LMDP in image formation.
Materials and Methods
Animals
The following strains of mice were used in this study: C56-BL6 (Jackson Laboratory, Bar Harbor, ME), TgN(GFPU)5Nagy (Hadjantonakis et al., 1998), Lim2Gt/Gt (Shiels et al., 2007), Cre-ER™ (Hayashi and McMahon, 2002), and Z/EG (Novak et al., 2000). Animals were killed by CO2 inhalation and lenses were dissected from the eye, as described (Bassnett, 2005). All procedures were approved by the Washington University Animal Studies Committee.
Fluorescence correlation spectroscopy
The diffusibility of GFP in the cytosol of lens cells located at various depths in the lens was measured directly using fluorescence correlation spectroscopy (FCS). FCS measures fluctuations in fluorescence intensity resulting from the diffusion of labeled molecules through a diffraction-limited detection volume. The statistical analysis of fluorescence fluctuations using autocorrelation techniques provides information on the concentration and the diffusion coefficient (D) of the fluorescent molecule (Bacia and Schwille, 2003). Lenses from tamoxifen-treated Cre-ER™;Z/EG mice were viewed using an LSM510 meta confocal microscope equipped with a Confocor2 FCS system (Carl Zeiss, Thornwood, NY). Lenses were oriented such that the lens equator rested on the coverslip base of the viewing chamber. Background fluorescence in non-expressing cells was of low intensity and did not correlate. Beginning with the equatorial epithelium, correlation spectra were collected from GFP-expressing cells at 25 μm intervals to a depth of 200 μm (the working distance of the 40× NA 1.2 C-apochromat lens used for the measurements). Spectra were fit using a two component model to account for the fraction of apparent bound molecules. The diffusion time (τD) determined by the fit was used to calculate the diffusion coefficient from the relationship τD=ω12/4D, where ω1 is the diameter of the confocal volume determined through instrument calibration.
Inducible gene expression
Induced expression of GFP was achieved by crossing Cre-ER™ mice with the Z/EG reporter strain, to generate mice heterozygous for both transgenes, as described (Shi and Bassnett, 2007). Three-week-old Cre-ER™;Z/EG animals were given two intraperitoneal injections of tamoxifen (Sigma) dissolved in corn oil (15 mg/ml) to a final dose of 0.15 mg tamoxifen per g body weight. The injections were given 8 hours apart. This protocol resulted in GFP expression in the lens within 24 hours. Typically, 1-5% of epithelial cells and young fiber cells were GFP positive (Shi and Bassnett, 2007). For some experiments, lenses from TgN(GFPU)5Nagy mice were used. In these animals, a GFP transgene is expressed spontaneously by approximately 25% of epithelial and fiber cells in the lens. The resulting fluorescence mosaicism within the tissue enables the formation of the LMDP to be visualized directly in intact living lenses (Shestopalov and Bassnett, 2003).
Histology and tissue array tomography
Cryosections of lens tissue were prepared essentially as described (Grey et al., 2003) except that incubation in sucrose cryoprotective solutions (10%, 20%, 30% sucrose) was increased to 12 hours, in each case. Frozen tissue was sectioned at 20 μm and incubated with either anti-Aqp0 (cat. no. AQP01-A; alphadiagnostic, San Antonio, TX) or anti-Lim2 (cat. no. 42-2600; Invitrogen, Carlsbad, CA) according to standard protocols.
Array tomography was performed essentially as described (Micheva and Smith, 2007). Briefly, lenses were fixed for 2 hours in 4% paraformaldehyde in PBS, dehydrated and embedded in LR-White resin according to standard procedures. Ribbons of 80-nm-thick serial sections were collected onto subbed glass slides and processed for immunofluorescence using a rabbit polyclonal antibody (Shiels et al., 1991) raised against aquaporin 0 (Aqp0), the most abundant integral protein of the lens fiber plasma membrane. Immunofluorescence was visualized using a 63× Apochromat (1.4 NA) objective lens.
Microscopy, 3D reconstruction and image processing
Intact lenses were placed in pre-warmed DMEM F12 culture medium in glass-bottomed Petri dishes. Lenses were viewed using an LSM510 confocal microscope (Carl Zeiss, Thornwood, NY), as described (Bassnett, 2005). To visualize GFP expression in cells scattered throughout the lens volume, stacks of optical sections were collected and collapsed to final, maximum intensity projections using software supplied with the instrument. GFP fluorescence was elicited using the 488 nm argon laser line and collected using 505-530 nm band-pass filter. Lenses were imaged in quadrants (using a 10× apochromat objective lens; 0.45 NA) and the resulting projections were compiled into a final montage using Photoshop image editing software. Cross-section profiles of GFP-expressing cells or clusters of cells were obtained from X-Z scans oriented orthogonal to the long axis of the expressing cells.
To visualize the three dimensional structure of the lens sutures and to chart the distribution of putative fusions between lens fiber cells, lenses were incubated in FM4-64 (Invitrogen, Carlsbad, CA). FM dyes are lipophilic styryl compounds commonly used in studies of plasma membrane dynamics. They are virtually non-fluorescent in aqueous media but become highly fluorescent following insertion into the outer leaflet of the plasma membrane. Lenses were imaged in the presence of 1 μM FM4-64 using a 40× water-immersion C-apochromat objective lens (1.2 NA).
Microinjection
The properties of LMDP- and gap-junction-mediated diffusion were probed by microinjecting tracers of various sizes into lens fiber cells. Lenses were immobilized in 4% low melting agarose dissolved in tissue culture medium and the microinjection pipette was inserted through the posterior capsule. Large molecule diffusion was assessed by injection of Alexa Fluor 488-dextran (MW 10 kDa; Invitrogen). The distribution of Alexa Fluor 488-dextran was visualized by confocal microscopy 10 minutes and 2 days after injection. Gap-junction-mediated communication was assessed by microinjection of the low molecular weight (367 Da) tracer, neurobiotin (Vector Laboratories, Burlingame, CA). Tracers were injected iontophoretically using a dual channel current generator (Model 260, World Precision Instruments, Sarasota, FL). After injection, lenses were fixed and sectioned using a vibratome tissue sectioner. The distribution of neurobiotin was visualized following incubation in Alexa Fluor 488-labeled streptavidin according to standard protocols.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/10/1607/DC1
We gratefully acknowledge the expert technical assistance of Seta Dikranian, Jean Jones and Belinda McMahan, and David Beebe for critically reading the manuscript. This work was supported by NEI grants EY09852 and EY018185 (S.B.), EY012284 (A.S.), EY13897 (J.M.P.) EY02687 (Core grant for vision research), and by an unrestricted grant to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness.
References
- Alcala, J., Lieska, N. and Maisel, H. (1975). Protein composition of bovine lens cortical fiber cell membranes. Exp. Eye Res. 21, 581-595. [DOI] [PubMed] [Google Scholar]
- Bacia, K. and Schwille, P. (2003). A dynamic view of cellular processes by in vivo fluorescence auto- and cross-correlation spectroscopy. Methods 29, 74-85. [DOI] [PubMed] [Google Scholar]
- Bassnett, S. (2005). Three-dimensional reconstruction of cells in the living lens: the relationship between cell length and volume. Exp. Eye Res. 81, 716-723. [DOI] [PubMed] [Google Scholar]
- Beebe, D. C., Vasiliev, O., Guo, J., Shui, Y. B. and Bassnett, S. (2001). Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Invest. Ophthalmol. Vis. Sci. 42, 727-734. [PubMed] [Google Scholar]
- Brock, R., Vamosi, G., Vereb, G. and Jovin, T. M. (1999). Rapid characterization of green fluorescent protein fusion proteins on the molecular and cellular level by fluorescence correlation microscopy. Proc. Natl. Acad. Sci. USA 96, 10123-10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, M. C. (1984). Measurement of refractive index in an intact crystalline lens. Vision Res. 24, 409-415. [DOI] [PubMed] [Google Scholar]
- Chen, E. H., Grote, E., Mohler, W. and Vignery, A. (2007). Cell-cell fusion. FEBS Lett. 581, 2181-2193. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Muller, J. D., Ruan, Q. and Gratton, E. (2002). Molecular brightness characterization of EGFP in vivo by fluorescence fluctuation spectroscopy. Biophys. J. 82, 133-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Korte, C. L., Van Der Steen, A. F., Thijssen, J. M., Duindam, J. J., Otto, C. and Puppels, G. J. (1994). Relation between local acoustic parameters and protein distribution in human and porcine eye lenses. Exp. Eye Res. 59, 617-627. [DOI] [PubMed] [Google Scholar]
- Donaldson, P., Kistler, J. and Mathias, R. T. (2001). Molecular solutions to mammalian lens transparency. News Physiol. Sci. 16, 118-123. [DOI] [PubMed] [Google Scholar]
- Ervin, L. A., Ball, L. E., Crouch, R. K. and Schey, K. L. (2005). Phosphorylation and glycosylation of bovine lens MP20. Invest. Ophthalmol. Vis. Sci. 46, 627-635. [DOI] [PubMed] [Google Scholar]
- Faulkner-Jones, B., Zandy, A. J. and Bassnett, S. (2003). RNA stability in terminally differentiating fibre cells of the ocular lens. Exp. Eye Res. 77, 463-476. [DOI] [PubMed] [Google Scholar]
- Fernald, R. D. and Wright, S. E. (1983). Maintenance of optical quality during crystalline lens growth. Nature 301, 618-620. [DOI] [PubMed] [Google Scholar]
- Galvan, A., Lampe, P. D., Hur, K. C., Howard, J. B., Eccleston, E. D., Arneson, M. and Louis, C. F. (1989). Structural organization of the lens fiber cell plasma membrane protein MP18. J. Biol. Chem. 264, 19974-19978. [PubMed] [Google Scholar]
- Gattegno, T., Mittal, A., Valansi, C., Nguyen, K. C., Hall, D. H., Chernomordik, L. V. and Podbilewicz, B. (2007). Genetic control of fusion pore expansion in the epidermis of Caenorhabditis elegans. Mol. Biol. Cell 18, 1153-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen, T., Grey, A. C., Jacobs, M. D., Donaldson, P. J. and Kistler, J. (2001). MP20, the second most abundant lens membrane protein and member of the tetraspanin superfamily, joins the list of ligands of galectin-3. BMC Cell Biol. 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey, A. C., Jacobs, M. D., Gonen, T., Kistler, J. and Donaldson, P. J. (2003). Insertion of MP20 into lens fibre cell plasma membranes correlates with the formation of an extracellular diffusion barrier. Exp. Eye Res. 77, 567-574. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis, A. K., Gertsenstein, M., Ikawa, M., Okabe, M. and Nagy, A. (1998). Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech. Dev. 76, 79-90. [DOI] [PubMed] [Google Scholar]
- Hayashi, S. and McMahon, A. P. (2002). Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305-318. [DOI] [PubMed] [Google Scholar]
- Jacobs, M. D., Donaldson, P. J., Cannell, M. B. and Soeller, C. (2003). Resolving morphology and antibody labeling over large distances in tissue sections. Microsc. Res. Tech. 62, 83-91. [DOI] [PubMed] [Google Scholar]
- Kuszak, J. R., Macsai, M. S., Bloom, K. J., Rae, J. L. and Weinstein, R. S. (1985). Cell-to-cell fusion of lens fiber cells in situ: correlative light, scanning electron microscopic, and freeze-fracture studies. J. Ultrastruct. Res. 93, 144-160. [DOI] [PubMed] [Google Scholar]
- Kuszak, J. R., Ennesser, C. A., Bertram, B. A., Imherr-McMannis, S., Jones-Rufer, L. S. and Weinstein, R. S. (1989). The contribution of cell-to-cell fusion to the ordered structure of the crystalline lens. Lens Eye Toxic. Res. 6, 639-673. [PubMed] [Google Scholar]
- Lynnerup, N., Kjeldsen, H., Heegaard, S., Jacobsen, C. and Heinemeier, J. (2008). Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS ONE 3, e1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi, S., Lee, X., Li, X., Veldman, G. M., Finnerty, H., Racie, L., LaVallie, E., Tang, X. Y., Edouard, P., Howes, S. et al. (2000). Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403, 785-789. [DOI] [PubMed] [Google Scholar]
- Micheva, K. D. and Smith, S. J. (2007). Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron 55, 25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak, A., Guo, C., Yang, W., Nagy, A. and Lobe, C. G. (2000). Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147-155. [PubMed] [Google Scholar]
- Oren-Suissa, M. and Podbilewicz, B. (2007). Cell fusion during development. Trends Cell Biol. 17, 537-546. [DOI] [PubMed] [Google Scholar]
- Ponnam, S. P., Ramesha, K., Tejwani, S., Matalia, J. and Kannabiran, C. (2008). A missense mutation in LIM2 causes autosomal recessive congenital cataract. Mol. Vis. 14, 1204-1208. [PMC free article] [PubMed] [Google Scholar]
- Potgens, A. J., Schmitz, U., Bose, P., Versmold, A., Kaufmann, P. and Frank, H. G. (2002). Mechanisms of syncytial fusion: a review. Placenta 23 Suppl. A, S107-S113. [DOI] [PubMed] [Google Scholar]
- Pras, E., Levy-Nissenbaum, E., Bakhan, T., Lahat, H., Assia, E., Geffen-Carmi, N., Frydman, M., Goldman, B. and Pras, E. (2002). A missense mutation in the LIM2 gene is associated with autosomal recessive presenile cataract in an inbred Iraqi Jewish family. Am. J. Hum. Genet. 70, 1363-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir, A., Avinoam, O., Podbilewicz, B. and Chernomordik, L. V. (2008). Viral and developmental cell fusion mechanisms: conservation and divergence. Dev. Cell 14, 11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalov, V. I. and Bassnett, S. (2000a). Expression of autofluorescent proteins reveals a novel protein permeable pathway between cells in the lens core. J. Cell Sci. 113, 1913-1921. [DOI] [PubMed] [Google Scholar]
- Shestopalov, V. I. and Bassnett, S. (2000b). Three-dimensional organization of primary lens fiber cells. Invest. Ophthalmol. Vis. Sci. 41, 859-863. [PubMed] [Google Scholar]
- Shestopalov, V. I. and Bassnett, S. (2003). Development of a macromolecular diffusion pathway in the lens. J. Cell Sci. 116, 4191-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. and Bassnett, S. (2007). Inducible gene expression in the lens using tamoxifen and a GFP reporter. Exp. Eye Res. 85, 732-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels, A., Griffin, C. S. and Muggleton-Harris, A. L. (1991). Immunochemical comparison of the major intrinsic protein of eye-lens fibre cell membranes in mice with hereditary cataracts. Biochim. Biophys. Acta 1097, 318-324. [DOI] [PubMed] [Google Scholar]
- Shiels, A., King, J. M., Mackay, D. S. and Bassnett, S. (2007). Refractive defects and cataracts in mice lacking lens intrinsic membrane protein-2. Invest. Ophthalmol. Vis. Sci. 48, 500-508. [DOI] [PubMed] [Google Scholar]
- Van Itallie, C. M. and Anderson, J. M. (2006). Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68, 403-429. [DOI] [PubMed] [Google Scholar]
- Vrensen, G. F., Graw, J. and De Wolf, A. (1991). Nuclear breakdown during terminal differentiation of primary lens fibres in mice: a transmission electron microscopic study. Exp. Eye Res. 52, 647-659. [DOI] [PubMed] [Google Scholar]
- White, T. W. (2002). Unique and redundant connexin contributions to lens development. Science 295, 319-320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.