Summary
Deviations in basal Ca2+ from normal levels interfere with receptor-mediated Ca2+ signaling as well as endoplasmic reticulum (ER) and mitochondrial function. While defective basal Ca2+ regulation has been linked to various diseases, the regulatory mechanism that controls basal Ca2+ is poorly understood. Here we performed a siRNA screen of the human signaling proteome to identify regulators of basal Ca2+ concentration and found STIM2 as the strongest positive regulator. In contrast to STIM1, a recently discovered signal transducer that triggers Ca2+-influx in response to receptor-mediated depletion of ER Ca2+ stores, STIM2 activated Ca2+ influx upon smaller decreases in ER Ca2+. STIM2, like STIM1, caused basal Ca2+ influx via activation of the plasma membrane Ca2+ channel Orai1. Our study places STIM2 at the center of a feedback module that keeps basal cytosolic and ER Ca2+ concentrations within tight limits.
Introduction
Ca2+ is a ubiquitous second messenger that regulates secretion, contraction, gene expression and other cell functions. In unstimulated cells, the basal cytosolic concentration of Ca2+ is kept constant at a concentration ~10,000 fold below the extracellular and endoplasmic reticulum (ER) Ca2+ concentration (Berridge et al., 2003). Receptor stimuli typically increase Ca2+ concentration up to ten-fold from basal by opening Ca2+ channels in the plasma membrane (PM) or ER membrane. These Ca2+ signals are generated by a dynamic system that relies on Ca2+ channels and pumps in the PM and ER (Figure 1A).
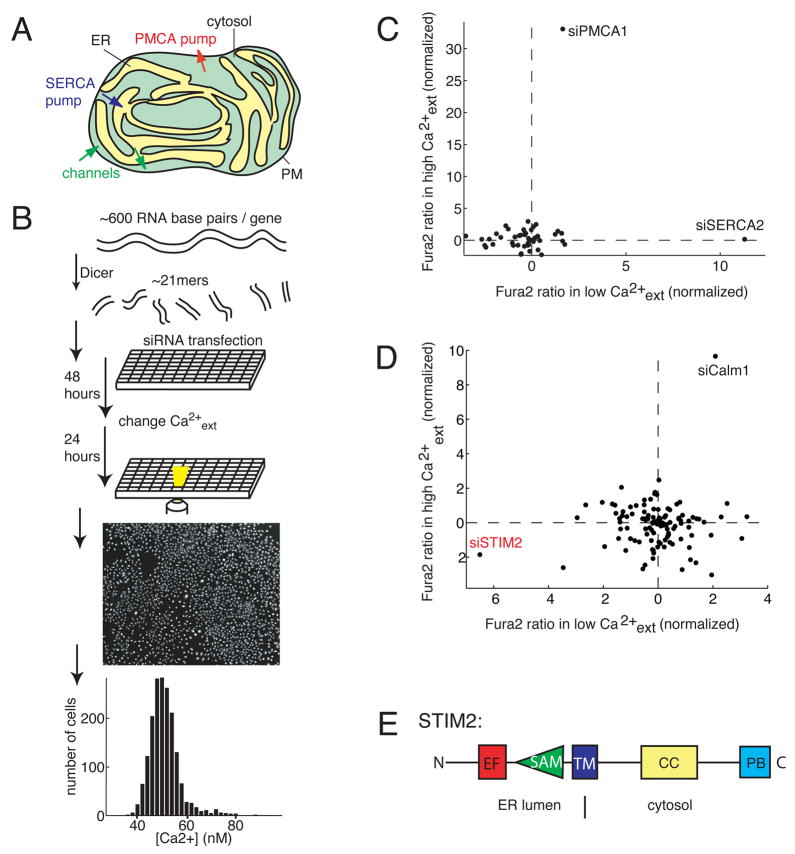
Figure 1. Identification of STIM2 as a regulator of basal Ca2+ concentration.
(A) Overview of intracellular Ca2+ homeostasis. Basal cytosolic Ca2+ concentration is controlled by PM as well as ER Ca2+ channels and pumps.
(B) Sensitized siRNA screening assay for basal Ca2+ regulation. 2304 diced siRNA constructs were individually transfected into HeLa cells and cultured in 384 well plates. High and Low extracellular Ca2+ exposure (+10 mM and ~0.1 mM) were used for sensitization. Single cell Ca2+ levels were measured using automated image analysis software.
(C) Test experiments using a siRNA set targeting Ca2+ pumps, channels, and exchangers (performed in duplicate). Deviations from control Ca2+ levels are shown in units of standard deviation.
(D) Result from the sensitized siRNA screen of the human signaling proteome highlighting STIM2 and Calm1 as primary hits (performed in triplicate).
(E) Schematic representation of modular domains found in STIM2. On the luminal side: EF-hand is a Ca2+ binding domain and SAM is a conserved protein interaction domain. On the cytosolic side: CC and PB are a coiled-coil and a polybasic region, respectively.
Tremendous progress has been made in recent years in understanding how receptor stimuli regulate different Ca2+-channels. However, less is known about regulatory feedback mechanisms that maintain a constant basal Ca2+ concentration. The importance of tight control of basal Ca2+ concentration is indicated by numerous diseases that have been associated with prolonged increases or decreases in basal Ca2+ concentration. For example, cells derived from Alzheimer patients with genetic mutations in presenilin have defects in Ca2+ homeostasis due to changes in the rate of basal Ca2+-flux out of the ER Ca2+ store (Tu et al., 2006). Changes in basal Ca2+ homeostasis have also been associated with diseases and conditions such as endothelial dysfunction (Shulman et al., 2005), kidney disease (Thebault et al., 2006), cardiac dysfunction (Ter Keurs and Boyden, 2007), Huntington disease (Bezprozvanny and Hayden, 2004), as well as other neurodegenerative and aging related diseases (Treves et al., 2005; Raza et al., 2007).
Mechanistically, these different disease states and conditions are believed to be caused by long-term increases or decreases in basal Ca2+ concentration that then result in defective Ca2+ signaling (Ter Keurs and Boyden, 2007), reduced ER Ca2+ concentration and ER stress (Zhang & Kaufman, 2006) or mitochondrial dysfunction (Campanella et al., 2004). Long term changes in basal Ca2+ also alter protein degradation (Spira et al., 2001) and transcription (Gallo, 2006) which may indirectly interfere with cell health.
The active components that maintain Ca2+ gradients in human cells are believed to be four different PM pump isoforms (PMCAs) (Strehler et al., 2007; Guerini et al., 2005) and three PM Ca2+ transporters (Na+/Ca2+-exchangers) (Philipson et al., 2002) as well as three different ER Ca2+ pump isoforms (SERCAs) (Periasamy, 2007). Less is known about the nature of basal Ca2+ influxes to the cytosol from outside of the cell and from ER Ca2+ stores, but some Ca2+ channels and other Ca2+ leak activities have been proposed to play a role (Tu et al., 2006; Pinton and Rizzuto, 2005; Camello et al., 2002). Remarkably, many researchers have observed that basal Ca2+ levels in cells change little in response to large changes in extracellular Ca2+ concentration, arguing that a highly effective feedback control exists to stabilize basal Ca2+ concentration (Kusters et al., 2005).
We set out to pursue a siRNA screening strategy to learn more about feedback mechanisms that ensure that basal Ca2+ levels are kept within narrow limits in cells. We used two sensitized screening conditions and live single cell Ca2+ imaging to enhance our ability to identify possible regulators. Since we were interested in signaling proteins, we made use of a Dicer-generated siRNA library of the human signaling proteome that we had previously generated (Liou et al., 2005). In addition to the expected findings that knocking down known Ca2+ pumps or calmodulin increase basal Ca2+ concentration, we found that knocking down STIM2 had the strongest effect on reducing basal Ca2+ concentration. We observed that over-expression of STIM2 but not its homolog STIM1 markedly enhanced basal Ca2+ concentration by regulating the plasma membrane Ca2+-channel Orai1. We also found that, upon Ca2+ store depletion, a YFP-STIM2 construct showed dynamic translocation from a uniform ER distribution to ER-PM junctions similar to the behavior of STIM1 (Liou et al., 2005). However, unlike the translocation of STIM1, STIM2 was able to translocate to ER-PM junctions with only small decreases in ER Ca2+ concentration, explaining why STIM2 but not STIM1 serves as a regulator of basal Ca2+.
Results
Design of a sensitized siRNA screen to identify regulators of basal Ca2+ concentration
We developed a siRNA screening protocol to identify human genes that regulate basal cytosolic Ca2+ concentration. HeLa cells were sensitized by subjecting their Ca2+ signaling system to two opposing pressures: 24 hours in high extracellular Ca2+ (+10 mM) and, in a separate experiment, 24 hours in low extracelluar Ca2+ (~0.1 mM). This strategy was devised to push the Ca2+ homeostatic control system towards its limits so that the effect of the RNAi perturbations could be more readily observed. We used the ratiometric Ca2+ indicator Fura-2 (Grynkiewicz et al., 1985) and an automated fluorescence imaging system to measure siRNA-mediated differences in basal Ca2+ concentration. An approximately 5 nM reduction was caused by the low and 2.5 nM increase by the high Ca2+ condition from a basal level of ~ 50 nM. Approximately 1000 individual cells in each well of a 384-well plate were analyzed and the mean single cell Fura-2 ratio was computed for each well (Figure 1B).
We tested the usefulness of our strategy by making a Dicer-generated Ca2+ siRNA set that included known and putative Ca2+ pumps, channels and exchangers (Figure 1C; Table S1 for a list of results). The strongest hit in the high external Ca2+ condition was the plasma membrane Ca2+-ATPase PMCA1. This is consistent with a role of plasma membrane Ca2+ pumps as a main avenue for Ca2+ extrusion out of these cells (Guerini et al., 2005). The strongest hit in the low external Ca2+ condition was the endoplasmic-reticulum Ca2+ pump SERCA2, which transports Ca2+ from the cytosol into the ER (Strehler et al., 2007). This effect was also expected since thapsigargin, an inhibitor of SERCA pumps, is known to trigger persistent increases in cytosolic Ca2+ concentration (Thastrup, 1990). These test measurements demonstrated that our assay system is able to identify different types of regulators of basal Ca2+ concentration.
Identification of STIM2 as a primary regulator of basal Ca2+ concentration
We then screened the Dicer-generated siRNA library targeting the human signaling proteome. 2304 signaling gene products were individually knocked down in duplicate for both the high and low external Ca2+ conditions. From this initial screen, we selected statistically significant positive and negative regulators of basal Ca2+ concentration and remade Dicer-generated siRNA constructs for the top 112 putative hits. We then re-screened these siRNAs in triplicate and found that the siRNAs targeting STIM2 and calmodulin 1 (Calm1) were the strongest positive and negative regulators, respectively (Figure 1D). Table S2 lists the statistically significant results from these two high and low Ca2+ screens.
Consistent with the known function of calmodulin to bind and activate PM Ca2+ pumps, Calm1 knockdown had a similar relative effect as PMCA1 knockdown for both sensitized conditions. Surprisingly, however, the STIM2 gene product was the number one hit in the low Ca2+ext condition. We were particularly intrigued by this finding since STIM2 shares 47% homology to the recently identified ER-Ca2+ sensor STIM1 which functions at the center of a signaling pathway that links receptor-mediated release of ER Ca2+ to the opening of plasma membrane Ca2+ channels (Roos et al., 2005; Liou et al., 2005). STIM2 is a multi-domain transmembrane protein that is at least partially localized to the ER, interfacing both the lumen of the ER and the cytosol (Figure 1E) (Liou et al., 2005; Dziadek and Johnstone, 2007). Conflicting results have been reported about a possible role of STIM2 in negatively regulating STIM1 (Soboloff et al., 2006a) or positively regulating store-operated Ca2+ influx (Liou et al., 2005; Soboloff et al., 2006b). We focused our subsequent studies on the role of STIM2 in regulating basal Ca2+ homeostasis.
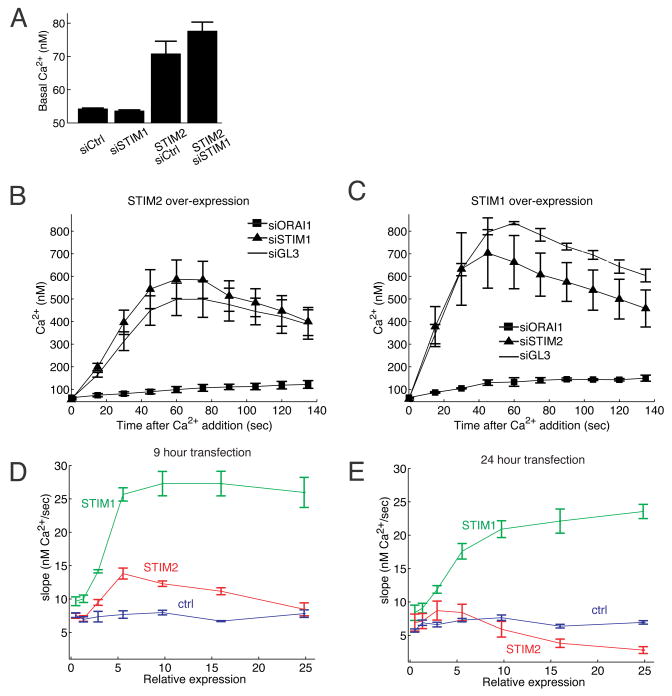
STIM2 knockdown selectively lowers basal cytosolic and ER Ca2+ concentrations
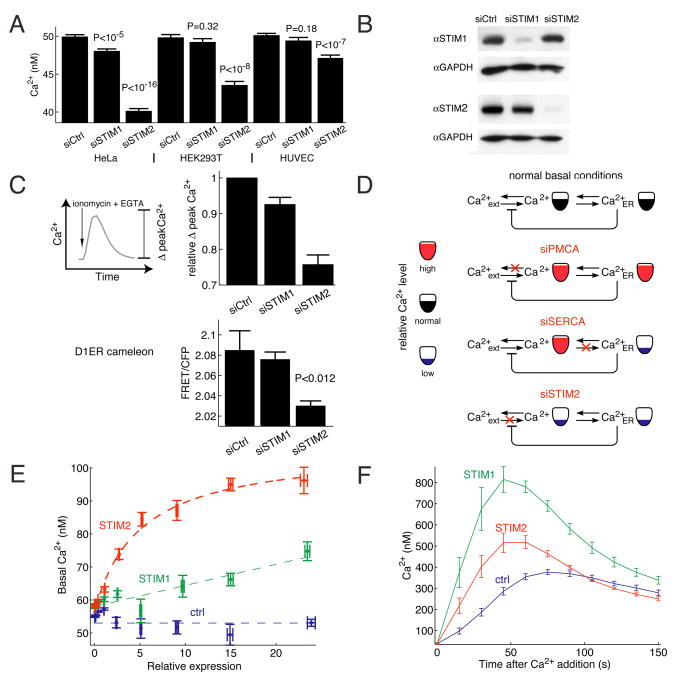
We used synthetic STIM2 siRNA to investigate the effect of STIM2 knockdown on basal Ca2+ concentration under normal external Ca2+ concentrations (1.5 mM). We also compared the effect of a knockdown of STIM2 to that of its isoform STIM1. Consistent with a unique role of STIM2 in regulating basal Ca2+, STIM2 knockdown, but not STIM1 knockdown, significantly lowered basal cytosolic Ca2+ in HeLa, HUVEC and HEK293T cells (Figure 2A). STIM1 and STIM2 knockdowns were effective and isoform-specific when assayed by Western blot (Figure 2B) in HeLa cells.
Figure 2. STIM2 controls basal cytosolic and ER Ca2+ concentration.
(A) Comparison of basal Ca2+ levels after siRNA knockdown of STIM2 compared to STIM1. HeLa, HUVEC, and HEK293T cells were transfected with synthetic siRNA against STIM2 and STIM1 as well as diced GL3 as a control. N=10 sites; error bars represent standard error.
(B) STIM1 and STIM2 siRNA specificity assayed by Western blot. HeLa cells were tranfected with siRNAs targeting STIM1, STIM2 or control for 3 days.
(C) STIM2 knockdown lowers basal ER Ca2+. ER Ca2+ levels were measured in two ways. First, as the Ca2+ pool released by addition of the Ca2+-ionophore ionomycin. 1 μM ionomycin + 3 mM EGTA were added to HeLa cells and the increase in cytosolic Ca2+ was measured (Δpeak). Single cell analysis from 3 wells each. In a second method, the ER targeted cameleon D1ER was transfected into cells two days after siRNA transfection and one day before imaging. FRET/CFP was then computed as described in Materials and Methods (8 sites).
(D) Schematic representation of the effects of PMCA1, SERCA2, and STIM2 knockdowns on basal Ca2+ levels in the ER and cytosol.
(E) Single cell analysis of basal Ca2+ concentration as a function of the expression level of YFP-STIM2 versus YFP-STIM1. Cells were transfected for 9 hours with YFP-STIM1, YFP-STIM2, or YFP (as a control). Ca2+ levels and YFP construct expression were measured for each cell. YFP fluorescence was normalized to the background in the YFP channel.
(F) Single cell analysis of Ca2+-influx triggered by ER Ca2+ store-depletion (Ca2+-add back experiments). Cells were depleted of ER Ca2+ by additions of 1 μM thapsigargin to block SERCA pumps and 3 mM external EGTA to prevent Ca2+ influx. Ca2+ was added back (to a free concentration of 0.75 mM) at t=0 to measure Ca2+ influx rates. Single cells were analyzed in 3 independent wells for each condition.
We also determined the consequence of STIM2 or STIM1 knockdown on the basal Ca2+ level in ER stores. We tested ER Ca2+ levels first by addition of the membrane permeant Ca2+ ionophore ionomycin together with external application of the Ca2+ chelator EGTA and by quantifying the induced cytosolic Ca2+ peak (Figure 2C). Ionomycin is known to rapidly release Ca2+ from internal Ca2+ stores and external EGTA was included to prevent Ca2+ influx from outside the cell. Since the ER is the primary Ca2+ store in the cell, it is therefore expected that the measured relative amplitude of the induced Ca2+ peak (Δpeak Ca2+) directly reflects the loading level of ER Ca2+ stores (full traces are shown in Figure S1). Using this approach, STIM2 knockdown led to a marked decrease in ER Ca2+ levels while STIM1 knockdown had a smaller effect.
In a second approach, we transfected HeLa cells with the ER targeted Ca2+ indicator D1ER (Palmer et al., 2004). Only siSTIM2 treated cells showed a significant decrease in ER Ca2+ as measured by the relative decrease in the ER FRET signal (Figure 2C). Thus, knockdown of STIM2 reduces both basal cytosolic as well as basal ER Ca2+ concentrations.
The effect of PMCA1, SERCA2, and STIM2 knockdowns on basal cytosolic Ca2+ levels can be parsimoniously explained by a simple model relating the extracellular, cytosolic, and ER Ca2+ pools (Figure 2D). In unstimulated cells with intact pumps and channels, long-term changes in cytosolic Ca2+ concentration are expected to be paralleled by changes in ER Ca2+ concentration. PMCA knockdown attenuates export of Ca2+ from the cell and would therefore result in an increase in cytosolic as well as ER Ca2+ concentrations. SERCA knockdown lowers ER Ca2+ levels, thereby activating store-operated Ca2+ influx which increases cytosolic Ca2+ concentration. In contrast, the finding that STIM2 knockdown lowers Ca2+ concentration both in the cytosol and ER suggests that it has a role as a positive regulator of basal Ca2+-influx that increases cytosolic Ca2+ concentration as well as ER Ca2+ concentration.
STIM2 and STIM1 have distinct roles in regulating basal Ca2+ influx versus receptor triggered store-operated Ca2+ influx
We next used over-expression of YFP-STIM1 and YFP-STIM2 fusion proteins to better understand the roles of the two isoforms. We used automated low magnification imaging to measure Ca2+ concentration together with the relative concentration of expressed YFP-STIM1 or YFP-STIM2 in thousands of individual cells. The YFP intensities were normalized using the autofluorescence of untransfected cells as a reference and we then grouped all the cells according to STIM expression. The cell-to-cell variability of expression levels guaranteed that we covered the full range of STIM expression in each experiment. Using this analysis, transient expression (9 hour) of YFP-STIM2 or YFP-STIM1 both increased basal Ca2+ as a function of the expression level. However, expression of YFP-STIM2 increased basal Ca2+ levels much more than expression of the same concentration of YFP-STIM1 (Figure 2E). Expression of YFP alone is shown as a control. This provides further support for a primary role of STIM2 but not of STIM1 in regulating basal Ca2+ concentration.
We then measured store operated Ca2+-influx (SOC) using Ca2+ add back experiments in cells where Ca2+ stores had been maximally depleted by thapsigargin in low external Ca2+. Expression of STIM2 increased Ca2+ influx, albeit less than expression of the same concentration of STIM1 (Figure 2F). This argues that both STIM1 and STIM2 can sense the depletion of ER Ca2+ levels and can trigger plasma membrane Ca2+ influx. As a control, expression of a STIM2 construct without a protein tag also increased Ca2+-influx after ER store depletion (Figure S2). This led to our working model that STIM2 serves as the primary regulator for basal Ca2+-influx while STIM1 and STIM2 both trigger Ca2+ influx following receptor-mediated ER store-depletion.
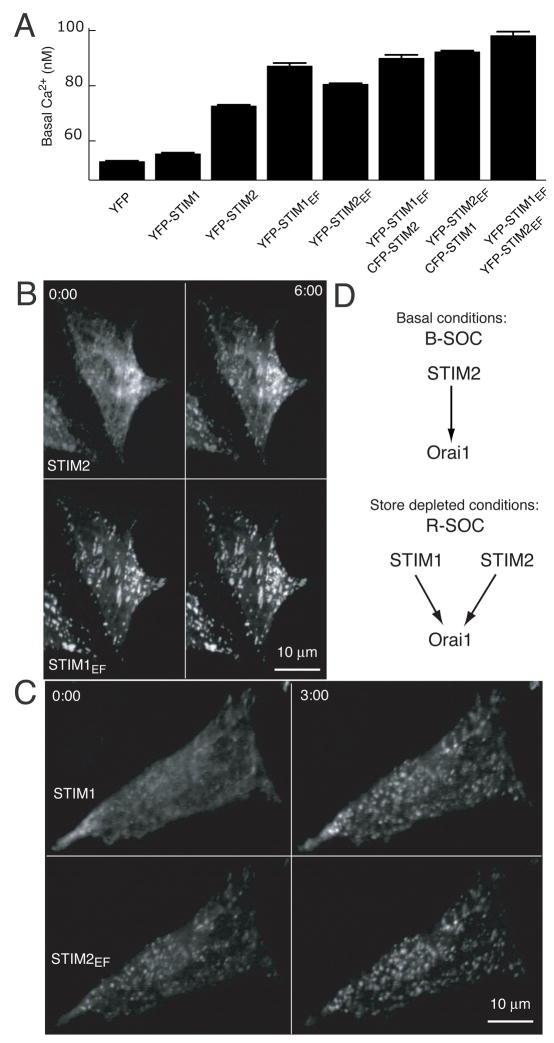
ER Ca2+ store depletion triggers STIM2 translocation to ER-PM junctions
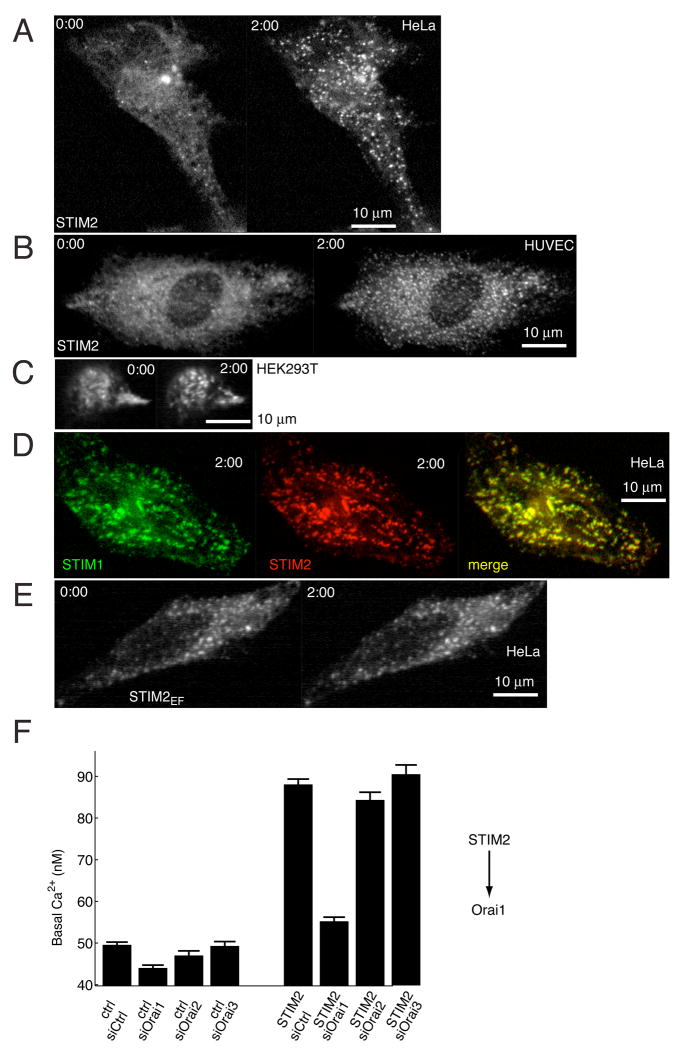
We used the YFP conjugated STIM2 construct to investigate whether it undergoes store-depletion triggered translocation as was shown previously for STIM1 (Liou et al., 2005). Strikingly, YFP-STIM2 was initially mostly distributed across the ER, and thapsigargin-induced ER Ca2+ store depletion led to a rapid translocation of YFP-STIM2 to PM localized puncta (Figure 3A). We observed the same YFP-STIM2 puncta formation in HeLa, HUVEC, and HEK293T cell lines (Figures 3A–C). To minimize expression artifacts, only cells with a low range of YFP-STIM2 expression are shown in these panels.
Figure 3. STIM2 translocates to ER-PM junctions following ER Ca2+ depletion and regulates Orai1.
(A–C) YFP-STIM2 was expressed (~24 hour) in HeLa (A), HUVEC, (B), and HEK293T (C) cells and confocal images were taken before and 2 minutes after 1 μM thapsigargin addition.
(D) Comparison of the distribution of CFP-STIM1 and YFP-STIM2 constructs 2 min after addition of thapsigargin.
(E) Ca2+-binding deficient YFP-STIM2 (point mutation in EF-hand) is prelocalized to ER-PM junction sites and does not alter its localization after Ca2+ store depletion.
(F) Knockdown of the PM Ca2+ channel Orai1 significantly reduces the increase in basal Ca2+ resulting from STIM2 expression. HeLa cells were transfected for two days with GL3, Orai1, Orai1, or Orai3 siRNA and then transfected for 24 hours with YFP-STIM2 or YFP as control. Cells used in the analysis expressed YFP at 7.5 to 15 fold above background. N=10 sites.
We next compared STIM1 and STIM2 localization by co-transfection of CFP-STIM1 and YFP-STIM2 followed by thapsigargin-induced depletion of ER Ca2+ stores. CFP-STIM1 and YFP-STIM2 were co-localized in the same puncta that have previously been characterized as ER-PM junction sites (Figure 3D) (Liou et al., 2005, Wu et al., 2006). Finally, we tested whether the punctate localization of STIM2 is driven by Ca2+ dissociation from its EF-hand by creating an EF-hand mutant of STIM2 that disrupts the EF-hand Ca2+ binding site (Asp 80 to Ala). The STIM2 EF-hand mutant (STIM2EF) was prelocalized to ER-PM junctions and did not significantly change distribution in response to thapsigargin (Figure 3E). This argues that STIM1 and STIM2 make use of a common regulatory mechanism that involves Ca2+ dissociation, oligomerization and translocation in order to signal from the lumen of the ER to the ER-PM junction sites (Liou et al., 2007). In order to facilitate the discussion of the distinguishing features of basal versus receptor-triggered Ca2+-influx, we refer to them in the text below as B-SOC and R-SOC, respectively.
Given the similarities in the observed STIM1 and STIM2 activation mechanism, we tested whether the effect of STIM2 on B-SOC is mediated by the PM Ca2+ channels Orai1, 2 or 3, as has been shown for R-SOC in the case of STIM1 (Feske et al., 2006; Peinelt et al. 2006; Luik et al., 2006; Yeromin et al., 2006; Mercer et al., 2006). We expressed STIM2 in cells pre-treated with siRNA targeting Orai1, Orai2, or Orai3 using isoform specific siRNAs. Orai1 knockdown abrogated the effect of STIM2 expression on basal Ca2+ by more than 80% (Figure 3F) while targeting of Orai2 or Orai3 had a smaller effect. This argues that HeLa cells make use of Orai1 Ca2+ channels for B-SOC as well as R-SOC Ca2+ influx. We note that in cells without STIM2 expression, knockdown of Orai isoforms lowered basal Ca2+ to a lesser extent compared to the knockdown of STIM2 (Figure S3). This lower suppression may be caused by insufficient knockdown, additive contributions from different Orai-isoforms or from other channels regulated by STIM2.
Translocation of STIM2 but not STIM1 in response to small reductions in ER Ca2+ concentration
Our imaging and functional studies suggest that key features of the molecular regulation of STIM2 and STIM1 are the same. A plausible mechanism explaining why they differentially regulate basal Ca2+ is a different sensitivity of STIM1 and STIM2 to ER Ca2+ concentration. STIM2 may already be partially active at basal ER Ca2+ concentrations and become more active by small reductions in ER Ca2+, while STIM1 may require much larger receptor-triggered reductions in ER Ca2+ to be activated.
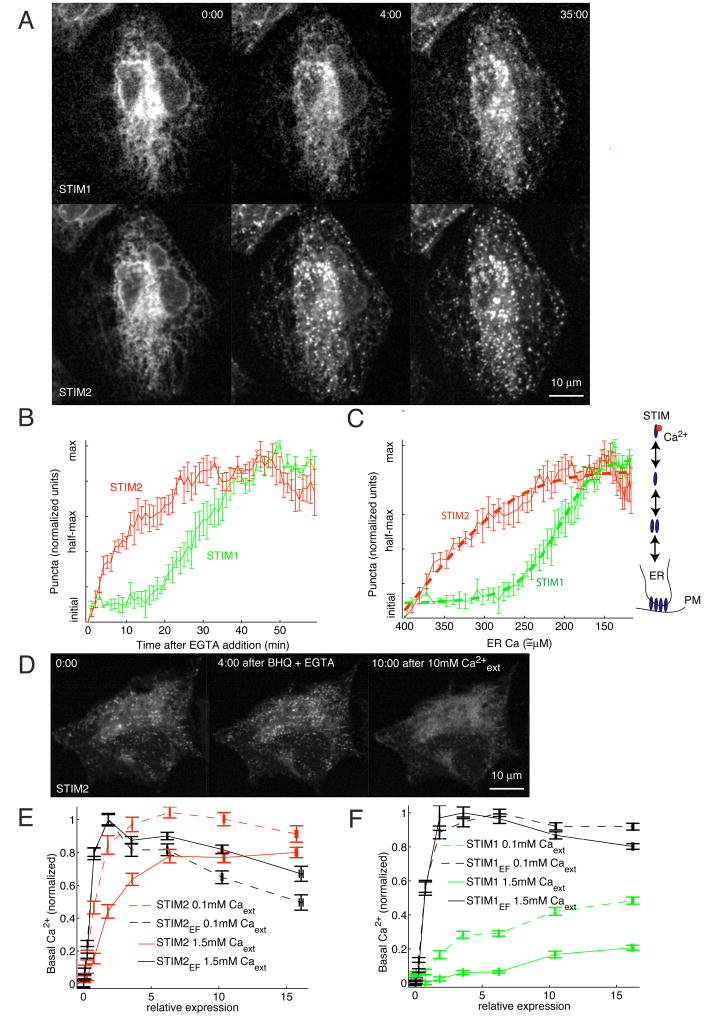
To test this hypothesis, HeLa cells were cotransfected with CFP-STIM1 and YFP-STIM2 and then subjected to slow ER Ca2+ store depletion by removing external Ca2+ using EGTA addition. Strikingly, YFP-STIM2 started to form puncta at ER-PM junctions before CFP-STIM1 following external EGTA addition (Figure 4A). While the time to reach half-maximal puncta for YFP-STIM2 was ~= 10 minutes, CFP-STIM1 puncta formation was half-maximal after only ~= 30 minutes (Figure 4A–B). When ER stores were depleted by thapsigargin, STIM2 formed puncta more rapidly, but again before STIM1 (Figure S4). The slower rate in STIM1 puncta formation is not due an intrinsic delay of the translocation process, since STIM1 puncta form within less than 1 minute upon rapid Ca2+ store depletion by ionomycin (Liou et al., 2005). These experiments strongly suggest that STIM2 is activated at higher ER Ca2+ concentrations and therefore will translocate to ER-PM junctions and activate Ca2+ influx even for small reductions in ER Ca2+ levels. In contrast, STIM1 requires a larger reduction in ER Ca2+ to be activated.
Figure 4. STIM2 translocation is cooperatively triggered by small decreases in ER Ca2+ concentration compared to larger decreases needed for STIM1.
(A) STIM2 translocates to ER-PM junctions for small decreases in ER Ca2+ concentrations compared to STIM1. YFP-STIM2 and CFP-STIM1 were co-tranfected (~24 hour) and imaged in the same cells. ER Ca2+ stores were depleted slowly by extracellular addition of 3 mM EGTA. YFP-STIM2 and CFP-STIM1 distributions are compared before, 4 minutes after and 35 minutes after 3 mM EGTA addition.
(B) Analysis of the kinetics of STIM1 and STIM2 translocation to ER-PM junctions upon EGTA addition. 3 mM EGTA was added to HeLa cells and imaged for 60 minutes. Cells were analyzed for puncta content as described in the Materials and Methods section. Average puncta intensity from N=5 cells.
(C) Calibration and quantitative model derived from the data in (B). ER Ca2+ concentration was calibrated as a function of time after EGTA addition (Figure S5A). The concentration dependence of translocation was then fit to a cooperative oligomerization and translocation model (thick dashed lines). The scheme shows the key features of our model that includes, in addition to the differential Ca2+ sensitivity, an oligomerization and translocation process with a cooperativity of 5 for STIM2 and 8 for STIM1 activation.
(D) ER Ca2+ overload reduces STIM2 puncta to sub-basal levels. 1 μM BHQ + 3 mM EGTA was added to YFP-STIM2 expressing cells and then washed out with extracellular buffer containing 10 mM Ca2+, causing a large Ca2+ influx and super-normal ER Ca2+ levels.
(E-F) Functional comparison supporting that STIM2 activity is suppressed at higher ER-Ca2+ levels compared to STIM1. Basal Ca2+ levels were measured as a function of the expression level of STIM1 and STIM2 constructs (9 h of transfection). Normal (dashed lines) and reduced (solid lines) ER Ca2+ levels were used to probe for the STIM1 and STIM2 Ca2+ sensitivities. Expression of EF-hand mutant STIM2 and STIM1 constructs (black) were employed as a reference of Ca2+-insensitive and constitutively active proteins and were used to normalize the wildtype data (each isoform of STIM was divided by the maximum basal level for the corresponding EF hand mutant in the same condition). In normal ER conditions, wildtype STIM1 (green) or STIM2 (red) expression showed a marked difference in basal Ca2+ profile compared the EF-hand mutants. In contrast, STIM2 but not STIM1 closely matched the profile of its EF-hand mutant at reduced ER Ca2+ levels, suggesting that reduced ER Ca2+ levels can still suppress STIM1 but not STIM2 activity. N=25 sites.
In order to determine the ER Ca2+ levels and the degree of cooperativity for translocation of STIM1 and STIM2, we used ionomycin triggered Ca2+ release to estimate the remaining Ca2+ in ER stores at different time points after EGTA addition. Alternatively, we measured the same time constant using the ER Ca2+ probe D1ER (Figure S5). Assuming that the basal ER Ca2+ concentration is approximately 400 μM (300–500 μM values derived in the literature: Brini et al., 1999; Yu and Hinkle, 2000; Montero et al., 2001), we plotted the degree of STIM1 and STIM2 translocation as a function of ER Ca2+ levels (Figure 4C) and fit the ER Ca2+ dependence of translocation to a cooperative activation model. Since the observed puncta formation has been shown to involve sequential Ca2+ dissociation, oligomerization, and translocation steps (Liou et al., 2007), our STIM activation model computes an overall cooperativity of the multi-step process (Figure 4C):
Where N is the cooperativity of the overall process, EC50 is the Ca2+ concentration for half-maximal translocation and α and β are normalization constants.
A best fit of this model was obtained with EC50 for STIM2 and STIM1 of 406 and 210 μM, respectively, and N = 5 and 8 for the cooperativity of STIM2 and STIM1, respectively (α=0.91, β=0.83 for STIM1; α= 0.84, β=1.5 for STIM2). This is consistent with a two-fold lower Ca2+ sensitivity of STIM2 compared to STIM1. An important finding from this analysis was that STIM1 translocation is regulated with high cooperativity in relation to the ER Ca2+ concentration. This high cooperativity, combined with its higher affinity for Ca2+, can explain how STIM1 is kept effectively inactive at basal ER Ca2+ concentrations.
Since this analysis predicts that STIM2 is partially active at basal conditions, we tested whether overloading the ER would reduce STIM2 puncta to levels below basal. We used the reversible SERCA inhibitor 2′,5′-di(tert-butyl)-1,4-benzohydroquinone (BHQ) followed by addition of 10 mM Ca2+ extracellular buffer to briefly hyperload the ER. As predicted, BHQ+EGTA caused STIM2 distribution to become more punctate in response to the lower ER Ca2+ (Figure 4D), and the subsequent addition of high extracelluar Ca2+ triggered a near complete loss of observable STIM2 puncta below the basal level. This result is consistent with STIM2 being partially punctate and active at basal conditions.
STIM2 but not STIM1 effectively activate Ca2+-influx in a partial Ca2+ store-depletion protocol
We also tested for different Ca2+ sensitivities of STIM1 and STIM2 by using the reduced ER Ca2+ loading protocol described in Figure 1 for the sensitized screen (0.1 mM external Ca2+). Cytosolic Ca2+ levels were compared as a function of YFP-STIM1 or YFP-STIM2 expression levels for normal as well as reduced ER loading (Figure 4E–F). We used mutant STIM proteins that cannot bind Ca2+ (constitutively active EF-hand mutants) as a reference and compared them to wildtype STIM proteins that can be inhibited by ER Ca2+.
Expression of increasing levels of EF-hand mutants of STIM1 or STIM2 triggered relative Ca2+ increases that were, as expected, insensitive as to whether we measured under normal or reduced ER loading conditions (Figure 4E–F). Also, as expected, for the condition of normal ER Ca2+, wildtype STIM1 and STIM2 were less potent in raising Ca2+ than their EF-hand mutant counterparts, albeit STIM2 was much more potent than STIM1 (Figure 4E–F). This is consistent with a strong inhibition of STIM1 and a weaker inhibition of STIM2 by normal ER Ca2+ levels. This differential regulation becomes even more clear for the measurements at the partially reduced ER Ca2+ concentration where STIM2 becomes nearly as potent as its EF-hand mutant in raising Ca2+, indicating that Ca2+ had almost completely dissociated from STIM2 (Figure 4E). STIM1, by contrast, was still much less effective compared to the STIM1 EF-hand mutant, suggesting that it was still mostly inhibited by Ca2+ (Figure 4F) (Non-normalized data is presented in Figure S6). This functional comparison provides additional evidence that STIM2 selectively activates Ca2+ influx for normal and partially depleted ER Ca2+ conditions for which STIM1 mediated Ca2+ influx is effectively suppressed.
Since these results pointed to possible differences in the EF hand Ca2+ binding affinity of STIM1 versus STIM2, we exchanged the three key sites that are different between the EF hands of STIM1 and STIM2 (positions 79,80,82 in STIM1 and 83,84,86 in STIM2). If the EF hand domain alone were responsible for the difference in sensitivity, the effect of expression of STIM1EF->STIM2 should be the same as that of STIM2 and vice-versa for the STIM2 EF->STIM1 construct. Indeed, expression of STIM1EF->STIM2 produced a construct with a higher Ca2+ sensitivity similar to that of STIM2 (Figure S7) that still senses ER calcium levels (Figure S6). Nevertheless, expression of STIM2 EF->STIM1 did not exhibit STIM1-like properties (data not shown). This argues that the activation threshold for STIM1 and STIM2 is in part regulated by the three amino acid difference in the EF hand region but must involve in addition other parts of the two proteins.
STIM2-regulation of Ca2+-influx does not require STIM1
Since STIM1 and STIM2 have been shown to be able to form hetero-oligomers (Dziadek and Johnstone, 2007; Stathopulos, 2006), it is plausible that they function as a complex and that STIM2 requires STIM1 to signal to Orai1 channels for both B-SOC and R-SOC type Ca2+ influx. We used over-expression of STIM2 combined with STIM1 knockdown to test for such a possible co-regulation. Figure 5A shows that STIM1 knockdown did not significantly alter the increase in basal Ca2+ resulting from STIM2 expression. Furthermore, STIM1 knockdown also did not interfere with the ability of YFP-STIM2 expression to enhance store-depletion-triggered Ca2+-influx (Figure 5B). For comparison, knockdown of Orai1 markedly suppressed the ability of expressed STIM2 to enhance store-operated Ca2+ influx. A reciprocal control experiment with the same result is shown in Figure 5C. This demonstrates that each of the two STIM isoforms can regulate Orai1 in the absence of the other.
Figure 5. STIM2 can regulate Ca2+ influx independent of STIM1.
(A) STIM2 expression-mediated increases in basal Ca2+ are not affected by knockdown of STIM1. Cells were transfected with YFP-STIM2 ~60 hours after STIM1 or GL3 control siRNA transfection and 9 hours before imaging. Cells selected expressing YFP at background autofluorescence (non transfected) or 4–8 times autofluorescense (transfected). N=15 sites.
(B-C) Experiments showing that STIM2-triggered R-SOC is not affected by STIM1 knockdown and STIM1-triggered R-SOC is not significantly affected by STIM2 knockdown. Ca2+ addback experiments were performed in cells prepared and selected as described in (A) expressing STIM2 (B) and STIM1 (C). 9 hour transfection. N=3 wells.
(D-E) Control experiment showing that prolonged overexpression of YFP-STIM2 downregulates the maximal attainable Ca2+ influx rate. Influx rates were measured in Ca2+ add-back experiments in the presence of thapsigargin. Expression at 9 hours (D) is compared to expression at 24 hours (E). N=4 sites from 2 wells.
While working with cells that overexpress YFP-STIM2, we noticed that longer expression (24 hours instead of 9 hours) led to a significant reduction in the maximal attainable store-operated Ca2+ influx (Figure 5D,E). This indicates that a slow adaptive mechanism downregulates store-operated Ca2+ influx upon prolonged supramaximal STIM2 signaling. This may explain an earlier finding that HEK293 cells with stably overexpressed STIM2 showed a similar inhibition (Soboloff et al., 2006a).
Synergistic regulation of Ca2+ influx by STIM1 and STIM2
While these experiments suggested that STIM1 and STIM2 can act independently of each other in regulating Ca2+ influx, we tested if they can also act synergistically by co-expression of STIM1 and STIM2 and their constitutively active mutants and using basal Ca2+ as a readout (Figure 6A). The observed higher basal Ca2+ levels in cells that express both constitutively active isoforms argues that STIM1 and STIM2 can synergize their activity when both isoforms are activated by lowering of ER Ca2+ concentration.
Figure 6. STIM2 and STIM1 act synergistically and independently of each other.
(A) Test for synergism and independence between STIM1 and STIM2. YFP tagged constructs were cotransfected with YFP or CFP constructs as indicated for 24 hours. Ca2+-levels are shown for cells expressing YFP signals 1–3 fold above autofluorescence.
(B) STIM2 can translocate in the presence of prelocalized STIM1EF. CFP-STIM2 and YFP-STIM1EF were cotransfected and imaged before and 6 minutes after addition of 1 μM thapsigargin.
(C) STIM1 can translocate in the presence of of prelocalized STIM2EF. CFP-STIM1 and YFP-STIM2EF were cotransfected and imaged before and 3 minutes after addition of 1 μM thapsigargin.
(D) Schematic representation of identified regulators of Ca2+-influx for basal versus receptor-triggered stimulation condition.
We further investigated the synergism and independent activation between STIM1 and STIM2 by imaging cells that had been co-transfected with wildtype CFP-STIM2 and constitutively active YFP-STIM1EF. Upon depletion of ER Ca2+ stores, STIM2 translocated to the existing STIM1EF puncta while the localization of STIM1EF did not change (Figure 6B). In the reciprocal experiment, transfecting CFP-STIM1 and YFP-STIM2EF showed similar results with STIM2EF being prelocalized to puncta and STIM1 translocating to the puncta upon store depletion.
These results support the conclusion that STIM1 and STIM2 can act synergistically if both are activated when Ca2+ stores are depleted by strong receptor stimuli and that STIM2 functions independently of STIM1 for basal ER Ca2+ levels or weak receptor stimuli (Figure 6D).
Discussion
STIM2 regulates basal cytosolic and ER Ca2+ levels
Our study shows that STIM2 functions as a feedback regulator that stabilizes basal cytosolic and ER Ca2+ concentrations. This conclusion is based on the finding that STIM2 knockdown reduced basal and ER Ca2+ levels (Figure 2A–C) while STIM2 over-expression increased basal Ca2+ levels (Figure 2D). In contrast, STIM1 had only minimal effects on basal ER and cytosolic Ca2+ concentration.
Despite these differences between STIM2 and STIM1, important steps in the activation of STIM2 were similar to that of STIM1. STIM2 translocates to ER-PM junctions upon ER Ca2+-store depletion (Figure 3A–E) and Orai1 knockdown sharply reduced the amplitude of the Ca2+ influx caused by STIM2 expression (Figure 3F). We also found that EF-hand mutants of STIM2 (deficient in Ca2+ binding) were no longer sensitive to ER Ca2+ concentration and were prelocalized to ER-PM junctions. This argues that STIM2, like STIM1, senses ER-Ca2+ levels, translocates to ER-PM junctions and regulates Orai1 PM Ca2+ channels.
We further found that while both STIM isoforms sense ER Ca2+ through their luminal EF-hand domain, the key difference is that STIM2 has a lower ER Ca2+ sensitivity than STIM1. Indeed, we found that STIM2 translocates to ER-PM junctions at a higher ER Ca2+ concentration compared to STIM1 (Figure 4A–C). This was supported by the finding that a STIM1 EF-hand switch of three amino acids from STIM1 to STIM2 created a STIM1 mutant with a lower Ca2+ sensitivity comparable to that of STIM2 (Figure S6C,D, S7). We also showed that STIM2 is partially active at basal ER Ca2+ levels and becomes more active once ER Ca2+ levels decline (Figure 4C). Markedly, this basal tuning point is near the linear range of STIM2 activation and can therefore provide cells with smooth homeostatic regulation rather than a switch like ON/OFF Ca2+ influx behavior. Finally, the surprisingly strong cooperativity in the Ca2+ dependence of STIM1 translocation provides for a potent mechanism that prevents STIM1 from being activated at basal ER Ca2+ concentrations. Together, these findings provide a molecular basis for the selective role of STIM2 in regulation basal Ca2+ concentration.
A STIM2-Orai1 control module tightly limits basal cytosolic and ER Ca2+ concentration
The long-term control of cytosolic Ca2+ levels is a function of the extracellular Ca2+ concentration and the balance between Ca2+ extrusion and Ca2+ influx at the plasma membrane. In turn, the long-term control of ER Ca2+ concentration is a function of cytosolic Ca2+ and the balance between Ca2+ pumping into the ER and Ca2+ flux out of the ER. If there would be no link between ER loading level and Ca2+ influx, deviations in basal Ca2+ concentration from normal caused by inadvertent cell stimuli or noise in gene expression would lead to harmful changes in ER Ca2+ concentration and cause ER stress. By using STIM2 to directly link basal ER Ca2+ levels to Ca2+ influx, cells can stabilize both cytosolic and ER Ca2+ concentration using a single feedback loop (Figure 7).
Figure 7. Model for STIM2 function in basal Ca2+ homoestasis.
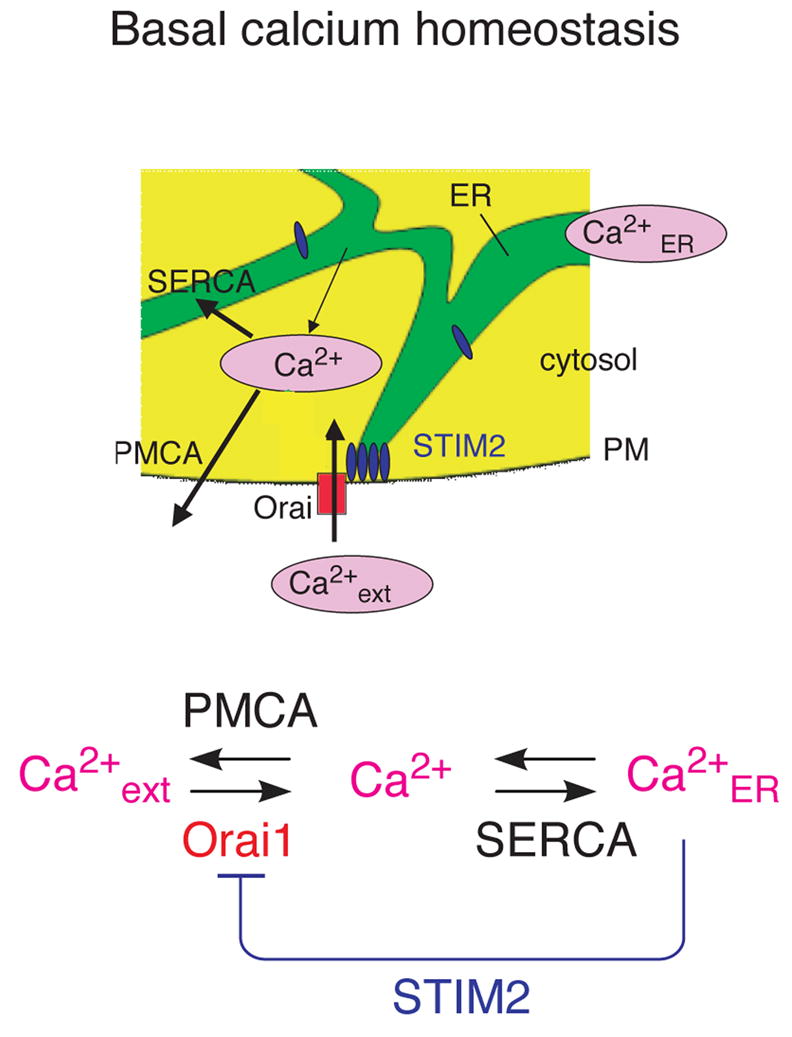
Schematic representations of the stabilization of basal cytosolic and ER Ca2+ concentrations by negative feedback.
Thus, STIM2-mediated regulation of basal Ca2+ is relevant for cell function in multiple ways. By having sufficient cytosolic and ER Ca2+, cells retain their ability to signal in response to receptor stimuli. By preventing excessive ER and cytosolic Ca2+ levels, cells reduce the likelihood for inadvertent activation of the signaling system. Indeed, as pointed out in the introduction, defective basal Ca2+ signaling has been linked to mitochondria dysfunction and ER-stress that can both be a secondary result of basal Ca2+ deregulation. In addition, different proteases and many transcription factors are Ca2+ sensitive, and changes in basal Ca2+ levels can directly induce changes in cell state. Together, this argues that STIM2-mediated feedback stabilization of basal ER and cytosolic Ca2+ levels serves as an important support mechanism for the long term health of cells.
Role of STIM2 in triggering persistent Ca2+ increases in response to submaximal receptor stimuli
We found that STIM2, like STIM1, can activate R-SOC type Ca2+ influx when Ca2+ stores are maximally depleted. The activation of Orai1 by STIM2 was independent of STIM1 (Figure 5A,B), suggesting that STIM2 can act alone or in conjunction with STIM1 to trigger R-SOC. Such a role of STIM2 in R-SOC is also consistent with our previous identification of STIM2 as a weak yet significant hit in a siRNA screen to identify regulators of store-depletion activated Ca2+ influx (Liou et al., 2005).
Since our study showed that STIM2 can be readily activated by submaximal reductions in ER Ca2+, it is likely that STIM2 has also a unique role in triggering persistent Ca2+ increases in response to submaximal receptor stimuli. It is therefore likely that STIM2 has two roles: First, to serve as a stabilizer of basal Ca2+ and, second, as a more sensitive regulator of R-SOC compared to STIM1 that can selectively open PM Ca2+ channels for submaximal receptor stimuli.
Evolution from a single STIM protein to a dual STIM1, STIM2 system
Given that Drosophila melanogaster has a single ubiquitously expressed STIM protein, we speculate that STIM proteins had an ancestral role reminiscent of STIM2 in regulating basal ER and cytosolic Ca2+ concentrations. This stabilization function may initially have been more important than its role in receptor-triggered Ca2+ signaling. Gene duplication in vertebrates may have enabled a dual use of STIM proteins, with a less sensitive STIM2 version that functions as a regulator of basal Ca2+ and a mediator of persistent Ca2+ increases for weak receptor stimuli. In contrast, the more sensitive STIM1 version became specialized to mediate persistent Ca2+ increases for strong receptor stimuli.
In summary, our study identified STIM2 in a siRNA screen of the human signaling proteome as the strongest positive regulator of basal cytosolic Ca2+ concentration. In contrast to STIM1, a recently identified signaling mediator that triggers Ca2+-influx in response to receptor-mediated Ca2+ release, STIM2 is active at basal ER Ca2+ concentrations and can be further activated by small reductions in ER Ca2+. Together, this defines a STIM2-Orai1 feedback module that keeps basal cytosolic as well as ER Ca2+ concentrations within a narrow range.
Materials and Methods
Cell Transfection, Plasmids, and Reagents
STIM1 and STIIM2 synthetic siRNA were purchased from Ambion and Dharmacon, respectively. Diced siRNA against GL3 luciferase was used as a transfection control. HeLa, HUVEC, and HEK293T cells were purchased from ATCC. DNA plasmids were transfected with Fugene 6 reagent (Roche). siRNA was transfected with Gene Silencer (Gene Therapy Systems) for HeLa, Lipofectamine 2000 (Invitrogen) for HEK293T, and Lipofectin (Invitrogen) for HUVEC. Full-length human STIM1 and STIM2 cDNA were isolated by PCR, sequenced, and cloned in to pDS_XB-YFP vector (ATCC). See Figure S9 for details on constructs. Briefly, the YFP-conjugated EF-hand mutants of STIM1, STIM1EF(D76A), and STIM2, STIM2 EF(D80A), STIM2EF->STIM1 (K83A, D84N, G86D), STIM1EF->STIM2 (A79K, N80D, D82G) were made by site-directed mutagenesis with the QuikChange Site-Directed Mutagenesis Kit (Stratagene). Thapsigargin (Invitrogen) and ionomycin (Sigma) and BHQ (EMD Bioscience) were used at 1 μM. Antibodies purchased from Prosci (STIM1, STIM2) and AbCam (GAPDH).
siRNA Library of Signaling Proteins
The synthesis of the Dicer generated siRNA library was described previously (Liou et al., 2005). In short, siRNAs were made targeting 2304 human signaling-related proteins selected from the NCBI (RefSeq database) on the basis of the presence of signaling domains, such as protein kinase, SH2, SAM, EF, and PH domains, as well as by text searches of signaling-related terms.
Ca2+ Measurements
HeLa cells were loaded with 1 μM Fura-2-AM in extracellular buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgCl2, 20 mM HEPES, 10 mM glucose, and 0.1, 1.5, 11.8 mM CaCl2 [pH 7.4]) for 30 min at room temperature. Fura-2 fluorescence was measured by exciting Fura-2 using alternating 340/380 nm illumination and measuring fluorescence intensity at 510 nm. Intracellular Ca2+ concentration was computed from the ratio of fluorescence intensity for excitation at 340 and 380 nm. For the RNAi screen, the values for each well of the 384 well plate have been subject to two normalization steps. First, regional background subtraction was performed between neighboring wells to correct for trends across the well plate. Second, the values are presented as fold standard deviations from the median of the screen. For all other figures, Fura-2 ratio was calibrated using:
Rmax was determined by measuring the maximal attainable fluorescence intensity in cells treated with ionomycin in high external Ca2+ (Rmax= 6.06). Rmin and Q values are dependent on autofluorescence, unspecific Fura-2 binding to cellular components as well as on Fura-2 sequestration inside cells. We adjusted them for HeLa cells so that the average basal Ca2+ level was 50 nM in calibration experiments (Rmin= 0.217 and Q = 9.71). The same Rmax, Rmin and Q values were used for all experiments shown. However, experiments that took a longer time to image required a final offset of −20 nM s due to presumed dye loading into stores.
For Ca2+ add-back experiments, 3 mM EGTA was added together with thapsigargin to remove extracellular Ca2+, and extracellular Ca2+ was changed to a final Ca2+ concentration of 0.75 mM 15 minutes later. Imaging-based single-cell Ca2+ measurements were performed with a 4x objective on an automated fluorescent microscope (ImageXpress 5000A, Molecular Devices). Fluorescence intensities of single cells were calculated using Matlab 7.1 software.
Extracellular Ca2+ manipulation
For the RNAi screen, low Ca2+ conditions were created by replacing normal media (Dulbecco’s Modified Eagle’s Medium (DMEM), 10% fetal bovine serum, penicillin, streptomycin, glutamate) with media made using DMEM without Ca2+ (Invitrogen). For other experiments, low Ca2+ media was created by adding 2 mM EGTA to normal media. High extracellular Ca2+ media was made by adding 10 mM Ca2+ to normal media.
Puncta Analysis
Puncta analysis for STIM1 and STIM2 translocation were performed by applying a bandpass filter and then taking the mean of the square of the pixels in the resulting image. Each time-series of values was then linearly transformed to range between 0 and 1.
FRET Imaging
CFP and FRET images were first background subtracted. The average FRET/CFP per pixel was computed for a mask created using the CFP channel. For Figure 2C, a 4x objective and EPI-fluorescence microscope was used to collect data from thousands of cells. For Figure S6, a 40x objective was used to most accurately measure change in FRET/CFP after EGTA for single cells.
Western Blot Conditions
Primary antibodies were used at 1:1000 dilution and 17.5 μg of protein samples/lane run on a 4–12% tris-glycine gel. Cells were lysed in modified RIPA buffer (specified below) and then spun down for 45 minutes to remove membranes. Samples were boiled for 5 minutes after addition of sample buffer.
Lysis buffer: 150 mM NaCl, 50 mM Tris-HCl (pH 7.4),1 mM EDT, 1% Triton X-100, 1% sodium deoxycholic acid, 0.1% SDS, 1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin.
Supplementary Material
Supplementary Figure S1
Single-well traces to indirectly assay ER Ca2+ concentration in Figure 2C. Addition of ionomycin and EGTA was used to rapidly release ER Ca2+ and the resulting cytosolic Ca2+ signal was measured using Fura-2. Cells were transfected with siRNA for STIM1, STIM2 or GL3 (control).
Supplemental Figure S2
A wildtype STIM2 construct (without YFP tag) also increases R-SOC Ca2+ influx. Cells were transfected with YFP or co-transfected with YFP and YFP-free STIM2 for 5 hours. Cells were selected expressing 7.5 to 15 times background. N=6 sites from 3 wells.
Supplementary Figure S3
Basal cytosolic Ca2+ measurements after 3 day transfection with siSTIM1-2 and, for comparison, siOrai1-3 and GL3 (control). N=10 sites.
Supplementary Figure S4
Time-course of STIM1 and STIM2 puncta formation upon thapsigargin addition. 1 μM thapsigargin was added to HeLa cells and imaged for 220 seconds. Images were then analyzed for puncta content as in described in Materials and Methods section. N=4 cells each.
Supplementary Figure S5
Calibration of the ER Ca2+ content at different time-points following external addition of EGTA. (A) 3 mM EGTA was added to wells at time = 0 min. Ionomycin was added to different wells at the indicated time points. The measured ΔCa2+ peak heights were fit to an exponential decay. (B) FRET measured using the D1ER cameleon probe. Average relative FRET signal for 6 cells imaged using a 40x confocal microscope. 1 μM ionomycin was added near the end of the timecourse.
Supplementary Figure S6
Ca2+ levels in cells expressing different concentrations of STIM1 and STIM2 constructs. Basal Ca2+ was measured for the reduced and normal ER conditions as described in the main text (reduced conditions are the low Ca2+ conditions from the siRNA screen). Both raw traces and traces normalized to constitutively active mutants are shown. EF hand switch mutant (STIM1EF->STIM2) is labeled with the subscript “3pt”.
Supplemental Figure S7
Basal Ca2+ levels in cells expressing a STIM1 construct with its EF hand mutated to be similar to STIM2 (STIM1EF->STIM2). Basal Ca2+ concentration is shown as a function of the expression level of YFP-STIM1EF->STIM2 and compared to that of YFP-STIM1, YFP-STIM2 and YFP control.
Supplementary Figure S8
Description of STIM constructs used in this study.
Supplementary Figure S9
Description of purchased siRNA constructs.
Acknowledgments
We thank the following people for contributions: Phil Vitorino, Mark Hammer and Dr. Marc Fivaz for helpful suggestions and careful reading of the manuscript. Drs. Rajat Rohatgi and Eric Humke for help with Western blots. Dr. Yigal Brandman for help with puncta analysis. Man Lyang Kim and Drs. Won Do Heo, Josh Jones, and Jason Myers for generating the siRNA library and JL. Jess Field for help with graphic design. This work was supported by a grant to TM from the Sandler Foundation and NIH grant GM030179. OB was funded by an NSF Predoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berridge MJ, Bootman MD, Roderick HL. Ca2+ signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Hayden MR. Deranged neuronal Ca2+ signaling and Huntington disease. Biochem Biophys Res Commun. 2004;322:1310–7. doi: 10.1016/j.bbrc.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Brini M, Pinton P, Pozzan T, Rizzuto R. Targeted recombinant aequorins: tools for monitoring [Ca2+] in the various compartments of a living cell. Microsc Res Tech. 1999;46:380–9. doi: 10.1002/(SICI)1097-0029(19990915)46:6<380::AID-JEMT6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Camello C, Lomax R, Petersen OH, Tepikin AV. Calcium leak from intracellular stores--the enigma of calcium signalling. Cell Calcium. 2002;32:355–61. doi: 10.1016/s0143416002001926. [DOI] [PubMed] [Google Scholar]
- Campanella M, Pinton P, Rizzuto R. Mitochondrial Ca2+ homeostasis in health and disease. Biol Res. 2004;37:653–60. doi: 10.4067/s0716-97602004000400022. [DOI] [PubMed] [Google Scholar]
- Dziadek MA, Johnstone LS. Biochemical properties and cellular localisation of STIM proteins. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.02.006. (epub Mar 21) [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in ORAI1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- Guerini D, Coletto L, Carafoli E. Exporting Ca2+ from cells. Cell Calcium. 2005;38:281–9. doi: 10.1016/j.ceca.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Kusters JM, Dernison MM, van Meerwijk WP, Ypey DL, Theuvenet AP, Gielen CC. Stabilizing role of calcium store-dependent plasma membrane calcium channels in action-potential firing and intracellular calcium oscillations. Biophys J. 2005;89:3741–56. doi: 10.1529/biophysj.105.062984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–6. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, KimMLHeo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–25. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, Bird GS, Putney JW., Jr Large store-operated Ca2+ selective currents due to co-expression of ORAI1 or ORAI2 with the intracellular Ca2+ sensor, STIM1. J Biol Chem. 2006;281:24979–90. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M, Barrero MJ, Torrecilla F, Lobaton CD, Moreno A, Alvarez J. Stimulation by thimerosal of histamine-induced Ca(2+) release in intact HeLa cells seen with aequorin targeted to the endoplasmic reticulum. Cell Calcium. 2001 Sep;30(3):181–90. doi: 10.1054/ceca.2001.0224. [DOI] [PubMed] [Google Scholar]
- Myers JW, Jones JT, Meyer T, Ferrell JE., Jr Recombinant Dicer efficiently converts large dsRNAs into siRNAs suitable for gene silencing. Nat Biotechnol. 2003;21:324–8. doi: 10.1038/nbt792. [DOI] [PubMed] [Google Scholar]
- Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17404–9. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet JP. Amplification of CRAC current by STIM1 and CRACM1 (ORAI1) Nat Cell Biol. 2006;8:771–3. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–42. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- Philipson KD, Nicoll DA, Ottolia M, Quednau BD, Reuter H, John S, Qiu Z. The Na+/Ca2+ exchange molecule: an overview. Ann N Y Acad Sci. 2002;976:1–10. doi: 10.1111/j.1749-6632.2002.tb04708.x. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–18. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- Raza M, Deshpande LS, Blair RE, Carter DS, Sombati S, Delorenzo RJ. Aging is associated with elevated intracellular Ca2+ levels and altered Ca2+ homeostatic mechanisms in hippocampal neurons. Neurosci Lett. 2007;418:77–81. doi: 10.1016/j.neulet.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman IH, Zachariah M, Raij L. Ca2+ channel blockers, endothelial dysfunction, and combination therapy. Clin Exp Res. 2005;17:40–5. [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Hewavitharana T, He LP, Xu W, Johnstone LS, Dziadek MA, Gill DL. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ Entry. Curr Biol. 2006a;16:1465–70. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. ORAI1 and STIM reconstitute store-operated Ca2+ channel function. J Biol Chem. 2006b;281:20661–5. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Spira ME, Oren R, Dormann A, Ilouz N, Lev S. Calcium, protease activation, and cytoskeleton remodeling underlie growth cone formation and neuronal regeneration. Cell Mol Neurobiol. 2001;21:591–604. doi: 10.1023/a:1015135617557. [DOI] [PubMed] [Google Scholar]
- Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–62. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- Strehler EE, Caride AJ, Filoteo AG, Xiong Y, Penniston JT, Enyedi A. Plasma membrane Ca2+ ATPases as dynamic regulators of cellular calcium handling. Ann N Y Acad Sci. 2007;1099:226–36. doi: 10.1196/annals.1387.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Keurs HE, Boyden PA. Ca2+ and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–70. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thebault S, Hoenderop JG, Bindels RJ. Epithelial Ca2+ and Mg2+ channels in kidney disease. Adv Chronic Kidney Dis. 2006;13:110–7. doi: 10.1053/j.ackd.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Treves S, Anderson AA, Ducreux S, Divet A, Bleunven C, Grasso C, Paesante S, Zorzato F. Ryanodine receptor 1 mutations, dysregulation of Ca2+ homeostasis and neuromuscular disorders. Neuromuscul Disord. 2005;15:577–87. doi: 10.1016/j.nmd.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–93. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–13. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of ORAI. Nature. 2006;443:226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Hinkle PM. Rapid turnover of calcium in the endoplasmic reticulum during signaling. Studies with cameleon calcium indicators. J Biol Chem. 2000;275:23648–53. doi: 10.1074/jbc.M002684200. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–9. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Single-well traces to indirectly assay ER Ca2+ concentration in Figure 2C. Addition of ionomycin and EGTA was used to rapidly release ER Ca2+ and the resulting cytosolic Ca2+ signal was measured using Fura-2. Cells were transfected with siRNA for STIM1, STIM2 or GL3 (control).
Supplemental Figure S2
A wildtype STIM2 construct (without YFP tag) also increases R-SOC Ca2+ influx. Cells were transfected with YFP or co-transfected with YFP and YFP-free STIM2 for 5 hours. Cells were selected expressing 7.5 to 15 times background. N=6 sites from 3 wells.
Supplementary Figure S3
Basal cytosolic Ca2+ measurements after 3 day transfection with siSTIM1-2 and, for comparison, siOrai1-3 and GL3 (control). N=10 sites.
Supplementary Figure S4
Time-course of STIM1 and STIM2 puncta formation upon thapsigargin addition. 1 μM thapsigargin was added to HeLa cells and imaged for 220 seconds. Images were then analyzed for puncta content as in described in Materials and Methods section. N=4 cells each.
Supplementary Figure S5
Calibration of the ER Ca2+ content at different time-points following external addition of EGTA. (A) 3 mM EGTA was added to wells at time = 0 min. Ionomycin was added to different wells at the indicated time points. The measured ΔCa2+ peak heights were fit to an exponential decay. (B) FRET measured using the D1ER cameleon probe. Average relative FRET signal for 6 cells imaged using a 40x confocal microscope. 1 μM ionomycin was added near the end of the timecourse.
Supplementary Figure S6
Ca2+ levels in cells expressing different concentrations of STIM1 and STIM2 constructs. Basal Ca2+ was measured for the reduced and normal ER conditions as described in the main text (reduced conditions are the low Ca2+ conditions from the siRNA screen). Both raw traces and traces normalized to constitutively active mutants are shown. EF hand switch mutant (STIM1EF->STIM2) is labeled with the subscript “3pt”.
Supplemental Figure S7
Basal Ca2+ levels in cells expressing a STIM1 construct with its EF hand mutated to be similar to STIM2 (STIM1EF->STIM2). Basal Ca2+ concentration is shown as a function of the expression level of YFP-STIM1EF->STIM2 and compared to that of YFP-STIM1, YFP-STIM2 and YFP control.
Supplementary Figure S8
Description of STIM constructs used in this study.
Supplementary Figure S9
Description of purchased siRNA constructs.