Abstract
The mechanisms underlying sudden cardiac death (SCD) are complex and diverse. Therefore, correct application of any marker to risk stratify patients for appropriate therapy requires knowledge regarding how the marker is reflective of a particular electro-anatomical substrate for arrhythmias. Non-invasive measurement of beat-to-beat alternation of the electrocardiographic T-wave, referred to as T-wave alternans (TWA), is an important marker of risk for sudden cardiac death (SCD). Is this relationship a mere association or is TWA mechanistically linked to SCD? Recent experimental evidence strongly supports a mechanistic relationship between TWA and SCD. This review will consider the underlying mechanisms of TWA derived from experimental studies, as they relate to clinical observations of TWA in humans, addressing the following questions derived from common clinical observations: 1) Where does TWA on the surface ECG come from? 2) Why is controlled heart rate elevation required to elicit TWA? 3) Why is TWA associated with risk for SCD? 4) Why is TWA associated with a broad range of ventricular arrhythmias? and 5) How do commonly used medications affect TWA?
Keywords: repolarization, electrophysiology, tachycardia, calcium
Sudden cardiac death (SCD) is the most devastating manifestation of heart disease, and most often occurs in patients with systolic heart failure (HF). Yet predicting which HF patients are at highest risk for SCD remains an important, and largely unresolved clinical challenge. There is compelling evidence to suggest that non-invasive detection of subtle (“microvolt-level”) beat-to-beat alternation of the T-wave on the surface electrocardiogram (ECG) is an important marker of risk for SCD27; 35. First described nearly 100 years ago, alternans of the ECG refers to a beat-to-beat variation in the amplitude and/or morphology of a component of the ECG, and has consistently been shown to be a marker of electrical instability in the heart13; 26. In particular, microvolt T-wave alternans (TWA) has recently been shown to be closely associated with risk for SCD in a wide range of clinical settings. Remarkably, the absence of TWA in patients with HF seems to indicate a resistance to SCD1. However, is the relationship between TWA and SCD a mere association or is TWA a mechanism for potentially fatal ventricular arrhythmias? The mechanisms underlying sudden cardiac death (SCD) are complex and diverse. Therefore, correct application of any marker to risk stratify patients for appropriate therapy requires knowledge regarding how the marker is reflective of a particular electro-anatomical substrate for arrhythmias.
Though caution must be taken when extrapolating results from experimental models to patients, recent experimental observations suggest that cardiac alternans is mechanistically linked to the pathogenesis of ventricular arrhythmias. In an effort to translate experimental observations to the bedside, this review will consider the underlying mechanisms of TWA derived from experimental studies, as they relate to clinical observations of TWA in humans. Specifically, the following questions derived from common clinical observations will be considered: 1) Where does TWA on the surface ECG come from? 2) Why is controlled heart rate elevation required to elicit TWA? 3) Why is TWA associated with risk for SCD? 4) Why is TWA associated with a broad range of ventricular arrhythmias? and 5) How do commonly used medications affect TWA?
Where does TWA on the surface ECG come from?
T-wave alternans is a beat-to-beat alternation of the amplitude and/or shape of the T-wave on the surface ECG. These fluctuations of the T-wave are primary and not related to alternans of other components of the ECG (i.e. QRS alternans). Also, TWA is a rate dependent phenomenon and once induced it is remarkably stable and persistent. Visually apparent TWA while relatively rare, have been described in a variety of clinical conditions such as; myocardial ischemia, long QT syndrome, and electrolyte imbalance48. In contrast, visually unapparent TWA (i.e. microvolt level fluctuations) is relatively common (i.e. detected in approximately 50% of patients with LVEF < 0.40), and is readily detectable with digital signal processing of the ECG and is a predictor of risk for SCD. Therefore, it is of utmost importance to explain the electrophysiological basis of TWA. Specifically, when TWA is manifest on the body surface ECG, what does it correspond to in terms of electrophysiological activity on the heart itself?
Originally, it was proposed that the electrophysiological basis for TWA is alternating conduction pathways on a beat by beat basis arising from spatial dispersion of refractoriness. This was based on the observations of Smith and Cohen40 using a simple finite-element model of ventricular conduction, which suggested that alternans occurs when the refractory period of subpopulations of cardiac myocytes exceeds the cycle length of pacing. Under these conditions, subpopulations of myocytes are depolarized on every other beat. However, this alternating conduction hypothesis predicts that TWA would be associated with equal or greater QRS alternans, which is inconsistent with clinical observations35 demonstrating that the magnitude of microvolt alternans tends to peak near the peak of the T wave, but is usually absent during the QRS complex.
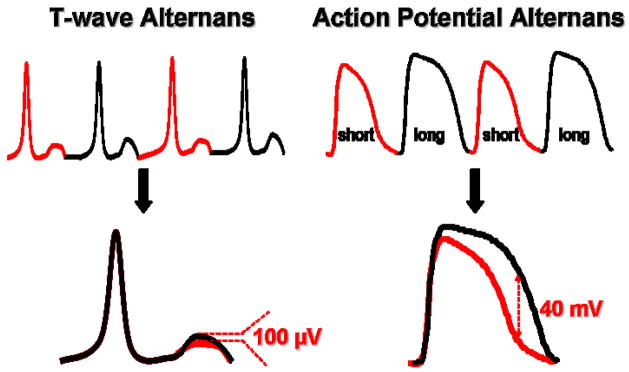
A compelling body of evidence now suggests that TWA emanates from beat-to-beat alternation of membrane repolarization (i.e. action potential duration) at the level of the cardiac myocyte. During the 1950’s, multiple investigations demonstrated alternans of the action potential in single myocardial cells, suggesting that alternans on the surface ECG reflects events occurring at the level of the single cell15; 22. This relationship was clearly established by Pastore et al. in 199928 using high-resolution optical mapping in the guinea pig model of pacing-induced TWA. Specifically, when heart rate increased, TWA developed on the surface ECG which corresponded to beat-to-beat alternation in the time course of cellular repolarization of the cardiac action potential duration (APD). Typically, beat to beat alteration in phase 2 and, to a greater extent, phase 3 of the action potential is readily observed when TWA is induced by progressive elevations in heart rate. Importantly, the magnitude of cellular alternans was several orders of magnitude larger than the corresponding magnitude of TWA on the surface ECG (Figure 1). This explained how it was possible that very subtle and visually undetectable TWA on the surface ECG of patients can be physiologically and clinically relevant. They also illustrated the important principle that TWA arises from dysregulation of repolarization at the level of individual myocytes.
Figure 1.
Cellular basis for T-wave alternans. Upper Panel: The onset of T-wave alternans on the ECG arises from beat-to-beat alternation of the action potential at the level of the single cardiac myocyte. Lower Panel: The magnitude of action potential alternans (millivolt) is several orders of magnitude larger than T-wave alternans (i.e. microvolt) on the ECG.
Understanding why repolarization alternans from single myocardial cells is exhibited as beat-to-beat fluctuation of the T-wave requires an understanding of the basic principles underlying the T-wave on the surface ECG. It is well established that the shape and polarity of the T-wave is a direct consequence of spatial gradients of repolarization across the heart 4. This suggests that for cellular alternans to be manifest on the surface ECG there must be spatial heterogeneity of repolarization alternans between myocytes throughout the heart. Recent experimental studies have demonstrated spatial heterogeneity of repolarization alternans between myocytes located in different ventricular locations 25; 50. For example, endocardial myocytes are more prone to repolarization alternans compared to epicardial myocytes such that, at a given pacing/heart rate, the magnitude of repolarization alternans is greater in endocardial compared to epicardial myocytes. These heterogeneities of repolarization alternans generate the spatial gradients of repolarization necessary for cellular alternans to manifest on the ECG as beat-to-beat fluctuations of the T-wave.
Why is controlled heart rate elevation required to elicit T wave alternans?
The most widely applied method for non-invasive measurement of TWA uses the fast Fourier Transform spectral method during graded exercise 16. Graded exercise is an essential component of non-invasive TWA testing because it produces a controlled elevation of heart rate (HR). The need for controlled HR elevation is based on the observation that TWA is a rate-dependent property of the heart, such that, above a specific threshold TWA increases monotonically, independent of autonomic state20. Importantly, patients at highest risk for SCD exhibit a leftward shift in the alternans-HR relation compared to patients at low risk for SCD20. Herein lies the need for graded elevation of HR. Too excessive and too rapid an increase would cause an overshoot of HR into ranges that could induce TWA in normal (low risk) individuals. Conversely, since TWA is rarely detectable at rest, it cannot be detected without some elevation of heart rate. A challenge for the individual performing the test is to elevate HR sufficiently to induce alternans (typically 95 – 110 bpm), to maintain an increased HR for a suitable period of time to make a reliable measurement of TWA (typically 1–3 minutes), but avoiding excessive (> 110 bpm) heart rates.
So why is TWA so dependent on HR? The HR dependence of TWA can be best understood by considering the cellular and molecular mechanisms underlying the development of repolarization alternans at the level of the single cardiac myocyte. There are two major hypotheses that have been proposed to explain the development of repolarization alternans, both of which can explain the HR dependence of TWA: 1) the APD restitution hypothesis and 2) the calcium cycling hypothesis.
Action potential restitution is the normal reduction of APD that occurs following increases in heart rate. This phenomenon is thought to be an adaptive mechanism to preserve diastolic filling time at faster heart rates. More specifically, restitution is a direct relationship between the APD of one beat and the diastolic interval (DI) of the preceding beat. Restitution can explain the self-perpetuating nature of APD alternans because according to restitution, a short DI is followed by APD shortening, which, in turn, will necessarily extend DI preceding the subsequent beat, and lengthen APD, which then leads to DI shortening in a repeated fashion. Theoretical models suggest that repolarization alternans occurs when the slope of the dynamic APD restitution curve (APD vs. DI) exceeds unity 7; 33; 51. In fact, according to simulation studies, the precise heart rate required to elicit TWA can be predicted directly from the restitution relationship. Although the restitution hypothesis provides a very useful theoretical framework for understanding the dynamics of TWA, experimental evidence supporting the restitution hypothesis is somewhat circumstantial. For example, flattening of the APD restitution curve by pharmacological ion channel blockade will decrease repolarization alternans8. However, there is also considerable experimental evidence that does not support the restitution hypothesis, suggesting alternative mechanisms. 32;9. Additionally, multiple clinical observations do not support the restitution hypothesis. For example, myocardial ischemia is well known to provoke TWA but markedly flattens the APD restitution relationship.43 Also, patients exhibiting greater susceptibility to TWA do not necessarily exhibit a steeper relationship between repolarization and heart rate2.
In contrast to restitution, there is considerable evidence for a primary role of alternating intracellular calcium cycling as a mechanism for cardiac alternans. The first clinical correlations between cardiac alternans and increased mortality were made in 1872 by Traube 47 from so called “pulsus alternans” of the arterial blood pressure; i.e. a clinical manifestation of alternating calcium flux through in sarcoplasmic reticulum release with each heart beat. Moreover, myocytes that are most susceptible to alternans do not exhibit the steepest restitution slopes, but rather have the slowest time course of cytosolic calcium reuptake32.
In support of the calcium cycling hypothesis, pharmacological blockade of the RyR or depleting SR calcium stores with caffeine suppresses repolarization alternans14; 36; 37. In contrast, delayed decay of the Ca2+ transient (i.e. impaired SR Ca2+ reuptake) has been shown to increase susceptibility to repolarization alternans25; 50. Finally, seminal observations by Chudin et al.3 showed that in isolated cardiac myocytes, Ca2+ alternans can develop under both current- (repolarization alternans present) and voltage-clamp (repolarization alternans absent) conditions. This proved that Ca2+ alternans is not dependent on repolarization alternans and supports the hypothesis that repolarization alternans arises from changes in SR Ca2+ cycling.
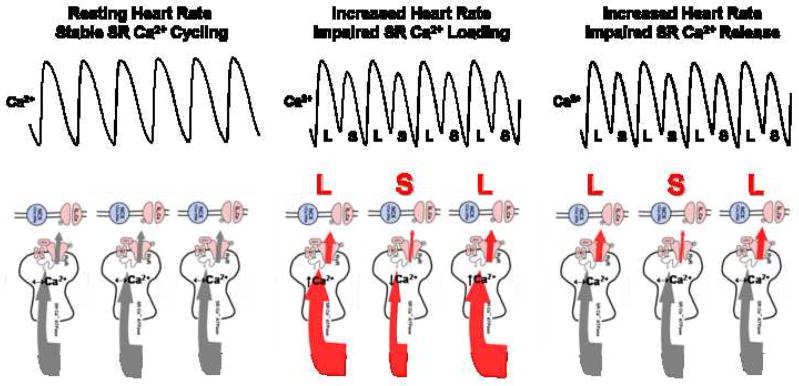
What are the mechanisms underlying the development of Ca2+ alternans? The calcium cycling hypothesis states that alternans occurs when the heart rate exceeds capabilities of the myocyte to cycle calcium on a beat by beat basis. Anything that impairs cellular calcium cycling will permit alternans to be initiated at slower heart rates. Hence, under this hypothesis, alternans is very much a rate-dependent process as well. Under normal conditions, Ca2+ is released from the SR through the large macromolecule ryanodine receptor (RyR) complex and activates myocardial contraction. Relaxation occurs primarily with reuptake of Ca2+ into the SR by sarcoplasmic reticulum Ca2+-ATPase (SERCA2a). Also, the sodium calcium exchanger (NCX) is involved in diastolic Ca2+ removal from cytosol. The calcium cycling hypothesis predicts that during steady-state (i.e. resting heart rate) the amount of Ca2+ released from the SR must equal SR reuptake, primarily by SERCA2a. Under these steady-state conditions, Ca2+ alternans will not develop (Figure 2: Panel 1). However, any sustained disturbances in the myocytes ability to load SR Ca2+ (i.e. impaired SR Ca2+ reuptake) or release SR Ca2+ can lead to the development of Ca2+ alternans (Figure 2: Panels 2 and 3). For example, Diaz et al.6 showed that beat to beat fluctuation in SR Ca2+ load is sufficient to produce alternans because of a steep relation between SR Ca load and SR Ca2+ release. In contrast, Picht et al.30 demonstrated that alternans can occur in the absence of fluctuations in SR Ca2+ load, supporting a primary role for alternating SR Ca2+ release dynamics as a mechanism for alternans.
Figure 2.
Calcium cycling hypothesis. Panel A: During steady-state SR Ca2+ cycling at resting heart rates Ca2+ transient alternans will not develop because SR Ca2+ release equals SR Ca2+ reuptake. Panel B: Impaired SR Ca2+ loading, leading to beat-to-beat fluctuation in SR Ca2+ content will produce Ca2+ transient alternans (i.e. large SR Ca2+ release followed by a small SR Ca2+ release). For example, with increasing HR the capacity of SERCA2a to pump Ca2+ into the SR becomes overwhelmed, creating a state in which subpopulations of SERCA2a only respond on alternating beats, leading to Ca2+ alternans. Panel C: Impaired SR calcium release in the absence of beat-to-beat fluctuation in SR Ca2+ content can also produce Ca2+ transient alternans. For example, as heart rate increases the RyR is not able to fully recover from inactivation such that, subpopulations of the RyR can at best release on alternating beats and lead to alternating large and small Ca2+ transients. SR = sarcoplasmic reticulum. RyR = ryanodine receptor. SERCA2a = sarcoplasmic reticulum Ca2+-ATPase. L = large calcium transient. S = small calcium transient.
Recent data have provided important insights into likely molecular targets responsible for the development of cellular alternans. Specifically, cardiac myocytes that are prone to cellular alternans exhibit a markedly different molecular profile for several Ca2+ handling proteins (i.e. relatively decreased SERCA2a and RyR expression) when compared to myocytes that are resistant to cellular alternans50. This suggests that the function of key calcium cycling proteins (specifically, SERCA2a and/or RyR) may account for the development of Ca2+ alternans and therefore, repolarization alternans. For example, it is proposed that with increasing HR, the capacity of SERCA2a to pump Ca2+ into the SR becomes overwhelmed, creating a state in which subpopulations of SERCA2a only respond on alternating beats, leading to Ca2+ alternans. This is supported by recent observations that overexpression of SERCA2a suppresses alternans in isolated myocytes under action potential clamp conditions 53. The calcium cycling hypothesis is further supported by observations from both experimental and theoretical models demonstrating a steep dependence of SR Ca2+ release on SR Ca2+ load as a mechanism for the development of Ca2+ alternans52.
Alternatively, instability of calcium release dynamics is also proposed to be an important mechanism in the development of Ca2+ alternans18. Recently, Picht et al.30 reported that beat-to-beat variations in recovery from inactivation of the RyR without variation in SR Ca2+ load could produce Ca2+ alternans. As such, it could be postulated that with increased pacing/heart rate the capability of the RyR to recover from inactivation is exceeded such that subpopulations of RyRs only recover from inactivation on alternating beats.
These observations highlight why non-invasive assessment of TWA requires graded HR elevation. The calcium cycling hypothesis can also explain why, once induced, TWA tends to persist even when HR is lowered to levels lower than those required to induce it 49.
HR elevation is not always required for the development of TWA. For example, in Long QT syndrome TWA mediated ventricular arrhythmias are associated with slow HRs38. The mechanism underlying this observation remains unclear, yet is likely related to a primary alteration of sarcolemmal ionic currents that are most evident at slow HRs.
Why is TWA associated with Sudden Cardiac Death?
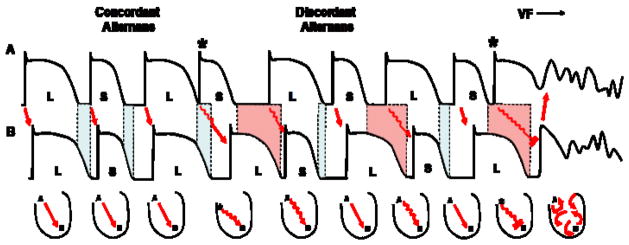
Numerous clinical investigations have shown that TWA is a marker of risk for SCD, although there have been some conflicting reports as well1; 5; 17; 27; 35. Yet, is there a mechanistic explanation for how beat-to-beat variation of the amplitude of the T-wave can lead to ventricular arrhythmias? Recently, spatially discordant repolarization alternans (i.e. repolarization alternans occurring with opposite phase between neighboring cells) was shown to be an important mechanism linking alternans to the genesis of ventricular arrhythmias (Figure 3)28. Initially, when repolarization alternans develops, all myocytes alternate in the same phase (i.e. long-short-long), referred to as concordant alternans. Concordant alternans alone is not particularly arrhythmogenic; however, it is usually requisite for the development of spatially discordant alternans. Specifically, as pacing/heart rate increases above a critical threshold or after a premature beat, a shift in the pattern of alternans can occur such that some cells begin to alternate in opposite phase (i.e. long-short vs. short-long), producing spatially discordant alternans33; 51
Figure 3.
Mechanism linking cardiac alternans to arrhythmogenesis. During concordant repolarization alternans, all myocytes alternate in the same phase (i.e. long-short vs. long-short). The occurrence of a premature beat (*) causes discordant alternans to develop in which some myocardial cells start alternating in opposite phase compared to neighboring cells (i.e. long-short vs. short long). Discordant alternans markedly increases dispersion of repolarization across the heart (shaded regions) such that a premature beat (*) during discordant alternans is sufficient to produce conduction block leading to ventricular fibrillation.
The onset of discordant alternans significantly alters the spatial organization of repolarization across the ventricle by markedly amplifying pre-existing heterogeneities of repolarization in the heart, producing a substrate prone to conduction block and reentrant excitation. For example, when an impulse (i.e. increasing HR or premature beat) propagates into refractory myocardium (secondary to enhanced dispersion of repolarization during discordant alternans) conduction block occurs, initiating reentrant excitation (Figure 3). In fact, in experimental models of TWA, rapid pacing-induced ventricular fibrillation (VF) was always preceded by discordant alternans.
Several mechanisms have been identified for causing the shift from concordant to discordant alternans: 1) conduction velocity restitution31; 33; 51, 2) intercellular uncoupling29 and 3) spatial heterogeneities of calcium cycling and sarcolemmal repolarization currents52.
In summary, these observations clearly demonstrate a mechanism by which TWA can increase susceptibility to SCD. Therefore, TWA may not just be associated with SCD, but may in fact reflect a specific electrophysiological substrate responsible for amplifying spatial heterogeneities of repolarization to produce conditions that favor conduction block and reentrant excitation.
Why is TWA associated with many types of ventricular arrhythmias?
Interestingly, TWA has been associated with a broad range of clinical conditions and ventricular arrhythmias. TWA has been demonstrated in both ischemic and non-ischemic cardiomyopathies over a broad range of systolic function and in patients with structurally normal hearts (i.e. Long-QT Syndrome)48. Abnormal calcium handling is a consistent finding in both diastolic (preserved LVEF) and systolic (reduced LVEF) HF and likely has a marked effect on susceptibility of the heart to TWA. Importantly, this effect is not necessarily dependent on LVEF and may explain why TWA is a marker of SCD beyond that of LVEF.
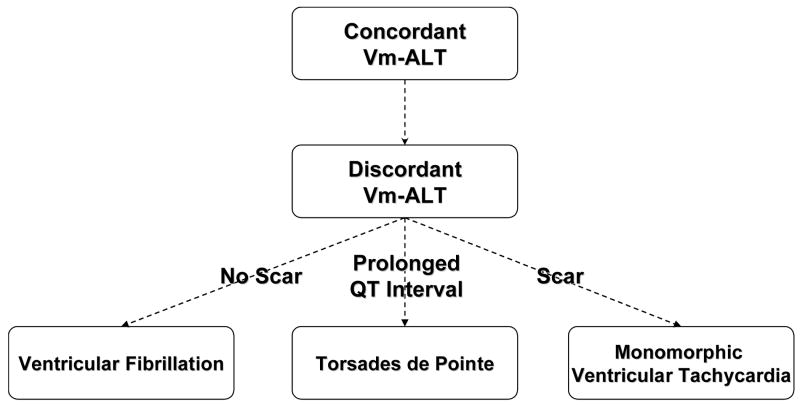
Both experimental and clinical studies have demonstrated an association between TWA and ventricular fibrillation, polymorphic ventricular tachycardia (i.e. Torsades de Pointe) and monomorphic ventricular tachycardia (Figure 4). All of these conditions share the common feature that TWA in them is closely associated with electrical instability. These observations suggest an intriguing hypothesis that TWA may be a common precursor to a broad range of arrhythmic conditions.
Figure 4.
Schematic of discordant alternans producing different types of ventricular arrhythmias depending on underlying structural (i.e. scar) and functional (i.e. long QT interval) characteristics. For example, when discordant alternans occurs in a structurally normal heart (i.e. no scar) ventricular fibrillations is the most common occurring ventricular arrhythmia. In contrast, discordant alternans in a structurally abnormal heart (i.e. scar) or in a heart with a prolonged QT interval on the ECG produces either monomorphic ventricular tachycardia or Torsades de Pointe, respectively.
As discussed earlier, experimental studies have clearly demonstrated that discordant alternans creates a myocardial substrate that is vulnerable to the development of conduction of block and reentrant VF28. However, does discordant repolarization alternans only create a substrate for VF or can it result in the development of polymorphic (Torsades de Pointe) and/or sustained monomorphic ventricular tachycardia? The classic experiment of Schwartz et al.38 demonstrated a relationship between TWA and Torsades de Pointe (polymorphic VT) in a model of Long-QT Syndrome. Also, Shimizu and Antzelevitch39 provided experimental evidence that the combination of prolongation of the QT interval and repolarization alternans can initiate polymorphic VT.
Recently, discordant repolarization alternans was implicated as a mechanism for the initiation of monomorphic ventricular tachycardia (VT). In particular, Pastore et al.29 demonstrated that creation of a structural barrier (epicardial laser lesion) in the heart increased susceptibility to the development of discordant repolarization alternans. Interestingly, following the development of discordant repolarization alternans, the most common arrhythmia seen in hearts with a structural barrier is sustained monomorphic VT. In contrast, discordant repolarization alternans in the absence of a structural barrier consistently causes VF. These observations suggest that when discordant repolarization alternans develops in the presence of a structural barrier (i.e. myocardial scar) this barrier may serve as an anchor to stabilize a reentrant rotor, producing monomorphic VT. In the absence of a structural barrier, repolarization alternans leads to ventricular fibrillation. Overall, these observations have important clinical relevance as they demonstrate that TWA and, more specifically, spatially discordant repolarization alternans produces a substrate that can lead to a variety of ventricular arrhythmias. Why one type of ventricular arrhythmia develops over another following discordant repolarization alternans seems to depend on the underlying structural and electrophysiological heterogeneities or lack thereof, inherent to a particular disease state.
How do commonly used medications affect TWA?
The landmark Cardiac Arrhythmia Suppression Trial clearly demonstrated the potential of antiarrhythmic drugs, in particular Class I agents, to increase mortality likely secondary to a proarrhythmic effect on cardiac repolarization45. As TWA is a marker of repolarization, how do these commonly used medications affect TWA and do these drug-induced changes in TWA make an individual patient more or less susceptible to SCD?
Antiarrhythmic drugs are the most extensively studied class of drug that can affect susceptibility to TWA. In particular Class I (sodium channel blockers) antiarrhythmic drugs have a variable affect on TWA. In particular, Procainamide decreases the magnitude of atrial pacing-induced TWA and flecainide increases susceptibility to TWA.21; 41 Interestingly, Tada et al.42 recently demonstrated that administration of the sodium channel blocker pilsicainide enhances susceptibility to TWA in a subset of patients with Brugada Syndrome. Importantly, in those patients where pilsicainide induced TWA, risk of spontaneous VF was markedly increased. This observation is clinically important because standard exercise-induced TWA is not a robust marker of SCD in Brugada Syndrome because exercise can attenuate the Brugada phenotype.
Sympathetic blockade with β-blockers decreases the magnitude of alternans23; 34. In particular, it was recently shown that the effect of β-blockers on TWA is greatest in patients with a history of VT when compared to patients without a prior history of VT24. Whether the decrease in susceptibility to TWA following β-blocker treatment can in part explain the mortality benefit that has been well described for β-blockers needs further investigation.
In general, both Class III (potassium channel blockers) and Class IV (calcium channel blockers) antiarrhythmic drugs have also been shown to decrease susceptibility to TWA11; 12; 23. For example, Groh, et al. 10 demonstrated that treatment with amiodarone in patients with either ischemic or non-ischemic cardiomyopathies decreased susceptibility to TWA. Moreover, treatment with d-sotalol or verapamil has also been shown to suppress TWA. However, there are published case reports documenting macroscopic TWA following administration of either sotalol or amiodarone19; 44; 46. Whether this was an indication of toxic levels of either drug needs further investigation.
In conclusion, commonly used cardiac medications have been shown to alter susceptibility to TWA. Whether a drug increases or decreases susceptibility to TWA appears to be drug class specific and in part dependent on the patient population treated. Whether these changes alter clinical outcomes (i.e. SCD risk) requires further investigation.
Unresolved Questions
Though much is known about the underlying mechanisms of TWA, several questions remain to be investigated regarding TWA as both a mechanism and marker of risk for SCD. For example, does TWA on the surface ECG represent concordant or discordant cellular alternans on the heart? Since it is discordant alternans that produces the substrate for conduction block and reentrant excitation, detecting differences in how concordant and discordant cellular alternans are exhibited on the surface ECG becomes important and could improve the positive predictive capability of non-invasive TWA testing. Also, if TWA is a rate dependent phenomenon and marker of SCD, why does SCD generally occur at ‘normal’ heart rates? Can hysteresis of the alternans explain this observation or are other factors involved, such as shifts in the alternans-HR relationship in various disease states (i.e. HF) leading to TWA at ‘normal’ heart rates? Future research that answers these questions could transform our ability to predict risk for SCD. Finally, the substrates underlying SCD in patients is no doubt complex and varied, whereas cardiac alternans represents just one component of this multifaceted disease substrate. Considerably more study is required to determine the specific role of cardiac alternans as a mechanism for triggering SCD in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloomfield DM, Steinman RC, Namerow PB, et al. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110:1885–9. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 2.Chow T, Gursoy S, Onufer JR, et al. The MASTER Trial Investigators: Clinical value of repeating indeterminate microvolt T-wave alternans tests. J Am Coll Cardiol. 2005;45:93A. (Abstract) [Google Scholar]
- 3.Chudin E, Goldhaber JI, Weiss J, et al. Intracellular Ca2 dynamics and the stability of ventricular tachycardia. Biophysical Journal. 77:2930–2941. 99. doi: 10.1016/S0006-3495(99)77126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen I, Giles W, Noble D. Cellular basis for the T-wave of the electrocardiogram. Nature. 1976;262:657–661. doi: 10.1038/262657a0. [DOI] [PubMed] [Google Scholar]
- 5.Costantini O, Hohnloser SH, Kirk MM, et al. The Alternans Before Cardioverter Defibrillator (ABCD) Trial: Strategies Using T-Wave Alternans to Improve Efficiency of Sudden Cardiac Death Prevention. Journal of the American College of Cardiology. 2008 doi: 10.1016/j.jacc.2008.08.077. In Press. [DOI] [PubMed] [Google Scholar]
- 6.Diaz ME, O’Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- 7.Fox JJ, McHarg JL, Gilmour RF., Jr Ionic mechanism of electrical alternans. Am J Physiol Heart Circ Physiol. 2002;282:H516–H530. doi: 10.1152/ajpheart.00612.2001. [DOI] [PubMed] [Google Scholar]
- 8.Garfinkel A, Kim YH, Voroshilovsky O, et al. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci USA. 2000;97:6061–6066. doi: 10.1073/pnas.090492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldhaber JI, Xie LH, Duong T, et al. Action potential duration restitution and alternans in rabbit ventricular myocytes: the key role of intracellular calcium cycling. Circ Res. 2005;96:459–66. doi: 10.1161/01.RES.0000156891.66893.83. [DOI] [PubMed] [Google Scholar]
- 10.Groh WJ, Shinn TS, Engelstein EE, et al. Amiodarone reduces the prevalence of T wave alternans in a population with ventricular tachyarrhythmias. J Cardiovasc Electrophysiol. 1999:1335–1339. doi: 10.1111/j.1540-8167.1999.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto H, Suzuki K, Miyake S, et al. Effects of calcium antagonists on the electrical alternans of the ST segment and on associated mechanical alternans during acute coronary occlusion in dogs. Circulation. 1983;68:667–672. doi: 10.1161/01.cir.68.3.667. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa T, Nagamoto Y, Ninomiya K, et al. Effects of heart rate and diltiazem hydrocholoride on alternans of ST segment elevation and ventricular arrhythmia during acute myocardial ischaemia in dogs. Cardiovasc Res. 1989;23:520–528. doi: 10.1093/cvr/23.6.520. [DOI] [PubMed] [Google Scholar]
- 13.Hering HE. Experimentelle studien an Saugertherien uber das elektrocardiogramm. II. Mittheilung. Z Exp Pathol Ther. 1910;7:363–378. [Google Scholar]
- 14.Hirata Y, Kodama I, Iwamura N, et al. Effects of verapamil on canine Purkinje fibers and ventricular muscle fibers with particular reference to the alternation of action potential duration after a sudden increase in driving rate. Cardiovasc Res. 1979;13:1–8. doi: 10.1093/cvr/13.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman BF, Suckling EE. Effect of heart rate on cardiac membrane potentials and unipolar electrogram. Am J Physiol. 1954;179:123–130. doi: 10.1152/ajplegacy.1954.179.1.123. [DOI] [PubMed] [Google Scholar]
- 16.Hohnloser SH, Klingenheben T, Bloomfield D, et al. J. Usefulness of microvolt T-wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: Results from a prospective observational study. J Am Coll Cardiol. 2003;41:2220–2224. doi: 10.1016/s0735-1097(03)00467-4. [DOI] [PubMed] [Google Scholar]
- 17.Hohnloser SH, Klingenheben T, Li YG, et al. T wave alternans as a predictor of recurrent ventricular tachyarrhythmias in ICD recipients: Prospective comparison with conventional risk markers. J Cardiovasc Electrophysiol. 1998;9:1258–1268. doi: 10.1111/j.1540-8167.1998.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 18.Hüser J, Wang YG, Sheehan KA, et al. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol(Lond) 2000;524:795–806. doi: 10.1111/j.1469-7793.2000.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaszala K, Kenigsberg DN, Krishnan SC. Drug-induced T wave alternans. J Cardiovasc Electrophysiol. 2006;17:332. doi: 10.1111/j.1540-8167.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman ES, Mackall JA, Julka B, et al. Influence of heart rate and sympathetic stimulation on arrhythmogenic T wave alternans. Am J Physiol Heart Circ Physiol. 2000;279:H1248–H1255. doi: 10.1152/ajpheart.2000.279.3.H1248. [DOI] [PubMed] [Google Scholar]
- 21.Kavesh NG, Shorofsky SR, Sarang SE, et al. The effect of procainamide on T wave alternans. J Cardiovasc Electrophysiol. 1999;10:649–654. doi: 10.1111/j.1540-8167.1999.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 22.Kleinfeld M, Stein E, Magin J. Electrical alternans in single ventricular fibers of the frog heart. Am J Physiol. 1956;187:139–142. doi: 10.1152/ajplegacy.1956.187.1.139. [DOI] [PubMed] [Google Scholar]
- 23.Klingenheben T, Grönefeld G, Li YG, et al. Effect of metoprolol and d, l-sotalol on microvolt-level T-wave alternans - Results of a prospective, double-blind, randomized study. J Am Coll Cardiol. 2001;38:2013–2019. doi: 10.1016/s0735-1097(01)01661-8. [DOI] [PubMed] [Google Scholar]
- 24.Komiya N, Seto S, Nakao K, Yano K. The influence of beta-adrenergic agonists and antagonists on T-wave alternans in patients with and without ventricular tachyarrhythmia. Pacing Clin Electrophysiol. 2005;28:680–4. doi: 10.1111/j.1540-8159.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 25.Laurita KR, Katra R, Wible B, et al. Transmural heterogeneity of calcium handling in canine. Circ Res. 2003;92:668–75. doi: 10.1161/01.RES.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- 26.Lewis T. Notes upon alternation of the heart. Quart J Med. 1910;4:141–144. [Google Scholar]
- 27.Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–81. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 28.Pastore JM, Girouard SD, Laurita KR, et al. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 29.Pastore JM, Rosenbaum DS. Role of structural barriers in the mechanism of alternans-induced reentry. Circ Res. 2000;87:1157–63. doi: 10.1161/01.res.87.12.1157. [DOI] [PubMed] [Google Scholar]
- 30.Picht E, DeSantiago J, Blatter LA, et al. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–8. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- 31.Poelzing S, Akar FG, Baron E, et al. Heterogeneous connexin43 expression produces electrophysiological heterogeneities across ventricular wall. Am J Physiol Heart Circ Physiol. 2004;286:H2001–9. doi: 10.1152/ajpheart.00987.2003. [DOI] [PubMed] [Google Scholar]
- 32.Pruvot EJ, Katra RP, Rosenbaum DS, et al. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–90. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 33.Qu Z, Garfinkel A, Chen P, et al. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102:1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 34.Rashba EJ, Cooklin M, MacMurdy K, et al. Effects of selective autonomic blockade on T-wave alternans in humans. Circulation. 2002;105:837–842. doi: 10.1161/hc0702.104127. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum DS, Jackson LE, Smith JM, et al. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–241. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 36.Saitoh H, Bailey J, Surawicz B. Alternans of action potential duration after abrupt shortening of cycle length: Differences between dog Purkinje and ventricular muscle fibers. Circ Res. 1988;62:1027–1040. doi: 10.1161/01.res.62.5.1027. [DOI] [PubMed] [Google Scholar]
- 37.Saitoh H, Bailey J, Surawicz B. Action potential duration alternans in dog Purkinje and ventricular muscle fibers. Circulation. 1989;80:1421–1431. doi: 10.1161/01.cir.80.5.1421. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz PJ, Malliani A. Electrical alternation of the T-wave: clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long Q-T syndrome. Am Heart J. 1975;89:45–50. doi: 10.1016/0002-8703(75)90008-3. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu W, Antzelevitch C. Cellular and ionic basis for T-wave alternans under long-QT conditions. Circulation. 1999;99:1499–1507. doi: 10.1161/01.cir.99.11.1499. [DOI] [PubMed] [Google Scholar]
- 40.Smith JM, Cohen RJ. Simple finite-element model accounts for wide range of ventricular dysrhythmias. Proc Nat Acad Sci USA. 1984;81:233–237. doi: 10.1073/pnas.81.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tachibana H, Yamaki M, Kubota I, et al. Intracoronary flecainide induces ST alternans and reentrant arrhythmia on intact canine heart - A role of 4-aminopyridine-sensitive current. Circulation. 1999;99:1637–1643. doi: 10.1161/01.cir.99.12.1637. [DOI] [PubMed] [Google Scholar]
- 42.Tada T, Kusano KF, Nagase S, et al. Clinical significance of macroscopic T-wave alternans after sodium channel blocker administration in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2008;19:56–61. doi: 10.1111/j.1540-8167.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 43.Taggart P, Sutton PMI, Boyett MR, et al. Human ventricular action potential duration during short and long cycles - Rapid modulation by ischemia. Circulation. 1996;94:2526–2534. doi: 10.1161/01.cir.94.10.2526. [DOI] [PubMed] [Google Scholar]
- 44.Tan HL, Wilde AAM. T wave alternans after sotalol: evidence for increased sensitivity to sotalol after conversion from atrial fibrillation to sinus rhythm. Heart. 1998;80:303–306. doi: 10.1136/hrt.80.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The cardiac arrhythmia suppression trial (CAST) investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 46.Tomcsányi J, Somlói M, Horváth L. Amiodarone-induced giant T wave alternans hastens proarrhythmic response. J Cardiovasc Electrophysiol. 2002;13:629. doi: 10.1046/j.1540-8167.2002.00629.x. [DOI] [PubMed] [Google Scholar]
- 47.Traube L. Ein Fall von Pulsus Bigeminus nebst Bemerkungen uber die Leberschwellungen bei Klappenfehlern und uber acute Leberatrophie. Berlin Klin Wochenschr. 1872;9:185–188. [Google Scholar]
- 48.Walker ML, Rosenbaum DS. Repolarization alternans: implications for the mechanism and prevention of sudden cardiac death. Cardiovasc Res. 2003;57:599–614. doi: 10.1016/s0008-6363(02)00737-x. [DOI] [PubMed] [Google Scholar]
- 49.Walker ML, Wan X, Kirsch GE, et al. Hysteresis effect implicates calcium cycling as a mechanism of repolarization alternans. Circulation. 2003;108:2704–9. doi: 10.1161/01.CIR.0000093276.10885.5B. [DOI] [PubMed] [Google Scholar]
- 50.Wan X, Laurita KR, Pruvot E, et al. Molecular correlates of repolarization alternans in cardiac myocytes. J Mol Cell Cardiol. 2005;39:419–428. doi: 10.1016/j.yjmcc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe MA, Fenton FH, Evans SJ, et al. Mechanisms for discordant alternans. J Cardiovasc Electrophysiol. 2001;12:196–206. doi: 10.1046/j.1540-8167.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 52.Weiss JN, Karma A, Shiferaw Y, et al. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98:1244–53. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 53.Xie LH, Sato D, Garfinkel A, et al. Intracellular Ca alternans: coordinated regulation by sarcoplasmic reticulum release, uptake, and leak. Biophys J. 2008;95:3100–10. doi: 10.1529/biophysj.108.130955. [DOI] [PMC free article] [PubMed] [Google Scholar]