Abstract
Cell cycle inhibition of neural stem and progenitor cells is critical for maintaining the stability of central nervous system in adults, but it may represent a significant hurdle for neural regeneration after injury. We have previously demonstrated that the cyclin-dependent kinase inhibitor (CKI) p21cip1/waf1 (p21) maintains the quiescence of neural stem-like cells under cerebral ischemia, as similarly shown for the hematopoietic stem cells. Here, we report the distinct role of another CKI member, p27kip1 (p27) in neural progenitor cells (NPCs) from adult brain (subventricular zone and hippocampal subgranular zone) under both homeostatic and ischemic conditions. The basal level of NPC proliferation in the p27−/− mice was higher than that in p27+/+ mice. Upon ischemia, the overall proliferation of NPCs continued to be higher in p27−/− mice than that in p27 +/+ mice. Moreover, the increase of NPC proliferation in p27−/− mice remained until 2 weeks after ischemia whereas it resumed back to the basal level in p27+/+ mice. As a result, newly generated neuronal cells in the granular layer of p27−/− brain were more abundant compared to p27 +/+ controls. These new data demonstrate that p27 functions as a distinct inhibitor for NPC proliferation under homeostatic as well as ischemic conditions.
Introduction
Adult stem and progenitor cells are critical cellular sources in tissue regeneration as evidenced in hematopoietic stem cell (HSC) transplantation for the treatment of many diseases 1. However, one of the major therapeutic hurdles for tissue regeneration is the restricted proliferative ability of the tissue precursor cells. In the central nervous system (CNS), neural stem cells (NSCs) and neural progenitor cells (NPCs) are restricted for their proliferation and reside only in relatively limited locations, such as subventricular zone (SVZ) and hippocampal subgranular zone (SGZ) 2. Unlike hematopoietic repopulation, neuronal replacement after brain injury or degeneration is inefficient, and it does not sufficiently repair the damaged tissue 3. This is perhaps largely due to a much broader range of cell cycle arrest or induced mitotic quiescence in the pools of NSC and NPC in contrast to that in the high turnover tissues such as intestinal epithelium, epidermal layer and hematopoietic system.
Mammalian cell cycle is driven in part by sequential activation and inactivation of the cyclin-dependent kinases (CDKs)/cyclin complexes 4, 5. In addition to regulation of CDK activity by tyrosine phosphorylation/dephosphorylation of the catalytic subunit and cyclin level, CDKs are also modulated by the CDK inhibitors (CKIs). There are two different families of CKIs which distinctively bind to different CDKs and regulate cell cycle progression through G1-S transition. One family includes p16INK4a, p15INK4b, p18INK4c and p19INK4d that specifically inhibit CDK4 and CDK6. Another family includes p21Cip1 (p21 hereafter), p27Kip1 and p57Kip2, which contain characteristic motifs within their amino-terminal moieties that enable them to mainly interfere with cyclin E/CDK2 or cyclin A/CDK2 to block or slow down the cell cycle movement.
Following our demonstration on the role of p21 in maintaining hematopoietic stem cell quiescence 6, we also assessed the role of p21 in regenerative response of mouse brain tissue following ischemic injury by middle cerebral artery occlusion (MCAO) 7. While steady state conditions revealed no difference, a significantly larger fraction of quiescent neural precursors was activated in SGZ and SVZ after MCAO in p21 −/− mice. The hippocampal precursors migrated and differentiated to a greater degree into mature neurons post-injury in p21−/−mice. Notably, however, the regeneration of NSC-like cells in p21 −/− ischemic brain was still limited 7, and furthermore p21−/− NSCs may ultimately undergo exhaustion under proliferative stress 8. Therefore, targeting p21 alone may not be able to provide clinically meaningful significance in ischemic brain. This underscores the importance of further exploring other CKIs experimentally.
Among all the CKIs, the level of p27 protein is most correlated with the cell cycle kinetics. An increase of p27kipl (p27 hereafter) can cause proliferating cells to exit from the cell cycle, while a decrease in p27 is required for quiescent cells to enter cell cycle in many cell types 9. In response to mitogenic stimuli, the level of p27 protein decreases, allowing CDK2 activation and entry into S phase 10, 11. Over expression of p27 leads to prolongation of G1 phase during neuroepithelium proliferation 12 and eventually lengthens the duration of the cell cycle. In contrast, lack of p27 increases NPCs 13 as well as hematopoietic progenitor cells (HPCs) 14 but does not seem to substantially affect the stem cell number 13, 14. As a consequence, loss of p27 increases the size of brain as well as other organs 15, 16. While the roles of p27 in developing brain and neurogenesis under homeostasis are relatively clear, its role following brain damage has not been examined.
In our current study, we show that p27 plays an important role in NPCs from adult brain. Unlike p21, it can inhibit proliferation of the NPCs from hippocampus or SVZ and more neuron-like cells can be regenerated in the absence of p27 either under a homeostasis or after MCAO. Therefore, the distinct role of p27 in NPCs offers a complementary molecular target with p21 or other CKIs for the enhancement of neural regeneration in brain after ischemia.
Materials and Methods
Animals and surgery
Animal care and experimental protocols complied with The Principles of Laboratory Animal Care (NIH's Guide for the Care and Use of Laboratory Animals) and small animal research center in Massachusetts General Hospital. p27 knockout mice were originally obtained from Andy Koff 16 or purchased from the Jackson Laboratories (Bar Harbor, Main). Ten to twelve week old mice were subjected to 20-minute middle cerebral artery occlusion (MCAO) as described in a previous model 7, 17. Briefly, mice were anesthetized with 1.5% isoflurane in 70% N2O and 30% O2 using a Fluotec 3 vaporizer (Colonial Medical Supply, Amherst, New Hampshire, USA). Regional cerebral blood flow was monitored by laser-Doppler flowmetry (PF2B; Perimed, Stockholm, USA). The left MCA was occluded with an 8−0 nylon monofilament (Ethicon Inc., Somerville, New Jersey, USA) coated with a mixture of silicone resin (Xantopren; Bayer Dental Nippon KK, Osaka, Japan) and hardener (Elastomer Activator; Bayer Dental Nippon KK). Twenty minutes later, the filament was withdrawn, and reperfusion was confirmed by laser-Doppler flowmetry.
Cell culture
NPCs were isolated from E-14 embryos by using 0.05% trypsin for tissue digestion. The cells were cultured in DMEM/F12 medium with 10ng/ml FGF-2, 10ng/ml EGF and 5μg/ml Heparin. The formed spheres were dissociated either mechanically or by using NeuroCult® Chemical Dissociation Kit (StemCell Technologies, Vancouver, BC, Canada). The NPCs were passaged once (p1) to passaged 6 (p6) were used in the experiments.
BrdU injections
5-Bromo-2’-deoxyuridine (BrdU) (Sigma-Aldrich) was dissolved in sterile PBS at concentration of 5mg/ml and prepared freshly for each daily use. A single dose of BrdU (50mg/kg) was given intraperitoneally on either day 7 or day 14 after MCAO for examining neural progenitor proliferation, or twice daily injection for two days at day 7 and 8 or day 14and 15 after MCAO for examining neural progenitor differentiation.
Laser capture microdissection (LCM) and quantitative real time RT-PCR
Thirty micromillimeter fresh frozen brain sections were prepared on membrane slides (Molecular Machines & Industries, Glattbrugg, Switzerland). The subgranular zone was captured by LCM (Smartcut, Molecular Machines & Industries, Glattbrugg, Switzerland) and RNA was isolated using RNAqueous-Micro (Ambion, Austin, TX). Reverse transcription was done by using Superscript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). p27 expression were analyzed by real time PCR. The primers and TaqMan FAM labeled probes for p27 was purchased from Applied Biosystems (Foster City, CA). Each sample was examined in triplicate.
Immunostaining
The animals were sacrificed at indicated time points and perfused transcardially with 4% paraformaldehyde in PBS under deep anesthesia. Immunostaining was performed using either 40-μm free-floating coronal sections or 5-μm paraffin sections. The sections were stained using the following antibodies and dilutions: mouse monoclonal anti-BrdU (1:400; Becton Dickinson Immunocytometry Systems, San Jose, California, USA), rat monoclonal anti-BrdU (1:400; Accurate Chemical & Scientific Corp., Westbury, New York, USA), anti–neuronal nuclear antigen (NeuN) (1:400; Chemicon International, Temecula, California, USA), anti-nestin (1:500; PharMingen, San Diego, California, USA), anti-p27 (1:200, NeoMarker, Fremont, CA), anti-GFAP (1:500; Sigma-Aldrich), anti-proliferating cell nuclear antigen (PCNA, 1:400) and anti-DCX (1:400; Santa Cruz Biotechnology Inc.). The second antibody was conjugated with biotin, cy2, cy5, or rhodamine red-X (1:400; Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA).
Quantification
All analyses were accomplished with stereologic counting methods as described previously 7. Briefly, the dentate gyrus evaluation in this study was focused on the region from bregma −1 to −2.9 mm. A systematic random sampling of every 6th in a series of 40-μm coronal sections (total 8 sections, 3 sections for SVZ and 5 sections for SGZ) was prepared from each animal and processed for immunohistochemistry. The granule cell layer area (mm2) was measured on adjacent sections stained with cresyl violet using an MCID Imaging Research Analysis System (Imaging Research Inc., St. Catherines, Ontario, Canada). Counting of BrdU+ cells was performed using a ×40 objective lens. The investigator who was counting the cells was blinded to all groups of animals. The density of BrdU+ cells and BrdU+/NeuN+ cells (cell number /mm3) within the subgranular zone was calculated by dividing into the total granular layer volume.
Statistics
Data were presented as mean ± SD. Statistical analyses were done by using ANOVA followed by a Hold-Sidak test. P < 0.05 was considered statistically significant.
Results
p27 is expressed in neural progenitor cells and up-regulated during neural differentiation in vitro
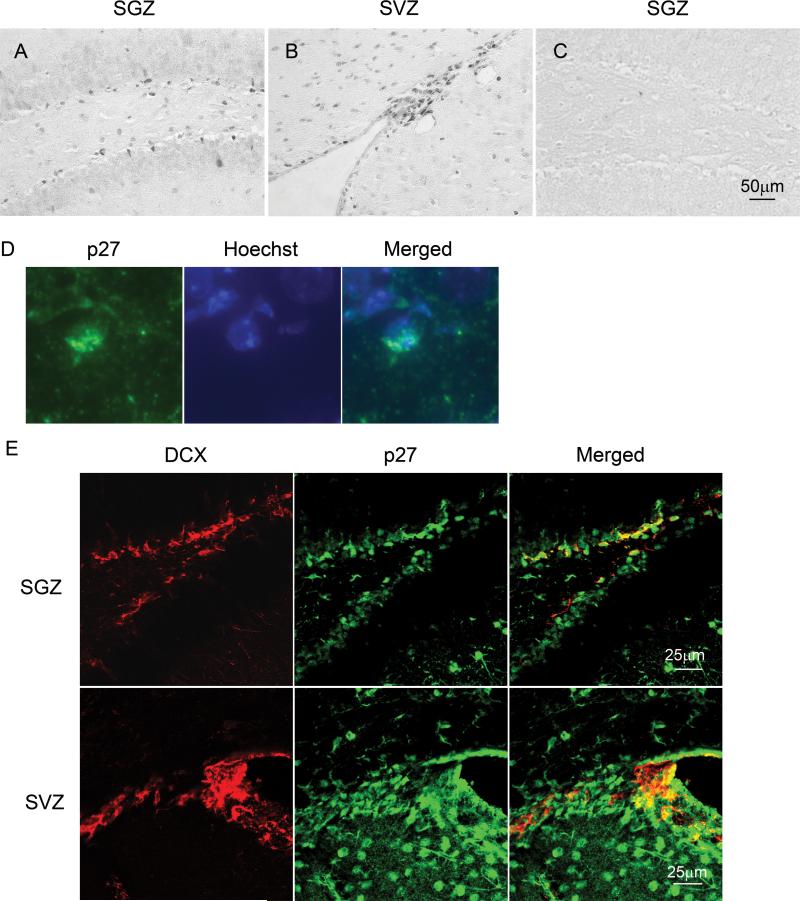
p27 in NPCs was first examined for its expression in SGZ and SVZ. p27 protein was readily detected with a considerable abundance within both regions (Fig. 1A & B). p27 staining appeared as a nuclear pattern (Fig. 1D), indicating an active form of p27 protein in the nuclei 18, 19. In SGZ, because NPCs can migrate into other regions to fulfill their functions during their differentiation 20, we investigated whether the marker for migratory neuroblasts, doublecortin (DCX) 21, 22 was co-expressed with p27 in SGZ. Clearly, many DCX+ cells were co-expressed with p27 in this region (Fig. 1E).
Figure 1. Expression of p27 protein in normal dentate gyrus and ventricular areas.
The histological sections of brain tissues derived from 8−10 weeks old normal mice (C57BL/6). The paraffin sections were stained with anti-p27 antibody and analyzed by microscopy. The representative images show that p27 positive cells reside at SGZ of dentate gyrus (A) as well as SVZ (B). A negative control for this staining (without primary antibody) was used and there was no staining on it (C). The scale bar is 50μm. D: High magnification of p27 and 4'-6-Diamidino-2-phenylindole (DAPI) staining in SGZ shows p27 staining is colocalized with nucleus. E: The normal brain sections were stained with anti-p27 and anti-doublecortin antibodies and analyzed by confocal microscopy. Upper and lower panels show the staining patterns in SGZ and SVZ respectively.
In addition, the expression of p27 protein in NPCs was further examined in cultured NPCs. NPCs were cultured in the DMEM/F12 medium with FGF-2 and EGF 7, 8. p27 was detected by RT-PCR as well as immunoblot in cultured neurospheres (Fig.2A-B), which can be differentiated into three major different neural cell phenotypes including neuron, astrocyte and oligodendrocyte. When FGF-2 and EGF are removed from the medium, the cells start to differentiate 23. p27 expression rapidly increased in these cells (Fig. 2C). This data confirms that the expression of p27 is correlated with NPC differentiation and cell cycle arrest.
Figure 2. p27 expression in cultured neurospheres and upregulation during neural differentiation.
NPCs were isolated from wild type E-14 embryos and cultured in DMEM/F12 medium supplied with FGF-2 and EGF. A. p27 expression was examined by RT-PCR in three independent neural progenitor cultures indicated as “1, 2, 3”. The graph on the right indicates the mean value of p27 mRNA levels examined by real time RT-PCR. B. p27 expression was examined by Western blot in two different passages of NPC culture marked as “p1 and p6”. C. Up-regulation of p27 expression during neural NPC differentiation in vitro. After the neurospheres were formed, the medium was switched to DMEM with 5% of FBS. The cultures were collected at different time points and the expression of p27 was examined by Western blot. The data demonstrate an increase of p27 expression during neural progenitor cell differentiation and the cell cycle exit.
Increased response to growth stimuli results in an enlarged pool of neural progenitor cells in the absence of p27
It has been previously suggested that NPCs rather than NSCs increase in p27−/− brain as determined by the morphological criteria for NSC and NPC in mouse brain 13, and this is consistent with our results on the hematopoietic system 24. To further define this point, we isolated the NPCs from SVZ or dentate gyrus of adult p27−/− or p27+/+ mice. A greater number of neurospheres were observed in p27−/− culture than in p27+/+ culture (Fig. 3A). Because neurosphere cultures mainly measure NPCs 25, 26, we conclude that the NPC pool is larger in p27−/− mice than that in p27+/+ mice, further confirming the previous claim 13 with this functional in vitro assay. All three major neural cell (neuron, astrocyte and oligodendrocyte) markers were observed in the differentiated spheres from both p27−/− and p27+/+ 25. Immunostaining for astrocytes (GFAP) and neurons (NeuN) showed no significant difference between p27−/− and p27+/+ groups, suggesting no significant impact of p27 absence on the lineage selection (Supplemental Fig. 1). This is also consistent with a previous demonstration by others 16.
Figure 3. Increased abundance and proliferation of neural progenitor cells in p27−/− mice.
A: NPCs were isolated from both sides of hippocampi or SVZ of adult p27+/+ or p27−/− mice, then cultured in DMEM/F12 medium with FGF-2 and EGF. The neurospheres were counted 10−14 days after culture. *, p<0.05. B: A single dose of BrdU was injected into uninjured p27−/− mice or their wild type littermates and the mice were sacrificed 30 min after BrdU injection and the BrdU incorporation was determined by BrdU immunostaining. The BrdU+ cells in the SVZ were counted under the microscope and calculated as described in the method section. There was a significant increase of BrdU+ cells in p27−/− mice compared to that in p27+/+ mice. *, p<0.05. C: The coronal brain sections from normal p27+/+ and p27−/− mice were stained with PCNA. The representative pictures shows more PCNA positive cells in SVZ and SGZ of p27−/− mice than in p27+/+ mice. Normal brain sections were stained with anti-PCNA antibody. The representative images show that PCNA positive cells (red) in SGZ and SVZ in normal adult p27−/− mice are more obvious than that in normal adult p27+/+ mice.
To attempt to elucidate whether the enlarged NPC pool in p27−/− adult brain are resulted from embryonic development or ongoing neurogenesis, a single dose of 5-Bromo-2-deoxyuridine (BrdU) (50mg/kg) was injected into uninjured mice and the mice were sacrificed 30 minutes after injection of BrdU 27. BrdU incorporation at the specific dose has been a standard method to examine NSC/NPC proliferation in developing or adult brain. When cells enter S phase, in which DNA replication occurs, BrdU can be integrated into newly generated DNA and thereby can be used to indicate the proliferating cells via immunostaining 7, 20. By this method, we found that the number of BrdU positive cells in SGZ or SVZ of p27−/− mice was greater than that in the regions of p27 +/+ mice (Fig. 3B), suggesting that lack of p27 promotes the proliferation of NPCs. As a result, NPCs were expanded. This result was further confirmed by the increased expression of the proliferating cell nuclear antigen (PCNA) in p27−/− brain (Fig. 3C). PCNA is expressed in the nuclei of cells during the DNA synthesis phase of the cell cycle. Therefore, absence of p27 enhances ongoing neurogenesis in adult brain.
Proliferation of neural progenitor cells in the absence of p27 is further enhanced after ischemia
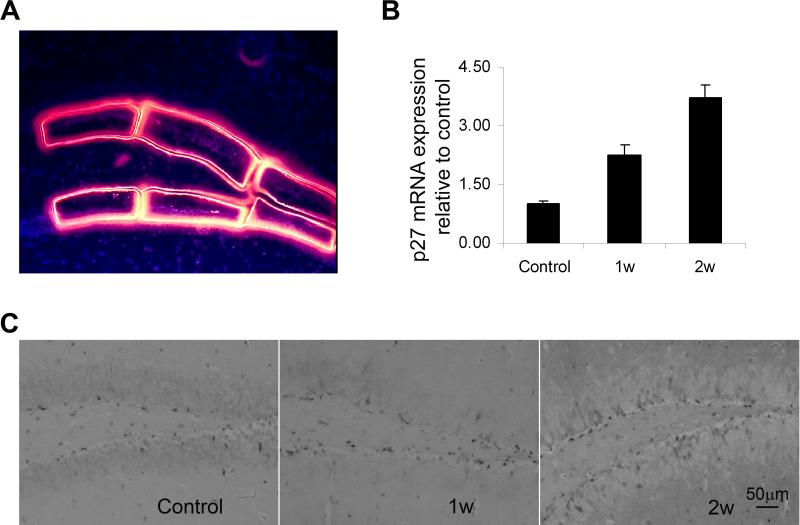
To define whether p27 is involved in neural regeneration specifically after acute brain damage, we first examined the expression of p27 in the MCAO model. We isolated the tissues specifically from SGZ of mouse brain with laser capture microdissection at day 7 and 14 after 20-min MCAO (Fig. 4A) and then quantified the mRNA expression of p27 in SGZ on the ischemic side (Fig. 4B) by real time RT-PCR. p27 was significantly increased during NPC regeneration in the ischemic brain. By day 14, mRNA expression of p27 was increased more than 3 times compared to the level under uninjured conditions. Also, the number of p27+ cells, as detected by immunohistochemistry, in the SGZ ipsilateral to the MCAO appeared increased at days 7 and 14 after ischemia (Fig. 4C). Therefore, like p21 expression 7, upregulation of p27 following MACO suggests a potential inhibitory effect of p27 upon neural regeneration after the ischemic insult. To further test this possibility, we then used the BrdU incorporation method to track the proliferating NPCs following MCAO.
Figure 4. Increased expression of p27 mRNA in neural regeneration after ischemia.
Subgranular tissues from sham-operated, ischemic mice one week and two weeks after MCAO were collected by laser capture microdissection (LCM) (A) and mRNA of p27 in these tissues was examined by real time RT-PCR (B). *: p< 0.05. Immunostaining further confirmed the presence of p27+ cells in the region (C).
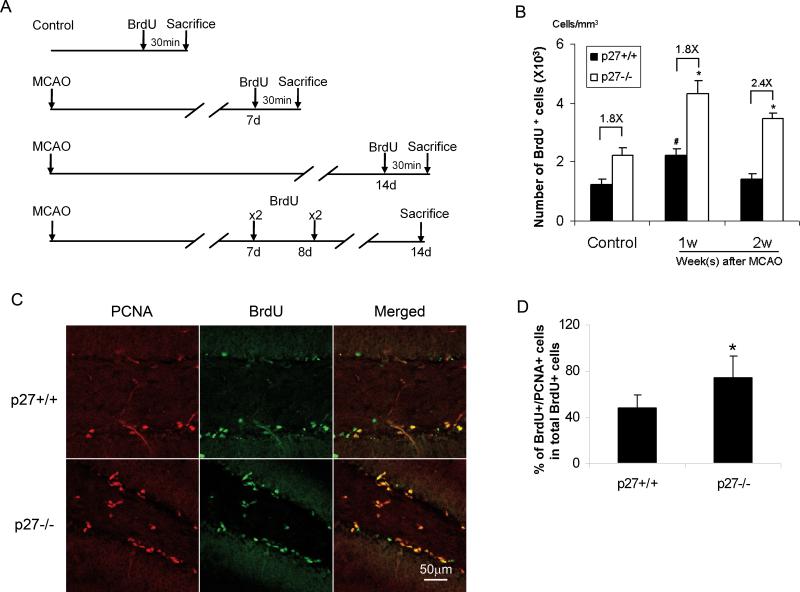
Both p27+/+ and p27−/− mice were subjected to transient MCAO for 20 minutes. To investigate NPC proliferation after ischemia, BrdU was injected intraperitoneally (i.p.) at day 7 after MCAO and the animals were sacrificed 30 minutes later after BrdU injection (Fig. 5A). We found that BrdU positive cells in SGZ were increased in p27 −/− mice as well as p27 +/+ littermates compared with their sham-operated control animals. Since the number of BrdU+ cells in normal SGZ of p27−/− mice was greater than that in normal SGZ of p27+/+ mice, we analyzed the ratio of the number of BrdU positive cells in p27−/− mice versus p27+/+ littermates. The ratio was 1.8 under uninjured conditions and remained similar at one week after MCAO. However, when BrdU was injected at day 14 after MCAO and the mice were sacrificed 30 minutes after BrdU injection (Fig. 5A), the ratio of BrdU positive cells between p27−/− and p27+/+ mice increased to 2.4 (Fig. 5B). To examine whether the cells divided one week after ischemia continued to proliferate in the following week, we injected BrdU twice daily at day 7 and 8 and the animals were sacrificed at day 14. BrdU and PCNA expression were examined. We found that 74% of BrdU+ cells also colabeled with PCNA in p27−/− SGZ whereas 48±12% of the cells in p27+/+ SGZ (p<0.05) (Fig. 5C-D), thereby suggesting that the divided NPCs at week one in p27−/−brain proliferated more actively than the cells in p27+/+ brain at week 2 after MCAO.
Figure 5. Increased proliferation of neural progenitors in p27−/− mice after ischemia.
The mice were subjected to 20-min MCAO, and BrdU was injected at either day 7 or day 14 (single dose). Mice were sacrificed 30 min thereafter. Control mice were sham-operated mice and receiving the same dose of BrdU as the ischemic mice. A. Schema of experimental design. B: The BrdU+ cells in SGZ were counted in 5 sections and calculated as described in Methods. *: p<0.05. C: The mice were subjected to 20-min MCAO, and BrdU was injected at day 7and 8. Animals were sacrificed at day 14 after MCAO. The coronal sections were stained with anti-BrdU and PCAN antibodies. The pictures show PCNA (red), BrdU (green) and merged staining in p27+/+ mice (upper penal) and p27−/− mice (lower penal). D. Quantitative data of Fig. 5C. BrdU+ cells and BrdU+/PCNA+ cells were counted. A higher percentage of BrdU+/PCNA+ cells in total BrdU+ cells were found in p27−/− mice compared with their littermates. The data is shown as mean ± SD, *: p<0.05.
More neuron-like cells can be regenerated in the absence of p27 under either homeostatic or ischemic conditions
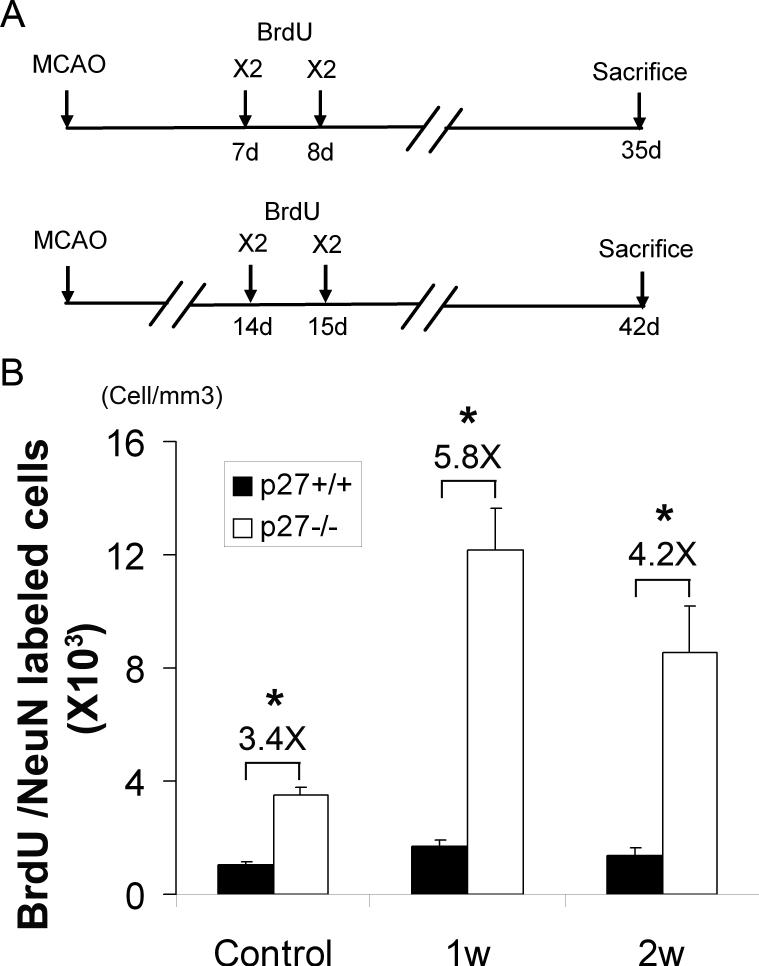
Differentiation potential toward neurons of the proliferated NPCs was examined with the mature neuronal marker, NeuN in the BrdU+ cells. BrdU was injected at day 7 and 8 or day 14 and 15 after ischemia, and mice were sacrificed 4 weeks after the last BrdU injection. BrdU and NeuN co-expression was analyzed by confocal microscopy (Fig. 6A). The number of BrdU positive cells in SGZ was reduced in both p27+/+ and p27−/− mice when sacrificed at 4 weeks rather than one day after the last BrdU injection (data not shown), suggesting that cell death had occurred during the cell proliferation. Most BrdU+ cells expressed NeuN and migrated to the granular zone of the dentate gyrus (Supplemental Fig. 2), showing that some of the BrdU+ cells had the ability to differentiate into neurons. Moreover, there were more BrdU+/NeuN+ cells in dentate gyrus in normal p27−/− mice than that in p27 +/+ mice as the ratio of BrdU+/NeuN+ cells in p27−/− mice versus the cells in p27+/+ mice was 3.4:1, indicating more active ongoing neurogenesis within p27−/− mice under homeostatic conditions (Fig. 6). Interestingly, when BrdU was injected day 7 and 8 or day 14 and day 15 after MCAO, the ratios went up to 5.8:1 and 4.2:1 respectively. Notably, however, no significant difference in the timing of acquiring the expression of NeuN was documented between p27+/+ and p27−/− mice, suggesting no delay of neuronal differentiation in p27−/− mice during four weeks period. In addition, there was no difference of cell death 24 hours after MCAO between p27+/+ mice p27−/− mice as examined by TUNEL assay (data not shown) as well as the infarct volume (Supplemental Fig. 3). Since the brain size of p27−/− mice was bigger than that of p27+/+ mice 16, 28, we used percentage hemisphere lesion volume to correct this issue (infarct volume vs. hemisphere volume 29). The percentages of infarct volumes were 19±4 in p27+/+ mice and 21±6 in p27−/− mice (mean ± SD, p>0.05), suggesting that ischemic damage was relatively equal between these two types of mice.
Figure 6. Increased production of neuron-like cells in dentate gyrus of p27−/− mice after ischemia.
A: BrdU was injected at either 1 week (day 7 and 8) or 2 weeks (day 14 and 15) after MCAO and the mice were sacrificed at 4 weeks after last BrdU injection. B: The number of BrdU and NeuN double stained cells was counted under confocal microscope and calculated as described in Methods. The data is shown as mean ± SD *: p<0.05.
Discussion
p27 has been extensively studied in neural developmental and in vitro culture models. However, its role in adult NPCs especially under conditions of brain injury is poorly understood. We show here that p27 was expressed in SGZ and SVZ in normal mouse brain as well as in the cultured differentiating NPCs. Although the ratio of BrdU positive cells in p27−/− mice compared to that in p27+/+ mice 1 week after MCAO was similar under homeostatic conditions, it increased significantly 2 weeks after MCAO. The BrdU+ cells in SGZ migrated to the granular zone and developed into neuron-like progeny expressing NeuN 4 weeks later. Thus, our current study demonstrates p27 as a strong cell cycle inhibitor for NPC proliferation under both homeostatic and ischemic conditions.
Efficient marking of NSCs or NPCs is dependent on the length of cell cycle and the timing of BrdU incorporation. Adult stem cells are relatively quiescent in cell cycle as opposed to progenitor cell populations 30. The cell cycle length of a murine HSC can be 30 days long 31 and only about 8 % of murine HSCs are cycling at a given time 32. Similarly, NSCs are also slow cycling while NPCs are more actively mitotic 33-35. NSCs therefore require a longer exposure of BrdU in order to be effectively marked. In the case where stem cell quiescence can be altered by p21 absence6-8, increased proliferation of NSCs in p21−/− adult brain was detected only in the long-term BrdU retention group (mice were sacrificed 30 day after the final injection of BrdU) 8 but not in the short-term retention group (mice were sacrificed 1 h or 1 day after BrdU treatment) 7, 8. In contrast, the half-life of NPCs is only about one week in C57BL/6J mouse strain 36. Given these facts, when BrdU is pulsed at a single or a few doses in a few days, the BrdU positivity should largely reflect the proliferative activity of NPCs rather than the quiescent NSCs. In consistence with the colocalization of DCX that indicates the migrating neuroblasts 21, 37 in multiple neurogenic regions and the data (Fig. 3A) from the neurosphere culture system that also largely reflect the acticity of NPCs in vitro 25, the increased BrdU incorporation in our current study (Fig. 3-5) mainly demonstrate that loss of p27 significantly increases the proliferation of NPCs. Interestingly, we noticed that the number of BrdU+ cells in both p27+/+ and p27−/− mice was reduced but the difference remained when the animals were sacrificed 4 weeks after last BrdU injection rather than the day after last BrdU injection. This suggests a sustained increase of NPC proliferation prior to its terminal differentiation in the absence of p27. Although we did not find the difference between infarct size or the number of TUNEL+ cells between p27 wild-type and knockout littermates after MCAO, we cannot rule out the possibility that newly divided NPCs in p27−/− mice may have less cell death at the late stage after ischemia. In fact, contrary to this possibility, it was reported that loss of p27 may increase apoptotic cell death during progenitor cell differentiation 38.
More newly regenerated neuron-like cells were observed in p27−/− mice in both non-ischemic and ischemic conditions compared to p27+/+ littermates. Notably, the ratio of BrdU+/NeuN+ cells in p27−/− mice versus BrdU+/NeuN+ cells in p27+/+ mice was higher under ischemic condition, suggesting an additional effect of p27 in neuronogeneis after ischemia as opposed to its role under homeostatic conditions. Downregulation of p27 not only increases NPC proliferation, but also increases regenerated neurons despite the fact that loss of p27 may increase cell apoptosis during cell differentiation 38. This result is also consistent with the finding that lack of p27 increases the size of multiple organs including brain 15, 16. Increased expression of p27 in differentiating NPCs (Fig. 2C and Fig. 4), seemingly suggested that p27 might be required for NPC differentiation. However, it has been reported that loss of p27 does not block the neural cell differentiation 15. Furthermore, according to our data, the BrdU positive cells in the SGZ in p27−/− mice and p27+/+ mice moved to the granular zone and readily expressed NeuN 4 weeks after BrdU injection. We did not observe significantly delayed neuronal differentiation in the granular and subgranular zones in p27−/− mice compared with p27+/+ mice. We thus conceive that p27 serves as an inhibitor primarily for NPC proliferation rather than an essential initiator for NPC differentiation in brain. It is known that ischemia induces NPC proliferation 7, 17, 39-41. Increased proliferation has been mainly observed in SGZ and SVZ, and the peak time point is around one week after MCAO 7, 17, 39-41. Our findings suggest that p27 functions at a relatively later stage after ischemia than other cell cycle modulators such as p21 (Fig. 5B). The late onset of the effect in this model may be attributed to the inhibitory effect of p27 more specifically in late HPCs although the mechanism by which ischemia induces HPC proliferation remains to be defined.
In light of our previous study on p21 7 involving the identical brain ischemia model, roles of p27 and p21 in adult neurogenesis appear to be quite distinct. p21 is expressed in a smaller portion of neurogenic cells than p27, including primitive NSC-like cells as well as some fully mature cell types. p21 activation appears to be move specifically associated with stress than p27 activation, as there was no difference of neurogenesis under homeostasis in the absence of p21 7. The response of p21−/− cells to an ischemic injury only occurs in the first week after MCAO. In contrast, p27 is expressed in the majority if not all the NPCs after ischemic injury. Therefore, p27 regulates NPCs under both homeostasis and stress conditions. Despite the different effects of p21 and p27, together with the previous demonstrations regarding the similar roles of p21 or p27 between the CNS and the hematopoietic system 6-8, 13, our current study further supports the notion that there is a molecular commonality mediated via CKIs among different stem cell populations in adults 30, 42.
In conclusion, p27 restricts NPC regeneration under both homeostatic and ischemic conditions, and such an inhibitory effect becomes more significant in a late stage after cerebral ischemia. Given the distinct impacts of other CKIs in neurogenesis and neural regeneration 7, 8, 30, 43, 44, it appears that the divergence of neural precursors in response to a variety of stimuli can be at least attributed to different CKIs. This rationalizes a complementary approach to enhance neural regeneration after brain damage. For example, suppressing p27 in conjunction with targeting p21 may be viewed as a potential therapeutic means to boost efficient neural regeneration following brain stroke. This strategy may also be applicable to other tissue regeneration given the broad expression of CKIs in most tissues. On the other hand, inhibitory roles of CKIs in NSCs or NPCs may also suggest their potential involvements in the development of brain tumor.
Supplementary Material
Acknowledgements
This work was supported by American Heart Association N0335154 (J.Q.), NIH grants P50 NS10828 (M.A.M.), NIH K08 NS049241 (J.R.S), NIH P30 NS045776 (Massachusetts General Hospital Neuroscience Center Core Facility), HL070561, ChangJiang Scholarship from the Ministry of Education, Technology Funds from the Tianjin City and Outstanding Young Scholar Award from the Natural Science Foundation of China (T.C.). We thank Masaki Nishimura and Igor Bagayev for their excellent technical assistance.
Footnotes
Disclosure of potential conflicts of interest
The authors indicate no potential conflicts of interest.
References
- 1.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 2.Gage FH. Cell therapy. Nature. 1998;392:18–24. [PubMed] [Google Scholar]
- 3.Snyder EY, Park KI. Limitations in brain repair. Nat Med. 2002;8:928–930. doi: 10.1038/nm0902-928. [DOI] [PubMed] [Google Scholar]
- 4.Pines J, Toldo L, Lafont F. Cytoskeleton. Curr Opin Cell Biol. 1998;10:11–12. [PubMed] [Google Scholar]
- 5.Sheaff RJ, Roberts JM. Regulation of G1 phase. Results Probl Cell Differ. 1998;22:1–34. doi: 10.1007/978-3-540-69686-5_1. [DOI] [PubMed] [Google Scholar]
- 6.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21(cip1/waf1). Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 7.Qiu J, Takagi Y, Harada J, et al. Regenerative response in ischemic brain restricted by p21cip1/waf1. J Exp Med. 2004;199:937–945. doi: 10.1084/jem.20031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loda M, Cukor B, Tam SW, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 10.Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- 11.Vidal A, Koff A. Cell-cycle inhibitors: three families united by a common cause. Gene. 2000;247:1–15. doi: 10.1016/s0378-1119(00)00092-5. [DOI] [PubMed] [Google Scholar]
- 12.Mitsuhashi T, Aoki Y, Eksioglu YZ, et al. Overexpression of p27Kip1 lengthens the G1 phase in a mouse model that targets inducible gene expression to central nervous system progenitor cells. Proc Natl Acad Sci U S A. 2001;98:6435–6440. doi: 10.1073/pnas.111051398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doetsch F, Verdugo JM, Caille I, et al. Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis. J Neurosci. 2002;22:2255–2264. doi: 10.1523/JNEUROSCI.22-06-02255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng T, Rodrigues N, Dombkowski D, et al. Stem cell repopulation efficiency but not pool size is governed by p27(kip1). Nat Med. 2000;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama K, Ishida N, Shirane M, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 16.Kiyokawa H, Kineman RD, Manova-Todorova KO, et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 17.Teramoto T, Qiu J, Plumier JC, et al. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest. 2003;111:1125–1132. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin I, Yakes FM, Rojo F, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 19.Liang J, Zubovitz J, Petrocelli T, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 22.Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciccolini F, Svendsen CN. Fibroblast growth factor 2 (FGF-2) promotes acquisition of epidermal growth factor (EGF) responsiveness in mouse striatal precursor cells: identification of neural precursors responding to both EGF and FGF-2. J Neurosci. 1998;18:7869–7880. doi: 10.1523/JNEUROSCI.18-19-07869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng T, Rodrigues N, Dombkowski D, et al. Stem cell repopulation efficiency but not pool size is governed by p27. Nature Medicine. 2000;6:1235–1240. doi: 10.1038/81335. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 26.Singec I, Knoth R, Meyer RP, et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3:801–806. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- 27.Zhang RL, Zhang ZG, Roberts C, et al. Lengthening the G(1) phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after stroke. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama K, Ishida N, Shirane M, et al. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Chen K, Huang PL, et al. bFGF ameliorates focal ischemic injury by blood flow-independent mechanisms in eNOS mutant mice. Am J Physiol. 1997;272:H1401–1405. doi: 10.1152/ajpheart.1997.272.3.H1401. [DOI] [PubMed] [Google Scholar]
- 30.Cheng T. Cell cycle inhibitors in normal and tumor stem cells. Oncogene. 2004;23:7256–7266. doi: 10.1038/sj.onc.1207945. [DOI] [PubMed] [Google Scholar]
- 31.Bradford GB, Williams B, Rossi R, et al. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp Hematol. 1997;25:445–453. [PubMed] [Google Scholar]
- 32.Cheshier SH, Morrison SJ, Liao X, et al. In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonfanti LGA, Galli R, Vescovi AL. Multipotent Stem Cells in the Adult Central Nervous System. In: MS R, editor. Stem Cells and CNS Development. Humana Press; Totowa, NJ: 2001. pp. 31–48. [Google Scholar]
- 34.Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 35.Morshead CM, Reynolds BA, Craig CG, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 36.Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 37.Kawauchi T, Chihama K, Nabeshima Y, et al. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- 38.Dyer MA, Cepko CL. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J Neurosci. 2001;21:4259–4271. doi: 10.1523/JNEUROSCI.21-12-04259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura S, Takagi Y, Harada J, et al. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshimura S, Teramoto T, Whalen MJ, et al. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J Clin Invest. 2003;112:1202–1210. doi: 10.1172/JCI16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 42.Boyer MJ, Cheng T. The CDK inhibitors: potential targets for therapeutic stem cell manipulations? Gene Ther. 2008;15:117–125. doi: 10.1038/sj.gt.3303064. [DOI] [PubMed] [Google Scholar]
- 43.Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16:2699–2712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zindy F, Soares H, Herzog KH, et al. Expression of INK4 inhibitors of cyclin D-dependent kinases during mouse brain development. Cell Growth Differ. 1997;8:1139–1150. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.