Abstract
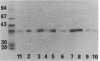
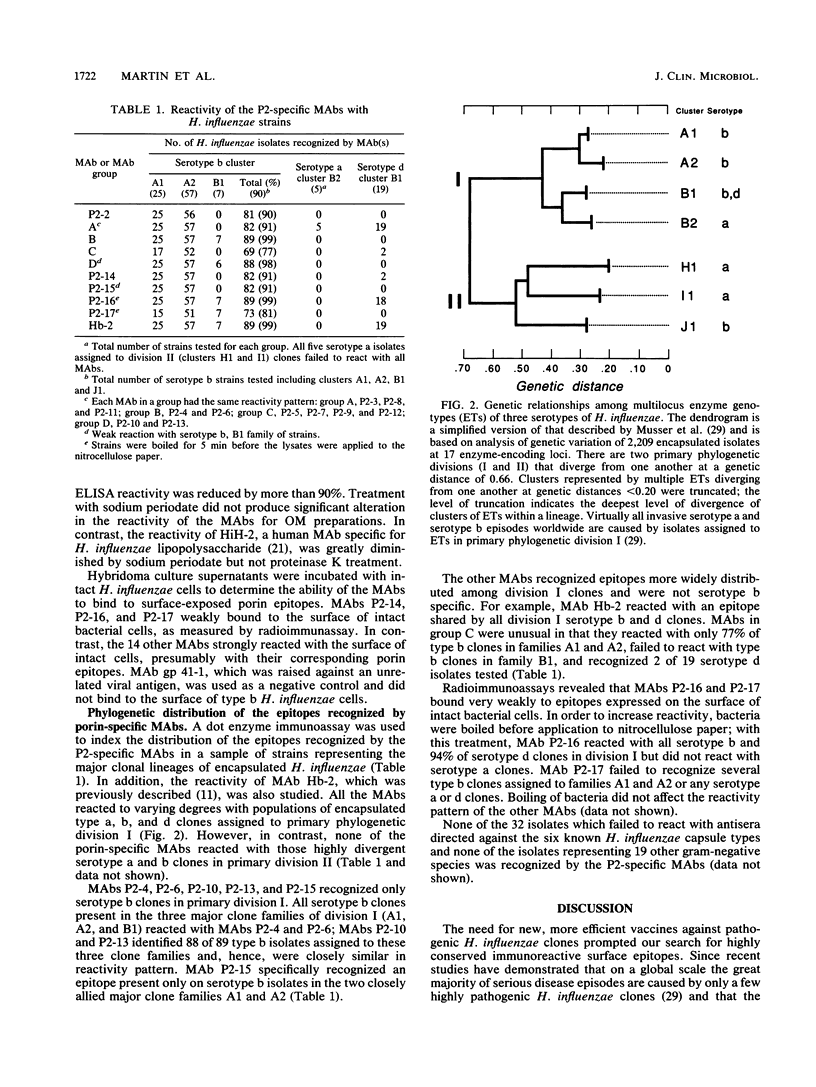
Monoclonal antibodies (Mabs) specific for Haemophilus influenzae were generated to identify antigenic determinants shared among encapsulated H. influenzae clones. Sixteen MAbs reacted by Western immunoblot with a protein of an approximate molecular size of 40 kilodaltons corresponding to the P2 major outer membrane protein (porin). These MAbs also reacted with purified and recombinant H. influenzae porin. Fourteen of the MAbs recognized cell surface-exposed epitopes, and two of the MAbs, P2-16 and P2-17, identified epitopes that are not present or are not accessible on the cell surface. The reactivity spectrum of the MAb panel was studied by dot immunoassay against 32 serologically nontypeable and 119 encapsulated H. influenzae strains recovered worldwide, representing the major serotype a, b, and d clone families. MAbs P2-4 and P2-6 recognized only serotype b clones assigned to primary phylogenetic division I. These clones account for more than 99% of all invasive episodes worldwide. MAbs P2-3, P2-8, and P2-11 reacted with division I serotype b isolates and also identified all genetically allied strains expressing serotype a and d polysaccharide capsules. In contrast, none of the 16 MAbs reacted with genetically divergent serotype a or b clones assigned to primary phylogenetic division II. These results demonstrate that, in general, the patterns of P2 protein surface epitope exposure are cognate with genetic lineages of encapsulated H. influenzae strains and support the hypothesis that the population structure of encapsulated H. influenzae is predominantly clonal.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan I., Loeb M. R., Moxon E. R. Limited genetic diversity of Haemophilus influenzae (type b). Microb Pathog. 1987 Feb;2(2):139–145. doi: 10.1016/0882-4010(87)90105-7. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Comparison of outer-membrane protein subtypes and biotypes of isolates of Haemophilus influenzae type b. J Infect Dis. 1981 Nov;144(5):480–480. doi: 10.1093/infdis/144.5.480. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Larose Y., Tsang P., Hamel J., Ashton F., Ryan A. Protection against infection with Neisseria meningitidis group B serotype 2b by passive immunization with serotype-specific monoclonal antibody. Infect Immun. 1985 Nov;50(2):510–516. doi: 10.1128/iai.50.2.510-516.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Smith A. L. A major outer-membrane protein functions as a porin in Haemophilus influenzae. J Gen Microbiol. 1987 May;133(5):1273–1277. doi: 10.1099/00221287-133-5-1273. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Wan D. T. The outer membrane of haemophilus influenzae type b: cell envelope associations of major proteins. Can J Microbiol. 1983 Feb;29(2):280–287. doi: 10.1139/m83-046. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Green B. A., Quinn-Dey T., Zlotnick G. W. Biologic activities of antibody to a peptidoglycan-associated lipoprotein of Haemophilus influenzae against multiple clinical isolates of H. influenzae type b. Infect Immun. 1987 Dec;55(12):2878–2883. doi: 10.1128/iai.55.12.2878-2883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel J., Brodeur B. R., Belmaaza A., Montplaisir S., Musser J. M., Selander R. K. Identification of Haemophilus influenzae type b by a monoclonal antibody coagglutination assay. J Clin Microbiol. 1987 Dec;25(12):2434–2436. doi: 10.1128/jcm.25.12.2434-2436.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel J., Brodeur B. R., Larose Y., Tsang P. S., Belmaaza A., Montplaisir S. A monoclonal antibody directed against a serotype-specific, outer-membrane protein of Haemophilus influenzae type b. J Med Microbiol. 1987 Mar;23(2):163–170. doi: 10.1099/00222615-23-2-163. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Gonzales F. R., Chamberlain N. R., Norgard M. V., Miller E. E., Cope L. D., Pelzel S. E., Gaddy B., Clausell A. Cloning of the gene encoding the major outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1988 Oct;56(10):2709–2716. doi: 10.1128/iai.56.10.2709-2716.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Pelzel S. E., Orth K., Moomaw C. R., Radolf J. D., Slaughter C. A. Structural and antigenic conservation of the P2 porin protein among strains of Haemophilus influenzae type b. Infect Immun. 1989 Nov;57(11):3270–3275. doi: 10.1128/iai.57.11.3270-3275.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Connelly C. J., Moxon E. R. Genetics of spontaneous, high-frequency loss of b capsule expression in Haemophilus influenzae. Infect Immun. 1985 Aug;49(2):389–395. doi: 10.1128/iai.49.2.389-395.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R. Protection of infant rats from Haemophilus influenzae type b infection by antiserum to purified outer membrane protein a. Infect Immun. 1987 Nov;55(11):2612–2618. doi: 10.1128/iai.55.11.2612-2618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Woodin K. A. Cross-reactivity of surface-exposed epitopes of outer membrane antigens of Haemophilus influenzae type b. Infect Immun. 1987 Dec;55(12):2977–2983. doi: 10.1128/iai.55.12.2977-2983.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier M., Brodeur B. R., Winston S. Detection of Neisseria gonorrhoeae by dot-enzyme immunoassay using monoclonal antibodies. J Immunoassay. 1989;10(4):373–394. doi: 10.1080/01971528908053248. [DOI] [PubMed] [Google Scholar]
- Martin D., Larose Y., Hamel J., Lagacé J., Brodeur B. R. Heterohybridomas secreting human monoclonal antibodies against Haemophilus influenzae type b. Eur J Immunol. 1988 Apr;18(4):601–606. doi: 10.1002/eji.1830180417. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Deich R. A., Connelly C. Cloning of chromosomal DNA from Haemophilus influenzae. Its use for studying the expression of type b capsule and virulence. J Clin Invest. 1984 Feb;73(2):298–306. doi: 10.1172/JCI111214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Bailey C., Grass S. Diversity of the outer membrane protein P2 gene from major clones of Haemophilus influenzae type b. Mol Microbiol. 1989 Dec;3(12):1797–1803. doi: 10.1111/j.1365-2958.1989.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Munson R., Jr, Grass S., Einhorn M., Bailey C., Newell C. Comparative analysis of the structures of the outer membrane protein P1 genes from major clones of Haemophilus influenzae type b. Infect Immun. 1989 Nov;57(11):3300–3305. doi: 10.1128/iai.57.11.3300-3305.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Tolan R. W., Jr Molecular cloning, expression, and primary sequence of outer membrane protein P2 of Haemophilus influenzae type b. Infect Immun. 1989 Jan;57(1):88–94. doi: 10.1128/iai.57.1.88-94.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Granoff D. M., Pattison P. E., Selander R. K. A population genetic framework for the study of invasive diseases caused by serotype b strains of Haemophilus influenzae. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5078–5082. doi: 10.1073/pnas.82.15.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Kroll J. S., Moxon E. R., Selander R. K. Clonal population structure of encapsulated Haemophilus influenzae. Infect Immun. 1988 Aug;56(8):1837–1845. doi: 10.1128/iai.56.8.1837-1845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Kroll J. S., Moxon E. R., Selander R. K. Evolutionary genetics of the encapsulated strains of Haemophilus influenzae. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7758–7762. doi: 10.1073/pnas.85.20.7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen K., Hjorth J. P., Kilian M. Limited diversity of the immunoglobulin A1 protease gene (iga) among Haemophilus influenzae serotype b strains. Infect Immun. 1988 Apr;56(4):987–992. doi: 10.1128/iai.56.4.987-992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk D. C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984 Aug;18(1):1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- Vachon V., Kristjanson D. N., Coulton J. W. Outer membrane porin protein of Haemophilus influenzae type b: pore size and subunit structure. Can J Microbiol. 1988 Feb;34(2):134–140. doi: 10.1139/m88-027. [DOI] [PubMed] [Google Scholar]
- Vachon V., Laprade R., Coulton J. W. Properties of the porin of Haemophilus influenzae type b in planar lipid bilayer membranes. Biochim Biophys Acta. 1986 Sep 25;861(1):74–82. doi: 10.1016/0005-2736(86)90373-1. [DOI] [PubMed] [Google Scholar]
- Weinberg G. A., Ghafoor A., Ishaq Z., Nomani N. K., Kabeer M., Anwar F., Burney M. I., Qureshi A. W., Musser J. M., Selander R. K. Clonal analysis of Hemophilus influenzae isolated from children from Pakistan with lower respiratory tract infections. J Infect Dis. 1989 Oct;160(4):634–643. doi: 10.1093/infdis/160.4.634. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Hopman C., Zanen H. C. Homogeneity of cell envelope protein subtypes, lipopolysaccharide serotypes, and biotypes among Haemophilus influenzae type b from patients with meningitis in The Netherlands. J Infect Dis. 1983 Jul;148(1):75–81. doi: 10.1093/infdis/148.1.75. [DOI] [PubMed] [Google Scholar]