Abstract
Invariant natural killer T (iNKT) cells are a unique subset of T lymphocytes that recognize glycolipid antigens in the context of the antigen-presenting molecule CD1d. Upon glycolipid antigen stimulation, iNKT cells rapidly produce copious amounts of immunomodulatory cytokines, leading to potent activation of a variety of innate and adaptive immune cells. These immune-potentiating properties of iNKT cells hold great promise for the development of vaccine adjuvants. This review aims to summarize the immunomodulatory activities of iNKT cell ligands and to discuss prospects for developing iNKT cell-based vaccine adjuvants.
Keywords: α-galactosylceramide, adjuvant, antigen presentation, CD1d, glycolipid, innate immunity, invariant natural killer T cell
One of the hallmarks of the adaptive immune system is its capacity to mount a more rapid and effective immune response to the second contact with a pathogen compared with the first contact, thereby preventing recurrent infection by the same pathogen [1]. This property of the adaptive immune system, referred to as immunological memory, is mediated by memory B and T lymphocytes that develop during the primary immune response against a given antigen. Vaccines are designed to act as surrogate primary antigens to induce development of memory cell types reactive to the target pathogenic organisms or tumor cells. In order to be effective, a vaccine needs to induce a strong immune response against the immunizing antigens. However, purified antigens alone are often insufficient for inducing long-lasting immune responses. Most acellular vaccines require the addition of adjuvants to elicit protective immune responses. Although adjuvants, also known as the immunologist’s ‘dirty little secret’ [2], have been employed for many decades, their mechanism of action has remained elusive for many years. The principal role of adjuvants in vaccine preparations is to provide a so-called ‘danger signal’ that activates the innate immune system [3]. The innate immune system relays these danger signals to cells of the adaptive system, ensuring robust responses that induce immunological memory. Most adjuvants work by activating antigen-presenting cells, most notably dendritic cells (DCs), which play a key role in initiating adaptive immune responses [4]. Prominent adjuvants include microbial products that interact with Toll-like receptors (TLRs) and other pattern-recognition receptors on DCs, resulting in cytokine secretion and induction of costimulatory molecules. In turn, activated DCs stimulate the activation and differentiation of antigen-specific T lymphocytes.
Based on our understanding of the biological activities of adjuvants, it is now possible to develop rational approaches for improving the activity of vaccines. One approach is to coadminister cytokines that potentiate the antigen-presenting properties of DCs. Another approach is to stimulate cells of the innate immune system that engage in intimate interactions with DCs. Invariant natural killer T (iNKT) cells represent such a cell type of the innate immune system that holds substantial promise as a target for the development of vaccine adjuvants.
Invariant natural killer T cells
Natural killer T (NKT) cells are a unique subset of T lineage cells that coexpress T-cell receptors (TCRs) and receptors of the natural killer (NK) cell lineage (FIGURE 1A). Although these cells express TCRs and share common developmental pathways with conventional T lymphocytes, many functional characteristics of these cells categorize them as components of the innate immune system. Most NKT cells express a semi-invariant TCR, Vα14-Jα18/Vβ8.2, Vβ7 or Vβ2 in mouse [5–7], and Vα24-Jα18/Vβ11 in human [8,9]. As such, these cells have a limited ligand repertoire, and are referred to as iNKT cells or type I NKT cells. The remaining NKT cells express noninvariant TCRs, exhibit diverse ligand specificities and are referred to as type II NKT cells [10]. Although there is growing evidence for an important immunoregulatory role of type II NKT cells [11–15], their immunological functions remain incompletely understood, and we focus on iNKT cells in this review.
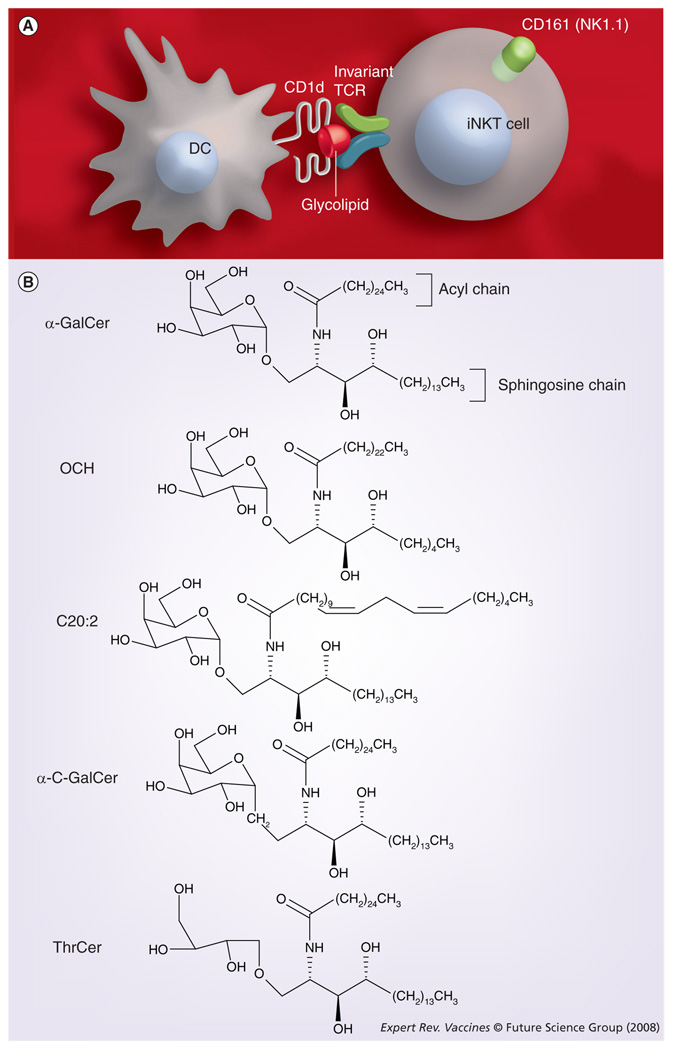
Figure 1. iNKT cells and immunomodulatory glycolipids with adjuvant activity.
(A) iNKT cells recognize glycolipids presented by CD1d. (B) α-GalCer and related glycolipids with promising adjuvant activities.
DC: Dendritic cell; GalCer: Galactosylceramide; iNKT: Invariant natural killer T cell; TCR: T-cell receptor; ThrCer: Threitolceramide.
The phenotype of iNKT cells shows a number of interesting features [16]. Several receptors commonly found on NK cells are also expressed on iNKT cells. Chief among these is the C-type lectin NK1.1 (also called Nkrp1c or CD161; FIGURE 1A), an activation receptor that biases cytokine secretion by iNKT cells toward IFN-γ [17]. A significant subset of iNKT cells also express NKG2D [18], a highly conserved C-type lectin-like membrane glycoprotein expressed on most NK cells and on subsets of T cells. NKG2D acts as an activating receptor for enhancing cytolytic activity and cytokine secretion, and has been implicated in T- and NK cell responses against viruses and tumors, as well as during autoimmunity [19]. In addition, some iNKT cells, particularly thymic iNKT cells, express various Ly49 receptors [20,21], most of which relay inhibitory signals. iNKT cells also express CD94 [20,21], a component of the inhibitory receptor CD94/NKG2A and the activating receptor CD94/NKG2C. Approximately 60% of iNKT cells express CD4 [22], which enhances TCR signaling in conventional, MHC class II-restricted T cells. Emerging evidence indicates that CD4 can enhance iNKT cell activation [23,24] and that CD4+ and CD4− iNKT cell subsets exhibit distinct functional properties [18,25,26]. Compared with conventional T cells, the levels of TCR expression on iNKT cells are substantially lower [22]. Furthermore, at baseline, iNKT cells exhibit an activated phenotype, with high expression levels of CD44, CD69 and CD122, and low levels of CD62L [22].
In sharp contrast with conventional T cells, which recognize peptide antigens presented by MHC class I or II proteins, iNKT cells are specific for glycolipid antigens presented by the MHC class I-related protein CD1d (FIGURE 1A) [22,27]. CD1d consists of a heterodimer of a glycosylated heavy chain and β2-microglobulin [28]. While related to MHC molecules, CD1d is structurally quite distinct, with a binding pocket adapted for glycolipid antigens [15,29–32]. CD1d is constitutively expressed on antigen-presenting cells, such as DCs, macrophages and B cells, which mediate activation of iNKT cells in the periphery, and also on cortical thymocytes, which are required for the development of iNKT cells during thymic selection [33–36]. In addition, high levels of CD1d are found on Kupffer cells, sinusoidal endothelial cells and hepatocytes in the liver [37]. CD1d is also expressed in the human intestine [38] and is upregulated on microglial cells during inflammation in the brain [39].
The distribution of iNKT cells has been well studied in mice. Quantitatively, murine iNKT cells represent approximately 0.5% of all thymocytes, 0.5% of T cells in peripheral lymph nodes, 2.5% of T cells in the spleen and peripheral blood, 5% of T cells in bone marrow and 30% of T cells in the liver [20,22]. While the distribution of iNKT cells in humans is less well characterized, they appear to be approximately ten-times less frequent in most organs, including the liver [40,41]. In addition, the frequency of iNKT cells in human peripheral blood is highly variable, differing up to 100-fold among individuals [42,43].
Glycolipid antigens for iNKT cells
All iNKT cells react with the glycolipid α-galactosylceramide (α-GalCer; FIGURE 1B), which was originally isolated from the marine sponge Agelas mauritianus during a screen for reagents with antimetastatic activities in mice [44–47]. α-GalCer bound with CD1d elicits extremely strong interactions with the murine iNKT cell receptor [48,49]. This interaction is somewhat weaker for the human iNKT cell receptor but remains robust.
More recently, it has become apparent that iNKT cells recognize several microbial glycolipids. In particular, iNKT cells react with α-anomeric glycosphingolipids, including α-glucuronosylceramide and α-galacturonosylceramide, derived from the cell wall of Sphingomonas bacteria [50–52]. Sphingomonas (also called Novosphingobium) species are Gram-negative, lipopolysaccharide-negative α-proteobacteria that are ubiquitously present in marine and soil environments. The Sphingomonas glycolipids are strong stimulators of iNKT cells and play an important role in the host defense against Sphingomonas. As these glycolipids are structurally similar to α-GalCer, and because marine sponges are often colonized by bacteria, including Sphingomonas species [53], it has been suggested that α-GalCer is derived from Sphingomonas or related microorganisms [54]. In addition to Sphingomonas antigens, α-galactosyl-diacylglycerols from the spirochete Borrelia burgdorferi, the etiologic agent of Lyme disease, can also activate iNKT cells [55]. Although iNKT cells play an important role in generating protective, marginal-zone B-cell-derived antibodies during Borrelia infection [56], the contribution of the Borrelia-derived iNKT cell antigens to this response remains unclear.
In addition to microbial iNKT cell ligands, much attention has been given to the identification of endogenous ligands. The idea that iNKT cells recognize an endogenous glycolipid(s) is based on the observation that both mouse and human iNKT cells exhibit autoreactivity to CD1d-expressing cells [57–59]. Moreover, activation of iNKT cells by certain ligands of TLRs requires reactivity toward CD1d [50,60]. Autoreactivity is also thought to mediate iNKT cell development in the thymus during positive and negative selection and to influence the maturation of new thymic iNKT cell emigrants in the periphery [36,61,62]. Recent findings have demonstrated that the glycosphingolipid isoglobotrihexosylceramide (iGb3) can activate the majority of mouse and human iNKT cells, but reactivity was weak compared with α-Galcer or the bacterial antigens [63,64]. Hexosaminidase B-deficient mice, which lack the lysosomal enzymatic activity to degrade a precursor lipid to iGb3, lacked iNKT cells [63]. However, a contradictory report demonstrated that deficiency in iGb3 synthase, a putative enzyme essential for iGb3 production, did not affect iNKT cell ontogeny and function [65]. Furthermore, it has been reported that humans lack functional iGb3 synthase [66]. Nevertheless, it is possible that an alternate synthesis pathway to iGb3 exists in vivo. One group of investigators was unable to detect iGb3 in the thymus and peripheral lymphoid organs [67] but a more recent study, employing highly sensitive detection techniques, reported the presence of iGb3 in human thymus [68], lending support for the relevance of this molecule as an endogenous iNKT cell antigen. Additional studies will be needed to investigate the physiological relevance of iGb3 and potentially other endogenous glycolipids, to iNKT cell biology.
iNKT cell responses to glycolipid antigens
Although a subset of the T-cell lineage, iNKT cells appear to play a pivotal role in bridging innate and adaptive immunity [69]. As the identity of physiological ligands has remained elusive, the function of iNKT cells has been studied predominantly with α-GalCer and its derivatives. While iNKT cells are capable of cytotoxic activity through the expression of perforins and granzymes as well as membrane-bound TNF family members, including Fas ligand, their primary effector function is to modulate immune responses by producing various cytokines [70]. Indeed, the hall-mark of iNKT cell activation is the rapid and robust secretion of a variety of cytokines, including IFN-γ, IL-2, -4, -5, -10, -13, -21, granulocyte–macrophage colony-stimulating factor, TNF-α and -β, immediately following TCR engagement [71,72]. This is in direct contrast to naive, conventional T cells, which require prolonged primary stimulation for cytokine secretion. Conventional CD4 T-cell responses usually also become biased towards either Th1 or Th2 cytokine production, but iNKT cells have the capacity to secrete both cytokines simultaneously [73]. iNKT cells initiate IFN-γ and IL-4 gene transcription during thymic development, resulting in the presence of abundant mRNA transcripts in antigen-inexperienced iNKT cells, allowing rapid cytokine production upon activation [74].
The cytokines secreted by activated iNKT cells amplify the immune response initiated by these cells through transactivation of other cell types, including DCs, macrophages, NK cells, B cells and conventional CD4 and CD8 T cells [75–79]. This is evidenced by rapid upregulation of the activation marker CD69 or NK and B and T cells, and by induction of various costimulatory molecules on DCs, macrophages and B cells, following in vivo injection of α-GalCer. Cytokine production by these cells further contributes to the burst of cytokines initiated by the activated iNKT cells. DCs activated by iNKT cells release significant levels of proinflammatory cytokines, such as IL-12 and TNF-α, as early as 4 h after intravenous injection of α-GalCer [80–82]. In response to IFN-γ produced by iNKT cells and IL-12 produced by DCs, NK cells rapidly begin to secrete large amounts of IFN-γ. The capacity to transactivate many other immune cell types identifies iNKT cells as key modulators of innate and adaptive immunity. These characteristics allow iNKT cells to efficiently amplify the adaptive immune response during vaccination, permitting iNKT cell ligands to act as potent adjuvants.
While iNKT cells can produce copious amounts of Th1 and Th2 cytokines, the balance between Th1 and Th2 cytokines varies according to the particular glycolipid antigen employed to stimulate these cells. Much like adjuvants that promote Th1 responses, such as bacterial CpG, and adjuvants that promote Th2 responses, such as alum, the diversity observed among glycolipid antigens provides flexibility in the application of iNKT cell ligands as vaccine adjuvants. While α-GalCer induces secretion of a mixture of Th1 and Th2 cytokines by iNKT cells [83], structural analogs of this reagent have been developed that preferentially elicit Th1 or Th2 cytokine production by iNKT cells [75,83–88]. For example, the C-glycoside analog of α-GalCer, α-C-GalCer (FIGURE 1B) preferentially induces Th1 cytokine secretion by iNKT cells [88,89]. On the other hand, glycolipids with shorter lipid chains, such as OCH, or less saturated lipid chains, such as C20:2 (FIGURE 1B), exhibit a Th2 bias in the cytokine production profile elicited by iNKT cells [76,84–86,89]. It will be interesting to determine whether iNKT cell antigens with variable structures derived from microbial pathogens can similarly induce differential responses by iNKT cells. For the purpose of developing vaccine adjuvants, the utility of structural variants of α-GalCer permits substantial flexibility in exploiting the adjuvant activities of iNKT cells.
Kinetics of iNKT cell responses
For naive, conventional peptide-reactive T cells, a primary antigenic stimulation in conjunction with adequate costimulation is followed by expansion and differentiation into effector cells, which typically takes several days. After clearance of the particular antigen, a population of memory T cells with the same antigen specificity emerges to mediate a more rapid and effective immune response during secondary challenge [1].
The response of glycolipid-reactive iNKT cells to antigenic stimulation is quite distinct from that of conventional T cells. A detailed analysis of the in vivo response of murine iNKT cells to α-GalCer has been reported by our laboratory and others. During the primary response to α-GalCer, iNKT cells rapidly produce cytokines, concomitant with a rapid downregulation of surface TCR expression, leading to an apparent ‘disappearance’ of these cells approximately 6–12 h after the initial α-GalCer treatment [90–93]. Surface TCR expression is largely restored by 24 h and these cells then undergo an extensive in vivo expansion, reaching maximal levels on approximately day 3, with a ten- to 15-fold increase in cellularity in the spleen [90–92]. In vivo expansion of iNKT cells is also observed in other organs, such as the lymph nodes, peripheral blood, liver and bone marrow, but not in the thymus. Following the peak response, iNKT cells gradually decrease in number to levels slightly lower than prechallenge. During this time period most iNKT cells lose NK1.1 expression, and this loss of NK1.1 expression persists up to 6 months following the initial α-GalCer challenge [94].
Invariant natural killer T cells also become activated following infection with many microorganisms [54]. While a few bacteria contain iNKT cell ligands, in most cases the precise mechanism of iNKT cell activation remains unclear. However, most evidence indicates that stimulation of TLRs and other pattern-recognition receptors on DCs by microbial products results in the production of cytokines (e.g., IL-12, IL-18 and type I interferons), which, in turn, activate iNKT cells [60,95]. iNKT cell activation in this manner usually also requires CD1d expression, possibly involving interactions of the iNKT cell receptor with endogenous glycolipid antigens, such as iGb3 [50,60,96]. In contrast to the mixed cytokine response of iNKT cells to bacteria that contain cognate iNKT cell antigens, this indirect mode of iNKT cell activation results in a Th1-biased cytokine production profile, often with undetectable levels of IL-4 [60]. Many microorganisms also induce a profound downregulation of NK1.1 on iNKT cells [97]. iNKT cell expansion is rarely observed, but some infectious agents, including Listeria monocytogenes [97] and lymphocytic choriomeningitis virus [98], induce substantial iNKT cell depletion in mice. Likewise, circulating iNKT cell numbers are also suppressed in humans during certain chronic infections, including infections with HIV [43] and Mycobacterium tuberculosis [99].
The secondary response of iNKT cells to α-GalCer challenge in mice is characterized by hyporesponsiveness [94,100,101]. For at least 1 month after the initial challenge, iNKT cells showed significantly suppressed capacity to proliferate and secrete cytokines in response to rechallenge with α-GalCer both ex vivo and in vivo. This decrease in cytokine production was associated with the inability of iNKT cells to transactivate DCs, B cells and NK cells, and these anergic iNKT cells were unable to demonstrate anti-tumor activities against B16 melanoma cells, indicating that α-GalCer-induced iNKT cell anergy might limit the utility of iNKT cell-based therapies, at least when α-GalCer treatment is performed repeatedly. Results from clinical trials of α-GalCer therapy in cancer patients are consistent with development of anergy in human iNKT cells following administration of free α-GalCer [102].
Similar to α-GalCer, several microorganisms that down-regulate NK1.1, including Salmonella typhimurium, Escherichia coli, Staphylococcus aureus, L. monocytogenes and Mycobacterium bovis, bacillus Calmette–Guérin induced a hyporesponsive state in iNKT cells [97,103,104]. iNKT cell hyporesponsiveness has also been observed in the context of sulfatide injection, which activates type II NKT cells and appears to indirectly influence iNKT cell functions through DCs [105]. Thus, induction of hyporesponsiveness appears to be a common theme following iNKT activation. iNKT cells from mice and humans with cancer also exhibit a hyporesponsive phenotype, but it is unclear whether this is due to iNKT cell activation or active suppression mediated by nitric oxide-producing macrophages [106,107]. This hyporesponsive phenotype of iNKT cells, which has been observed in multiple settings, should be taken into consideration when designing iNKT cell-based vaccine adjuvants.
Enhancement of adaptive cellular immunity by iNKT cells
The maturation of DCs by activated iNKT cells is central to the adjuvant activity of iNKT cells. Initial studies demonstrated the capacity of α-GalCer to improve CD8 T-cell stimulation by peptide-pulsed DCs ex vivo, but this only occurred at unusually high doses of peptide [108]. It has been suggested that this vaccine strategy immobilizes DCs within the sinusoids of the splenic red pulp, where iNKT cells are localized, and not in T-cell-rich areas where adaptive immune responses are generated [80].
Several groups have reported that in vivo injection of α-GalCer induces phenotypic and functional maturation of DCs leading to the priming of CD4 and CD8 effector T cells in mice [81,109–111]. As early as 4 h after intravenous injection of 1–2 µg α-GalCer, profound upregulation of costimulatory molecules, including CD40, CD80 and CD86, as well as molecules involved in antigen capture and presentation, such as MHC class II and DEC205, was evident on DCs [81,82]. Unlike other maturation stimuli for DCs, the effects of α-GalCer were indirect because DCs from Jα18-deficient mice that lack iNKT cells failed to mature in response to α-GalCer [81,109]. Furthermore, splenic DCs matured by α-GalCer in vivo efficiently captured ovalbumin (OVA) protein antigens and exhibited enhanced stimulation of naive CD4 and CD8 T cells in culture [81,109,111]. This maturation of splenic DCs was accompanied by increased IL-12 and TNF-α secretion by DCs and IFN-γ secretion by NK cells [80–82]. Blockade of TNF-α or IFN-γ during α-GalCer treatment prevented the upregulation of costimulatory molecules and enhancement of immune responses induced by α-GalCer [80,111]. However, expression of IFN-γR was not required for the adjuvant activities of α-GalCer [109]. Instead, CD40–CD40L interactions between DCs and iNKT cells were crucial for the induction of CD4 and CD8 T-cell immunity by α-GalCer-matured DCs. CD40L was upregulated on iNKT cells following α-GalCer challenge and induced CD40 signaling in DCs [82]. In the absence of either CD40 or CD40L, α-GalCer no longer acted as an adjuvant for T-cell-mediated immunity [109,112]. This critical dependence on the delivery of CD40 signals to DCs is surprising because antigen presentation and expression of costimulatory molecules by DCs from CD40- and CD40L-deficient mice were largely unaffected [112]. Likewise, the improved adjuvant activity of α-C-GalCer compared with α-GalCer correlated with the degree of CD40L upregulation on iNKT cells and antigen presentation by DCs, but not with upregulation of CD86 on DCs [113]. Additional reports further showed that the adjuvant activities of iNKT cells require CD40 signaling and antigen presentation to CD8 T cells on the same DCs [109,114]. Furthermore, it has been demonstrated that CD40 signaling on DCs by activated iNKT cells results in upregulation of CD70 on DCs [114]. CD70 interacts with CD27, a costimulatory receptor on CD8 T cells, and blocking the interaction between CD70 and CD27 abrogated the capacity of iNKT cells to promote CD8 T-cell-mediated immune responses [114].
Additional studies showed that, among DC subsets, the CD8α− subset was most important for T-cell priming induced by soluble α-GalCer, as this subset was largely responsible for IL-12 secretion [81]. Likewise, CD8α− DCs in mediastinal lymph nodes were shown to play a critical role in priming naive T-cell responses during the intranasal coadministration of a protein antigen with α-GalCer [110].
Enhancement of adaptive humoral immunity by iNKT cells
Induction of a robust humoral immune response is crucial to the development of effective vaccines against many pathogens. Therefore, the capacity of iNKT cells to enhance antibody production by B cells has been closely scrutinized. Early studies demonstrated that α-GalCer treatment induced an increase in total serum IgE antibody levels in mice [115,116]. Another research group reported that human peripheral blood iNKT cells stimulated ex vivo with α-GalCer promoted autologous memory B-cell responses [117]. Interestingly, iNKT cells promoted antibody production even in the absence of α-GalCer, suggesting that autoreactivity of iNKT cells conveyed by an endogenous ligand can provide B-cell help [117]. These investigators further showed that murine iNKT cells enhanced antibody responses to protein antigens in vivo, even in the absence of exogenous stimuli [118]. Likewise, another group of investigators demonstrated that activation of iNKT cells by α-GalCer enhanced antibody responses to coadministered T-cell-dependent antigens through the induction of persistent plasma cell responses [119]. Another study showed that coadministration of intranasal allergen with α-GalCer resulted in increased allergen-specific IgE production and exacerbation of asthma induction [120]. Finally, additional studies showed that, in the setting of contact hypersensitivity, iNKT cells were required for IgM production by marginal-zone B cells [121,122].
Invariant natural killer T cells may influence antibody production by B cells in several ways. During protein antigen challenge, cognate interaction of CD4+ T cells with naive B cells is critically important for driving affinity maturation, class switching, plasma cell differentiation and memory B-cell development [123]. Similarly, the adjuvant activity of iNKT cells on the generation of CD4 T-cell responses indirectly enhanced T-cell-dependent humoral responses [118]. Interestingly, in addition to their effects on conventional CD4 T cells, it has been demonstrated that iNKT cells can substitute for conventional CD4 T cells in providing B-cell help. For example, α-GalCer was able to induce significant antibody production against coadministered protein antigens in MHC class II-deficient mice [118]. Similar findings were obtained in an independent study, but these investigators only observed antibody production when agonistic anti-CD40 antibodies were coadministered with α-GalCer [124]. This group also reported that α-GalCer enhanced production of antibodies against the T-cell-independent antigen Ficoll [124]. Recent studies have shown an absolute requirement for CD1d expression by B cells for the adjuvant activity of α-GalCer following immunization with a T cell-dependent antigen [125], indicating that direct activation of B cells by iNKT cells is probably the primary means of the adjuvant activity of α-GalCer. This principle has been exploited in studies that have targeted glycolipid antigens to B cells via the B-cell receptor, resulting in the recruitment of cognate iNKT cell help for antigen-specific antibody production by B cells [126,127].
An additional mechanism by which iNKT cells might contribute to the generation of antibody responses is through their capacity to induce IFN-γ production by NK cells, thus promoting IgG antibody production by B cells [128].
The effector mechanisms of iNKT cells that are responsible for the adjuvant activities of α-GalCer on antibody responses remain unclear. While CD69 upregulation by B cells in response to α-GalCer stimulation was dependent on IL-4 secretion by iNKT cells [78], α-GalCer retained its adjuvant activities in IL-4-deficient mice, suggesting that other cytokines such as IL-13 can take its place [118]. Moreover, IFN-γ production by iNKT cells was also dispensable, but was required for the Th1-like component (i.e., IgG2a) of the humoral immune response [118]. However, akin to the capacity of iNKT cells to promote DC maturation, CD40–CD40L interactions between B cells and iNKT cells were a critical component for the adjuvant activity of α-GalCer on antibody production [118].
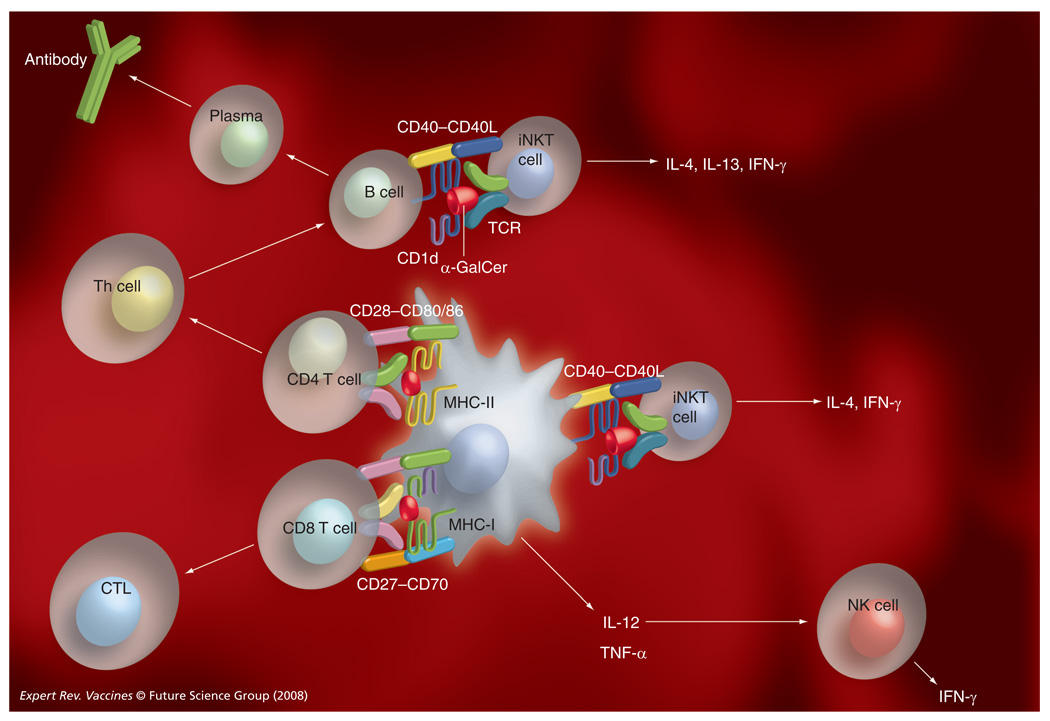
Collectively, these studies have provided strong evidence for potent adjuvant activities of α-GalCer and related glycolipid antigens. Our current understanding of the mechanisms involved is depicted in FIGURE 2.
Figure 2. Enhancement of humoral and cellular immunity by iNKT cells.
Upon coadministration of α-GalCer and a vaccine antigen, α-GalCer is presented by dendritic cells (DCs) and B cells to iNKT cells, resulting in upregulation of CD40 on these cells. DCs also upregulate other costimulatory molecules, including CD80, CD86 and CD70, and start producing cytokines, such as IL-12 and TNF-α. Activation of iNKT cells also results in the transactivation of NK cells, which start producing IFN-γ. The adjuvant activities of α-GalCer for adaptive cellular immune responses require presentation of α-GalCer and MHC class I- or class II-restricted antigens by the same DCs. The adjuvant activities of α-GalCer for adaptive humoral immunity are mediated indirectly through the adjuvant activities of α-GalCer on Th cell responses, and directly by interactions between antigen-specific B cells and iNKT cells.
CTL: Cytotoxic T lymphocyte; GalCer: Galactosylceramide; iNKT: Invariant natural killer T cells; NK: Natural killer cell; TCR: T-cell receptor.
iNKT cell-based adjuvants for vaccines against infectious diseases
The adjuvant activities of iNKT cells have been tested in vaccines against several pathogens. Although the physiological role of iNKT cells appears to be dispensable for inducing an antibody response against pre-erythrocytic stages of malaria parasites, Tsuji and colleagues found that α-GalCer greatly enhanced the protective antimalaria immunity induced by irradiated sporozoites or recombinant viruses expressing a malaria antigen in mice [129]. This adjuvant activity of α-GalCer was dependent on IFN-γ production by iNKT cells. Extensive studies investigating the immune response against malaria have demonstrated a central role for CD8 T-cell immunity during the liver stages of the parasites [130], thus explaining the requirement for IFN-γ. These results further suggested that enhanced IFN-γ production might benefit the adjuvant activities of iNKT cells. Consistently, Tsuji and colleagues found a 1000-fold potentiation of the antimalaria immune response with α-C-GalCer, an α-GalCer analog that induces more prolonged production of IFN-γ and IL-12, with decreased production of IL-4 compared with α-GalCer [89].
Kang and colleagues evaluated the utility of α-GalCer as a mucosal adjuvant in different vaccine formulations directed against an influenza virus [131]. Intranasal administration of purified hemagglutinin or a hemagglutinin-expressing adenovirus, together with α-GalCer, enhanced mucosal as well as systemic humoral immune responses to the virus. Additional studies by Seino and colleagues showed that this immunization strategy stimulated mucosal IgA production and afforded cross-protection against different influenza virus strains [132]. Furthermore, this vaccine provided long-term protective immunity without redirecting antigens into the CNS, thus confirming the safety of the proposed vaccine strategy [133].
α-GalCer was also effective in enhancing the immunogenicity of HIV-1 DNA vaccine [134]. In a DNA prime–DNA boost regimen, this adjuvant activity was maximal when α-GalCer was administered during the initial priming but not during the boost phase. Interestingly, in addition to enhancing CD4 and CD8 T-cell responses, α-GalCer promoted a tenfold increase in humoral immune responses, which is typically induced only at low levels by DNA vaccines.
iNKT cell-based adjuvants for tumor vaccines
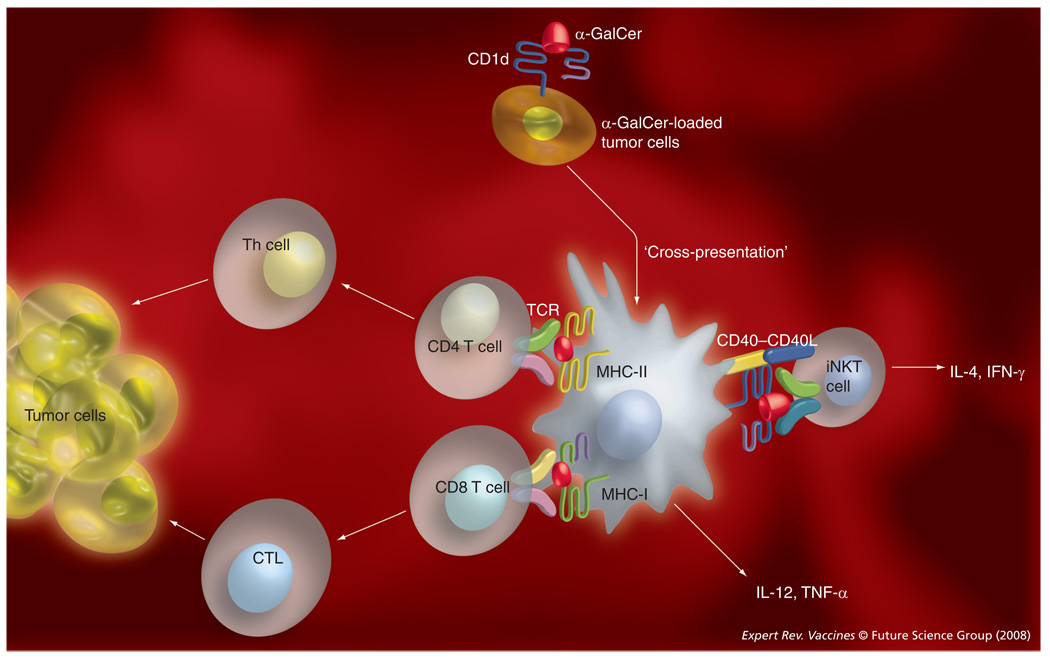
α-GalCer was first identified while screening for a marine sponge-derived molecule with anti-tumor activity against B16 melanoma cells in mice. Promising preclinical studies with α-GalCer, α-GalCer analogs and α-GalCer-loaded DCs in mice [135] have led the way to several clinical trials in cancer patients [102,136–138]. Although significant anti-tumor efficacy has not yet been achieved, these studies have demonstrated the safety of α-GalCer treatment in humans. Several studies have investigated the capacity of iNKT cell ligands to promote the efficacy of cancer vaccines [139]. Cerundolo and colleagues immunized mice with a viral vector containing the OVA antigen [111]. α-GalCer significantly enhanced priming and boosting of CD8 T cells to OVA, which resulted in the eradication of established tumors bearing OVA antigens. Similar results were obtained with the human cancer/testis antigen NY-ESO-1, which is normally expressed in tumor tissue but not in adult somatic tissue [111]. α-GalCer significantly enhanced T-cell immunity against a clinically relevant HLA-A2-restricted peptide epitope of the NY-ESO-1 antigen. Steinman and colleagues have observed that intravenous coadministration of α-GalCer with irradiated MHC class I-negative plasmacytoma cells in mice led to improved T-cell immunogenicity through maturation of splenic DCs that captured and presented tumor antigens [140]. This vaccine strategy conferred tumor resistance for at least 2 months. The adjuvant activity of α-GalCer against the irradiated plasmacytoma vaccine required CD4 and CD8 T cells, as the depletion of these cells abrogated tumor resistance. Consistently, α-C-GalCer, which is more effective than α-GalCer in inducing Th1 responses and DC maturation, was superior to α-GalCer in promoting antitumor activity [113]. Fujii and colleagues further showed that intravenous delivery of α-GalCer-loaded B16 melanoma cells transduced with CD1d provided protective immunity against subcutaneous rechallenge of the melanoma cells [141]. These findings were reproduced with three additional, poorly immunogenic tumors. Importantly, immunity was long lived, persisting for 6–12 months. In addition, this vaccine strategy was effective during intravenous and subcutaneous tumor challenge, whereas α-GalCer-loaded DCs only demonstrated significant efficacy when the tumor cells were challenged intravenously [142]. Mechanistic studies further showed that the glycolipid-loaded tumor cells conferred tumor resistance through activation of CD8α+ DCs that captured these tumor cells and cross-presented the glycolipid to iNKT cells (FIGURE 3) [141]. Tumor resistance in this vaccine model required CD4 and CD8 T cells [141,143].
Figure 3. α-GalCer-loaded tumor cells as therapeutic tumor vaccines.
In this promising vaccine strategy, tumor cells expressing CD1d (endogenously or following transfection with the CD1d gene) are loaded ex vivo with α-GalCer and then administered to tumor-carrying subjects. The tumor cells are then taken up by dendritic cells (DCs), which cross-present α-GalCer to iNKT cells, and tumor antigens to MHC-I- and -II-restricted T cells. The adjuvant activity of α-GalCer in this model is mediated through activation of tumor antigen-presenting DCs, with a key role for CD40–CD40L interactions between DCs and iNKT cells.
CTL: Cytotoxic T lymphocytes; GalCer: Galactosylceramide; iNKT: Invariant natural killer T cells; TCR: T-cell receptor.
Expert commentary
The preclinical studies demonstrating robust adjuvant activities of iNKT cell ligands in a variety of vaccine settings raises enthusiasm for developing these reagents as a new class of adjuvants. As iNKT cell ligands are distinct in their mechanism of action from currently employed vaccine adjuvants, their utility might complement that of other adjuvants. Furthermore, variations in the capacity of distinct iNKT cell ligands to promote Th1 or Th2 immune responses offers flexibility in tailoring vaccine strategies according to the specific cytokine profiles required for inducing protective immunity against target microorganisms or tumors. For instance, α-C-GalCer induces a Th1-polarized cytokine production profile by iNKT cells, mediates more effective maturation of DCs and induces stronger cellular immune responses against tumors and viruses than α-GalCer [113]. In vaccine settings where a Th2 cytokine environment is critical for induction of effective humoral immunity, reagents such as OCH or C20:2 might provide a better adjuvant activity. In addition, α-GalCer has demonstrated efficacy in both systemic and mucosal vaccine regimens [131], further emphasizing the versatility of this glycolipid.
Nonetheless, a better understanding of the immunological functions of iNKT cells will be needed to develop iNKT cell antigens as effective vaccine adjuvants. Acquisition of an anergic phenotype by murine iNKT cells following strong stimulation with α-GalCer is of practical concern in utilizing these cells as adjuvant targets, because the vaccine regimens will need to take into consideration previous exposure to iNKT cell antigens. In this context, the recent development of a nonglycosidic iNKT cell antigen, threitolceramide (FIGURE 1B), exhibiting reduced activation-induced anergy while retaining the capacity to mature DCs and prime T and B cells [144], provides a potential solution to overcoming the problem of iNKT cell anergy. Another possibility is to utilize low doses of iNKT cell ligands that fail to induce iNKT cell anergy, a method employed by Galli and colleagues in a vaccine strategy that involved priming and boosting in the presence of α-GalCer [118]. Nevertheless, this approach might also be flawed because continued activation of iNKT cells triggered by Sphingomonas ligands released in small amounts during persistent infection resulted in destruction of bile ducts, resembling primary biliary cirrhosis in humans [145]. Thus, repeated low-dose treatment with iNKT cell antigens, in conjunction with vaccine antigens, might induce unexpected side effects resulting from chronic immune system activation.
Of more significant concern is the induction of iNKT cell hyporesponsiveness by microorganisms themselves. Aside from routine infection, which may impact iNKT cell functions, vaccines derived from inactivated microorganisms might also interfere with the adjuvant activities of these cells during subsequent vaccination. iNKT cells in cancer-bearing individuals also exhibit hyporesponsiveness [106,107], potentially limiting the utility of iNKT cell-based adjuvants in cancer patients. We currently have limited understanding of the impact of prior iNKT cell stimulation on the functional responses of these cells in humans, but it probably reflects our results in mice [102]. iNKT cell numbers and functions have wide individual variability in humans, probably reflecting genetic polymorphisms, as well as environmental influences such as infection, cancer or various other chronic diseases [20,146]. In many cases iNKT cell functions were significantly suppressed. Clinical trials in terminal cancer patients have further demonstrated that activation of the biological functions of iNKT cells with free α-GalCer is challenging [102,147]. Interestingly, in a study where α-GalCer was administered to patients at three consecutive 7-day intervals, decreased biological activity was obtained upon each subsequent treatment [102], probably reflecting iNKT cell anergy. Additional studies with α-GalCer-loaded DCs have demonstrated not only improved mobilization of iNKT cell functions, but also lack of iNKT cell anergy [136,138,142]. However, it is unlikely that the latter approach can be applied on a routine basis during therapeutic tumor vaccination. Alternative methods of activating iNKT cells that avoid the induction of iNKT cell anergy will be needed for developing effective human vaccines.
Another potential obstacle is that iNKT cell antigens might induce a tolerogenic environment that can adversely affect their adjuvant activity. Complete Freund’s adjuvant, which is commonly used in experimental animals, promotes the development of tolerogenic macrophages that has impacted its utility as a vaccine adjuvant [148]. In the case of iNKT cells, the tolerogenic activities of these cells have been associated with Th2 cytokine secretion [70,83], suggesting that induction of a tolerogenic environment can be avoided with iNKT cell ligands that promote Th1 cytokine secretion by iNKT cells.
Although α-GalCer has been well tolerated during a number of clinical trials for cancer and hepatitis C virus infection [102,136–138,149], this glycolipid has been associated with a number of side effects in mice, including hepatotoxicity, abortion, atherosclerosis and allergy [83]. As more effective ways to mobilize iNKT cell functions are being developed, we will need to recognize the possibility that similar adverse drug reactions might occur in humans.
Five-year view
Future studies in the field should be focused on optimizing iNKT cell-based adjuvant strategies and overcoming obstacles to clinical application. The key objective should be to effectively mobilize the adjuvant activities of iNKT cells in humans containing variable numbers and functions of these cells while avoiding detrimental side effects. Major research efforts should be focused on identifying structural variants of α-GalCer or novel iNKT cell antigens with superior adjuvant activities. Furthermore, it will be important to identify iNKT cell antigens or vaccine strategies that minimally affect the secondary response in order to retain the capacity to employ iNKT cell adjuvants during priming and boosting.
Another important area for future investigation is to explore combination adjuvants, combining the adjuvant activities of iNKT cell antigens with other adjuvants. For instance, because α-GalCer acts independently of the TLR adaptor protein MyD88 [81] it might synergize with TLR ligands in promoting the maturation of DCs. One study has already provided evidence for cooperation between the adjuvant activities of α-GalCer and bacterial monophosphoryl lipid A, which binds with TLR4 [111]. However, such synergistic effects were not observed when α-GalCer was combined with several other TLR ligands [119]. Combination vaccine adjuvants might be able to employ low doses of iNKT cell antigens, thus avoiding some of the obstacles associated with treatments that require high doses of iNKT cell antigens.
Key issues.
Invariant natural killer T (iNKT) cells express a semi-invariant T-cell receptor that is reactive towards glycolipid antigens presented by the CD1d protein.
iNKT cells have potent adjuvant properties that can be harnessed with cognate glycolipid antigens, such as α-galactosylceramide (α-GalCer).
iNKT cell activation with α-GalCer results in rapid secretion of Th1 and Th2 cytokines, leading to transactivation of various other immune cells.
iNKT cell ligands promote adaptive cellular immunity by inducing the maturation and antigen-presenting properties of dendritic cells.
iNKT cell ligands promote adaptive humoral immunity by interacting with B cells and through their capacity to promote CD4 T-cell responses.
Preclinical studies have demonstrated the efficacy of α-GalCer as an adjuvant in vaccines directed against microorganisms and tumors.
Future studies should focus on optimizing the adjuvant activities of iNKT cell ligands while minimizing their side effects, using α-GalCer analogs and/or by combining iNKT cell-based adjuvants with other adjuvants.
Financial & competing interests disclosure
Work in the authors’ laboratory was supported by grants from the NIH and a pilot and feasibility grant from the Diabetes Research and Training Center at Vanderbilt Medical Center. VV Parekh is supported by a post-doctoral fellowship from the National Multiple Sclerosis Society. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Sungjune Kim, Department of Microbiology and Immunology, Vanderbilt University School of Medicine, Room A-5301, Medical Center North, Nashville, TN 37232, USA, Tel.: +1 615 343 2708, Fax: +1 615 343 2972, sungjune.kim@vanderbilt.edu.
Saif Lalani, Department of Microbiology and Immunology, Vanderbilt University School of Medicine, Room A-5301, Medical Center North, Nashville, TN 37232, USA, Tel.: +1 615 343 2708, Fax: +1 615 343 2972, saif.lalani@vanderbilt.edu.
Vrajesh V Parekh, Department of Microbiology and Immunology, Vanderbilt University School of Medicine, Room A-5301, Medical Center North, Nashville, TN 37232, USA, Tel.: +1 615 343 2708. Fax: +1 615 343 2972, vrajesh.v.parekh@vanderbilt.edu.
Lan Wu, Department of Microbiology and Immunology, Vanderbilt University School of Medicine, Room A-5301, Medical Center North, Nashville, TN 37232, USA, Tel.: +1 615 322 1290, Fax: +1 615 322 2926, lan.wu@vanderbilt.edu.
Luc Van Kaer, Department of Microbiology and Immunology, Vanderbilt University School of Medicine, Room A-5301, Medical Center North, Nashville, TN 37232, USA, Tel.: +1 615 343 2707, Fax: +1 615 343 2972, luc.van.kaer@vanderbilt.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Murphy K, Travers P, Walport M. Janeway’s Immunobiology (7th Edition) NY, USA: Garland Science; 2007. [Google Scholar]
- 2.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J. Clin. Invest. 2002;109(12):1519–1526. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH. Activation events during thymic selection. J. Exp. Med. 1992;175(3):731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendelac A, Schwartz RH. CD4+ and CD8+ T cells acquire specific lymphokine secretion potentials during thymic maturation. Nature. 1991;353(6339):68–71. doi: 10.1038/353068a0. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa K, Lin BT, Hardy RR. Murine thymic CD4+ T cell subsets: a subset (Thy0) that secretes diverse cytokines and overexpresses the Vβ8 T cell receptor gene family. J. Exp. Med. 1992;176(1):269–274. doi: 10.1084/jem.176.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Vα24-JαQ/Vβ11 T cell receptor is expressed in all individuals by clonally expanded CD4–8− T cells. J. Exp. Med. 1994;180(3):1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4–8− αβ T cells demonstrates preferential use of several Vβ genes and an invariant TCRα chain. J. Exp. Med. 1993;178(1):1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat. Rev. Immunol. 2004;4(3):231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 11.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16(4):583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 12.Terabe M, Swann J, Ambrosino E, et al. A nonclassical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J. Exp. Med. 2005;202(12):1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect. Immun. 2005;73(1):181–192. doi: 10.1128/IAI.73.1.181-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 2004;199(7):947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zajonc DM, Maricic I, Wu D, et al. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J. Exp. Med. 2005;202(11):1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 2005;26:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 17.Arase H, Arase N, Saito T. Interferon γ production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 1996;183(5):2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 2002;195(5):625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 2003;3(10):781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 20.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 21.Vincent MS, Gumperz JE, Brenner MB. Understanding the function of CD1-restricted T cells. Nat. Immunol. 2003;4(6):517–523. doi: 10.1038/ni0603-517. [DOI] [PubMed] [Google Scholar]
- 22.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 23.Thedrez A, de Lalla C, Allain S, et al. CD4 engagement by CD1d potentiates activation of CD4+ invariant NKT cells. Blood. 2007;110(1):251–258. doi: 10.1182/blood-2007-01-066217. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Wang X, Besra GS, Gumperz JE. Modulation of CD1d-restricted NKT cell responses by CD4. J. Leuk. Biol. 2007;82(6):1455–1465. doi: 10.1189/jlb.0307163. [DOI] [PubMed] [Google Scholar]
- 25.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Vα24 natural killer T cells. J. Exp. Med. 2002;195(5):637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowe NY, Coquet JM, Berzins SP, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 2005;202(9):1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 28.Brutkiewicz RR, Bennink JR, Yewdell JW, Bendelac A. TAP-independent, β2-microglobulin-dependent surface expression of functional mouse CD1.1. J. Exp. Med. 1995;182(6):1913–1919. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu D, Zajonc DM, Fujio M, et al. Design of natural killer T cell activators: structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc. Natl Acad. Sci. USA. 2006;103(11):3972–3977. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zajonc DM, Cantu C, 3rd, Mattner J, et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat. Immunol. 2005;6(8):810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch M, Stronge VS, Shepherd D, et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nat. Immunol. 2005;6(8):819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 32.Silk JD, Salio M, Brown J, Jones EY, Cerundolo V. Structural and functional aspects of lipid binding by CD1 molecules. Annu. Rev. Cell. Dev. Biol. 2008;24:369–395. doi: 10.1146/annurev.cellbio.24.110707.175359. [DOI] [PubMed] [Google Scholar]
- 33.Mandal M, Chen XR, Alegre ML, et al. Tissue distribution, regulation and intracellular localization of murine CD1 molecules. Mol. Immunol. 1998;35(9):525–536. doi: 10.1016/s0161-5890(98)00055-8. [DOI] [PubMed] [Google Scholar]
- 34.Brossay L, Jullien D, Cardell S, et al. Mouse CD1 is mainly expressed on hemopoieticderived cells. J. Immunol. 1997;159(3):1216–1224. [PubMed] [Google Scholar]
- 35.Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J. Immunol. 1998;160(7):3121–3127. [PubMed] [Google Scholar]
- 36.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 1995;182(6):2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geissmann F, Cameron TO, Sidobre S, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3(4):650–661. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blumberg RS, Terhorst C, Bleicher P, et al. Expression of a nonpolymorphic MHC class I-like molecule, CD1D, by human intestinal epithelial cells. J. Immunol. 1991;147(8):2518–2524. [PubMed] [Google Scholar]
- 39.Larsson LC, Anderson P, Widner H, Korsgrent O. Enhanced survival of porcine neural xenografts in mice lacking CD1d1, but no effect of NK1.1 depletion. Cell Transplant. 2001;10(3):295–304. [PubMed] [Google Scholar]
- 40.Karadimitris A, Gadola S, Altamirano M, et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc. Natl Acad. Sci. USA. 2001;98(6):2950–2952. doi: 10.1073/pnas.051604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emoto M, Kaufmann SH. Liver NKT cells: an account of heterogeneity. Trends Immunol. 2003;24(7):364–369. doi: 10.1016/s1471-4906(03)00162-5. [DOI] [PubMed] [Google Scholar]
- 42.Sandberg JK, Bhardwaj N, Nixon DF. Dominant effector memory characteristics, capacity for dynamic adaptive expansion, and sex bias in the innate Vα24 NKT cell compartment. Eur. J. Immunol. 2003;33(3):588–596. doi: 10.1002/eji.200323707. [DOI] [PubMed] [Google Scholar]
- 43.Motsinger A, Haas DW, Stanic AK, Van Kaer L, Joyce S, Unutmaz D. CD1d-restricted human natural killer T cells are highly susceptible to human immunodeficiency virus 1 infection. J. Exp. Med. 2002;195(7):869–879. doi: 10.1084/jem.20011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol. Res. 1995;7(10–11):529–534. [PubMed] [Google Scholar]
- 45.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 46.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 47.Borg NA, Wun KS, Kjer-Nielsen L, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448(7149):44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 48.Sidobre S, Naidenko OV, Sim BC, Gascoigne NR, Garcia KC, Kronenberg M. The Vα14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J. Immunol. 2002;169(3):1340–1348. doi: 10.4049/jimmunol.169.3.1340. [DOI] [PubMed] [Google Scholar]
- 49.Cantu C, Benlagha K, Savage PB, Bendelac A, Teyton L. The paradox of immune molecular recognition of α-galactosylceramide: low affinity, low specificity for CD1d, high affinity for αβ TCRs. J. Immunol. 2003;170(9):4673–4682. doi: 10.4049/jimmunol.170.9.4673. [DOI] [PubMed] [Google Scholar]
- 50.Mattner J, DeBord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434(7032):525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 51.Kinjo Y, Wu DY, Kim G, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 52.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur. J. Immunol. 2005;35(6):1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 53.Dieckmann R, Graeber I, Kaesler I, Szewzyk U, von Dohren H. Rapid screening and dereplication of bacterial isolates from marine sponges of the Sula Ridge by intact-cell-MALDI-TOF mass spectrometry (ICM-MS) Appl. Microbiol. Biotechnol. 2005;67(4):539–548. doi: 10.1007/s00253-004-1812-2. [DOI] [PubMed] [Google Scholar]
- 54.Tupin E, Kinjo T, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 2007;5(6):405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 55.Kinjo Y, Tupin E, Wu D, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 2006;7(9):978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 56.Belperron AA, Dailey CM, Bockenstedt LK. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J. Immunol. 2005;174(9):5681–5686. doi: 10.4049/jimmunol.174.9.5681. [DOI] [PubMed] [Google Scholar]
- 57.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268(5212):863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 58.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J. Exp. Med. 1997;186(1):109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park SH, Roark JH, Bendelac A. Tissue-specific recognition of mouse CD1 molecules. J. Immunol. 1998;160(7):3128–3134. [PubMed] [Google Scholar]
- 60.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 2003;4(12):1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 61.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296(5567):553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 62.Zimmer MI, Colmone A, Felio K, Xu H, Ma A, Wang CR. A cell-type specific CD1d expression program modulates invariant NKT cell development and function. J. Immunol. 2006;176(3):1421–1430. doi: 10.4049/jimmunol.176.3.1421. [DOI] [PubMed] [Google Scholar]
- 63.Zhou D, Mattner J, Cantu C, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306(5702):1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 64.Xia C, Yao Q, Schumann J, et al. Synthesis and biological evaluation of α-galactosylceramide (KRN7000) and isoglobotrihexosylceramide (iGb3) Bioorg. Med. Chem. Lett. 2006;16(8):2195–2199. doi: 10.1016/j.bmcl.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 65.Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ. Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc. Natl Acad. Sci. USA. 2007;104(14):5977–5982. doi: 10.1073/pnas.0611139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christiansen D, Milland J, Mouhtouris E, et al. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 2008;6(7):e172. doi: 10.1371/journal.pbio.0060172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Speak AO, Salio M, Neville DC, et al. Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc. Natl Acad. Sci. USA. 2007;104(14):5971–5976. doi: 10.1073/pnas.0607285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y, Teneberg S, Thapa P, Bendelac A, Levery SB, Zhou D. Sensitive detection of isoglobo and globo series tetraglycosylceramides in human thymus by ion trap mass spectrometry. Glycobiology. 2008;18(2):158–165. doi: 10.1093/glycob/cwm129. [DOI] [PubMed] [Google Scholar]
- 69.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat. Rev. Immunol. 2001;1(3):177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 70.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 2004;114(10):1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Kaer L. Regulation of immune responses by CD1d-restricted natural killer T cells. Immunol. Res. 2004;30(2):139–153. doi: 10.1385/IR:30:2:139. [DOI] [PubMed] [Google Scholar]
- 72.Coquet JM, Chakravarti S, Kyparissoudis K, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4−NK1.1− NKT cell population. Proc. Natl Acad. Sci. USA. 2008;105(32):11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsuda JL, Gapin L, Baron JL, et al. Mouse Vα14i natural killer T cells are resistant to cytokine polarization in vivo. Proc. Natl Acad. Sci. USA. 2003;100(14):8395–8400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stetson DB, Mohrs M, Reinhardt RL, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 2003;198(7):1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parekh VV, Lalani S, Van Kaer L. The in vivo response of invariant natural killer T cells to glycolipid antigens. Int. Rev. Immunol. 2006;26(1–2):31–48. doi: 10.1080/08830180601070179. [DOI] [PubMed] [Google Scholar]
- 76.Parekh VV, Singh AK, Wilson MT, et al. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J. Immunol. 2004;173(6):3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 77. Nishimura T, Kitamura H, Iwakabe K, et al. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int. Immunol. 2000;12(7):987–994. doi: 10.1093/intimm/12.7.987. • First paper demonstrating that α-GalCer promotes the differentiation of cytotoxic T lymphocytes.
- 78.Kitamura H, Ohta A, Sekimoto M, et al. α-galactosylceramide induces early B-cell activation through IL-4 production by NKT cells. Cell. Immunol. 2000;199(1):37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 79.Carnaud C, Lee D, Donnars O, et al. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 1999;163(9):4647–4650. [PubMed] [Google Scholar]
- 80.Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Vα14+ natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol. Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 81. Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 2003;198(2):267–279. doi: 10.1084/jem.20030324. •• Demonstrates that α-GalCer promotes dendritic cell (DC) maturation in vivo and enhances CD4 and CD8 T-cell responses to coadministered protein antigens.
- 82. Kitamura H, Iwakabe K, Yahata T, et al. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 1999;189(7):1121–1128. doi: 10.1084/jem.189.7.1121. •• First demonstration that α-GalCer promotes IL-12 production by DCs in a manner dependent on CD40–CD40 ligand interactions between DCs and invariant natural killer T (iNKT) cells.
- 83.Van Kaer L. α-galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat. Rev. Immunol. 2005;5(1):31–42. doi: 10.1038/nri1531. [DOI] [PubMed] [Google Scholar]
- 84.Goff RD, Gao Y, Mattner J, et al. Effects of lipid chain lengths in α-galactosylceramides on cytokine release by natural killer T cells. J. Am. Chem. Soc. 2004;126(42):13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 85.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing Th2 bias of natural killer T cells. Nature. 2001;413(6855):531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 86.Yu KO, Im JS, Molano A, et al. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc. Natl Acad. Sci. USA. 2005;102(9):3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: tuning the adjuvant versus immunosuppression activity. J. Am. Chem. Soc. 2006;128(28):9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 88.Yang G, Schmieg J, Tsuji M, Franck RW. The C-glycoside analogue of the immunostimulant α-galactosylceramide (KRN7000): synthesis and striking enhancement of activity. Angew. Chem. Int. Ed. Engl. 2004;43(29):3818–3822. doi: 10.1002/anie.200454215. [DOI] [PubMed] [Google Scholar]
- 89.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J. Exp. Med. 2003;198(11):1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harada M, Seino KI, Wakao H, et al. Down-regulation of the invariant Vα14 antigen receptor in NKT cells upon activation. Int. Immunol. 2004;16(2):241–247. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- 91.Crowe NY, Uldrich AP, Kyparissoudis K, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NKT cells. J. Immunol. 2003;171(8):4020–4027. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 92.Wilson MT, Johansson C, Olivares-Villagomez D, et al. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc. Natl Acad. Sci. USA. 2003;100(19):10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3ɛ-or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9(3):345–353. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 94.Parekh VV, Wilson MT, Olivares-Villagomez D, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J. Clin. Invest. 2005;115(9):2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr. Opin. Immunol. 2007;19(3):354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 96.Van Kaer L, Joyce S. Innate immunity: NKT cells in the spotlight. Curr. Biol. 2005;15(11):R429–R431. doi: 10.1016/j.cub.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 97.Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions and therapeutic activities of iNKT cells in mice. J. Clin. Invest. 2008;118(6):2301–2315. doi: 10.1172/JCI33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin Y, Roberts TJ, Wang CR, Cho S, Brutkiewicz RR. Long-term loss of canonical NKT cells following an acute virus infection. Eur. J. Immunol. 2005;35(3):879–889. doi: 10.1002/eji.200425495. [DOI] [PubMed] [Google Scholar]
- 99.Snyder-Cappione JE, Nixon DF, Loo CP, et al. Individuals with pulmonary tuberculosis have lower levels of circulating CD1d-restricted NKT cells. J. Infect. Dis. 2007;195(9):1361–1364. doi: 10.1086/513567. [DOI] [PubMed] [Google Scholar]
- 100.Ulrich AP, Crowe NY, Kyparissoudis K, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, BIM-dependent contraction, and hyporesponsiveness to further antigenic challenge. J. Immunol. 2005;175(5):3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ikarashi Y, Iizuka A, Koshidaka Y, et al. Phenotypical and functional alterations during the expansion phase of invariant Vα14 natural killer T (Vα14i NKT) cells in mice primed with α-galactosylceramide. Immunology. 2005;116(1):30–37. doi: 10.1111/j.1365-2567.2005.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giaccone G, Punt CJA, Ando Y, et al. A Phase I study of natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer. Res. 2002;8(12):3702–3709. [PubMed] [Google Scholar]
- 103.Chiba A, Dascher CC, Besra GS, Brenner MB. Rapid NKT cell responses are self-terminating during the course of microbial infection. J. Immunol. 2008;181(4):2292–2302. doi: 10.4049/jimmunol.181.4.2292. [DOI] [PubMed] [Google Scholar]
- 104.Choi HJ, Xu H, Geng Y, Colmone A, Cho H, Wang CR. Bacterial infection alters the kinetics and function of iNKT cell responses. J. Leuk. Biol. 84 doi: 10.1189/jlb.0108038. DOI: 10.1189/jlb.0108038 (2008) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J. Clin. Invest. 2007;117(8):2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yanagisawa K, Exley MA, Jiang X, Ohkochi N, Taniguchi M, Seino K. Hyporesponsiveness to natural killer T-cell ligand α-galactosylceramide in cancer-bearing state mediated by CD11b+ Gr-1+ cells producing nitric oxide. Cancer Res. 2006;66(23):11441–11446. doi: 10.1158/0008-5472.CAN-06-0944. [DOI] [PubMed] [Google Scholar]
- 107.Tahir SM, Cheng O, Shaulov A, et al. Loss of IFN-γ production by invariant NK T cells in advanced cancer. J. Immunol. 2001;167(7):4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 108.Stober D, Jomantaite I, Schirmbeck R, Reimann J. NKT cells provide help for dendritic cell-dependent priming of MHC class I-restricted CD8+ T cells in vivo. J. Immunol. 2003;170(5):2540–2548. doi: 10.4049/jimmunol.170.5.2540. [DOI] [PubMed] [Google Scholar]
- 109. Hermans IF, Silk JD, Gileadi U, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 2003;171(10):5140–5147. doi: 10.4049/jimmunol.171.10.5140. •• Demonstrates that α-GalCer enhances CD4+and CD8+ T-cell responses against coadministered protein antigen, in a manner dependent on CD40–CD40L interactions and presentation of protein antigen and α-GalCer by the same DCs.
- 110.Ko SY, Lee KA, Youn HJ, et al. Mediastinal lymph node CD8α− DC initiate antigen presentation following intranasal coadministration of α-GalCer. Eur. J. Immunol. 2007;37(8):2127–2137. doi: 10.1002/eji.200636909. [DOI] [PubMed] [Google Scholar]
- 111. Silk JD, Hermans IF, Gileadi U, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J. Clin. Invest. 2004;114(12):1800–1811. doi: 10.1172/JCI22046. • Demonstrates the adjuvant properties of α-GalCer against specific tumor antigens.
- 112.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 2004;199(12):1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fujii S, Shimizu K, Hemmi H, et al. Glycolipid α-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc. Natl Acad. Sci. USA. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taraban VY, Martin S, Attfield KE, et al. Invariant NKT cells promote CD8+ cytotoxic T cell responses by inducing CD70 expression on dendritic cells. J. Immunol. 2008;180(7):4615–4620. doi: 10.4049/jimmunol.180.7.4615. [DOI] [PubMed] [Google Scholar]
- 115.Singh N, Hong S, Scherer DC, et al. Cutting edge: activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 1999;163(5):2373–2377. [PubMed] [Google Scholar]
- 116.Burdin N, Brossay L, Kronenberg M. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 1999;29(6):2014–2025. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 117.Galli G, Nuti S, Tavarini S, et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J. Exp. Med. 2003;197(8):1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Galli G, Pittoni P, Tonti E, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc. Natl Acad. Sci. USA. 2007;104(10):3984–3989. doi: 10.1073/pnas.0700191104. • Provides evidence that α-GalCer enhances memory B-cell responses, even in MHC class II-deficient mice.
- 119.Devera TS, Shah HB, Lang GA, Lang ML. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur. J. Immunol. 2008;38(4):1001–1011. doi: 10.1002/eji.200738000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim JO, Kim DH, Chang WS, et al. Asthma is induced by intranasal coadministration of allergen and natural killer T-cell ligand in a mouse model. J. Allergy Clin. Immunol. 2004;114(6):1332–1338. doi: 10.1016/j.jaci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 121.Campos RA, Szczepanik M, Itakura A, et al. Cutaneous immunization rapidly activates liver invariant Vα14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact hypersensitivity. J. Exp. Med. 2003;198(12):1785–1796. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Campos RA, Szczepanik M, Lisbonne M, Itakura A, Leite-de-Moraes M, Askenase PW. Invariant NKT cells rapidly activated via immunization with diverse contact antigens collaborate in vitro with B-1 cells to initiate contact sensitivity. J. Immunol. 2006;177(6):3686–3694. doi: 10.4049/jimmunol.177.6.3686. [DOI] [PubMed] [Google Scholar]
- 123.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381(6585):751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 124. Lang GA, Exley MA, Lang ML. The CD1d-binding glycolipid α-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006;119(1):116–125. doi: 10.1111/j.1365-2567.2006.02413.x. • Demonstrates that α-GalCer enhances antibody production against T-cell-dependent and -independent antigens.
- 125.Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008;111(4):2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barral P, Eckl-Dorna J, Harwood NE, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc. Natl Acad. Sci. USA. 2008;105(24):8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leadbetter EA, Brigl M, Illarionov P, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc. Natl Acad. Sci. USA. 2008;105(24):8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gray JD, Horwitz DA. Activated human NK cells can stimulate resting B cells to secrete immunoglobulin. J. Immunol. 1995;154(11):5656–5664. [PubMed] [Google Scholar]
- 129. Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, et al. Natural killer T cell ligand α-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med. 2002;195(5):617–624. doi: 10.1084/jem.20011889. •• First paper demonstrating the efficacy of α-GalCer as a vaccine adjuvant against a microbial pathogen, the malaria parasite.
- 130.Schmieg J, Gonzalez-Aseguinolaza G, Tsuji M. The role of natural killer T cells and other T cell subsets against infection by the pre-erythrocytic stages of malaria parasites. Microbes Infect. 2003;5(6):499–506. doi: 10.1016/s1286-4579(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 131.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. α-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J. Immunol. 2005;175(5):3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 132.Kamijuku H, Nagata Y, Jiang X, et al. Mechanism of NKT cell activation by intranasal coadministration of α-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunol. 2008;1(3):208–218. doi: 10.1038/mi.2008.2. [DOI] [PubMed] [Google Scholar]
- 133.Youn HJ, Ko SY, Lee KA, et al. A single intranasal immunization with inactivated influenza virus and α-galactosylceramide induces long-term protective immunity without redirecting antigen to the central nervous system. Vaccine. 2007;25(28):5189–5198. doi: 10.1016/j.vaccine.2007.04.081. [DOI] [PubMed] [Google Scholar]
- 134. Huang Y, Chen A, Li X, et al. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, α-galactosylceramide. Vaccine. 2008;26(15):1807–1816. doi: 10.1016/j.vaccine.2008.02.002. • Demonstrates that α-GalCer enhances the efficacy of a DNA vaccine against HIV-1.
- 135.Seino K, Motohashi S, Fujisawa T, Nakayama T, Taniguchi M. Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97(9):807–812. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ishikawa A, Motohashi S, Ishikawa E, et al. A Phase I study of α-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. 2005;11(5):1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 137.Nieda M, Okai M, Tazbirkova A, et al. Therapeutic activation of Vα24+Vβ11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103(2):383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 138.Chang DH, Osman K, Connolly J, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosylceramide loaded mature dendritic cells in cancer patients. J. Exp. Med. 2005;201(9):1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fujii S. Exploiting dendritic cells and natural killer T cells in immunotherapy against malignancies. Trends Immunol. 2008;29(5):242–249. doi: 10.1016/j.it.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 140. Liu K, Idoyaga J, Charalambous A, et al. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J. Exp. Med. 2005;202(11):1507–1516. doi: 10.1084/jem.20050956. • Demonstrates that coadministration of dying tumor cells with α-GalCer induces tumor immunity, in a manner that involves capture of the tumor cells by iNKT cell-activated DCs.
- 141.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with α-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J. Exp. Med. 2007;204(11):2641–2653. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat. Immunol. 2002;3(9):867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 143. Shimizu K, Goto A, Fukui M, Taniguchi M, Fujii S. Tumor cells loaded with α-galactosylceramide induce innate NKT and NK cell-dependent resistance to tumor implantation in mice. J. Immunol. 2007;178(5):2853–2861. doi: 10.4049/jimmunol.178.5.2853. •• Demonstrates that α-GalCer-loaded tumor cells induce long-lived adaptive immunity via cross-presentation by DCs.
- 144.Silk JD, Salio M, Reddy BG, et al. Cutting edge: nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J. Immunol. 2008;180(10):6452–6456. doi: 10.4049/jimmunol.180.10.6452. [DOI] [PubMed] [Google Scholar]
- 145.Mattner J, Savage PB, Leung P, et al. Liver autoimmunity triggered by NKT cells. Cell Host Microbe. 2008;3(5):304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilson MT, Van Kaer L. Natural killer T cells as targets for therapeutic intervention in autoimmune diseases. Curr. Pharm. Des. 2003;9(3):201–220. doi: 10.2174/1381612033392080. [DOI] [PubMed] [Google Scholar]
- 147.Nieda M, Nicol A, Koezuka Y, et al. Activation of human Vα24 NKT cells by α-glycosylceramide in a CD1d-restricted and Vα24 TCR-mediated manner. Hum. Immunol. 1999;60(1):10–19. doi: 10.1016/s0198-8859(98)00100-1. [DOI] [PubMed] [Google Scholar]
- 148.Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leuk. Biol. 2001;70(6):849–860. [PubMed] [Google Scholar]
- 149.Veldt BJ, van der Vliet HJ, von Blomberg BM, et al. Randomized placebo controlled Phase I/II trial of α-galactosylceramide for the treatment of chronic hepatitis C. J. Hepatol. 2007;47(3):356–365. doi: 10.1016/j.jhep.2007.04.018. [DOI] [PubMed] [Google Scholar]