Abstract
To create ligands for the estrogen receptor that contain pendant groups for tethering to a poly(amido)amine (PAMAM) dendrimer, we have explored a class of N-substituted 2-phenyl indoles. Attachment of tethers of different length and chemical nature to this non-steroidal indole scaffold gave high affinity ligand-tether conjugates that can be easily functionalized. To further explore the utility of this system, an indole-conjugated dendrimer was prepared and evaluated as an estrogen receptor ligand.
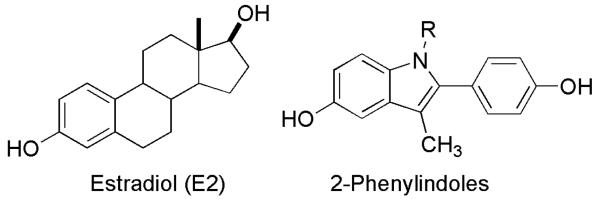
The estrogen receptor (ER) is a member of the nuclear hormone receptor superfamily of ligand-regulated transcription factors. The ER is regulated primarily by the endogenous estrogen, estradiol (E2, Figure 1), but it is also the target of pharmaceutical agents, including estrogen agonists, antagonists, and selective estrogen receptor modulators (SERMs) that activate the subtypes ERα and ERβ.1 The binding of E2 or other agonists to ER results in conformational changes that affect its ability to recruit coactivators or corepressors and controls ER interaction with DNA-regulatory sequences, termed estrogen response elements.2
Figure 1.
Estradiol (E2) and 2-phenylindole estrogens
In addition to its nuclear role to regulate gene transcription, ER can also have non-genomic actions by directly activating kinase cascades. These non-genomic effects are more rapid than the transcriptional ones and are mediated through ER from membrane or other extranuclear sites, where it acts as a classical activator of signal transduction.3 Extranuclear ER is most likely the same protein as nuclear ER, yet it represents only a few percent of total cellular ER, so rigorous characterization of membrane ER has been difficult.
Estrogens conjugated to poly(amido)amine (PAMAM) dendrimers have been used to study the non-genomic, extranuclear effects of the estrogen receptor.4,5 We have found that when an estrogen is conjugated to the highly charged, abiotic PAMAM macromolecule, this estrogen-dendrimer conjugate remains outside of the nucleus, allowing the ligand to activate signaling of only non-nuclear, non-genomic pathways.
PAMAMs are available in a number of generations that correspond to progressively increasing molecular weights and surface functionalities. PAMAMs contain multiple surface amine substituents onto which ligands can be covalently attached. Generation-6 PAMAM has a nominal molecular weight of 58 kDa, similar to bovine serum albumin, which has been used as an estradiol carrier6; the dendrimer also has nominally 256 surface primary amines. A distinct advantage of a PAMAM over a protein as a macromolecule for hormone conjugation is that the PAMAM can withstand organic solvents and extraction protocols needed to remove any remaining free ligand, which can confound biological experiments.4-6
Production of ER ligand-macromolecule conjugates involves four design steps: (I) identifying a good ligand scaffold with a position for covalent attachment of the tether, (II) determining an optimal tether length and chemical nature, (III) locating an appropriate linker type for synthetic ease in attachment to the dendrimer, and (IV) attachment of the dendrimer.
Regarding the first design step, adding a tether to various positions on E2 generally reduces its affinity, although substitution at 7α, 11β, or 17α is typically well tolerated.7 Estradiol-macromolecular conjugates attached through 7α or 17α that we prepared bind well to ER and were useful as biological probes.4,5 Although these steroidal estrogen conjugates were useful, many interesting non-steroidal ligands for ER are known. While originally of interest because of their ease of synthesis, non-steroidal ligands have noteworthy biological profiles and have provided ER subtype-selective ligands and supplied new therapeutic agents.2,8,9 Thus, we were intrigued at the possibility of building functional ER-non-steroidal ligand conjugates, particularly those with macromolecules.
To investigate the tolerance of ER to macromolecule conjugates of non-steroidal ligands, we chose a series of 2-phenylindole ligands (Fig. 1). Many members of this class have pharmacological activity,10-18 and attachment of alkyl groups to the indole nitrogen generally provided favorable ER binding.10,17 The 2-phenylindole estrogens have been tested as inhibitors of mammary tumor growth15 and have been successfully attached to pendant chemotherapy agents to target ER+ tumors.11
Recent reports show that the second design step can often be best achieved with short tethers.4,5,19 Addition of long or moderate-length tethers to ER ligands can sometimes decrease their accessibility to the receptor. Particularly when the ligand is conjugated to a macromolecule, shorter, more rigid tethers result in better exposure of the ligand to the receptor, by projecting the ligand outward, away from the conjugated molecule.5 Thus, ligands with shorter tethers often have affinity similar to that of the analogous ligands without these tethers.7 Thus, we prepared a small group of N-substituted 3-methyl-2-phenylindoles with tethers of various lengths and determined their binding affinities.
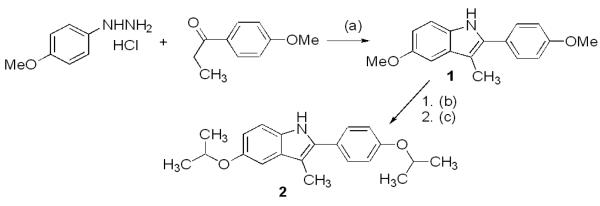
Using the Fischer indole synthesis (Scheme 1), 4-methoxyphenylhydrazine hydrochloride was reacted with 4-methoxypropiophenone to obtain dimethoxy protected 3-methyl-2-phenylindole 1. Deprotection of 1 with BBr3 gave a dihydroxy indole intermediate, which was reprotected as the di-isopropyl ether 2. This was necessary to avoid incompatibility during eventual removal of protecting groups from the indole and substituents placed on the indole nitrogen.
Scheme 1.
(a) HCl, EtOH, 80 °C, 6 h, 84%; (b) BBr3, CH2Cl2, −78 °C to r.t., 18 h, 82%; (c) 2-bromopropane, TBAH, acetone, 60 °C, 24 h, 46%.
The protected indoles 1 and 2 were used to prepare tether-containing indoles to determine which attachment gives the highest affinity ER ligand (Table 1). Indoles 1 or 2 were treated with NaH followed by the appropriate electrophile to introduce N-benzyl or N-alkyl groups. The isopropyl groups were selectively cleaved with AlCl3 to give products 3-4 and 6-7, whereas treatment of the trimethoxy indoles with BBr3 gave 5 and 8.
Table 1.
Synthesis and evaluation of estrogen receptor ligands.
 | |||
|---|---|---|---|
| Compound # |
SM/deprotection scheme |
R2 | ERα, ERβ relative binding affinity (RBA)* |
| 3 | 2/(b) | 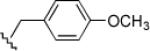 |
11, 6.5 |
| 4 | 2/(b) | 27, 1.2 | |
| 5 | 1/(c) | 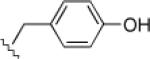 |
10, 1.0 |
| 6 | 2/(b) | 11, 2.0 | |
| 7 | 2/(b) | 41, 3.0 | |
| 8 | 1/(c) | 13, 1.4 | |
Binding affinities are expressed as relative binding affinity (RBA) values, where the affinity of the tracer and native ligand estradiol is set at 100. The Kd of estradiol is 0.2 nM for ERα and 0.5 nM for ERβ.
The affinity of these modified indoles for ERα and ERβ was measured using a radiometric binding assay with [3H]estradiol as tracer.20 The binding is expressed as relative binding affinity (RBA) values, which represent a percent of the binding affinity of the standard, E2 = 100% (Table 1). The affinities of these compounds are quite promising. Especially noteworthy are N-alkyl indole 7 and N-benzyl indole 4, with ERα RBAs of 41% and 27%, respectively. These two compounds show that the ER is able to tolerate flexible tethers of some length on the N-1 position; thus, such compounds are viable candidates for further study. The ERα binding affinity of the other N-substituted indoles is also high, but in all cases, their ERβ affinity is less.
Some indoles were assayed for their agonistic and antagonistic character as regulators of transcription by co-transfection reporter gene assays in human endometrial cancer cells, at ligand concentrations from 10−10 to 10−6 M. Agonist activity was measured with the ligand alone, antagonistic activity with 10−9 M estradiol. None of the compounds showed full agonist activity; all were mixed agonist/antagonists of modest potency, consistent with the behavior of other N-substituted-2-phenylindoles.10-17 They also showed only limited selectivities for ERα and ERor β (data not shown).
As this class of tether-containing indoles was promising, we prepared another group to address the third design challenge of locating an appropriate chemical linker for attachment to the N-1 position. We utilized either ether-based phenolic or amine-based aniline linkages. These molecules ended in an amine, which can be used to anchor the PAMAM dendrimer.
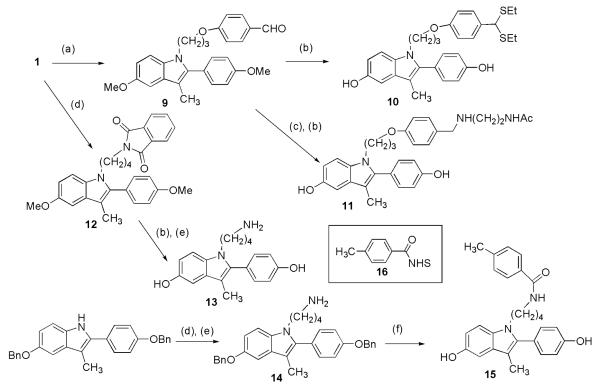
For the ether linkage 9 (Scheme 2), we used 4-(3-iodopropoxy)benzaldehyde to add a three-carbon linker to 1. Compound 9 was deprotected with EtSH to give 10, modifying the aldehyde as well. This compound had low ER affinity (Table 2). From intermediate 9 we made 11, using reductive amination to attach a linker that mimics the attachment site to the PAMAM dendrimer. Compound 11 (Table 2) again shows a preference for the ERα.
Scheme 2.
(a) NaH, 4-(3-iodopropoxy)benzaldehyde, DMF, 79%; (b) EtSH, AlCl3, r.t. 89%-quant; (c) – i.) NH2(CH2)2NHAc, MeOH, 65 °C; ii.) NaBH4, MeOH; (d) NaH, 4-iodobutylphthalimide, r.t., 62-83%; (e) H2NNH2, CH2Cl2CHCl2, MeOH, r.t., quant; (f) – i.) 16, CH2Cl2, quant; ii.) Pd/C, MeOH, quant.
Table 2.
RBA values for tethered indole ligands
| Compound # | Structure | ERα, ERβ relative binding affinity (RBA) |
|---|---|---|
| 10 | (See Scheme 2) | 0.188, 0.084 |
| 11 | (See Scheme 2) | 2.17, 0.24 |
| 13 | (See Scheme 2) | 0.314, 0.046 |
| 15 | (See Scheme 2) | 0.226, 0.048 |
| 19 | 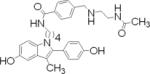 |
0.5±0.1, 0.1±0.1 |
| 20 | (See Scheme 3) | 1.3±0.2a, <0.007a |
RBA values for the dendrimer conjugate are based on the concentration of ligand, not the dendrimer conjugate. Based on the dendrimer, RBA values would be 25-fold higher.
For the amide linkage, we added 4-iodobutylphthalimide to 1 or to a benzyl-protected indole. Deprotection of 12 and treatment with hydrazine gave 13. An N-hydroxysuccinimide (NHS) ester (16) was added to amine 14, and the product was deprotected to give 15. Compounds 13 and 15 also show selectivity for ERα. Thus, we determined that ether or amine linkages would be acceptable for further design of an indole-PAMAM conjugate. For synthetic ease, we choose a 4-carbon linker to a substituted benzamide (like 15) because it would provide the most direct route to the desired conjugate.
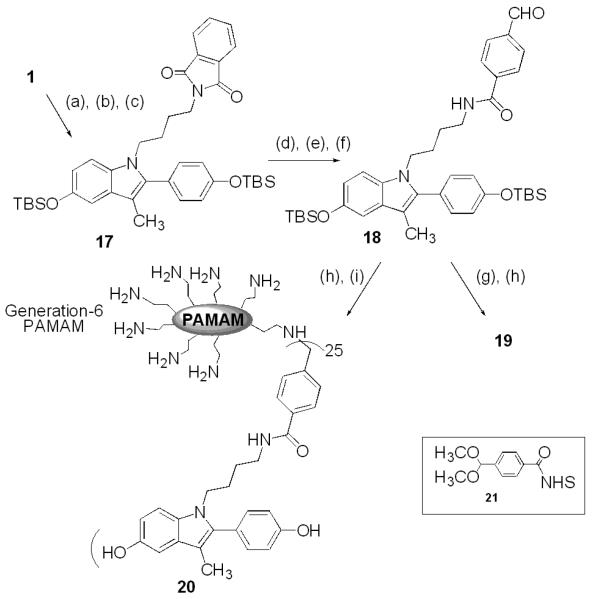
For the fourth and final design step, we created an indole compound 18 having an aldehyde-substituted phenyl ring through which it could be conjugated to a PAMAM dendrimer (Scheme 3). Compound 18 was to be reacted with N-acetyl ethylenediamine to afford conjugate 19, a reference compound whose structure mimics the functionality of last unit of a G-6 PAMAM, or with G-6 PAMAM itself to form the dendrimer conjugate 20.
Scheme 3.
(a) NaH, 4-iodobutylphthalimide, DMF, 0 °C to r.t., 83%; (b) EtSH, AlCl3, r.t., 85%; (c) TBDMSCl, imidazole, DMF, 91%; (d) H2NNH2, CH2Cl2, MeOH, r.t., 80%; (e) 21, CH2Cl2, THF, r.t., 80%; (f) H+, acetone, r.t., 72%; (g) - i.) NH2CH2CH2NHAc, CH2Cl2, ii.) NaBH4; (h) 1N HCl/MeOH, r.t., quant; (i) - i.) G-6 PAMAM, MeOH, Δ; ii.) NaBH4, quant.
To synthesize the indole conjugates, methyl-protected indole 1 was treated with NaH followed by 4-iodobutylphthalimide. Deprotection of the methyl ethers with AlCl3 and EtSH followed by reprotection with TBDMS gave indole 17. Hydrazinolysis gave the primary amine, and a protected benzaldehyde was obtained by treatment with NHS ester 21. The acetal protecting group was removed with acid to give intermediate 18. Reductive amination with N-acetyl ethylenediamine followed by cleavage of the silyl protecting groups gave reference indole 19.
Protecting group cleavage of 18 followed by reductive amination was also performed with the G-6 PAMAM dendrimer to provide the indole-PAMAM conjugate 20, after removal of unreacted indole by membrane filtration, as we have previously described.4 Because the reductive amination reaction is highly efficient, the degree of dendrimer substitution by the indole ligand directly reflects the indole-to-PAMAM stoichiometric ratio.4,5 The uniformity of substitution was determined by MALDI MS, which showed an average of 25 indole substituents per PAMAM molecule, leaving approximately 230 free amines.21
The binding affinity analyses of 19 and 20 are given in Table 2. As an ER ligand, the reference compound 19 had relatively low affinity for both ERα and ERβ. By contrast, the indole-PAMAM conjugate 20 showed respectable binding to ERα, with very high affinity preference (ca. 200 fold) for ERα over ERβ. Notably, this is the first ligand-dendrimer conjugate that shows such a large ERα-selectivity, a finding that supports further study within this compound class.
In summary, we have prepared a series of N-substituted indoles having good binding affinities for the estrogen receptor and show the high tolerance of the ER for tethers of various lengths on N-1 of the 2-phenylindole. A dendrimer-conjugated indole was produced that had good affinity and selectivity for ERα. This novel compound can be used in further studies to characterize the biological functions of extranuclear ER.
Acknowledgements
This research was supported by a Grant from the NIH (PHS 5R37 DK15556). We thank Drs. F. Stossi and B. Katzenellenbogen for performing co-transfection assays and Kathryn Carlson for the binding assays.
References and Notes
- 1.Katzenellenbogen BS, Katzenellenbogen JA. Science. 2002;295:2380. doi: 10.1126/science.1070442. [DOI] [PubMed] [Google Scholar]
- 2.Lin X, Huebner V. Curr. Opin. Drug Discovery Dev. 2000;3:383. [PubMed] [Google Scholar]
- 3.Levin ER. Steroids. 2002;67:471. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Katzenellenbogen JA. Angew. Chem. Int. Ed. 2006;45:7243. doi: 10.1002/anie.200601923. [DOI] [PubMed] [Google Scholar]
- 5.Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Mol. Endocrinol. 2006;20:491. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- 6.Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE. Endocrinology. 1999;140:5455. doi: 10.1210/endo.140.11.7247. [DOI] [PubMed] [Google Scholar]
- 7.Anstead GM, Carlson KE, Katzenellenbogen JA. Steroids. 1997;62:268. doi: 10.1016/s0039-128x(96)00242-5. [DOI] [PubMed] [Google Scholar]
- 8.Veeneman GH. Curr. Med. Chem. 2005;12:1077. doi: 10.2174/0929867053764662. [DOI] [PubMed] [Google Scholar]
- 9.Buijsman RC, Hermkens PH, van Rijn RD, Stock HT, Teerhuis NM. Curr. Med. Chem. 2005;12:1017. doi: 10.2174/0929867053764671. [DOI] [PubMed] [Google Scholar]
- 10.von Angerer E, Prekajac J, Strohmeier J. J. Med. Chem. 1984;27:1439. doi: 10.1021/jm00377a011. [DOI] [PubMed] [Google Scholar]
- 11.Rink SM, Yarema KJ, Solomon MS, Paige LA, Tadayoni-Rebek BM, Essigmann JM, Croy RG. Proc. Natl. Acad. Sci. U.S.A. 1996;93:15063. doi: 10.1073/pnas.93.26.15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberger LM, Annable T, Collins KL, Komm BS, Lyttle CR, Miller CP, Satyaswaroop PG, Zhang Y, Frost P. Clin. Cancer Res. 2001;7:3166. [PubMed] [Google Scholar]
- 13.Miller CP, Collini MD, Tran BD, Harris HA, Kharode YP, Marzolf JT, Moran RA, Henderson RA, Bender RHW, Unwalla RJ, Greenberger LM, Yardley JP, Abou-Gharbia MA, Lyttle CR, Komm BS. J. Med. Chem. 2001;44:1654. doi: 10.1021/jm010086m. [DOI] [PubMed] [Google Scholar]
- 14.Biberger C, von Angerer E. J. Steroid Biochem. Mol. Bio. 1998;64:277. doi: 10.1016/s0960-0760(97)00197-0. [DOI] [PubMed] [Google Scholar]
- 15.Biberger C, von Angerer E. J. Steroid Biochem. Mol. Bio. 1996;58:31. doi: 10.1016/0960-0760(96)00004-0. [DOI] [PubMed] [Google Scholar]
- 16.Golob T, Biberger C, Walter G, von Angerer E. Arch. Pharm. 2000;333:305. doi: 10.1002/1521-4184(20009)333:9<305::aid-ardp305>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.von Angerer E, Strohmeier J. J. Med. Chem. 1987;30:131. doi: 10.1021/jm00384a022. [DOI] [PubMed] [Google Scholar]
- 18.von Angerer E, Knebel N, Kager M, Ganss B. J Med Chem. 1990;33:2635. doi: 10.1021/jm00171a045. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed N, Dubuc C, Rousseau J, Benard F, van Lier JE. Bioorg. Med. Chem. Lett. 2007;17:3212. doi: 10.1016/j.bmcl.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Carlson KE, Choi I, Gee A, Katzenellenbogen BS, Katzenellenbogen JA. Biochemistry. 1997;36:14897. doi: 10.1021/bi971746l. [DOI] [PubMed] [Google Scholar]
- 21.MALDI-TOF (DHB) Mn 57117, Mw 58260, PI 1.02. (Reference G6 PAMAM dendrimer, Mn 46825, Mw 47573, PI 1.02: theoretical MW 58047). 1H NMR (500 MHz, D2O) 1.65 (Indole, CH2), 2.26 (G6), 2.42 (G6), 2.62 (G6), 2.85 (G6), 3.10 (G6), 3.23 (G6), 6.40 – 7.40 (m, Indole).