Abstract
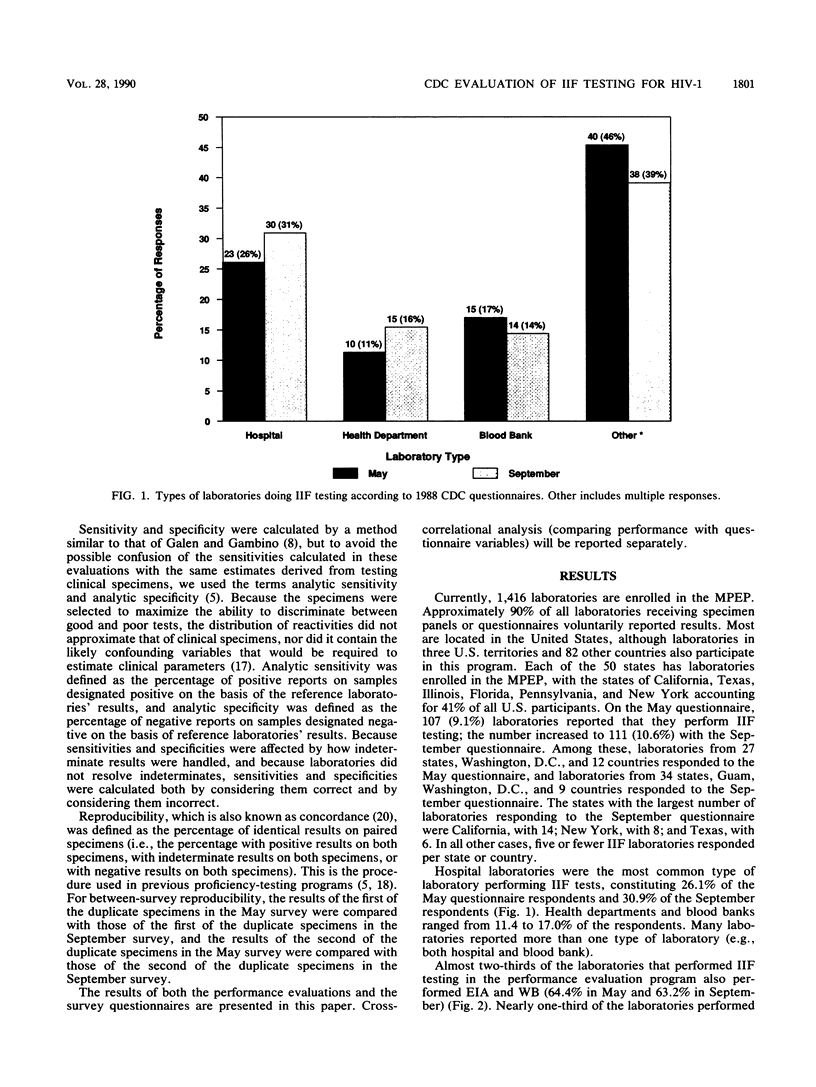
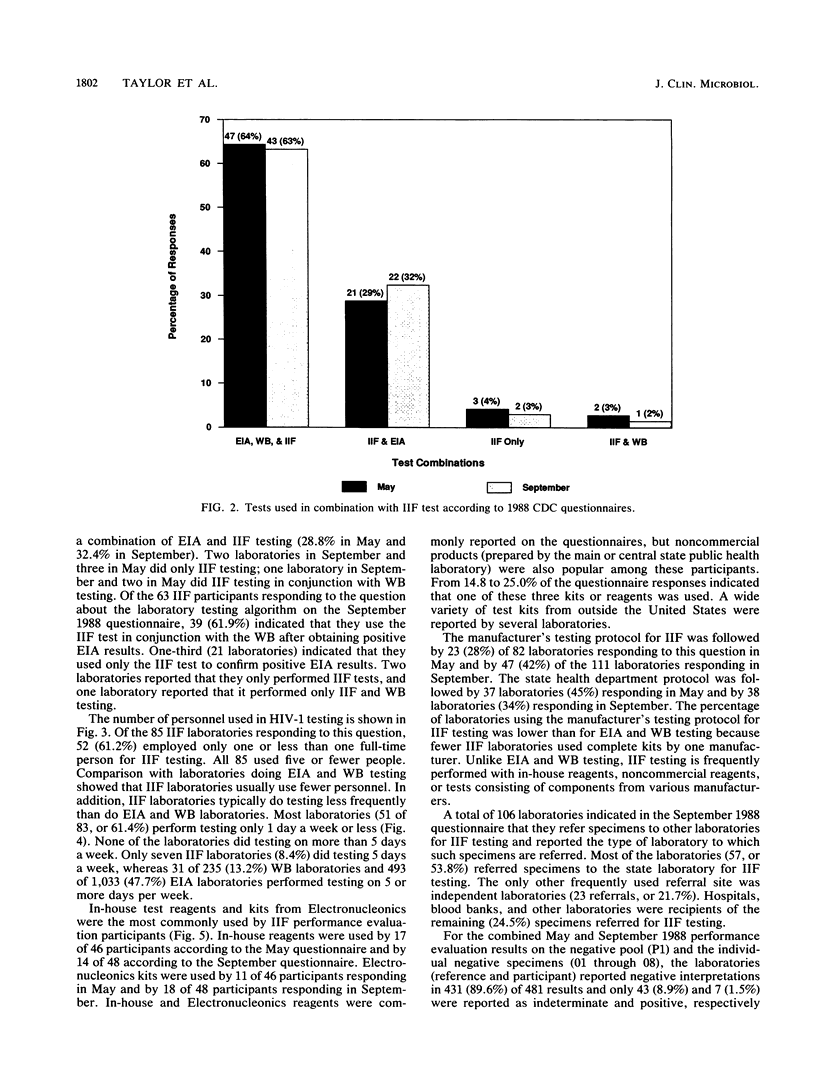
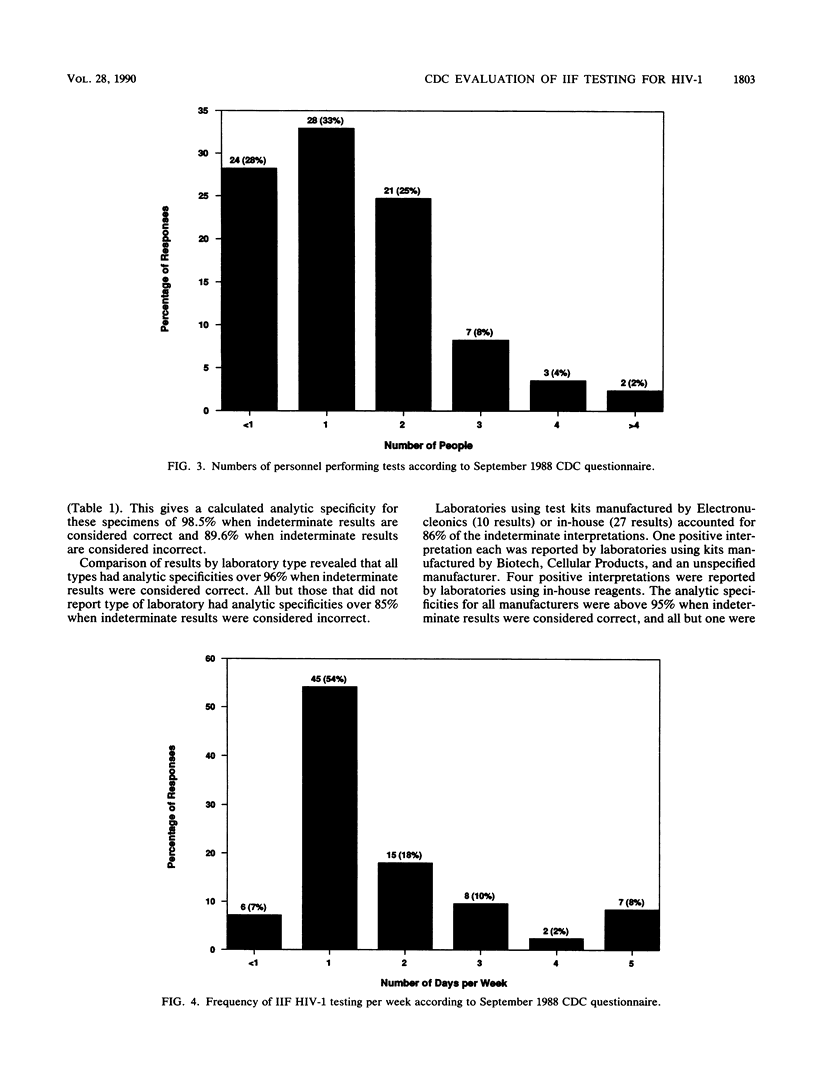
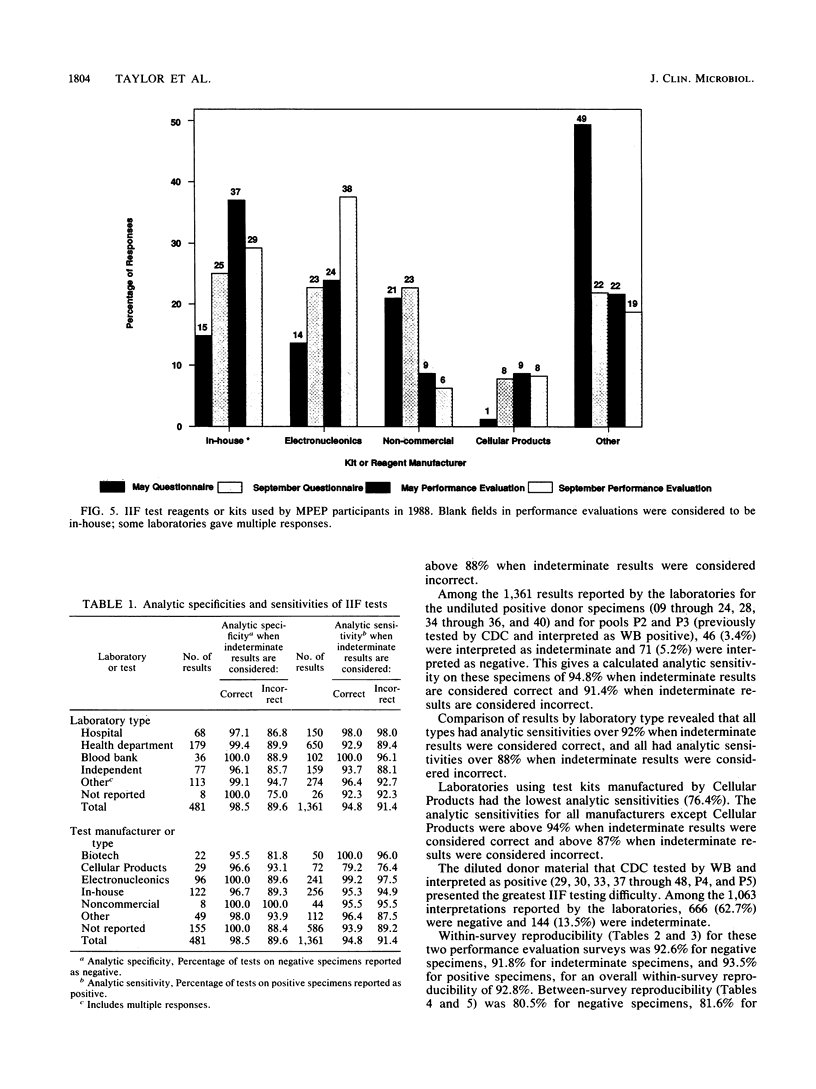
Results from laboratories performing indirect immunofluorescence (IIF) testing for human immunodeficiency virus type 1 antibody and participating in the Centers for Disease Control Model Performance Evaluation Program in 1988 are presented. Approximately 90% of all laboratories receiving specimen panels or questionnaires furnished results to the Centers for Disease Control. In September 1988, 111 reports were received from IIF laboratories from 34 states and nine countries; most of these laboratories did IIF testing in conjunction with other antibody tests. Hospital laboratories were the most common type of laboratory participating in the program. Laboratories that performed IIF employed fewer personnel and performed testing less frequently than did laboratories that performed enzyme immunoassays or Western blot (immunoblot) tests and were likely to use a commercial test kit. Most of the laboratories that referred specimens for IIF testing sent them to the state laboratory. The analytic specificity for the Model Performance Evaluation Program specimens was 98.5% when indeterminate results on a negative specimen were considered correct (negative) and 89.6% when indeterminate results on a negative specimen were considered incorrect; analytic sensitivity was 94.8% when indeterminate results on a positive specimen were correct (positive) and 91.4% when indeterminate results on a positive specimen were considered incorrect. When indeterminate results were considered correct, all types of laboratories (blood bank, state, hospital, independent, and other) had analytic specificities over 96%, and all manufacturers had analytic specificities above 95%. All types of laboratories had analytic sensitivities over 92%, and analytic sensitivities were above 94% for all manufacturers and reagent sources except Cellular Products. Comparison of percentages of correct responses between IIF and Western blot assays on those samples for which there was good agreement on the target interpretation revealed no significant differences. Both individual donor and diluted materials were included in the evaluations; the diluted donor material presented the greatest testing difficulty. Within-survey reproducibility was about 93% overall and by specimen type. Between-survey reproducibility was about 81% for negative and indeterminate specimens and 88.5% for positive specimens, for an overall between-survey reproducibility of 84.3%. Differences in performance were noted when results were compared by type of laboratory and test manufacturer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Broder S., Gallo R. C. A pathogenic retrovirus (HTLV-III) linked to AIDS. N Engl J Med. 1984 Nov 15;311(20):1292–1297. doi: 10.1056/NEJM198411153112006. [DOI] [PubMed] [Google Scholar]
- Carlson J. R., Yee J., Hinrichs S. H., Bryant M. L., Gardner M. B., Pedersen N. C. Comparison of indirect immunofluorescence and Western blot for detection of anti-human immunodeficiency virus antibodies. J Clin Microbiol. 1987 Mar;25(3):494–497. doi: 10.1128/jcm.25.3.494-497.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J. W., Jaffe H. W., Hardy A. M., Morgan W. M., Selik R. M., Dondero T. J. Epidemiology of HIV infection and AIDS in the United States. Science. 1988 Feb 5;239(4840):610–616. doi: 10.1126/science.3340847. [DOI] [PubMed] [Google Scholar]
- Dondero T. J., Jr, Pappaioanou M., Curran J. W. Monitoring the levels and trends of HIV infection: the Public Health Service's HIV surveillance program. Public Health Rep. 1988 May-Jun;103(3):213–220. [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Sarin P. S., Gelmann E. P., Robert-Guroff M., Richardson E., Kalyanaraman V. S., Mann D., Sidhu G. D., Stahl R. E., Zolla-Pazner S. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):865–867. doi: 10.1126/science.6601823. [DOI] [PubMed] [Google Scholar]
- Goedert J. J. Testing for human immunodeficiency virus. Ann Intern Med. 1986 Oct;105(4):609–610. doi: 10.7326/0003-4819-105-4-609. [DOI] [PubMed] [Google Scholar]
- Hedenskog M., Dewhurst S., Ludvigsen C., Sinangil F., Rodriguez L., Wu Y. T., Volsky D. J. Testing for antibodies to AIDS-associated retrovirus (HTLV-III/LAV) by indirect fixed cell immunofluorescence: specificity, sensitivity, and applications. J Med Virol. 1986 Aug;19(4):325–334. doi: 10.1002/jmv.1890190405. [DOI] [PubMed] [Google Scholar]
- Lennette E. T., Karpatkin S., Levy J. A. Indirect immunofluorescence assay for antibodies to human immunodeficiency virus. J Clin Microbiol. 1987 Feb;25(2):199–202. doi: 10.1128/jcm.25.2.199-202.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh T. M., Stites D. P., Casavant C. H., Carlson J. R., Yee J., McVay P. A., Busch M. P., Levy J. A. Evaluation of the indirect immunofluorescence assay as a confirmatory test for detecting antibodies to the human immunodeficiency virus. Diagn Immunol. 1986;4(5):233–240. [PubMed] [Google Scholar]
- Schalla W. O., Hearn T. L., Taylor R. N., Eavenson E., Valdiserri R. O., Essien J. D. CDC's Model Performance Evaluation Program: assessment of the quality of laboratory performance for HIV-1 antibody testing. Public Health Rep. 1990 Mar-Apr;105(2):167–171. [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. S., Dans P. E., Kinosian B. P. Human immunodeficiency virus test evaluation, performance, and use. Proposals to make good tests better. JAMA. 1988 May 6;259(17):2574–2579. [PubMed] [Google Scholar]
- Taylor R. N., Przybyszewski V. A. Summary of the Centers for Disease Control human immunodeficiency virus (HIV) performance evaluation surveys for 1985 and 1986. Am J Clin Pathol. 1988 Jan;89(1):1–13. doi: 10.1093/ajcp/89.1.1. [DOI] [PubMed] [Google Scholar]
- Valdiserri R. O., Taylor R. N., Hearn T. L., Schalla W. O., Muir H. W. Centers for Disease Control perspective on quality assurance for human immunodeficiency virus type 1 antibody testing. Model Performance Evaluation Program. Arch Pathol Lab Med. 1990 Mar;114(3):263–267. [PubMed] [Google Scholar]
- Vercauteren G., van der Groen G., Piot P. Comparison of enzyme immunoassays and an immunofluorescence test for detection of antibody to human immunodeficiency virus in African sera. Eur J Clin Microbiol. 1987 Apr;6(2):132–135. doi: 10.1007/BF02018193. [DOI] [PubMed] [Google Scholar]


