Abstract
Background
Tuberculosis (TB) is a disease which kills two million people every year and infects approximately over one-third of the world's population. The difficulty in managing tuberculosis is the prolonged treatment duration, the emergence of drug resistance and co-infection with HIV/AIDS. Tuberculosis control requires new drugs that act at novel drug targets to help combat resistant forms of Mycobacterium tuberculosis and reduce treatment duration.
Methodology/Principal Findings
Our approach was to modify the naturally occurring and synthetically challenging antibiotic thiolactomycin (TLM) to the more tractable 2-aminothiazole-4-carboxylate scaffold to generate compounds that mimic TLM's novel mode of action. We report here the identification of a series of compounds possessing excellent activity against M. tuberculosis H37Rv and, dissociatively, against the β-ketoacyl synthase enzyme mtFabH which is targeted by TLM. Specifically, methyl 2-amino-5-benzylthiazole-4-carboxylate was found to inhibit M. tuberculosis H37Rv with an MIC of 0.06 µg/ml (240 nM), but showed no activity against mtFabH, whereas methyl 2-(2-bromoacetamido)-5-(3-chlorophenyl)thiazole-4-carboxylate inhibited mtFabH with an IC50 of 0.95±0.05 µg/ml (2.43±0.13 µM) but was not active against the whole cell organism.
Conclusions/Significance
These findings clearly identify the 2-aminothiazole-4-carboxylate scaffold as a promising new template towards the discovery of a new class of anti-tubercular agents.
Introduction
The disease tuberculosis (TB), once considered eradicated, has again become a major global health concern. M. tuberculosis, the causative organism, produces a chronic infection in the lungs that can become disseminated. Over the past decade, at least 30 million individuals have died from the disease and estimates indicate that one-third of the world's population is infected with latent or persistent M. tuberculosis. Moreover, globally more than 8 million people develop active TB every year, and if trends continue there will be a total of 36 million disease-related deaths by the year 2020 [1], [2].
The resurgence in the disease is caused by an inadequate and extended chemotherapy that relies on drugs developed in the mid-twentieth century. The associated poor patient compliance and emergence of drug resistant forms of TB, coupled with a strong epidemiological co-existence with HIV/AIDS highlights the fundamental need for new, more effective drugs to treat the disease [3]–[5].
The mycobacterial cell wall of M. tuberculosis is rich with many unique key structural components that are necessary for the mycobacteria to survive and grow within the human host, and has long been a target for anti-TB drug development. Essential to the cell wall are the mycolic acids, which are high molecular weight 2-alkyl, 3-hydroxy fatty acids that exist in several forms of differing chemical functionality. Indeed, the first line anti-tubercular drug isoniazid (INH) works by inhibiting their biosynthesis. The complete sequencing of the TB genome [6] has revealed significant biochemical and genetic insight into mycolic acid biosynthesis that will aid the search for new druggable targets. These unique lipids are biosynthesised by both fatty acid synthase enzyme systems I and II (FAS I and FAS II) to produce C56–64 meromycolic acids and the C26 α-branch [7], [8] after a series of biostransformations [9], [10].
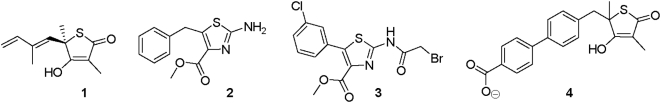
The naturally occurring antibiotic thiolactomycin 1 (TLM, figure 1) [11]–[13] primarily acts by inhibiting the FAS-II β-ketoacyl-ACP synthase condensing enzymes, halting mycolic acid biosynthesis and subsequently to M. tuberculosis cell death [14]–[17]. TLM is also orally available and non-toxic in the mouse model, which makes it an attractive compound for development. Conversely, the chemical scaffold of TLM possesses a chiral centre at the 5-position which makes the synthesis of series of TLM analogues lengthy and costly, and complicates the optimisation process. Such factors need to be considered carefully when developing economically viable drugs for developing countries.
Figure 1. The chemical structures of thiolactomycin 1, its analogue 4, and inhibitors 2 and 3.
This issue of synthetic tractability has focused researchers' efforts towards the synthesis of either racemic analogues or derivatives that contain simple modifications, and has yielded limited improvements in activity against M. tuberculosis and modest activity against mtFabH [18]–[22]. We have focussed on identifying alternative, easily accessible 5-membered ring isosteres to generate large compound libraries targeted against the condensing enzyme mtFabH and M. tuberculosis. Herein, we demonstrate that 2-aminothiazole-4-carboxylate offers a promising template for the development of new anti-tubercular agents, and report the design and synthesis of methyl 2-amino-5-benzylthiazole-4-carboxylate 2 as our most potent inhibitor of M. tuberculosis H37Rv, with an MIC of 0.06 µg/ml (0.24 µM), which is more effective than both TLM and INH (MICs of 13 µg/ml (62.5 µM) [18] and 0.25 µg/ml (1.8 µM) [19] respectively (figure 1). We also show that methyl 2-(2-bromoacetamido)-5-(3-chlorophenyl)thiazole-4-carboxylate 3 inhibits mtFabH with an IC50 of 0.95 µg/ml (2.43 µM), which compares well with TLM 1 and its most potent analogue 4 (IC50 values of 16 µg/ml (75 µM) and 1.1 µg/ml (3.0 µM) respectively [22] (figure 1).
Results and Discussion
Ligand design
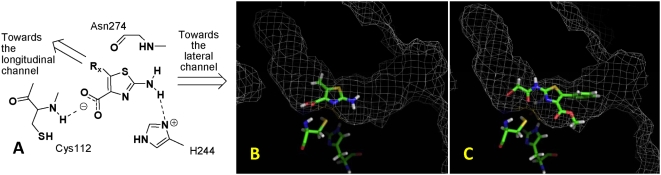
The initial focus of our ligand design was based on the active site geometry and mechanism of action of the target enzyme mtFabH, a homodimer (PDB: 1M1M) that converts C12–20 acyl-CoA substrates to the corresponding β-ketoacyl-AcpM product after reaction with mal-AcpM in a two step process [23]. At the molecular level, the acyl-CoA substrate enters an “L” shaped binding pocket consisting of a lateral and longitudinal channel, with the active site catalytic triad of Cys112-His244-Asn274 located at the junction. Transacylation of the Cys122 residue occurs when the adjacent His244 deprotonates the thiol group (directly or via a molecule of water, as postulated by Brown et al. [23]) to generate a thiolate nucleophile that attacks the carbonyl group of the acyl chain occupying the longitudinal channel, and releases CoA-SH from the lateral channel through which the substrate entered. The second substrate, mal-AcpM, then enters the lateral channel, and is decarboxylated by the catalytic residues His244 and Asn274, and condensed with the thioester formed at Cys112 to generate the β-ketoacyl-AcpM, which also dissociates via the lateral channel. Recently, Sachdeva et al. have postulated that the overall reaction may occur simultaneously in both active sites of the dimer [24].
As no inhibitor-mtFabH co-crystal structures have been solved to date, we investigated the binding pattern of TLM with the closely related analogue ecFabB from E.coli [25]. TLM reversibly inhibits ecFabB by forming a number of non-covalent interactions: the methyl group at carbon 3 of TLM is positioned in a hydrophobic pocket defined by residues Phe229 and Phe392; the 5-isoprenoid moiety is wedged between two peptide bonds - from above by residues Val271 and Phe272 and from below by Gly391 and Phe392, which are important for specificity [23]; the carbonyl oxygen forms two H-bonds with the two histidines in the active site; the 4-hydroxyl group H-bonds to the carbonyl oxygen of Val270 and the amide NH of Gly305 through a lattice of three water molecules; and the sulfur is adjacent to the active site Cys residue, although without any obvious interaction. The strong H-bonding between the active site His residues of ecFabB and the TLM carbonyl group is believed to be crucial for effective inhibitory activity against this enzyme. Based on this analysis, we postulated that the closely related active site of mtFabH could be expected to form an equivalent H-bonding network with TLM, and any new isosteric scaffold would need to maintain many of these important interactions. The equivalent condensing enzyme from E. coli (ecFabH) is also closely related to mtFabH, and has been co-crystalised with the very potent inhibitor 2-hydroxy-6-(3-phenoxy-4-phenyl-benzamido) benzoic acid [26]. This complex revealed an important role for a carboxylic acid moiety in the ligand as it forms specific interactions in the active site with the His250 residue from ecFabH [26]. We considered inclusion of this moiety to be important for our inhibitors and proposed the achiral 2-amino thiazole-5-carboxylate scaffold as an alternative to the TLM substructure to combine pharmacodynamic potency with essential pharmacokinetic considerations such as solubility. Finally, synthetic tractability and subsequent diverse library generation were considered possible using the simple procedure described by Barton et al. [27].
Using GOLD, docking studies were performed to investigate the poses for our scaffold in the mtFabH active site [28]. We observed that the carboxyl group of the thiazole ring forms H-bonds with the NH of Cys112, whilst the NH2 is proximal to and H-bonds with the imidazole ring of His244. This allowed us to generate a hypothetical template for the development of inhibitors of mtFabH (Figure 2A). Further docking studies were carried out with phenyl, m-chlorophenyl (figure 2B) and benzyl substituents in the 5-position in both the 4-methyl ester and free acid forms of the thiazoles. In these cases, the longitudinal channel appears to be the preferred residence for the 5-substituents, whilst the 2-amino group and potentially amide derivatives thereof could be accommodated in the lateral channel. We also noted that a 2-bromoacetamido substituent in this position would place the thiol group of Cys112 in a position to become alkylated via a nucleophilic SN2 reaction, and could lead to irreversible inhibition of the enzyme (figure 2C). Whilst such a strategy would normally be performed once a selective and relatively potent inhibitor had been found, we postulated that it would be reasonable at this early stage of inhibitor design to attempt this in order to establish if ligand inhibition was at all possible before addressing selectivity in later rounds of ligand optimisation. Based on this rationale, we prepared a series of thiazoles that included the substituents studied for evaluation against the enzyme and M. tuberculosis.
Figure 2. The modeling studies of the 2-aminothiazole-4-carboxylate analogues with mtFabH.
(A) The hypothetical template of the 2-aminothiazole-4-carboxylates for mtFabH inhibitor development. This illustrates the key H-bonding interactions with the catalytic triad amino acid residues. (B) The binding pose of methyl 2-amino-5-methylthiazole-4-carboxylate in the active site of mtFabH showing the NH2 group proximal to His 244 and directed towards the lateral channel, with the 5-methyl group directed towards the longitudinal channel. (C) The binding pose of methyl 2-(2-bromoacetamido)-5-(3-chlorophenyl)thiazole-4-carboxylate with the bromomethylene portion in the vicinity of the Cys112 thiol group.
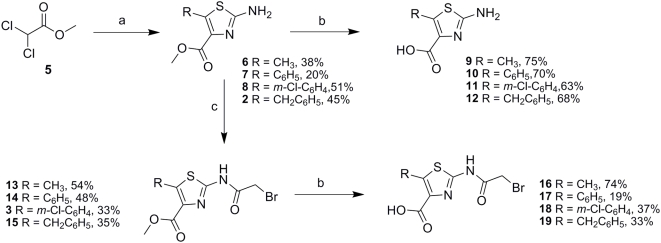
Chemical Synthesis
Using the flexible synthetic procedure described by Barton et al. [27], the synthesis of the thiazole derivatives (figure 3) was achieved starting with the Darzens reaction between methyl dichloroacetate 5 and the appropriate aldehyde. This afforded a mixture of the α-chloro glycidic ester and β-chloro α-oxoester, which was extracted with diethylether and immediately reacted with thiourea dissolved in methanol to generate the methyl ester thiazoles 2, 6–8. To investigate whether a free carboxylic acid functionality would increase binding within the active site through facilitating electrostatic interactions proposed by the modeling studies, we hydrolysed the esters with 0.1 M sodium hydroxide solution followed by workup with dilute hydrochloric acid to generate compounds 9–12. In order to generate 2-bromoacetamido analogues, the free amines 2, 6–12 were reacted with 2-bromoacetylchloride in anhydrous THF at 0°C to afford the amides 3, 13–15. As described previously, hydrolysis with 0.1 M sodium hydroxide gave the corresponding carboxylic acids 16–19 for comparison.
Figure 3. Synthesis of the 2-aminothiazole-4-carboxylate analogues.
In vitro structure activity relationships
Inhibition of mtFabH
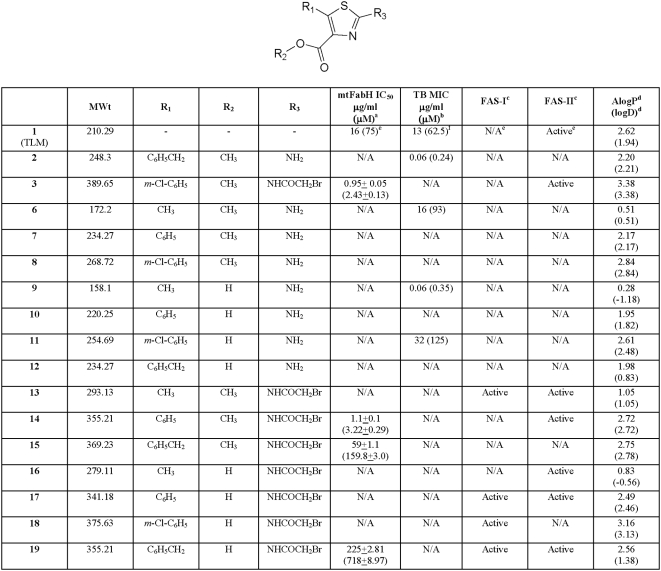
The compounds were first assessed against the target enzyme mtFabH using the procedure developed by Brown et al. [23] (figure 4). Despite many of them not demonstrating any inhibitory activity at a concentration of 200 µg/ml, it was pleasing to see that the bromoacetamido analogues that were prepared to facilitate an SN 2 type substitution between the ligand and the Cys112 residue, were active. The bromoacetamido esters 3, 14 and 15 inhibited the enzyme with IC50 values of 0.95±0.05 (2.43±0.13 µM), 1.1±0.1 µg/ml (3.22±0.29 µM) and 59±1.1 µg/ml (159.8±3.0 µM) respectively, whilst the corresponding carboxylic acid 19 inhibited the enzyme at 225±2.81 µg/ml (718±8.97 µM). Interestingly, the ester 13 and carboxylic acids 16, 17 and 18 failed to inhibit the enzyme.
Figure 4. The in vitro activity and molecular properties of the 2-aminothiazole-4-carboxylates.
a, b Compounds regarded as not active (N/A) if no inhibition is observed at 200 µg/ml. c FAS-I/II assay conducted at 200 µg/ml and compounds regarded as not active is <50% inhibition observed. d AlogP and logD calculated using Pipeline Pilot (SciTegic) software. eFrom reference [18]. fFrom reference [22].
It is clear that whilst the electrophilic bromomethyl substituent establishes activity against the enzyme, its effect is modified by different substituents at the 4- and 5- positions. Assuming that the inhibition observed involves reaction with the Cys112 residue, then the ligands must be situated in the vicinity of the catalytic triad. As the longitudinal and lateral tunnels are composed of lipophilic amino acid residues, we suggest that 13 and 16, which possess methyl groups at position 5, are unable to maximize the hydrophobic interactions with these channels necessary to facilitate enzyme inhibition. However, inserting a phenyl group at position 5 and augmenting it with a m-Cl, as in 14 and 3 respectively, enables the appropriate hydrophobic interactions with the enzyme to occur and enables effective inhibition. The flexibility of the ligand also appears to be an important factor; the m-Cl phenyl carboxylic acid analogue 18 is inactive whereas its 5-benzyl counterpart 19, which is less lipophilic, inhibits the enzyme with an IC50 of 225±2.81 µg/ml (718±8.97 µM). Comparison with its benzyl ester 15, which inhibits mtFabH with an IC50 of 59±1.1 µg/ml (159.8±3.0 µM), suggests that both hydrophobicity and flexibility at the 5-position of the thiazole ring are instrumental in orientating the ligand to achieve effective inhibition. It is interesting that 13 fails to inhibit mtFabH as it appears that the free acids are weaker inhibitors than the esters. This may be accounted for by the molecule possessing a methyl group at position 5 which does not enable it to maximize the hydrophobic interactions necessary to bind to the enzyme. Conversely, such interactions may be permitted by the flexible benzyl substituent on the free acid 19 which enables weak inhibition.
Inhibition of M. tuberculosis H37Rv
Whilst it was encouraging to find that a small number of compounds inhibited the target enzyme, it was important to know if this activity would lead to inhibition of the whole cell organism. From the data obtained it was clear that all of the bromoacetamido analogues failed to inhibit M. tuberculosis H37Rv (figure 4). We do not know, at this stage, whether these compounds did not inhibit the mycobacteria through an inability to access the target enzyme due to inappropriate physicochemical properties, or because the bromomethyl moiety was chemically or metabolically inactivated by the Mycobacterium.
In contrast to the 2-bromoacetamido analogues, four of the free amine compounds (2, 6, 9 and 11) inhibited M. tuberculosis H37Rv with MIC values of 0.06, 16, 0.06 and 32 µg/ml (0.24, 93, 0.35 and 125 µM) respectively, whilst the other analogues of this group showed no activity. Given that these compounds did not inhibit mtFabH, their mechanism of action must involve other targets within the organism.
Although these compounds exhibit excellent activity, in the absence of any recognisable trends in the series, it is difficult to ascertain clear structure activity relationships. The best activity was obtained with 2 with a benzyl group in the 5-position and a methyl ester in the 4-position, whereas the carboxylic acid analogue was shown to be inactive. The opposite observations were seen with the inactive methyl ester analogue 8 possessing an m-Cl phenyl group at the 5-position and the corresponding active acid 11. A similar trend was observed for the 5-methyl analogues 9 and 6 with MIC values of 0.06 and 16 µg/ml (0.35 and 93 µM) respectively. We speculate that these observations may result from the compounds' ability to enter the cell. In all cases, the primary amine at the 2-position would be associated with a dissociation equilibrium at physiological pH which could penetrate cellular membranes in the unionized state. Compounds 9 and 11 on the other hand, with both carboxylic acid and amino substituents, would exist as zwitterions and would not normally be expected to penetrate the lipophilic cell wall. The uptake of these compounds could involve a cellular uptake mechanism, such as mycobacterial porins that the inactive zwitterionic compounds 10 and 12 are not substrates for. The inactivity of the 2-amino analogue 7 is more likely due to its inability to interact structurally with the target in the organism.
Specificity of the 2-amino thiazole-4-carboxylates
The molecules under investigation were designed to inhibit mtFabH in the mycobacterial FAS-II system, with selectivity over the related mammalian FAS-I system. To examine selectivity, the compounds were assessed using the procedures of Brown et al. [23] and Slayden et al. [13].
No inhibition of the FAS enzymes was observed, either as the acid or ester, when the 2-position was the free amine (figure 5). These data support the possibility that activity against M. tuberculosis H37Rv of compounds 2, 6, 9 and 11 involves a target other than mtFabH, or indeed the other FAS-II enzymes. However, with the exception of 15 all of the compounds possessing the bromoacetamido group at the 2-position showed activity against the FAS enzymes although no obvious trends were observed across this series. This is perhaps not surprising as these compounds may be inhibiting other enzymes in the FAS-II system or indeed at different sites in the multifunctional FAS-I complex. However, it was evident from the data that the phenyl 14 and m-Cl-phenyl 3 analogues were the only compounds active against mtFabH and selective against FAS-II. The mtFabH inhibitor 19 inhibited both FAS-I and FAS-II, whereas 16 inhibited only FAS-II and 18 FAS-I. Intriguingly, compound 15 failed to inhibit the FAS enzymes although inhibited mtFabH with an IC50 of 59±1.1 µg/ml (159.8±3.0 µM). While 15 may have shown activity against purified mtFabH, its inhibitory potential against mtFabH in the crude FAS-II assay may be difficult to detect as the related enzyme KasA (present in the crude reaction mix) has been shown to have FabH-type activity and could have had a compensatory effect [17].
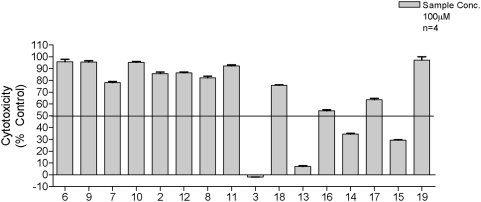
Figure 5. The cytotoxic effects of the compounds against HS-27 human fibroblast cells.
The data indicates that only the carboxyl esters of the bromoacetamido analogues were toxic.
Cytoxicity was investigated against human foreskin fibroblast HS-27 cells (Figure 5) to establish toxicity profiles for our compounds. The results indicated that none of the 2-amino analogues or the free carboxylic analogues of the bromoacetamido compounds showed significant cytotoxicity at a concentration of 100 µg/ml. Conversely, the carboxylic esters of the bromoacetamido compounds 3, 13, 14 and 15 all showed signs of significant cytotoxicity. We speculate that this may be due to the indiscriminate alkylation of essential cellular components rather than the increased ability of these esters to penetrate the cells over the carboxylic acids. This is supported by the fact that the non-cytotoxic acids 17, 18 and 19 have comparable logD values to those of the cytotoxic esters 13, 14 and 15 and thus possess similar physicochemical characteristics.
Considerations for future development of the 2-aminothiazole-4-carboxylate scaffold
Our data clearly indicate that the 2-aminothiazole-4-carboxylate scaffold offers a promising new lead for further development. Methyl 2-amino-5-benzylthiazole-4-carboxylate 2 inhibits M. tuberculosis with an MIC of 0.06 µg/ml (240 nM) and is not cytoxic against HS-27 cells at 100 µg/ml concentrations. These compounds do not appear to inhibit the enzyme mtFabH, and it is important that their mode of action is elucidated for optimisation of the ligand to be carried out effectively. In doing so, care must be taken to retain the non-toxic and oral bioavailability properties of the molecule. However, whilst the zwitterionic, low molecular weight compound 9 has a structure that is highly hydrophilic, which has implications for both absorption and excretion by patients, this should be viewed in the context of INH, a successful and routinely administered anti-TB drug, which also has a low molecular weight and a logP value of −1.1.
Materials and Methods
Computational methods
Definition of active site
The active site determines the volume taken into consideration during the docking process and was defined as all protein atoms within 20 Å of the sulphur atom of Cys112.
Ligand and protein flexibility
All rotatable bonds of the ligand were randomized at the beginning of the GOLD docking and treated as completely flexible during the docking runs. Bond lengths, angles and torsions associated with non-rotatable bonds in the ligand were fixed in their initial configurations. The protein in GOLD is normally treated as rigid, with partial flexibility applied to hydroxyl protons and NH3 + groups, to allow rotation for hydrogen bond optimization.
GOLD docking
For each independent genetic algorithm (GA) run, a maximum number of 100,000 operations were performed on a population of 5 islands of 100 individuals. Operator weights for crossover, mutation and migration were set to 95, 95 and 10 respectively. Limits for van der Waals contacts and hydrogen bonds were assigned as 4.0 Å and 2.5 Å respectively and “GOLD Score” was chosen to assess the fitness of docked ligand-protein poses. Fifty poses were saved and visualized inside the active site to provide incite into potential binding patterns. Water molecules were removed from the pdb file and ligands were docked directly without any further preparation of the protein as recommended by the vendor.
Experimental methods
Reagents and apparatus
Melting point determination was performed using a Stuart Scientific Melting Point SMP1 apparatus with degrees Celsius (°C) as the unit. Infra-red spectra were run on Jasco FT-IR-4200 ATR (Attenuated Total Reflection Mode) and Mattson Genesis Series FT-IR spectrometers with samples compressed with KBr into disks. Wavenumbers, (ν max) are expressed as cm−1. Proton nuclear magnetic resonance (1H NMR) and carbon (13C NMR) spectra were run on JEOL EX 270 (270 MHz), Bruker AMX-400 (400 MHz) and JEOL Lambda delta 400 (400 MHz) spectrometers. Chemical shifts are stated in parts per million (ppm) and multiplicity indicated as singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). Coupling constants (J) are quoted in Hertz (Hz) and deuterated solvents specified for each of the compounds. High-resolution mass spectroscopy (MS) was obtained using Fourier transform electrospray ionisation (FTMS-ESI) and fast atom bombardment (3-nitrobenzyl alcohol matrix) (FAB-NOBA) ionizations on a JEOL JMS-700 Dual-sector High-resolution Mass Spectrometer. Mass to charge ratio (m/z) and relative abundance stated for molecular ion radicals is M+.. Unless otherwise stated, all reagents and solvents were obtained from commercial sources. Solvents were dried according to standard procedures when deemed necessary.
Synthesis of methyl 2-amino-5-benzylthiazole-4-carboxylate (2) as a general method for the synthesis of 2-amino-5-derivatized 4-carboxylate thiazoles (General procedure A)
Phenylcetaldehyde (1.6 g, 36 mmol, 1.0eq) was added to a stirring solution of methyl dichloroacetate 5 (5 g, 36 mmol, 1.0eq) dissolved in anhydrous ether (150 ml) at 0°C before adding NaOMe (1.65 g, 54 mmol, 1.5eq) dissolved in anhydrous MeOH (20 ml) dropwise over a period of 45 minutes. The mixture was allowed to stir for a further one hour at 0°C before adding brine (150 ml), extracting the organic phase with ether (150 ml), drying over MgSO4, and reducing in vacuo. To the crude residue was added thiourea (1.9 g, 36 mmol, 1.0eq) dissolved in MeOH (100 ml) and the mixture refluxed for 4 hours before concentrating in vacuo and neutralizing with concentrated ammonium hydroxide solution. The mixture was washed with dichloromethane (DCM) (3×, 50 ml), dried over anhydrous MgSO4, and purified by re-crystallization using chloroform and methanol (1∶3) to give 2 as a yellow powder (4.0 g, 44.9%). m.p 92–94°C; 1H NMR (270 MHz, DMSO-d 6) δ 3.74 (s, 3H), 4.32 (s, 2H), 7.02 (s, 2H), 7.32 (m, 5H); 13C NMR (270 MHz, DMSO-d 6) δ 31.7, 51.0, 126.1, 127.9, 128.8, 135.5, 136.4, 139.7, 162.3, 164.3; υmax (cm−1): 3465, NH stretch, amine; 1711, C = O stretch; FAB/NOBA-MS calculated C12H13N2O2S (M+H) 249.0698, found 249.0696.
Synthesis of methyl 2-(2-bromoacetamido)-5-(3-chlorophenyl)thiazole-4-carboxylate (3) as a general procedure for the synthesis of the bromoacetamido derivatives (general procedure B)
Compound 8 (3.0 g, 17.4 mmol, 1.0eq) was added to a stirring solution of anhydrous tetrahydrofuran (THF) (100 ml) and triethylamine (TEA) (3.5 g, 34.8 mmol, 2.0eq) at 0°C before adding acetyl chloride (3.45 g, 17.4 mmol, 1.0eq) dropwise over a period of 30 minutes. The mixture was left to stir for a further 30 minutes at 0°C before allowed to warm to room temperature for one hour. THF was reduced in vacuo and the crude residue was re-dissolved in a mixture of DCM and water before the pH of the solution was adjusted to 3.0 using 0.1 M HCl. The mixture was washed with DCM (3×50 ml), dried over anhydrous MgSO4, and purified by re-crystallization using chloroform and methanol (1∶6) to give 3 as a white powder (1.4 g, 33.1%). m.p 218–220°C. 1H NMR (400 MHz, DMSO-d 6) δ 3.80 (s, 3H), 4.11 (s, 2H), 7.36–7.39 (m, 3H), 7.47 (s, 1H); 13C NMR (400 MHz, DMSO-d 6) δ 27.7, 52.2, 128.4–131.7, 134.2, 139.0, 156.0, 162.3, 164.9; υmax (cm−1) 3216, NH stretch, 1699, C = O stretch, conjugated ester; 1684, C = O stretch, primary amide; FTMS-ESI calculated C13H11N2O3SClBr (M+H) 388.9362, found 388.9323.
Methyl 2-amino-5-methylthiazole-4-carboxylate (6)
The title compound was obtained as a pale yellow powder (1.7 g, 38.3%) using general procedure A. m.p 165–168°C. 1H NMR (270 MHz, DMSO-d6) δ 2.49 (s, 3H), 3.70 (s, 3H), 6.97 (s, 2H); 13C NMR (270 MHz, DMSO-d6) δ 12.7, 52.1, 135.2, 137.1, 164.3, 167.5; υmax (cm−1) 3433, NH stretch, amine; 1688, C = O stretch, conjugated ester; FTMS-ESI calculated C6H9N2O2S (M+H) 173.0385, found 173.0379.
Methyl 2-amino-5-phenylthiazole-4-carboxylate (7)
The title compound was obtained as a pale yellow powder (1.7 g, 20.4%) using general procedure A. m.p 218–221°C. (Lit: 223°C) [28]. 1H NMR (270 MHz, DMSO-d 6) δ 3.65 (s, 3H), 7.25 (s, 2H), 7.29–7.39 (m, 5H); 13C NMR (270 MHz, DMSO-d 6) δ 128.35, 128.68, 129.88, 131.65, 131.98, 137.54, 164.19, 166.13; υmax (cm−1) 3411, NH stretch, amine; 1696, C = O stretch, conjugated ester. FAB/NOBA-MS calculated C11H11N2O2S (M+H) 235.0541, found 235.0545.
Methyl 2-amino-5-(3-chlorophenyl)thiazole-4-carboxylate (8)
The title compound was obtained as a pale yellow powder (2.3 g, 50.7%) using general procedure A. mp 229–232°C. 1H NMR (400 MHz, DMSO-d 6) δ 3.70 (s, 3H), 7.76 (m, 3H), 7.81 (s, 1H); 13C NMR (400 MHz, DMSO-d 6) δ 52.0, 128.19, 128.8, 129.01, 129.95, 130.54, 132.68, 133.13, 136.1, 162.26, 166.1; υmax (cm−1) 3417, NH stretch; 1699, C = O stretch; FAB/NOBA-MS calculated C11H10N2O2SCl (M+H) 269.0152, found 269.0163.
Synthesis of 2-amino-5-methylthiazole-4-carboxylic acid (9) as a general method for the hydrolysis of 2-amino-5-derivatized 4-carboxylic acid thiazoles (general procedure C)
Compound 6 (1.0 g, 6.3 mmol, 1.0eq) was added to a stirring solution of NaOH (150 ml, 85 mM) at 50–60°C over a period of 30 minutes before a clear solution formed, cooled and acidified with 1 M HCl to pH 3–4. A precipitate was formed and collected in a Buchner funnel, which was re-crystallized using methanol to give 9 (0.8 g, 75.0%). mp 320–324°C; 1H NMR (270 MHz, DMSO-d6) δ 2.49 (s, 3H), 6.97 (s, 2H); 13C NMR (270 MHz, DMSO-d6) δ 12.7, 135.2, 137.1, 164.3, 167.5; υmax (cm−1) 3433, NH stretch, amine; 1688, C = O stretch, ester; 1688, COO stretch, carboxylic acid; FTMS-ESI calculated C5H7N2O2S (M+H) 159.0228, found 159.02138.
2-amino-5-phenylthiazole-4-carboxylic acid (10)
The title compound was obtained as a white needle-like crystals (2.4 g, 70%) using general procedure C. mp 242–243°C; 1H NMR (400 MHz, DMSO-d 6) δ 7.2 (bs, 1H), 7.35 (m, 4H), 12.5 (bs, 1H); 13C NMR (400 MHz, DMSO-d 6) δ 128.35, 128.68, 129.88, 131.66, 131.98, 137.54, 164.2, 166.13; υmax (cm−1) 3364, NH stretch, primary amine; 1701, C = O stretch, carboxylic acid; FTMS-ESI calculated C10H9N2O2S (M+H) 221.0385, found 221.0377.
2-amino-5-(3-chlorophenyl)thiazole-4-carboxylic acid (11)
The title compound was obtained as a white powder (2.4 g, 63.3%) using general procedure C. mp 242–244°C; 1H NMR (400 MHz, DMSO-d 6) δ 7.30–7.37 (m, 3H), 7.48 (s, 1H); 13C NMR (400 MHz, DMSO-d 6) δ 128.2, 129.02, 131.4, 133.2, 134.0, 138.2, 163.9, 166.5; υmax (cm−1) 3274, NH stretch, primary amine; 1684, C = O stretch, carboxylic acid; FAB/NOBA-MS calculated C10H8N2O2SCl (M+H) 254.9995, found 254.9982.
2-amino-5-benzylthiazole-4-carboxylic acid (12)
The title compound was obtained as a yellow powder (0.6 g, 68.0%) using general procedure C. m.p 300–302°C; 1H NMR (400 MHz, DMSO-d 6) δ 4.32 (s, 2H), 7.02 (s, 2H), 7.32 (m, 5H); 13C NMR (400 MHz, DMSO-d 6) δ 32.6, 127.0, 128.8, 129.06, 136.4, 137.6, 140.9, 164.2, 165.1; υmax (cm−1) 3465, NH stretch, amine; 1711, C = O stretch, carboxylic acid.; FAB/NOBA-MS calculated C11H11N2O2S (M+H) 235.0541, found 235.0529.
Methyl 2-(2-bromoacetamido)-5-methylthiazole-4-carboxylate (13)
The title compound was obtained as an off-white powder (2.6 g, 53.7%) using general procedure B. mp 220–222°C; 1H NMR (400 MHz, DMSO-d 6) δ 2.62 (s, 3H), 3.79 (s, 3H), 4.12 (s, 2H), 12.8 (s, 1H); 13C NMR (400 MHz, DMSO-d 6) δ 12.66, 28.7, 52.19, 135.9, 138.7, 153.7, 163.0, 166.0; υmax (cm−1) 3235, NH stretch, primary amide; 1717, C = O stretch, conjugated ester; 1699, C = O stretch, primary amide; FTMS-ESI calculated (M+H) 292.9595, found 292.9582.
Methyl 2-(2-bromoacetamido)-5-phenylthiazole-4-carboxylate (14)
The title compound was obtained as a brown powder (0.8 g, 47.9%) using general procedure B. mp 218–220°C; 1H NMR (400 MHz, DMSO-d 6) δ 3.69 (s, 3H), 4.16 (s, 2H), 7.48 (m, 5H), 13.08 (s, 1H); 13C NMR (400 MHz, DMSO-d 6) δ 28.6, 52.3, 128.9–130.5, 135.1, 139.5, 155.6, 162.6, 166.4; υmax (cm−1) 3230, NH stretch, primary amide; 1697, C = O stretch, conjugated ester; 1697, C = O stretch, primary amide; FAB/NOBA-MS calculated C13H12BrN2O3S (M+H) 354.9752, found 354.9752.
Methyl 5-benzyl-2-(2-bromoacetamido)thiazole-4-carboxylate (15)
The title compound was obtained as a brown powder (1.5 g, 34.5%) using general procedure B. mp 218–220°C; 1H NMR (400 MHz, DMSO-d 6) δ 3.96 (s, 3H), 4.05 (s, 2H), 4.54 (s, 2H), 7.32 (m, 5H), 9.90 (s, 1H); 13C NMR (400 MHz, DMSO-d 6) δ 28.6, 32.5, 52.3, 127.3, 129.11, 129.25, 135.6, 140.5, 143.2, 154.7, 163.5, 166.0; υmax (cm−1) 3231, NH stretch, primary amide; 1701, C = O stretch, conjugated ester; 1701, C = O stretch, primary amide; FAB/NOBA-MS calculated C14H14N2O3SBr (M+H) 368.9908, found 368.9902.
2-(2-Bromoacetamido)-5-methylthiazole-4-carboxylic acid (16)
The title compound was obtained as an off-white powder (0.7 g, 73.5%) using general procedure C. mp 218–220°C; 1H NMR (400 MHz, DMSO-d 6) δ 2.65 (s, 3H), 4.11 (s, 2H), 12.8 (s, 1H); 13C NMR (400 MHz, DMSO-d 6) δ 12.77, 28.82, 137.0, 138.5, 153.0, 164.0, 166.0; υmax (cm−1) 3185, NH stretch, primary amide; 1699, C = O stretch, carboxylic acid; 1662, C = O stretch, primary amide. FTMS-ESI calculated C7H8BrN2O3S (M+H) 278.9439, found 278.9425.
2-(2-Bromoacetamido)-5-phenylthiazole-4-carboxylic acid (17)
The title compound was obtained as a brown powder (0.1 g, 18.6%) using general procedure C. mp 208–210°C; 1H NMR (400 MHz, DMSO-d 6) δ 4.16 (s, 2H), 7.41 (m, 5H); 13C NMR (400 MHz, DMSO-d 6) δ 28.7, 128.8, 129.21, 130.31, 137.8, 139.1, 155.0, 164.0, 167.0; υmax (cm−1) 3216, NH stretch, primary amide; 1675, C = O stretch, carboxylic acid; 1675, C = O stretch, primary amide. FAB/NOBA-MS calculated C12H10BrN2O3S (M+H) 340.9595, found 340.9600.
2-(2-Bromoacetamido)-5-(3-chlorophenyl)thiazole-4-carboxylic acid (18)
The title compound was obtained as a white powder (0.3 g, 37.2%) using general procedure C. mp 228–230°C; 1H NMR (400 MHz, DMSO-d 6) δ 4.17 (s, 2H), 7.46 (m, 3H), 7.61 (s, 1H); 13C NMR (400 MHz, DMSO-d 6) δ 28.6, 114.98, 123.96, 128.46, 129.04, 131.32, 133.59, 136.7, 137.2, 155.7, 163.4, 166.4; υmax (cm−1) 3180, NH stretch, primary amide; 1679, C = O stretch, carboxylic acid; 1679, C = O stretch, primary amide. FAB/NOBA-MS calculated C12H9N2O3S (M+H) 374.9206, found 374.9194.
5-Benzyl-2-(2-bromoacetamido)thiazole-4-carboxylic acid (19)
The title compound was obtained as a brown powder (0.3 g, 33.3%) using general procedure C. mp 220–222°C; 1H NMR (400 MHz, DMSO-d 6) δ 4.10 (s, 2H), 4.49 (s, 2H), 7.32 (m, 5H), 12.80 (s, 1H); 13C NMR (400 MHz, DMSO-d 6) δ 28.7, 32.5, 127.24, 129.08, 129.21, 136.8, 140.3, 142.4, 154.3, 164.0, 166.0; υmax (cm−1) 3185, NH stretch, primary amide; 1695, C = O stretch, carboxylic acid; 1661, C = O stretch, primary amide; FTMS-ESI calculated C13H12N2O3SBr (M+H) 354.9752, found 354.9736.
mtFabH inhibition assay
The condensing activity of mtFabH was assayed by mixing 100 µM holo-AcpM, 1 mM β-mercaptoethanol, 0.1 M sodium phosphate buffer pH 7.0, 50 µM malonyl-CoA, 45 nCi of [2-14C]malonyl-CoA (specific activity 53 Ci/mol), 12.5 µM of palmitoyl-CoA, and 0.3 µg mtFabD in a final volume of 40 µl. The mtFabD protein was added to generate the malonyl-AcpM substrate for the reaction in situ. A mixture of AcpM, 1 mM β-mercaptoethanol, and the buffer was incubated at 37°C for 30 min to ensure complete reduction of AcpM, and then the remaining components (except mtFabH) were added. The mixture was then dispensed into microcentrifuge tubes along with the test compound (0.5–200 µg/ml), and the reaction was initiated by the addition of 0.5 µg of mtFabH. The reaction mixture was held at 37°C for 40 min. The reaction was quenched by adding 5 mg/ml NaBH4 in 100 mM K2HPO4, 100 mM KCl, 30% tetrahydrofuran, resulting in the liberation of β-ketoacyl groups from their respective thioesters as acyl-1,3-diols. These products were extracted three times with 2 ml of water-saturated toluene, and the extracts were pooled and washed with an equal volume of water. The organic component was transferred to scintillation vials, the solvent was removed by evaporation, 5 ml EcoScintA (National Diagnostics, UK) was added, and the radiolabeled products were quantified by liquid scintillation counting.
FAS-I and FAS-II Assays
FAS-I and FAS-II experiments were conducted as per Slayden et al. (1996) using the 40–80% ammonium sulphate fraction. The standard reaction mixture for the incorporation of radioactivity from [2-14C]malonyl-CoA into C16 to C24 fatty acids catalysed by FAS-I was composed as follows: 100 mM Tris.HCl pH 7.9, 5 mM EDTA, 5 mM dithiothreitol, 300 µM acetyl-CoA, 100 µM NADPH, 100 µM NADH, 1 µM flavin mononucleotide, 500 µM α-cyclodextrin, 300 µM CoA-SH, 100,000 cpm of [2-14C] malonyl-CoA (specific activity 53 Ci/mol), test compound (0.5–200 µg/ml) and 2 mg of cytosolic enzyme preparation in a total volume of 500 µl. Similarly, the standard reaction mixture for incorporation of radioactivity from [2-14C]malonyl-CoA into C24 to C30 fatty acids catalysed by FAS-II contained the following: 100 mM potassium phosphate buffer (pH 7.0), 5 mM EDTA, 5 mM dithiothreitol, 10 µM palmitoyl-CoA, 140 µM NADPH, 140 µM NADH, 100 µg of AcpM, 40 µM malonyl-CoA, 200,000 cpm of [2-14C]malonyl-CoA (specific activity 53 Ci/mol), test compound (0.5–200 µg/ml) and 200 mg of cytosolic enzyme preparation in a total volume of 250 µl. In both the FAS-I and FAS-II assays, reactions were performed in triplicate at 37°C for 30 min and terminated by the addition of 250 µl of 20% potassium hydroxide in 50% methanol at 100°C for 45 min. Following acidification with 150 µl of 6 M HCl, the resultant 14C-labelled fatty acids were extracted three times with petroleum ether. The organic extracts were pooled, washed once with an equal volume of water, and dried in a scintillation vial prior to counting.
M. tuberculosis H37Rv inhibition assays
All compounds were screened for their in vitro antimycobacterial activity against M. tuberculosis H37Rv by an in-house broth macrodilution method. The activity of compounds was confirmed by MIC determination by different methods against M. tuberculosis H37Rv, which included: broth microdilution assay, resazurin microdilution assay, agar dilution proportion method and the MB/Bactec 3D system. Positive and negative growth controls were run in each experiment run and ciprofloxacin was used as an antibiotic control (MIC = 0.025–0.5 µg/ml would indicate that the experiment is successful). Unless stated otherwise, the inoculum was 100 µl of 1 McFarland broth during log phase. A 21 gauge fine-needled syringe was used to flush the broth 5 times to break up any clumps. Blood agar plates were inoculated to check contamination. Here is a brief description of each method:
Broth macrodilution assay
A stock solution of each compound (1 mg/ml) was diluted in sterile distilled water to test the range (0.06–32 µg/ml). Each tube contained 4 ml sterile Middlebrook 7H9 broth containing albumin-dextrose-catalase (ADC supplement, Oxoid), Tween 80, glycerol and 4 ml of the compound solution was added to make serial double dilutions. Tubes were incubated at 37°C for 7 days and then read visually. MIC was determined as the lowest concentration of antibiotic that prevented turbidity.
Resazurin microdilution assay
96-well microtitre plates with U-bottomed wells (Nunclon, Denmark) were used and stock solutions (1 mg/ml) were diluted to four fold the final highest concentration tested [29]. Three ranges were tested in duplicate: 0.2–0.025 µg/ml, 0.3–0.037 µg/ml and 0.5–0.06 µg/ml. A two-fold dilution series was prepared with 100 µl antibiotic solution in sterile distilled water. The microtitre plates were covered with lids and placed inside a safety box (Nalgene, USA) and incubated at 37°C for seven days. On the seventh day, 30 µl resazurin (prepared as 0.01% and store at 4°C until use within one week) was added and incubated at 37°C for another 24 h. Plates were read on the following day and MIC was determined as the lowest concentration of antibiotic that prevented colour change (blue to pink).
Microdilution broth assay
Microdilution broth assay was performed simultaneously with the resazurin assay using same method but without the 1∶20 dilution step. The MIC was determined after fourteen days by reading the microtitre plate visually.
Agar proportion method
The MIC of each compound was tested by agar dilution in triplicate as recommended by the National Committee for Clinical Laboratory Standards (NCCLS). The compounds dilution range was (0.3–0.025 µg/ml) and stock solutions were diluted to tenfold the final highest concentration tested. Plates were prepared as by adding 2 ml of the compound solution to 18 ml of Middlebrook 7H10 agar containing oleic acid-albumin-dextrose-catalase supplement (OADC, Oxoid), sealed and stored at 4°C to be used within one week of preparation. Each plate was inoculated with 20 µl 1 McFarland broth in the log phase diluted to different concentrations (10−1 to 10−6 dilutions). Plates were incubated at 37°C for fourteen days. The MIC was considered the lowest concentration that showed no visible colonies at all dilutions.
MIC measurement by MB/Bactec 3D system
Stock solutions of the compounds were prepared as 10,000 times the desired concentration and diluted accordingly with the liquid medium provided in the MB bottles. Each bottle was inoculated with 0.5 ml Middlebrook 7H9 broth culture in the log phase with growth of 1.0 McFarland turbidity. A positive (1%) and a negative control and isoniazid control were included. Bottles were kept in the automated system for 56 days. MIC was considered as the lowest concentration of compound that did not grow or gave growth signal later than the (1%) control.
HS27 toxicity studies
The HS27 cells (grown in Dulbecco's modified eagle medium, supplemented with FBS, penicillin/streptomycin, L-glutamine and sodium pyruvate (Invitrogen) were plated in 96 well plates from Helena Bioscience at 1×10e4 cells per well (80 ul/well) and incubated for 24 hrs at 37°C, 5% CO2. The samples (10 ul/well) and alamar blue (10 ul/well) were added, the cells incubated in the presence of sample at 37°C, 5% CO2 for 24 hrs before reading in the fluorescence mode (560/590 nm).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Bloom BR, Small PM. The evolving relation between humans and mycobacterium tuberculosis. N Engl J Med. 1998;338:677–678. doi: 10.1056/NEJM199803053381008. [DOI] [PubMed] [Google Scholar]
- 3.Mitchison DA. The search for new sterilizing anti-tuberculosis drugs. Front Biosci. 2004;9:1059–1072. doi: 10.2741/1293. [DOI] [PubMed] [Google Scholar]
- 4.Selwyn PA, Sckell BM, Alcabes P, Friedland GH, Klein RS, et al. High risk of active tuberculosis in HIV-infected drug users with cutaneous anergy. JAMA. 1992;268:504–509. [PubMed] [Google Scholar]
- 5.Migliori GB, Ortmann J, Giradi E, Besozzi G, Lange C, et al. Extensively drug-resistant tuberculosis, Italy and Germany. Emerg Infect Dis. 2007;13:780–782. doi: 10.3201/eid1305.060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 7.Takayama K, Wang C, Besra GS. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin Microbiol Rev. 2005;18:81–101. doi: 10.1128/CMR.18.1.81-101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portevin D, De Sousa-D'Auria C, Houssin C, Grimaldi C, Chami M, et al. A polyketide synthase catalyzes the last condensation step of mycolic acid biosynthesis in mycobacteria and related organisms. Proc Natl Acad Sci USA. 2004;101:314–319. doi: 10.1073/pnas.0305439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kremer L, Baulard AR, Besra GS. Genetics of mycolic acid biosynthesis. Molecular Genetics of Mycobacteria. ASM Press; 2000. p. 173. [Google Scholar]
- 10.Yuan Y, Lee RE, Besra GS, Belisle JT, Barry CE. Identification of a gene involved in the biosynthesis of cyclopropanated mycolic acids in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:6630–6634. doi: 10.1073/pnas.92.14.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oishi N, Noto T, Sasaki H, Suzuki K, Hayashi T, et al. Thiolactomycin, a new antibiotic. Taxonomy of the producing organism, fermentation and biological properties. Antibiot. 1982;35:391–395. doi: 10.7164/antibiotics.35.391. [DOI] [PubMed] [Google Scholar]
- 12.Noto T, Miyakawa S, Oishi H, Endo H, Okazaki H. Thiolactomycin, a new antibiotic. In vitro antibacterial activity. J Antibiot. 1982;35:401–410. doi: 10.7164/antibiotics.35.401. [DOI] [PubMed] [Google Scholar]
- 13.Slayden RA, Lee R, Armour JW, Cooper AM, Orme IM, et al. Antimycobacterial action of thiolactomycin: an inhibitor of fatty acid and mycolic acid synthesis. Antimicro Agents Chemother. 1996;40:2813–2819. doi: 10.1128/aac.40.12.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi KH, Kremer L, Besra GS, Rock CO. Identification and substrate specificity of beta -ketoacyl (acyl carrier protein) synthase III (mtFabH) from Mycobacterium tuberculosis. J Biol Chem. 2000;275:28201–28207. doi: 10.1074/jbc.M003241200. [DOI] [PubMed] [Google Scholar]
- 15.Kremer L, Douglas JD, Baulard AR, Morehouse C, Guy MR, et al. Thiolactomycin and related analogues as novel anti-mycobacterial agents targeting KasA and KasB condensing enzymes in Mycobacterium tuberculosis. J Biol Chem. 2000;275:16857–16864. doi: 10.1074/jbc.M000569200. [DOI] [PubMed] [Google Scholar]
- 16.Schaeffer ML, Agnihotri J, Volker C, Kallender H, Brennan PJ, et al. Purification and biochemical characterization of the Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein synthases KasA and KasB. J Biol chem. 2001;276:47029–47037. doi: 10.1074/jbc.M108903200. [DOI] [PubMed] [Google Scholar]
- 17.Kremer L, Dover LG, Carrere S, Nampoothiri KM, Lesjean S, et al. Mycolic acid biosynthesis and enzymic characterization of the beta-ketoacyl-ACP synthase A-condensing enzyme from Mycobacterium tuberculosis. Biochem J. 2002;364:423–430. doi: 10.1042/BJ20011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim P, Zhang Y-M, Shenoy G, Hguyen Q-A, Boshoff HI, et al. structure-activity relationships at the 5-position of thiolactomycin: An intact (5R)-isoprene unit is required for activity against the condensing enzymes from Mycobacterium tuberculosis and Escherichia coli. J Med Chem. 2006;49:159–171. doi: 10.1021/jm050825p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamal A, Shaik A, Sinha R, Yadav JS, Arora SK. Antitubercular agents Part 2: new thiolactomycin analogues active against Mycobacterium tuberculosis. Bioorg Med Chem Lett. 2005;15:1927–1929. doi: 10.1016/j.bmcl.2005.01.084. [DOI] [PubMed] [Google Scholar]
- 20.Senior SJ, Illarionov PA, Gurcha SS, Campbell IB, Schaeffer ML, Minnikin DE, Besra GS. Biphenyl-based analogues of thiolactomycin, active against Mycobacterium tuberculosis mtFabH fatty acid condensing enzyme. Bioorg Med Chem Letts. 2003;13:3685–3688. doi: 10.1016/j.bmcl.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Senior SJ, Illarionov PA, Gurcha SS, Campbell IB, Schaeffer ML, Minnikin DE, Besra GS. Acetylene-based analogues of thiolactomycin, active against Mycobacterium tuberculosis mtFabH fatty acid condensing enzyme. Bioorg Med Chem Letts. 2003;14:373–376. doi: 10.1016/j.bmcl.2003.10.061. [DOI] [PubMed] [Google Scholar]
- 22.Bhowruth V, Brown AK, Senior SJ, Snaith JS, Besra GS. Acetylene-based analogues of thiolactomycin, active against Mycobacterium tuberculosis mtFabH fatty acid condensing enzyme. Bioorg Med Chem Letts. 2007;17:5643–5646. [Google Scholar]
- 23.Brown AK, Sridharan S, Kremer L, Lindenberg S, Dover LG, et al. Probing the mechanism of the Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein synthase III mtFabH: factors influencing catalysis and substrate specificity. J Biol Chem. 2005;280:32539–32547. doi: 10.1074/jbc.M413216200. [DOI] [PubMed] [Google Scholar]
- 24.Sachdeva S, Musayev F, Alhamadsheh M, Scarsdale NJ, Wright TH, et al. Separate entrance and exit portals for ligand traffic in Mycobacterium tuberculosis FabH. Chem Biol. 2008;15:402–12. doi: 10.1016/j.chembiol.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Price AC, Choi K-H, Heath RJ, Li Z, White SW, et al. Inhibition of beta -Ketoacyl-Acyl Carrier Protein Synthases by Thiolactomycin and Cerulenin, structure and mechanism. J Biol Chem. 2001;276:6551–6559. doi: 10.1074/jbc.M007101200. [DOI] [PubMed] [Google Scholar]
- 26.Ashek A, Cho SJ. A combined approach of docking and 3D QSAR study of [beta]-ketoacyl-acyl carrier protein synthase III (FabH) inhibitors. Bio Med Chem. 2006;14:1474–1482. doi: 10.1016/j.bmc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Barton A, Breukelman SP, Kaye PT, Meakins DG, Morgan DJ. The preparation of thiazole-4- and -5-carboxylates, and an infrared study of their rotational isomers. J Chem Soc, Perkin Trans. 1982;1:159–164. [Google Scholar]
- 28.Jones G, Willett P, Glen RC. Molecular Recognition of Receptor Sites using a Genetic Algorithm with a Description of Desolvation. J Mol Biol. 1995;245:43–53. doi: 10.1016/s0022-2836(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 29.Palomino JC, Martin A, Camacho M, Guerra H, Swings J, et al. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2002;46:2720–2722. doi: 10.1128/AAC.46.8.2720-2722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]