The concept of hemodynamics was born in 1628 with Harvey’s description of the circulation, but its growth was limited for centuries by the inability to measure pressure and flow accurately. The work of Starling, Wiggers, and other hemodynamic physiologists in the first part of the twentieth century, along with introduction of cardiac catheterization by Cournand and Richards in the 1940s ushered in the golden era of hemodynamics. From the late 1970s to early 1990s, there was an explosion of clinical and basic research as new methods were developed to quantify ventricular systolic and diastolic properties more definitively. Enthusiasm for such characterization subsequently waned, however, as therapies directly targeting hemodynamic derangements, such as inotropes, were found to hasten mortality. This change coincided with a paradigm shift in the way heart failure was conceptualized, from a disease of abnormal hemodynamics to one of neurohormonal derangements, abnormal cell signaling, and maladaptive remodeling. As such, many “gold-standard” methods for characterizing load, contractility, diastole, and, ventricular-arterial interaction were not adopted into clinical practice. A working understanding of each element remains paramount to interpret properly the hemodynamic changes in patients who have acute and chronic heart failure, however.
Routine cardiac catheterization provides data on left heart, right heart, systemic and pulmonary arterial pressures, vascular resistances, cardiac output, and ejection fraction. These data are often then applied as markers of cardiac preload, after-load, and global function, although each of these parameters reflects more complex interactions between the heart and its internal and external loads. This article reviews more specific, gold standard assessments of ventricular and arterial properties and how these relate to the parameters reported and used in practice, and then discusses the re-emerging importance of invasive hemodynamics in the assessment and management of heart failure.
CARDIAC CONTRACTILITY: LOOKING BEYOND THE EJECTION FRACTION
The most universally accepted index of contractility used in practice, the EF, unfortunately is also one of the least specific.1 As with any parameter measuring the extent of muscle shortening or thickening, it is highly sensitive to afterload and really is an expression of ventricular–arterial coupling rather than of contractility alone. EF also is affected by heart size, because its denominator is end-diastolic volume (EDV), leading many to propose that EF is more a parameter of remodeling than of contractility. EF commonly is used to classify different “forms” of heart failure (low versus preserved EF).2 This approach is appealing, given its binary nature and ease of application in practice, but the realities of how a patient develops signs and symptoms of heart failure are far more complex,3 and in this regard EF serves as a somewhat arbitrary marker.
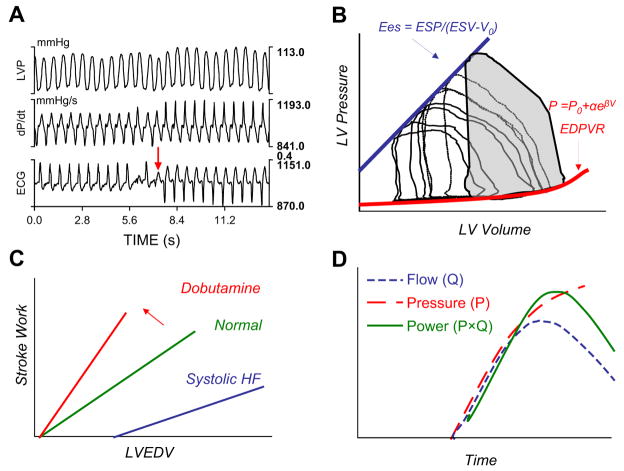
More specific measures of contractility have been developed but because of their complexity remain used principally in research. The maximal rate of pressure rise during isovolumic contraction (dP/dtmax) can be assessed using a high-fidelity micromanometer and is used widely as a measure of contractility. dP/dtmax, however, is dependent on cardiac filling (ie, is preload dependent) and heart rate, and it may not always reflect contractile function that develops after cardiac ejection is initiated.4 In patients who have cardiac dyssynchrony, the lack of coordinated contraction in early-systole reduces dP/dtmax because the force developed by the early activated wall is dissipated by stretching of the still relaxed opposite wall.5 dP/dtmax is quite sensitive to this phenomenon (Fig. 1), but in this case it reflects chamber mechanics rather than intrinsic muscle function.
Fig. 1.
(A) Time plot of left ventricular pressure (LVP), first derivative of pressure (dP/dt), and EKG in a patient who has heart failure with left bundle-type conduction delay. At the arrow, the patient received bi-ventricular stimulation resulting in an abrupt rise in dP/dtmax. (B) PV loops obtained at baseline and during transient caval occlusion (decreasing LV volumes—loops moving right to left). The slope of the EDSPV derived from multibeat analysis defines ventricular Ees, a load-independent measure of contractility. By measuring diastolic pressure and volume during diastasis at variably loaded beats, the end-diastolic PV relationship (EDPVR) is obtained. The shaded area subtended by the baseline loop represents the stroke work performed by the ventricle. ESP, end-systolic pressure; ESV, end-systolic volume; V0, volume axis intercept of ESPVR. (C) The slope of the relation between systolic chamber performance (stroke work) and preload (left ventricle end-diastolic volume, LVEDV) determines the preload recruitable stroke work. This relationship shifts up and to the left, as indicated by the arrow, with an increase in contractility, as with dobutamine, or down and to the right with systolic heart failure (HF). (D) LV power (P × Q, solid line) is determined by the product of simultaneously measured pressure (P, dashed line) and flow (Q, dotted line). When indexed to preload, this calculation produces another load-independent measure of LV chamber contractility.
An ideal parameter of contractility would assess inotropic state independently of preload, afterload, heart rate, and remodeling.1 This assessment still remains somewhat elusive, but parameters derived from relations between cardiac pressure and volume have come the closest to achieving it. As shown in Fig. 1B, a series of variably loaded pressure–volume (PV) loops can be obtained to assess systolic, diastolic, coupling, and energetic properties. Stroke work, dP/dtmax, maximal ventricular power, elastance, efficiency, and other parameters are assessed, and by examining these variables over a range of preload volumes, one can derive more load-independent, cardiac-specific measures.6
The relationship between end-systolic pressure and volume from a variety of variably loaded cardiac contractions yields the end-systolic pressure–volume relationship (ESPVR),7 its slope being the end-systolic elastance (Ees) (see Fig. 1B). The Ees conveys information about both contractile function and myocardial constitutive properties; that is, it is a measure of chamber stiffness. It can be related easily to arterial vascular load to assess ventricular–arterial interaction8 and provides important information about how a patient’s blood pressure and flow will respond to loading changes.9 Although traditionally Ees was assessed from multiple cardiac cycles requiring simultaneous measurement of cardiac pressure and volume (or flow), algorithms have been generated and tested to derive these parameters noninvasively from single steady-state data.10–12 These parameters have been used to characterize systolic properties in various forms of heart failure and in larger populations.6,13,14 The diastolic correlate of Ees is the end-diastolic elastance, the lower PV boundary that is parameterized most often by a mono-exponential stiffness coefficient. Both the Ees and diastolic curve fits are dependent on chamber size and remodeling.
Several other parameters commonly are derived for indexing systolic function. The relationship between stroke work and preload, measured from multiple beats under different loading generates a “Sarnoff curve,” and its slope, often termed “preload recruitable stroke work” (PRSW) provides a contractile index (see Fig. 1C).15 Maximal ventricular power (PWRmax) is the peak instantaneous product of ventricular outflow and pressure and also is quite preload dependent (see Fig. 1D). Regression over a range of preloads yields a more specific index, however, and because the intercept of this relation often is near zero for normal-sized ventricles, the ratio of PWRmax/EDV can be used. In failing ventricles, normalization to EDV2 seems to reduce load dependence better.16,17
DIASTOLE—MORE THAN END-DIASTOLIC PRESSURE
Diastolic function is determined from active and passive processes, and both contribute to relaxation and chamber filling.3 Left heart filling pressures, either pulmonary artery occlusion (wedge pressure) or left ventricle (LV) end-diastolic pressure (EDP), are central to standard cardiac catheterization, and their elevation is taken to reflect abnormal loading and/or abnormal chamber compliance. One cannot determine whether the elevation reflects abnormal loading or abnormal chamber compliance from the pressure parameter alone, but diastolic function also is assessed by a variety of noninvasive methods to characterize it better.
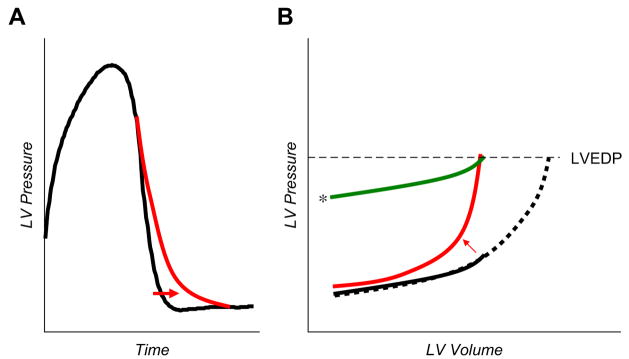
Aortic valve closure marks the onset of ventricular diastole, where relaxation is quantified invasively by the rate of pressure decay, typically expressed by a time constant (τ) that is estimated using a number of mathematical fits (Fig. 2).18 Even this seemingly straightforward assessment has technical pitfalls. These model fits–usually mono-exponential decays (ie, P = P∞ + Poe−t/τ or the same equation without P∞)–may or may not describe adequately the actual fall of pressure. When used in a setting where they do not fit properly, such as in dilated heart failure, they can lead to erroneous conclusions.18 An alternative model based on a modified logistic equation, P = PA/(1 + et/τ) + PB,19 fits such failure data better than the exponential. Chung and Kovacs20 recently explored both model fits in contrast to a more physics-based approach, showing that relaxation is best described using both resistive and elastic elements. This intriguing approach deserves further attention in clinical studies.
Fig. 2.
(A) The kinetics of LV pressure decay during isovolumic relaxation (the time between aortic valve closure and mitral opening) can be modeled by various equations to derive the time constant, τ. The curve indicated by the arrow shows prolonged relaxation, often seen with heart failure, aging, and hypertension. (B) Groups of DPVR. The solid black line shows the curve in a normal person. The curve that shifts up and to the left (arrow) indicates the effects of increased passive chamber stiffness. Filling pressures (LVEDP) may be elevated because of the increased passive chamber stiffness, but similar elevations also can be seen in the absence of increased stiffness, as when there is with increased extrinsic restraint (line indicated by the asterisk). Note that the shape (stiffness) of the DPVR with enhanced restraint is similar to that in the normal patient. Finally, EDP may be elevated simply because of the overfilling of a structurally normal ventricle (dotted black line). There is evidence to support each of these possible contributors to elevated EDP in HFpEF.
Delayed relaxation is common. It frequently accompanies normal aging,21 but it becomes even more prominent in cardiac failure and with hypertrophy. It has multiple determinants starting with dissociation of the cross-bridge, calcium-handling, and elasticity-restoring forces resulting from the recoil of compressed macromolecules such as titin.3 The extent to which delayed relaxation alters mean and late-diastolic pressures remains controversial,22 because diastole generally is long enough that even delayed relaxation is completed before late-diastolic filling occurs. It clearly can affect early filling and pressures, particularly at faster heart rates. Analysis requires invasive high-fidelity micro-manometer recordings, although it may be approximated by the time between aortic valve closure and mitral valve opening or inferred from mitral Doppler flow patterns.23
Similar to isovolumic relaxation, passive diastolic chamber stiffness rarely is measured directly in standard clinical practice. As noted, the LV diastolic pressure–volume relationship (DPVR; see Fig. 1B) is curvilinear; thus compliance most often is expressed by a mono-exponential stiffness coefficient.24 Linear approximations have been used, but one must be careful to compare these approximations within similar loading ranges. The DPVR most often is estimated from a single heart-beat, although this estimation combines features that reflect early relaxation and resistive (viscous) properties and extra-chamber (eg, pericardial) loading. A more accurate approach is to assess multiple PV loops over a loading range (varying preload) and to connect points at late-diastole from these cycles (see Fig. 1B). The apparent chamber stiffness derived from single versus multiple loops can vary substantially, as shown particularly in patients who have genetic hypertrophic cardiomyopathy; in these patients the use of a single loop markedly underestimated LV stiffness.25
LVEDP may be increased because chamber is stiffer or is subject to higher preload or because the entire DPVR is shifted upwards (see Fig. 2B).3 All three variables play a role in heart failure with a reduced EF. The first and last seem to contribute mostly to heart failure with a preserved EF (HFpEF),14,26,27 and although some have found increased LV filling volumes as well,28 this phenomenon has not been seen by others.14,29 Approximately 40% of a measured intracavitary LV pressure stems from extrinsic forces applied to the LV, mediated by the pericardium and right heart across the interventricular septum.30 This phenomenon of diastolic ventricular interaction becomes more important as heart size increases (ie, as pericardial space decreases).31 Even though the LV chamber size usually is normal in HFpEF, total epicardial heart size can be enlarged substantially because of increased atrial size and cardiac hypertrophy, so the pericardial constraint can be relevant.29 This effect is supported by invasive data from patients who have HFpEF in which the DPVR shifted upwards in patients subjected to exertional stress.32 In heart failure with low EF, cardiac enlargement in almost all chambers exacerbates pericardial constraint.31,33 Overfilling of right-sided chambers can increase left-sided pressures via right ventricular (RV)–LV crosstalk as the distending (transmural) pressure that drives LV filling becomes impaired.34 This mechanism can explain how acute unloading of the right heart paradoxically can increase LV filling and output in patients who have advanced systolic heart failure or cor pulmonale.33
AFTERLOAD AND VENTRICULAR–ARTERIAL INTERACTION
Adequate pressure and flow to the body depends both on cardiac performance and on the nature of the vascular load into which it ejects. This load traditionally has been conceived of as equivalent to mean or systolic blood pressure, although this notion can lead to ambiguous interpretations. Unlike isolated muscle (for which the term “after-load” was first defined), where one can fix a constant force during contraction, the intact heart generates varying stress (and pressures) during ejection, and the blood pressure generated is determined as much by the heart’s properties as by those of the vasculature.35 A useful alternative parameter is aortic input impedance, which characterizes the mean and pulsatile properties of the vascular loading circuit and is independent of the heart. Impedance is derived from Fourier analysis of aortic pressure and flow waves,36,37 traditionally assessed by invasive catheters although noninvasive methods also have been described.
Coupling of impedance to a heart property is mathematically complicated, because impedance is described in the frequency domain (ie, Fourier spectra), and the heart property is described in the time domain. In the early 1980s, however, Sunagawa and colleagues38,39 developed the parameter effective arterial elastance (Ea), based on LV pressure–volume loop analysis. Ea, like Ees, is measured in units of elastance and can be calculated more easily to study ventricular–arterial interaction. Ea is not a measure of a specific vascular property per se but combines both mean and pulsatile loading (and heart rate influences), providing a lumped parameter reflecting the net impact of this load on the heart. Kelly and colleagues40 showed that the simple ratio of end-systolic pressure to stroke volume accurately estimates Ea in both hypertensive and normal humans. Graphically Ea is identified by the negative slope running through the end-systolic PV coordinates and EDV at zero pressure (Fig. 3). As discussed earlier, LV Ees is determined by the slope of the ESPVR. The Ees reflects chamber contractility but also is influenced by chamber size and remodeling. Knowledge of Ees, Ea, and EDV allows one to predict blood pressure, stroke volume and stroke work, and EF.8
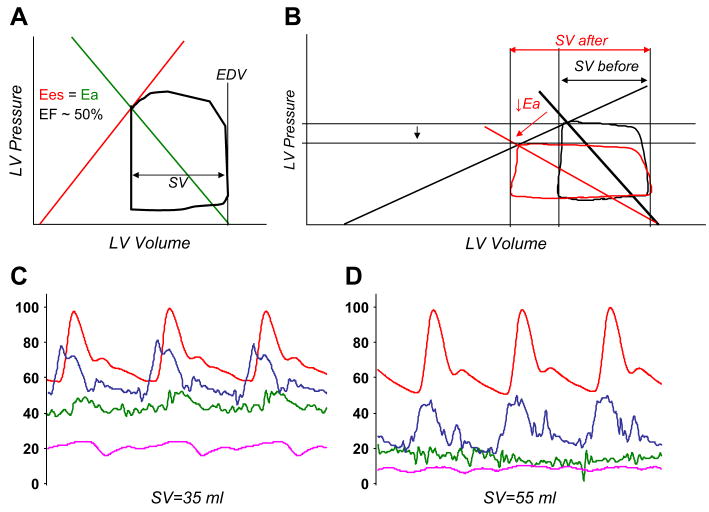
Fig. 3.
(A) Normal steady-state PV loop. Ea is defined by the negative slope (green line) running between the end-systolic PV point and EDV at P = 0. In healthy persons, Ees (red line) and Ea are matched to maintain optimal coupling and efficiency, with an EF of around 50% to 60% when the volume intercept is near zero. (B) In systolic heart failure, the heart is dilated (increased EDV), Ees is low (shallow ESPVR), and the EF is reduced. Acute reduction of Ea with a vasodilator (red) leads to a marked 50% increase in stroke volume (SV after) with very little reduction in blood pressure. (C) Radial artery (red), pulmonary artery (blue), pulmonary wedge (green), and right atrial (pink) pressure versus time in a patient who has dilated cardiomyopathy. There is severe pulmonary arterial and venous hypertension with borderline systemic hypotension. SV, stroke volume. (D) The same patient on a high dose (7 μg/kg/min) of sodium nitroprusside. Note the near-normalization of cardiac filling pressures with marked increase in stroke volume (SV), with little change in systolic blood pressure.
Ees and Ea are matched in healthy persons to provide optimal mechanical efficiency in the transfer of blood from the heart to the body, so that the coupling ratio (Ea/Ees) approaches unity.41 It can be shown mathematically that the EF varies inversely with the Ea/Ees ratio.8 In systolic heart failure, contractility (Ees) is low, whereas afterload (Ea) usually is high in the setting of vasoconstriction and neurohormonal activation. This condition produces “afterload mismatch” so that the coupling ratio increases, meaning that the EF decreases.42 With advanced systolic dysfunction, it usually is difficult to increase contractility (Ees) effectively because of limited inotropic reserve, but therapies reducing Ea to very low levels have been extremely useful in optimizing ventricular ejection in such patients.
Conceptualizing systolic heart failure in terms of ventricular–arterial interaction can help explain the clinical response to vasodilator therapy of patients who have dilated cardiomyopathy.9 Fig. 3B shows a typical resting PV loop from such a patient. Because of the shallow slope of the ESPVR (low Ees), there is little change in blood pressure despite a marked improvement in stroke volume for a given dose of vasodilator. Fig. 3C and D shows pressure tracings as part of transplant evaluation for a patient who has advanced dilated cardiomyopathy. Filling pressures are high at baseline, pulmonary vascular resistance is prohibitively elevated, and there is systemic hypotension. With the administration of sodium nitroprusside there is marked improvement in cardiac output, filling pressures, and pulmonary vascular resistance and no significant reduction in systolic pressures–all as predicted based on the principals of ventricular–arterial coupling.
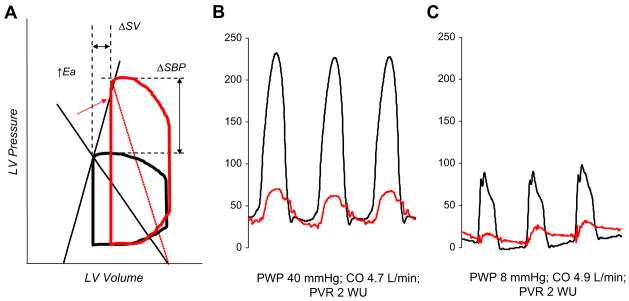
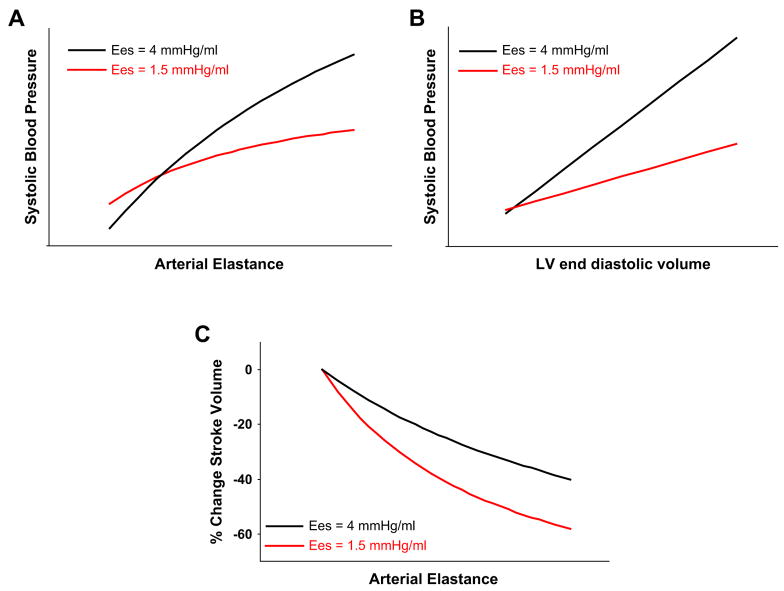
Although the coupling ratio is useful for determining stroke volume and the pressure generated during systole, the absolute magnitude of both the numerator and denominator is equally important. In normal aging, systolic hypertension, and renal disease, there are exaggerated increases in both ventricular and vascular stiffness.8 The stiff ventricle-artery unit creates a “high-gain” system in which there are much larger changes in pressure with relatively little change in stroke volume (Fig. 4). This situation is essentially the opposite of that seen in systolic heart failure, where loading changes result in relatively minor alterations in blood pressure, despite more dramatic changes in stroke volume. The importance in ventricular-arterial stiffening becomes most dramatic in many patients who have HFpEF, who can have quite high Ea and Ees.32 Fig. 4B and C shows LV and pulmonary artery pressures from a typical patient who has HFpEF and increased ventricular-arterial stiffness. At rest (see Fig. 4B), there is severe systemic and pulmonary artery hypertension. In contrast to the patient who has heart failure and low EF shown in Fig. 3, there is a marked hypotensive effect after the administration of a very low dose of nitroprusside (see Fig. 4C) with little or no change in stroke volume and cardiac output. In this way, high resting stiffness greatly amplified the change in blood pressure for a given change in preload or afterload while minimizing changes in stroke volume. Increased ventricular-arterial stiffening helps explain the clinical behavior of patients who have HFpEF, who often oscillate between hypertensive crisis and symptomatic hypotension with relatively minor perturbations. Treatments targeting combined stiffening may allow better regulation of PV responses during stress in such patients and are being explored in upcoming clinical trials.3 Fig. 5 contrasts the two types of patients schematically, using model-based analyses.
Fig. 4.
(A) With aging, hypertension, and in HFpEF, ventricular (Ees) and arterial (Ea) stiffness increases. Although the Ea/Ees ratio may remain normal, combined ventricular and vascular stiffening leads to marked fluctuations in blood pressure with relatively small changes in preload or afterload. This condition is in striking contrast to heart failure with low EF (see Fig. 3B). (B) LV (black) and pulmonary artery (red) pressure tracings from an 81-year-old woman who has HFpEF demonstrating severe systemic and pulmonary artery hypertension, with markedly elevated LVEDP and wedge pressures (not shown). (C) In response to a very low dose of sodium nitroprusside (2 μg), filling pressures normalize, but severe hypotension develops. Note that there is little change in cardiac output (stroke volume) with vasodilation, again in striking contrast to heart failure with reduced EF. CO, cardiac output; PVR, pulmonary vascular resistance; PWP, pulmonary wedge pressure; WU, Wood units.
Fig. 5.
Increased ventricular systolic stiffness leads to a greater rise in blood pressure for a given increase in (A) afterload or (B) preload. (C) Although isolated increases in afterload lead to a predictable reduction in stroke volume for a given level of contractility, this afterload dependence is more marked in patients who have lower Ees, as seen in patients who have heart failure with reduced EF.
Arterial–ventricular interaction also is important in affecting diastolic processes. Acutely increased vascular load, particularly applied in late ventricular systole,43 prolongs relaxation in humans and animals.3,44 Such load dependence becomes more pronounced in heart failure, perhaps related in part to abnormal phosphorylation of sarcomeric proteins. Troponin I phosphorylation by protein kinase A attenuates afterload-induced impairment in early-diastolic relaxation, and mice lacking such phosphorylation sites have enhanced load-dependent relaxation delay.45 Acute increases in Ea also have been shown to increase LV diastolic stiffness in an aged canine model of HFpEF.46 LV early-diastolic relaxation varies inversely with net afterload and vascular stiffness in humans with and without hypertensive heart disease43 and is correlated most closely with pulsatile components, particularly late-systolic load, determined by returning pressure wave reflections and arterial stiffening.
THE RIGHT HEART
Pulmonary hypertension and accompanying right heart dysfunction is increasingly common in patients who have heart failure, regardless of EF, and potently affect exercise capacity and clinical outcome.47,48 Pulmonary hypertension generally is defined as a mean pulmonary arterial pressure higher than 25 mm Hg at rest (30 mm Hg with exercise), whereas pulmonary arterial hypertension (ie, pulmonary vascular disease) further requires an elevated pulmonary vascular resistance while maintaining a normal pulmonary capillary wedge pressure.49 The presence of pulmonary vascular resistance and the ability to reduce it with vasodilators are used commonly to establish eligibility for cardiac transplantation. Drug testing uses nitric oxide donators or milrinone and, more recently, the phosphodiesterase 5 inhibitor sildenafil and the natriuretic peptide nesiritide.50 Although pulmonary artery pressures can be estimated by echo-Doppler methods, invasive assessment is required for definitive diagnosis and to guide treatment decisions.49
With the wide use of echo-Doppler cardiography, pulmonary hypertension now is being recognized increasingly, particularly among the older patients presenting with dypsnea.51 The prevalence of suspected idiopathic pulmonary hypertension of the elderly is increasing, and in many cases the condition may be a forme fruste of HFpEF. Recent studies have found that these patients tend to have higher left heart filling pressures than younger patients who have more traditional isolated pulmonary arterial hypertension.51 Invasive hemodynamic assessment in the catheterization laboratory can be extremely useful in evaluating such patients, many of whom complain of exertional dyspnea and whose symptoms could be explained by multiple, potentially competing causes (eg, diastolic dysfunction, pulmonary vascular disease, obesity, deconditioning, and others). Interpreting elevated pulmonary wedge pressures in such patients often is difficult, because in the setting of right heart and left atrial enlargement enhanced extrinsic pressure may be applied via the pericardium.31
Compared with the left side, the assessment of right ventricular function remains fairly primitive. Standard two-dimensional echocardiographic views often are difficult to standardize to provide reproducible parameters, and the shape of the RV limits the use of simple geometric models to derive accurate volumetric data. As with the LV, RV pressures are not determined exclusively by the ventricle or vasculature but rather result from the dynamic interaction of the two. The RV PV loop normally is triangular, reflecting the lower resistive load and relatively higher compliance of the pulmonary vascular circuit, although it becomes more rectangular (like the LV) in patients who have pulmonary hypertension (perhaps making application of standard LV approaches and the assumptions behind them more justified in this setting). Assessment of RV diastolic stiffness is quite rare in the literature and is affected greatly by pericardial restraint and biventricular remodeling. RV vascular impedance consists of mean pulmonary resistance, the proximal stiffness of pulmonary conduit arteries, characteristic impedance, distal vascular compliance, and reflected waves. There now is renewed interest in how these properties of impedance can impose late-systolic loads on the RV52 (much as they do on the LV), impacting RV remodeling, relaxation delay, and inefficiency.
The importance of the right heart–pulmonary vascular interaction on symptoms in chronic heart failure is appreciated increasingly. Traditional vasodilators used to treat heart failure (eg, converting enzyme inhibitors and angiotensin receptor blockers) tend to have less effect on the pulmonary circuit. Treatments used to target the pulmonary vasculature, such as prostacyclin and endothelin antagonists, also have systemic effects and have not yet been evaluated larger-scale clinical trials in patients who have predominantly left-sided heart failure. Phosphodiesterase inhibitors such as sildenafil reduce pulmonary vascular resistance while having mild systemic vascular effects, and these drugs are helping elucidate RV-pulmonary pathophysiology in heart failure. In an elegant series of recent studies performed in humans who had primary left-sided systolic heart failure, sildenafil acutely and chronically reduced pulmonary resistance, correlating with enhanced exercise capacity, without much systemic change.53,54 In another study, investigators found sildenafil improved endothelial function (flow-mediated dilation) in addition to reducing pulmonary resistance, and this improvement also was coupled with improved exercise capacity.55 Given the lack of obvious left-sided heart or arterial resistive effects, these studies highlight the importance of enhancing pulmonary vascular throughput and normal vasodilator reserve in patients who have heart failure.
INVASIVE HEMODYNAMICS: A RE-EMERGING ROLE IN THE EVALUATION OF PATIENTS WHO HAVE POSSIBLE HEART FAILURE AND PRESERVED EJECTION FRACTION
Most cardiologists are fairly confident in making the diagnosis of heart failure when a patient who has severe LV enlargement and an EF of 25% presents with dyspnea, but a significant group of patients present with exertional dyspnea, clinical euvolemia (or only mild hypervolemia), and a normal EF. The differential diagnosis is fairly broad, including noncardiac causes (deconditioning, obesity, anemia, and other possibilities) and a variety of cardiogenic sources. These conditions may include valvular disease, isolated right heart failure, pulmonary vascular disease, constrictive pericarditis, restrictive cardiomyopathy, or “garden variety” HFpEF. The correct diagnosis often can be made from the combination of physical examination, comprehensive echo-Doppler evaluation, and plasma natriuretic peptide levels. In many patients, particularly the elderly, the picture is not so clear, because diastolic dysfunction seen on echo-Doppler imaging is common in this cohort,21 and natriuretic peptide levels may be mildly elevated even in the absence of true heart failure.56
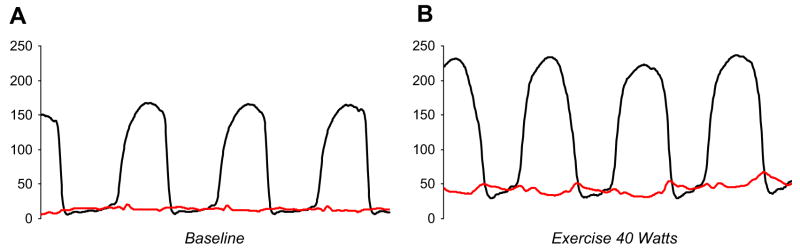
Invasive hemodynamic assessment in the catheterization laboratory can be clinically useful in these cases. Fig. 6 shows hemodynamics from an 80-year-old woman who has class II–III dyspnea, normal EF, mild diastolic dysfunction, and mild to moderate pulmonary hypertension on echocardiogram. Filling pressures are normal at rest (see Fig. 6A), suggesting that the patient’s symptoms may not be related to heart failure. Supine exercise at low workload (see Fig. 6B), however, reveals a marked increase in cardiac filling pressures associated with severe symptoms of dyspnea. Pulmonary artery pressures increase in proportion to the increase in pulmonary wedge, indicating that the patient’s symptoms probably are caused primarily by HFpEF. Other patients may show filling pressures and cardiac output that are normal both at rest and at maximal workload, arguing against a diagnosis of HFpEF. Finally, others develop pulmonary arterial hypertension with exercise in the absence of an increase in left heart filling pressures, identifying a more isolated lesion at the level of the pulmonary vasculature. In practice, individual patients may embody any of these conditions or, more commonly, present with some combination of all three, and future research is required to understand how best to treat patients who have each type of response.
Fig. 6.
(A) LV (black) and pulmonary wedge (red) pressures at rest in a patient who has symptoms of New York Heart Association class II–III dyspnea and normal LV size and function on echocardiogram. Despite mild to moderate systemic hypertension, cardiac filling pressures are normal, arguing against heart failure. (B) With low-level (40 W) supine exercise in the catheterization laboratory, there is a dramatic increase in cardiac filling pressures (to 45–50 mm Hg) associated with significant dyspnea, suggesting that HFpEF indeed is the cause of the patient’s symptoms.
Patients who have had cardiac surgery or radiation therapy may present months to years later with predominant right-sided heart failure, and high-fidelity cardiac catheterization focusing on relationships between intrathoracic–intracardiac pressure dissociation and diastolic ventricular interaction can identify whether symptoms are caused predominantly by constrictive physiology, valvular disease, or restrictive cardiomyopathy.57,58 Administration of arterial vasodilators such as nitroprusside can be useful to determine whether elevated filling pressures are caused by a partially load-dependent process, such as diastolic dysfunction, or an irreversible myopathic process, such as restrictive cardiomyopathy.
Right heart catheterization can be useful in the management of patients who have acute decompensated heart failure, particularly in the setting of right-sided congestion, low cardiac output, and worsening renal function, when central hemodynamic status remains uncertain. In addition, central hemodynamics may provide independent prognostic value in patients who have heart failure. Each of these topics is discussed elsewhere in this issue.
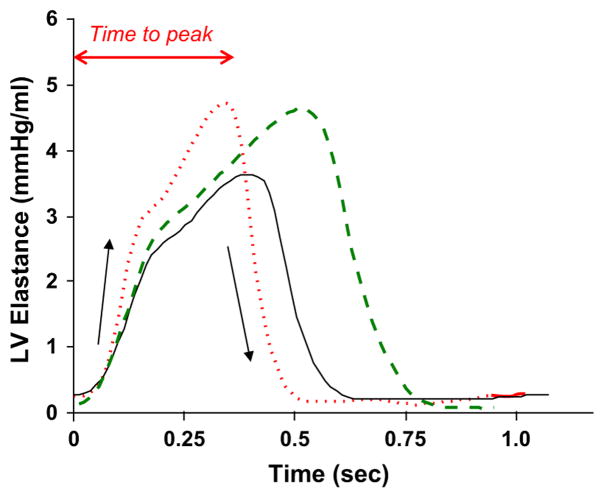
The time course of ventricular stiffening (ie, elastance varying over time, long a bio-engineering concept59 but with little apparent interest to physicians) could become clinically important in the near future. Novel therapies targeting myofilament calcium sensitivity and/or the filaments (activators) themselves to increase force generation without altering activator calcium or stimulating cAMP/protein kinase A (PKA) cascades are in development.60 Although phosphorylation of myosin-binding protein C augments early dP/dtmax downstream of β-adrenergic stimulation,4 sensitizers/activators do not work in this manner. Instead of affecting isovolumic contraction, these drugs often enhance myocardial stiffening (elastance), prolonging the time for elastance to reach its peak and thus making the ejection time longer. The difference in mechanisms is not easily discerned from traditional methods of analysis but is shown readily by the elastance curves. Fig. 7 shows a schematic of such curves comparing the effects of a beta-agonist to a Ca2+-sensitizer. The beta-agonist increases the rate of rise and fall of myocardial stiffening but shortens systole. In contrast, the sensitizer has little effect on the rates of rise and fall but prolongs ejection. The lure of sensitizers is their potential to provide inotropy without the increases in heart rate, risk of arrhythmia, or metabolic demand seen with traditional cAMP/PKA-mediated agonists. Such drugs are being developed, so this type of hemodynamic analysis, or a simplified version of it, ultimately may provide ways to index and follow the effects of these drugs in individual patients.
Fig. 7.
Time varying elastance curves obtained at baseline (solid line), after β-adrenergic stimulation (dotted line), and in response to an agent that enhances myofilament calcium sensitivity (dashed line). Although the calcium sensitizer has less effect on the early rise in elastance, there is an increase in the time to peak elastance and systolic duration. See text for details.
SUMMARY
A few years after fading from the forefront of cardiology, interest in cardiovascular hemodynamics is returning, especially as newer devices are developed that help measure these parameters in patients chronically. Invasive assessment of cardiovascular properties provides greater insight into the mechanisms of disease in disorders such as HFpEF and can explain how patients who have different forms of heart failure respond to various therapies or to certain forms of stress. This information may be useful for treating individual patients and in understanding group differences and treatment effects. Invasive hemodynamic assessment remains the reference standard for assessing systolic and diastolic function and ventricular–arterial interaction and can allow more definitive diagnosis of heart failure, especially in patients where the diagnosis of HF that is based upon clinical and oninvasive evaluation alone remains uncertain.
References
- 1.Kass DA, Maughan WL, Guo ZM, et al. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation. 1987;76(6):1422–36. doi: 10.1161/01.cir.76.6.1422. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Kass DA. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med. 2006;16(8):273–9. doi: 10.1016/j.tcm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Nagayama T, Takimoto E, Sadayappan S, et al. Control of in vivo left ventricular [correction] contraction/relaxation kinetics by myosin binding protein C: protein kinase A phosphorylation dependent and independent regulation. Circulation. 2007;116(21):2399–408. doi: 10.1161/CIRCULATIONAHA.107.706523. [DOI] [PubMed] [Google Scholar]
- 5.Spragg DD, Kass DA. Pathobiology of left ventricular dyssynchrony and resynchronization. Prog Cardiovasc Dis. 2006;49(1):26–41. doi: 10.1016/j.pcad.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Baicu CF, Zile MR, Aurigemma GP, et al. Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation. 2005;111(18):2306–12. doi: 10.1161/01.CIR.0000164273.57823.26. [DOI] [PubMed] [Google Scholar]
- 7.Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res. 1974;35(1):117–26. doi: 10.1161/01.res.35.1.117. [DOI] [PubMed] [Google Scholar]
- 8.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin. 2008;4(1):23–36. doi: 10.1016/j.hfc.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kass DA, Maughan WL. From ‘Emax’ to pressure-volume relations: a broader view. Circulation. 1988;77(6):1203–12. doi: 10.1161/01.cir.77.6.1203. [DOI] [PubMed] [Google Scholar]
- 10.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38(7):2028–34. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 11.Lee WS, Huang WP, Yu WC, et al. Estimation of preload recruitable stroke work relationship by a single-beat technique in humans. Am J Physiol Heart Circ Physiol. 2003;284(2):H744–50. doi: 10.1152/ajpheart.00455.2002. [DOI] [PubMed] [Google Scholar]
- 12.Borlaug BA, Melenovsky V, Marhin T, et al. Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation. 2005;112(17):2642–9. doi: 10.1161/CIRCULATIONAHA.105.540500. [DOI] [PubMed] [Google Scholar]
- 13.Redfield MM, Jacobsen SJ, Borlaug BA, et al. Age-and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112(15):2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 14.Lam CS, Roger VL, Rodeheffer RJ, et al. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from olmsted county, Minnesota. Circulation. 2007;115(15):1982–90. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glower DD, Spratt JA, Snow ND, et al. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985;71(5):994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]
- 16.Kass DA, Beyar R. Evaluation of contractile state by maximal ventricular power divided by the square of end-diastolic volume. Circulation. 1991;84(4):1698–708. doi: 10.1161/01.cir.84.4.1698. [DOI] [PubMed] [Google Scholar]
- 17.Sharir T, Feldman MD, Haber H, et al. Ventricular systolic assessment in patients with dilated cardio-myopathy by preload-adjusted maximal power. Validation and noninvasive application. Circulation. 1994;89(5):2045–53. doi: 10.1161/01.cir.89.5.2045. [DOI] [PubMed] [Google Scholar]
- 18.Senzaki H, Fetics B, Chen CH, et al. Comparison of ventricular pressure relaxation assessments in human heart failure: quantitative influence on load and drug sensitivity analysis. J Am Coll Cardiol. 1999;34(5):1529–36. doi: 10.1016/s0735-1097(99)00362-9. [DOI] [PubMed] [Google Scholar]
- 19.Matsubara H, Takaki M, Yasuhara S, et al. Logistic time constant of isovolumic relaxation pressure-time curve in the canine left ventricle. Better alternative to exponential time constant. Circulation. 1995;92(8):2318–26. doi: 10.1161/01.cir.92.8.2318. [DOI] [PubMed] [Google Scholar]
- 20.Chung CS, Kovacs SJ. Physical determinants of left ventricular isovolumic pressure decline: model prediction with in vivo validation. Am J Physiol Heart Circ Physiol. 2008;294(4):H1589–96. doi: 10.1152/ajpheart.00990.2007. [DOI] [PubMed] [Google Scholar]
- 21.Redfield MM, Jacobsen SJ, Burnett JC, Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Glantz SA, Parmley WW. Factors which affect the diastolic pressure-volume curve. Circ Res. 1978;42(2):171–80. doi: 10.1161/01.res.42.2.171. [DOI] [PubMed] [Google Scholar]
- 23.Oh JK, Hatle L, Tajik AJ, et al. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47(3):500–6. doi: 10.1016/j.jacc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Kass DA. Assessment of diastolic dysfunction. Invasive modalities Cardiol Clin. 2000;18(3):571–86. doi: 10.1016/s0733-8651(05)70162-4. [DOI] [PubMed] [Google Scholar]
- 25.Pak PH, Maughan L, Baughman KL, et al. Marked discordance between dynamic and passive diastolic pressure-volume relations in idiopathic hypertrophic cardiomyopathy. Circulation. 1996;94(1):52–60. doi: 10.1161/01.cir.94.1.52. [DOI] [PubMed] [Google Scholar]
- 26.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 27.Westermann D, Kasner M, Steendijk P, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117(16):2051–60. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 28.Maurer MS, Burkhoff D, Fried LP, et al. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;49(9):972–81. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 29.Melenovsky V, Borlaug BA, Rosen B, et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49(2):198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 30.Dauterman K, Pak PH, Maughan WL, et al. Contribution of external forces to left ventricular diastolic pressure. Implications for the clinical use of the Starling law. Ann Intern Med. 1995;122(10):737–42. doi: 10.7326/0003-4819-122-10-199505150-00001. [DOI] [PubMed] [Google Scholar]
- 31.Frenneaux M, Williams L. Ventricular-arterial and ventricular-ventricular interactions and their relevance to diastolic filling. Prog Cardiovasc Dis. 2007;49(4):252–62. doi: 10.1016/j.pcad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi M, Hay I, Fetics B, et al. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714–20. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 33.Atherton JJ, Moore TD, Lele SS, et al. Diastolic ventricular interaction in chronic heart failure. Lancet. 1997;349(9067):1720–4. doi: 10.1016/S0140-6736(96)05109-4. [DOI] [PubMed] [Google Scholar]
- 34.Moore TD, Frenneaux MP, Sas R, et al. Ventricular interaction and external constraint account for decreased stroke work during volume loading in CHF. Am J Physiol Heart Circ Physiol. 2001;281(6):H2385–91. doi: 10.1152/ajpheart.2001.281.6.H2385. [DOI] [PubMed] [Google Scholar]
- 35.Kass DA, Kelly RP. Ventriculo-arterial coupling: concepts, assumptions, and applications. Ann Biomed Eng. 1992;20(1):41–62. doi: 10.1007/BF02368505. [DOI] [PubMed] [Google Scholar]
- 36.Milnor WR. Arterial impedance as ventricular after-load. Circ Res. 1975;36(5):565–70. doi: 10.1161/01.res.36.5.565. [DOI] [PubMed] [Google Scholar]
- 37.Murgo JP, Westerhof N, Giolma JP, et al. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62(1):105–16. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 38.Sunagawa K, Maughan WL, Burkhoff D, et al. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245(5 Pt 1):H773–80. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 39.Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res. 1985;56(4):586–95. doi: 10.1161/01.res.56.4.586. [DOI] [PubMed] [Google Scholar]
- 40.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86(2):513–21. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 41.De Tombe PP, Jones S, Burkhoff D, et al. Ventricular stroke work and efficiency both remain nearly optimal despite altered vascular loading. Am J Physiol. 1993;264(6 Pt 2):H1817–24. doi: 10.1152/ajpheart.1993.264.6.H1817. [DOI] [PubMed] [Google Scholar]
- 42.Asanoi H, Sasayama S, Kameyama T. Ventriculoarterial coupling in normal and failing heart in humans. Circ Res. 1989;65(2):483–93. doi: 10.1161/01.res.65.2.483. [DOI] [PubMed] [Google Scholar]
- 43.Borlaug BA, Melenovsky V, Redfield MM, et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50(16):1570–7. doi: 10.1016/j.jacc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 44.Gillebert TC, Leite-Moreira AF, De Hert SG. Load dependent diastolic dysfunction in heart failure. Heart Fail Rev. 2000;5(4):345–55. doi: 10.1023/a:1026563313952. [DOI] [PubMed] [Google Scholar]
- 45.Bilchick KC, Duncan JG, Ravi R, et al. Heart failure-associated alterations in troponin I phosphorylation impair ventricular relaxation-afterload and force-frequency responses and systolic function. Am J Physiol Heart Circ Physiol. 2007;292(1):H318–25. doi: 10.1152/ajpheart.00283.2006. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro BP, Lam CS, Patel JB, et al. Acute and chronic ventricular-arterial coupling in systole and diastole. Insights from an elderly hypertensive model. Hypertension. 2007;50(3):503–11. doi: 10.1161/HYPERTENSIONAHA.107.090092. [DOI] [PubMed] [Google Scholar]
- 47.Haddad F, Doyle R, Murphy DJ, et al. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117(13):1717–31. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 48.Kjaergaard J, Akkan D, Iversen KK, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol. 2007;99(8):1146–50. doi: 10.1016/j.amjcard.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 49.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S–34S. doi: 10.1378/chest.126.1_suppl.14S. [DOI] [PubMed] [Google Scholar]
- 50.Alaeddini J, Uber PA, Park MH, et al. Efficacy and safety of sildenafil in the evaluation of pulmonary hypertension in severe heart failure. Am J Cardiol. 2004;94(11):1475–7. doi: 10.1016/j.amjcard.2004.07.157. [DOI] [PubMed] [Google Scholar]
- 51.Shapiro BP, McGoon MD, Redfield MM. Unexplained pulmonary hypertension in elderly patients. Chest. 2007;131(1):94–100. doi: 10.1378/chest.06-1571. [DOI] [PubMed] [Google Scholar]
- 52.Kussmaul WG, 3rd, Altschuler JA, Matthai WH, et al. Right ventricular-vascular interaction in congestive heart failure. Importance of low-frequency impedance. Circulation. 1993;88(3):1010–5. doi: 10.1161/01.cir.88.3.1010. [DOI] [PubMed] [Google Scholar]
- 53.Lepore JJ, Maroo A, Bigatello LM, et al. Hemodynamic effects of sildenafil in patients with congestive heart failure and pulmonary hypertension: combined administration with inhaled nitric oxide. Chest. 2005;127(5):1647–53. doi: 10.1378/chest.127.5.1647. [DOI] [PubMed] [Google Scholar]
- 54.Lewis GD, Lachmann J, Camuso J, et al. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation. 2007;115(1):59–66. doi: 10.1161/CIRCULATIONAHA.106.626226. [DOI] [PubMed] [Google Scholar]
- 55.Guazzi M, Samaja M, Arena R, et al. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50(22):2136–44. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 56.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47(2):345–53. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hurrell DG, Nishimura RA, Higano ST, et al. Value of dynamic respiratory changes in left and right ventricular pressures for the diagnosis of constrictive pericarditis. Circulation. 1996;93(11):2007–13. doi: 10.1161/01.cir.93.11.2007. [DOI] [PubMed] [Google Scholar]
- 58.Talreja DR, Nishimura RA, Oh JK, et al. Constrictive pericarditis in the modern era: novel criteria for diagnosis in the cardiac catheterization laboratory. J Am Coll Cardiol. 2008;51(3):315–9. doi: 10.1016/j.jacc.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 59.Suga H, Sagawa K, Demer L. Determinants of instantaneous pressure in canine left ventricle. Time and volume specification. Circ Res. 1980;46(2):256–63. doi: 10.1161/01.res.46.2.256. [DOI] [PubMed] [Google Scholar]
- 60.Kass DA, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation. 2006;113(2):305–15. doi: 10.1161/CIRCULATIONAHA.105.542407. [DOI] [PubMed] [Google Scholar]