Abstract
Cocaine’s ability to block the dopamine transporter (DAT) is crucial for its reinforcing effects. However the brain functional consequences of DAT blockade by cocaine are less clear since they are confounded by its concomitant blockade of norepinephrine and serotonin transporters. To separate the dopaminergic from the non-dopaminergic effects of cocaine on brain function we compared the regional brain metabolic responses to cocaine between dopamine transporter deficient (DAT−/−) mice with that of their DAT+/+ littermates. We measured regional brain metabolism (marker of brain function) with 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) and microPET (μPET) before and after acute cocaine administration (i.p. 10 mg/kg). Scans were conducted 2 weeks apart. At baseline DAT−/− mice had significantly greater metabolism in thalamus and cerebellum than DAT+/+. Acute cocaine decreased whole brain metabolism and this effect was greater in DAT+/+ (15%) than in DAT−/− mice (5%). DAT+/+ mice showed regional decreases in the olfactory bulb, motor cortex, striatum, hippocampus, thalamus and cerebellum whereas DAT−/− mice showed decreases only in thalamus. The differential pattern of regional responses to cocaine in DAT−/− and DAT+/+ suggests that most of the brain metabolic changes from acute cocaine are due to DAT blockade. Cocaine-induced decreases in metabolism in thalamus (region with dense noradrenergic innervation) in DAT−/− suggest that these were mediated by cocaine’s blockade of norepinephrine transporters. The greater baseline metabolism in DAT−/− than DAT+/+ mice in cerebellum (brain region mostly devoid of DAT) suggests that dopamine indirectly regulates activity of these brain regions.
Keywords: PET, drug abuse, ADHD, DAT, insulin
INTRODUCTION
The rewarding properties of cocaine have been shown to result from temporary blockade of dopamine transporters (DAT), and subsequent increases in extracellular dopamine (DA) levels (Jones et al., 1999; Koob and Nestler, 1997; Kuhar et al., 1991). Chronic enhanced DA signaling is also believed to trigger the plastic changes that are responsible for cocaine addiction (Jones et al., 1999).
DAT knockout (DAT−/−) mice exhibit unique phenotypes that include hyperactivity, dwarfism, neuroen-docrine dysfunction, and cognitive deficits (Gainetdinov and Caron, 2003; Gainetdinov et al., 1999). The basal behavioral profile of DAT−/− mice was similar to DAT+/+ mice under the influence of psychostimulants (Jones et al., 1998). Microdialysis studies have reported significantly greater extracellular DA in DAT−/− mice than in DAT+/+ mice in the striatum and nucleus accumbens (Jones et al., 1998) with estimates that the lifetime extracellular DA in DAT−/− mice is 300 times greater than in DAT+/+ littermates (Gainetdinov and Caron, 2003). Contrary to expected, while DAT−/− mice are resistant to cocaine’s locomotor effects (Giros et al., 1996) they are sensitive to its rewarding effects (Rocha et al., 1998; Sora et al., 2001; Sora et al., 1998). Although these results led some to question the role of DAT in cocaine’s reinforcing effects, the recent findings that cocaine’s rewarding effects were abolished in transgenic mice with cocaine-insensitive DAT demonstrate the necessary role of DAT in cocaine’s rewarding effects (Chen et al., 2006). It appears that in DAT−/− mice cocaine increases extracellular DA in the nucleus accumbens (NAc) through its blockade of the norepinephrine transporter (NET) (Carboni et al., 2001).
Although much emphasis has been placed on cocaine’s blockade of DAT because of its involvement on its reinforcing effects the functional significance of cocaine’s acute blockade of other transporters for Nor-epinephrine (NET) and Serotonin (SERT) is much less understood. To separate the dopaminergic from the non-dopaminergic effects of cocaine on brain function we compared the regional brain glucose metabolic responses to cocaine between DAT−/− mice with that of their DAT+/+ littermates. To assess the effects of cocaine on brain function we measured glucose metabolism, which serves as a marker of brain function (Kelly et al., 1982) and is sensitive to the regional effects of acute drug administration (Macey et al., 2004; Porrino and Lucignani, 1987; Porrino et al., 2002; Williams-Hemby et al., 1996; Whitlow et al., 2002; McCulloch et al., 1982). For this purpose we used the radiotracer 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) (Sokoloff et al., 1977; Phelps et al., 1979; Reivich et al., 1979) in conjunction with μPET technology which allows one to measure glucose metabolism in the rodent brain non-invasively (Thanos et al., 2002a,2002b; Thanos et al., 2004). We hypothesized that the metabolic changes induced by cocaine would be mostly ascribed to its blockade of DAT. In addition, the assessment of baseline metabolism in DAT−/− mice, which are hyperdopaminergic offers a different window to evaluate the effects of enhanced DA activity in baseline brain glucose metabolism.
MATERIALS AND METHODS
Subjects
Adult male mice DAT+/+ (n = 7) and DAT−/− (n = 7) mice were obtained from Duke University (M. Caron). Animals were individually housed in clear acrylic cages with wire covers under standard laboratory conditions (22°C ± 2°C, 50% ± 10% relative humidity) and a normal 12-h/12-h light/dark cycle with lights on at 0700 and off at 1900. Rodent chow (Purina) and tap water were available ad libitum and all animals were weighed daily. Experiments were conducted in conformity with the National Academy of Sciences Guide for the Care and Use of Laboratory Animals (NAS and NRC, 1996) and Brookhaven National Laboratory Institutional Animal Care and Use Committee protocols.
Materials
FDG was purchased from a commercially available radiopharmaceutical supplier (Cardinal Health, Franklin Sq., NY). Cocaine hydrochloride was purchased from Sigma Aldrich (St. Louis, MO).
Scanning procedures
PET scans were performed using a Concorde μPET R4 tomograph (Concorde Microsystems, Inc). Total acquisition time was 80 min (static single-frame) and data was acquired in fully three-dimensional mode with maximum axial acceptance angle (±28°). Images were reconstructed using the OSEM/3D algorithm provided by the manufacturer.
Five DAT+/+ and three DAT−/− mice underwent two repeated FDG μPET scans (within-group design) on different days: a control and cocaine challenge FDG scan. Additional mice from each genotype were supplemented (between-group design) in order to reach the desired sample size but with the limitation that the supplemental mice were scanned using saline or cocaine and not both. In the control scans, mice were injected i.p. with saline followed by i.p. injection of 200–300 μCi FDG 30 min later. In the cocaine scan, the protocol was repeated with an acute i.p. injection of 10 mg/kg cocaine. Following the 30-min uptake of FDG, each animal was anesthetized i.p. with a mixture of Ketamine/Xylazine (100/10 mg/kg). The anesthetized animal was placed in a prone position on the scanner bed. Final orientation of the head was within 3 cm of the center of the scanning area for maximum spatial resolution. Immediately after placement in the scanning field, acquisition was initiated.
Image analysis
Image processing and quantification was completed using PMOD v.2.75 software (PMOD Technologies Ltd.) (Fig. 1). μPET images were co-registered to a T1 weighted isotropic MRI image template from an age matched C57/BL6 mouse. Eight Regions of Interest (ROI) were drawn on the olfactory bulb (OB), frontal cortex (FR), motor cortex (MC), cingulate cortex (CC), striatum (ST), thalamus (TH), hippocampus (HP), and cerebellum (CB). These ROIs were identified using the MRI template and the Paxinos mouse brain atlas as a reference (Paxinos and Franklin, 2001). Standardized uptake values normalized for body weight (SUVBW) were calculated by normalizing each ROI value by radioactivity injected and animal weight (Marsteller et al., 2006; Wang et al., 2004) using the following equation:
Fig. 1.
Masked FDG μPET brain images averaged (n = 7/group), corrected for injected dose and co-registered to μMRI using the Image Algebra and Image Fusion functions implemented within the PMOD v2.75 software environment. The first column from the left shows the μMRI template used for coregistration. Each of the three remaining columns (from left to right) shows the μMRI-μPET coregistered images of the mouse brain in horizontal cross sections: (a) DAT+/+ vehicle, (b) DAT+/+ cocaine (i.p. 10 mg/kg), (c) DAT−/− vehicle, (d) DAT−/− cocaine (i.p. 10 mg/kg).
Note that the weight of the animals as had previously been reported differed significantly and corresponded for the DAT+/+ to 38.09 ± 0.66 g and for the DAT−/− mice to 25.66 ± 0.47 g [F(1,28) = 40.74; P < 0.001].
RESULTS
Differences in brain glucose metabolism in response to cocaine
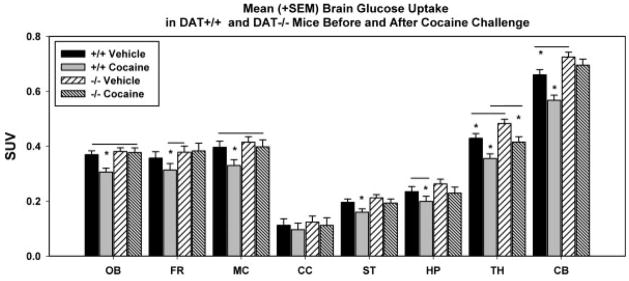
The ANOVA on the baseline metabolic measures detected significant main effects for the Experimental Group [F(3,1985) = 27.21; P < 0.001] and for the Brain Region [F(7,1985) = 319.57; P < 0.001]. Pairwise comparisons (Holm-Sidak) to isolate the direction of these differences revealed that baseline metabolic activity was significantly higher in DAT−/− than in DAT+/+ mice in the thalamus (12.5% greater; t = 2.310; Fig. 2) and in the cerebellum (10% greater; t = 2.535) (Fig. 2). The differences in the other six brain regions examined, was not significant.
Fig. 2.
Standardized uptake value (SUV) in selected brain regions (mean (+SEM) regional brain glucose metabolism corrected for injected dose and body weight).
Cocaine significantly decreased BGluM in DAT+/+ mice in olfactory bulb (21%; t = 3.175), motor cortex (20%; t = 2.161), striatum (23%; t = 2.041), thalamus (21%; t = 3.035) and cerebellum (16.5%; t = 3.519). In contrast, the thalamus was the only brain region that was significantly decreased by cocaine in the DAT−/− mice (16.4%; t = 2.709). Indeed, cocaine accentuated the differences in regional brain glucose metabolism between DAT−/− and DAT+/+ mice. Comparison of BGluM after cocaine showed that DAT−/− mice had significantly greater metabolism than DAT+/+ mice not just in thalamus (17%; 2.296) and cerebellum (22.5%; 4.423) as seen during baseline but also in olfactory bulb (23.2%; 3.257) and motor cortex (21%; 2.040).
Differences in regional metabolism within experimental groups and treatments
Pair-wise multiple comparison procedures (using the Holm-Sidak method; P < 0.05) were also used to examine differences in BGluM across the eight brain regions examined between the four experimental groups (Fig. 2). For all groups and experimental conditions the cerebellum was the brain regions with the highest metabolic activity. Specifically DAT+/+ mice treated with saline_showed greatest levels of BGluM in the CB compared to all other brain regions: ST (t = 20.72), CC (t = 18.224), HP (t = 16.142), OB (t = 12.453), FR (10.096), MC (t=9.136), TH (t = 9.056). DAT+/+ mice treated with cocaine showed greatest levels of BGluM in the CB compared to: ST (t = 18.192), CC (t = 15.666), HP (t = 13.972), OB (t = 11.190), FR (8.378), MC (t = 8.346) and TH (t = 8.427). DAT−/− mice treated with saline showed greatest levels of BGluM in the CB compared to all other brain regions: ST (t = 24.426), CC (t = 21.379), HP (t = 18.735), OB (t = 15.732), FR (t = 12.331), MC (t = 11.649), TH (t = 10.337). Finally DAT−/− mice treated with cocaine showed greatest levels of BGluM in the CB compared to all other brain regions: ST (t = 18.932), CC (t = 16.397), HP (t = 14.936), OB (t = 11.505), FR (t = 8.777), MC (t = 8.825), TH (t = 9.468).
DISCUSSION
This study revealed that: (1) DAT−/− mice showed greater baseline BGluM compared to DAT+/+ mice in the TH and CB (2) cocaine decreases BGluM in DAT+/+ mice in the OB, MC, ST, HP, TH, CB and (3) cocaine’s effects on decreasing BGluM are selective to the TH in DAT−/− mice.
DAT−/− mice have significantly greater baseline BGluM than DAT+/+mice in the TH and CB
The difference in baseline BGluM between the DAT−/− and the DAT+/+ mice in the TH and CB suggests that DAT in these brain regions may be involved in regulating their baseline levels of glucose utilization. Though DAT are found in both brain regions the concentration in cerebellum is low (Glaser et al., 2006; Sanchez-Gonzalez et al., 2005). However, DA regulation of metabolism in these brain regions could be indirect via striato-thalamic or striato-cerebellar pathways (Hoshi et al., 2005). Inasmuch as DAT regulate not only the concentration of extracellular DA but also the duration in the extrasynaptic space, these findings could be interpreted to indicate that chronically high DA concentrations as occur in DAT−/− lead to increases in BGluM in thalamus and cerebellum. To our knowledge there are no studies on the role of DAT in modulating baseline brain glucose metabolic rate. Also even though there are multiple studies showing that DA agonists or antagonists change baseline regional brain metabolism (see introduction) the relevance for our findings is unclear since most studies involve acute drug administration whereas the knockout model reflects a stable chronic state. Similarly, human studies have previously reported that chronic stimulant drug use, is associated with changes in brain glucose metabolism, but extrapolation is limited by the fact that drug abuse in humans involves an intermittent situation with periods of drug use and withdrawal and polysubstance abuse (nicotine and alcohol) whereas in knockout animals the condition is stable and not confounded by co-morbid abuse of other drugs.
DAT−/− mice were proposed as an animal model of ADHD (Viggiano et al., 2003) and in this respect our findings of increased basal BGluM levels in TH and CB may suggest that increased BGluM due to persistent elevated DA levels in these brain regions may be a factor contributing to the phenotype associated with ADHD.
Cocaine decreased regional BGluM differentially in DAT+/+and DAT−/− mice
Our results corroborate previous findings of decreases in metabolism after acute cocaine administration in two different strains of mice (C57 and DBA) (Zocchi et al., 2001). However, the effects of acute cocaine on brain glucose metabolism have been shown to differ across species; in rats, it increases metabolism (Zocchi et al., 2001) whereas in monkeys (Lyons et al., 1996) and in humans (London et al., 1990) it decreases it. This would suggest that the effects of acute cocaine on brain glucose metabolism in mice may be more similar to those in primates than to those in rats. Differences in the response to cocaine between DAT−/− and DAT+/+ mice suggests that the decrements in metabolism from cocaine are driven mostly by its dopaminergic effects. However, the fact that in DAT KO cocaine decreases thalamic metabolism suggest that these effects are driven by cocaine’s noradrenergic and serotonergic effects as well as its local anesthetic effects. Indeed, we recently showed that acute cocaine administration at a dose equivalent to that used in this study induced significant increases in intracellular calcium concentration in the rat brain and this effect was driven by its local anesthetic actions (Du et al., 2005).
Since DAT−/− mice have been shown to have five-fold higher levels of extracellular DA than their DAT+/+ counterparts, we expected some regional differences in brain metabolic activity between strains (Gainetdinov and Caron, 2003). More specifically we predicted that the changes in metabolism in DAT−/− animals would be attenuated when compared to that of DAT+/+. Observing such differences suggests that the main drive for the decrements in response to cocaine reflect dopaminergic effects of cocaine.
DAT plays a critical role in maintaining dopaminergic homeostasis and normal synaptic re-uptake (Jones et al., 1998). However, in interpreting the hyperdopaminergic state in DAT−/− mice one needs to consider potential compensatory responses in other neurotransmitter systems (i.e. NE or 5HT), which may have an impact on brain glucose metabolism (Rocha et al., 1998). Indeed, disulfiram, which is an inhibitor of the NE biosynthetic enzyme dopamine beta-hydroxylase, has demonstrated promising efficacy in the treatment of cocaine dependence in preliminary clinical trials (Weinshenker and Schroeder, 2007) and 5HT receptor-specific pharmacological compounds have been shown to reduce addictive properties of cocaine (Bubar and Cunningham, 2006). Furthermore NE/5HT uncoupling has recently been proposed to be a common neurochemical consequence of repeated consumption of drugs of abuse that is unrelated with DA release (Lanteri et al., 2007). Finally, it is important to consider previous findings reporting differences in D1, D2, and D3 mRNA in several brain regions between wild type and DAT deficient mice (Fauchey et al., 2000).
In summary, here we show that, DAT−/− mice had similar baseline brain glucose metabolism compared to DAT+/+, except in the TH and CB, which implicates a role for DA in modulating baseline levels of glucose metabolism in these brain regions. The finding of significant differences in cocaine induced reduction in regional BGluM between DAT−/− and DAT+/+ mice indicates that the acute effects of cocaine in regional metabolic activity are mostly driven by its dopaminergic actions.
Acknowledgments
The authors thank Dr. Marc Caron for helping them by kindly providing them with the DAT mice.
Contract grant sponsor: NIAAA; Contract grant numbers: AA 11034 and AA07574, AA07611; Contract grant sponsor: U.S. Department of Energy; Contract grant number: DE-AC02-98CH10886; Contract grant sponsor: NIH; Contract grant numbers: DA-013511, NS-019576.
Footnotes
This article is a US Government work and, as such, is in the public domain in the United States of America.
References
- Carboni E, Spielwoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J Neurosci. 2001;21:RC141. doi: 10.1523/JNEUROSCI.21-09-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci USA. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Thanos PK, Yu M, Rivera SN, Benveniste H. Optical brain monitoring of cocaine-induced cerebrovascular and intracellular calcium effects in the living rat. Proceedings of the International Narcotics Research Conference 2005; July 10–15 2005; Annapolis, MD. 2005. [Google Scholar]
- Fauchey V, Jaber M, Caron MG, Bloch B, Le Moine C. Differential regulation of the dopamine D1, D2 and D3 receptor gene expression and changes in the phenotype of the striatal neurons in mice lacking the dopamine transporter. Eur J Neurosci. 2000;12:19–26. doi: 10.1046/j.1460-9568.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Caron MG. Monoamine transporters: From genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Jones SR, Caron MG. Functional hyperdopaminergia in dopamine transporter knock-out mice. Biol Psychiatry. 1999;46:303–311. doi: 10.1016/s0006-3223(99)00122-5. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Glaser PE, Surgener SP, Grondin R, Gash CR, Palmer M, Castellanos FX, Gerhardt GA. Cerebellar neurotransmission in attention-deficit/hyperactivity disorder: Does dopamine neurotransmission occur in the cerebellar vermis? J Neurosci Methods. 2006;151:62–67. doi: 10.1016/j.jneumeth.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nature Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Jones S, Gainetdinov R, Jaber M, Giros B, Wightman R, Caron M. Profound neuronal plasticity in response to inactivation of the dopamine transpoter. Proc Natl Acad Sci USA. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- Kelly PA, Graham DI, McCulloch J. Specific alterations in local cerebral glucose utilization following striatal lesions. Brain research. 1982;233:157–172. doi: 10.1016/0006-8993(82)90937-4. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HN., Jr Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry. 1990;47:567–574. doi: 10.1001/archpsyc.1990.01810180067010. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16:1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Rice WN, Freedland CS, Whitlow CT, Porrino LJ. Patterns of functional activity associated with cocaine self-administration in the rat change over time. Psychopharmacology. 2004;172:384–392. doi: 10.1007/s00213-003-1676-7. [DOI] [PubMed] [Google Scholar]
- Marsteller DA, Barbarich-Marsteller NC, Fowler JS, Schiffer WK, Alexoff DL, Rubins DJ, Dewey SL. Reproducibility of intra-peritoneal 2-deoxy-2-[18F]-fluoro-D-glucose cerebral uptake in rodents through time. Nucl Med Biol. 2006;33:71–79. doi: 10.1016/j.nucmedbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- McCulloch J, Savaki HE, McCulloch MC, Jehle J, Sokoloff L. The distribution of alterations in energy metabolism in the rat brain produced by apomorphine. Brain Res. 1982;243:67–80. doi: 10.1016/0006-8993(82)91121-0. [DOI] [PubMed] [Google Scholar]
- NAS, NRC. Guide for the care and use of laboratory animals. Washington D.C: National Academy Press; 1996. [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- Phelps ME, Huang SC, Hoffman EJ, Selin C, Sokoloff L, Kuhl DE. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: Validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lucignani G. Different patterns of local brain energy metabolism associated with high and low doses of methyl-phenidate. Relevance to its action in hyperactive children. Biol Psychiatry. 1987;22:126–138. doi: 10.1016/0006-3223(87)90223-x. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, Casella V, Fowler J, Hoffman E, Alavi A, Som P, Sokoloff L. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res. 1979;44:127–137. doi: 10.1161/01.res.44.1.127. [DOI] [PubMed] [Google Scholar]
- Rocha B, Fumagalli F, Gainetdinov R, Jones S, Ator R, Giros B, Miller G, Caron M. Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: Combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;98:5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, Lesch KP, Murphy DL, Uhl GR. Cocaine reward models: Conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 1998;95:7699–7704. doi: 10.1073/pnas.95.13.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Taintor NB, Alexoff D, Vaska P, Logan J, Grandy DK, Fang Y, Lee JH, Fowler JS, Volkow ND. In vivo comparative imaging of dopamine D2 knockout and wild-type mice with (11)C-raclopride and microPET. J Nucl Med. 2002a;43:1570–1577. [PubMed] [Google Scholar]
- Thanos P, Taintor N, Umegaki H, Ikari H, Roth GS, Ingram DK, Volkow ND. Dopamine D2 receptor upregulation reduces cocaine self-administration. p Program No. 119.114. Washington, DC: Society for Neuroscience; 2002b. 2002 Abstract viewer/itinerary planner. [Google Scholar]
- Thanos PK, Taintor N, Rivera SN, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R, Fowler JS, Gatley J, Wang G-J, Volkow ND. DRD2 Gene transfer into the nucleus accumbens of the alcohol preferring (P) and non preferring (NP) rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28:720–728. doi: 10.1097/01.alc.0000125270.30501.08. [DOI] [PubMed] [Google Scholar]
- Viggiano D, Ruocco LA, Sadile AG. Dopamine phenotype and behaviour in animal models: In relation to attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2003;27:623–637. doi: 10.1016/j.neubiorev.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A, Fowler JS. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Freedland CS, Porrino LJ. Metabolic mapping of the time-dependent effects of delta 9-tetrahydrocannabinol administration in the rat. Psychopharmacology. 2002;161:129–136. doi: 10.1007/s00213-002-1001-x. [DOI] [PubMed] [Google Scholar]
- Williams-Hemby L, Grant KA, Gatto GJ, Porrino LJ. Metabolic mapping of the effects of chronic voluntary ethanol consumption in rats. Pharmacol Biochem Behav. 1996;54:415–423. doi: 10.1016/0091-3057(95)02060-8. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Conti G, Orzi F. Differential effects of cocaine on local cerebral glucose utilization in the mouse and in the rat. Neurosci Lett. 2001;306:177–180. doi: 10.1016/s0304-3940(01)01898-5. [DOI] [PubMed] [Google Scholar]