Abstract
Context: Calcium binding to the Ca-sensing receptor (CASR) expressed in thick ascending limb inhibits the Na,K,2Cl cotransporter, which decreases sodium reabsorption and secondarily decreases Ca reabsorption. CASR gene variants could influence blood pressure (BP) by affecting Na retention.
Objective: The objective of the study was to determine whether variations in CASR associated with BP in African-Americans, an ethnic group at high risk for hypertension.
Design: Population- and family-based association studies of single-nucleotide polymorphisms (SNPs) in CASR with BP measured over the age range 5.6–25 yr (14 biannual visits per subject on average) were carried out. In a cross-sectional study where urinary Ca excretion had been measured, Ca excretion was used as an additional phenotype of CASR influence on Na,K,2Cl cotransporter activity.
Participants: Subjects were normotensive. In the longitudinal study, there were 223 subjects (mean age 14 yr) and 123 families (one or both parents provided a DNA sample); in the cross-sectional study, there were 106 subjects (mean age 23 yr) and 88 families.
Results: Three SNPs in linkage disequilibrium associated with systolic BP at P < 0.005 (the significance threshold corrected for multiple comparisons) in the population-based longitudinal study. In the cross-sectional study, SNPs contained in the same linkage disequilibrium block associated with urinary Ca excretion in both population- and family-based association studies.
Conclusion: The findings suggest that in African-Americans, functional heterogeneity of the CASR in thick ascending limb may influence BP.
This study suggests that common variants in the calcium-sensing receptor affect blood pressure.
The G protein-coupled calcium-sensing receptor (CASR) expressed in parathyroid gland functions to maintain Ca homeostasis by inhibiting the secretion of PTH in response to increases in Ca (1). CASR is also expressed in the thick ascending limb (TAL) of the kidney at the basolateral surface (2,3), where upon activation by Ca, CASR conveys an inhibitory influence on the Na,K,2Cl cotransporter (NKCC2) (4) at the apical surface. Inhibition of the cotransporter leads secondarily to a more negatively charged lumen that favors urinary excretion over paracellular uptake of cations, including Ca and Na. Through its capacity to affect NKCC2 activity, CASR serves as a significant regulator of not only the extracellular concentration of Ca, but the net retention of Na and possibly the level of blood pressure (BP). Gain-of-function mutations in CASR are one cause of Bartter syndrome, a salt-losing nephropathy associated with low BP and an increase in Ca excretion due to reduced NKCC2 activity (5,6). An infusion of Ca into healthy men (raising levels by 25%), which would be expected to activate CASR in the TAL, was shown to increase Na excretion by 150% (7), and diets higher in Ca are accompanied by lower BP in some studies (8), although not all (9).
We report here on the finding of a significant association of common variants [single-nucleotide polymorphisms (SNPs)] in CASR with BP in young African-Americans, an ethnic group that demonstrates increased retention of Na (10,11). They were from a cohort study where BP was assessed repeatedly over an age range that extended from childhood to young adulthood. A loss-of-function variation in CASR could increase BP by increasing NKCC2-mediated Na reabsorption. This mechanism was explored in a separate cross-sectional study where urinary excretion of Ca, an intermediate phenotype more proximal to the activity level of CASR, had been measured.
Subjects and Methods
Subjects
Subjects were normotensive and in good health; none were taking medication with the exception that some of the female subjects used oral contraceptives. Ethnicity was African-American based on subject’s self-report. In the longitudinal study, there were 223 subjects. They were from a cohort study of BP regulation (12). The age range over which measurements were made was 5.6–25 yr. Parents of 123 of the subjects provided a DNA sample for family studies. There were 106 subjects (nine sib pairs) ranging in age from 18–36 yr who provided cross-sectionally collected data. They had been participants in an inpatient study of ethnic differences in Na reabsorption (13); they were recruited either from the cohort or by way of advertisements posted in the hospital or on campus. There were 19 subjects who participated in both the cross-sectional and the longitudinal studies. Parental DNA was available for 88 of the subjects in the cross-sectional study. The studies were approved by the Indiana University-Purdue University of Indianapolis Institutional Review Board. Each subject provided consent or assent with a parent or guardian providing informed consent.
Phenotype measurements
In the longitudinal study, weight, height, and BP were measured approximately every 6 months, either at the school they attended or in an outpatient extension of the General Clinical Research Center (GCRC). BP was measured by trained research personnel in the right arm three times using a random zero sphygmomanometer (Hawksley and Sons, Lancing, West Sussex, UK). For each visit, the average of the last two readings was used in the analyses. The number of BP measurements used in the study ranged from two to 27 per subject with a mean of 14. At each visit, height and weight were also measured for determination of body mass index (BMI).
In the cross-sectional study, subjects were admitted to the GCRC in the afternoon where they consumed a standard diet for dinner, evening snack, and breakfast. The next morning after breakfast, no additional food or liquid was consumed, and normal saline was infused (60 ml/h) over 2 h to facilitate collection of urine samples. The Na and Ca excretion rates were determined from the urine sample collected over the second hour. Urinary Na and Ca concentrations were measured using a COBAS MIRA analyzer (Roche Diagnostics, Indianapolis, IN).
SNP selection and genotyping
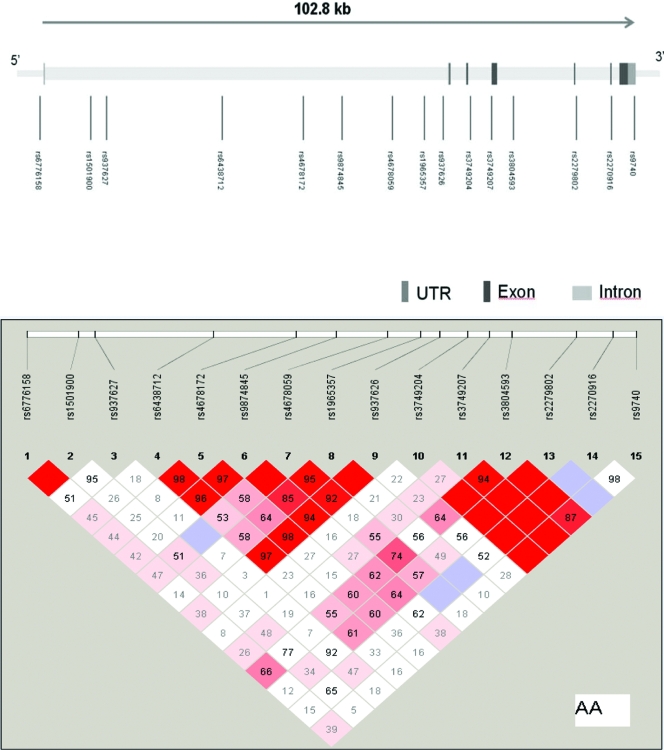
Fifteen SNPs distributed throughout CASR (Fig. 1) were selected from public databases, primarily dbSNP (http://www.ncbi.nlm.nih.gov/SNP/). To determine allele frequencies and test the assays, SNPs were genotyped in two sets of samples, each consisting of 40 unrelated individuals from the Coriell African-American samples. SNPs with more than 10% heterozygosity were preferentially genotyped; all SNPs were assessed for significant (P < 0.001) deviations from Hardy-Weinberg equilibrium. SNP location was determined from the annotations in the NCBI human genome assembly Build 36.2.
Figure 1.
Top, Genomic structure of CASR. The gene structure of CASR is based on transcript NM_0003888. The direction of transcription and the exons are indicated by the arrow and the number, respectively. The size of the gene is indicated in scale at the top of the figure. Bottom, Pairwise LD (D′) estimates are provided in each box. No D′ estimates is in indicated by D′ = 0. More intensely colored boxes depict stronger LD. AA, African-American; UTR, untranslated region.
Genotyping was carried out using a modified single-nucleotide extension reaction with allele detection by mass spectrometry (Sequenom MassArray system; Sequenom, San Diego, CA). The assays were designed and run with either of two formats, hME or iPLEX. The success rate of all genotypes was 90% or higher, and minor allele frequencies were higher than 5%. DNA was available from parents of 123 families in the primary longitudinal cohort, and SNP genotypic data were screened for Mendelian errors using mega2. In addition, genotypic data from CASR and 13 other unlinked genes were used to verify reported family relationships using PREST (14) and RELPAIR (15).
Statistical analysis
SNP coverage across CASR was evaluated using the program HAPLOVIEW (16), which examined the extent of linkage disequilibrium (LD) between pairs of SNPs. The program Tagger (http://www.broad.mit.edu/mpg/tagger/) was used to estimate how well the selected SNPs represented the genetic information contained in nongenotyped SNPs. HapMap YRI (Yoruba from Nigeria) data were applied to represent the coverage for African-American subjects.
Longitudinal study
A semiparametric mixed-effect model for repeated-measurement analysis of the longitudinal data were used to test for association of each SNP with systolic and diastolic BP (17,18,19). The model incorporates genetic (SNP) effects on BP as a fixed effect at the population level and subject-specific growth curves of BP over age as random effects. The subject-specific growth curve was fitted using the standard nonparametric smoothing function of penalized splines, and additive genetic effect (20,21) was tested after adjusting for a time-invariant (sex) and time-varying covariates (BMI). The approach allows flexible utility of measurement of BPs at the individual level.
Family-based analysis (22) was performed using 123 families. Information with respect to transmitted and nontransmitted alleles of a single SNP was inferred from the parent’s genotype, except when all family members had heterozygous genotypes. The transmitted allele was treated as a fixed effect variable in the semiparametric mixed model to test whether the transmitted allele was associated with BP (23) after allowing subject-specific growth curves of BP over age.
Cross-sectional study
No significant correlation of phenotypes within siblings was observed based on the Spearman correlation test, and thus both siblings were used in subsequent analyses. A mixed-effect model was used to test additive genetic effects of each SNP with urinary Ca after adjusting for sex, BMI, and age. Logarithms of urinary Ca were used because normality assumptions were not met.
Adjustments for multiple comparisons
Many of the SNPs were highly correlated (in high LD) and provided nonindependent evidence of association; therefore, a Bonferroni correction was considered to be overly conservative. Instead, we estimated a gene-wide significance (threshold) using Nyholt’s method (24) that takes into account LD among SNPs (on the basis of the spectral decomposition of matrices of pairwise LD between SNPs) and calculates an effective number of independent tests. The estimated threshold obtained by gene-wide adjustment was a P value ≤0.005 for both the longitudinal and the cross-sectional studies.
Results
Characteristics of subjects
Table 1 depicts characteristics of the subjects in both longitudinal and cross-sectional studies. In the longitudinal study, systolic BP was significantly higher in males (P < 0.006). In the cross-sectional study, systolic and diastolic BP and urinary Ca excretion were all significantly higher in males (P < 0.005), whereas BMI was higher in females (P = 0.006). Urinary Na excretion was not significantly different in males and females. Males and females were of similar ages in both study groups. In the longitudinal study, the age ranges were the same in males (5.68–24.91 yr) and females (5.68–24.64 yr).
Table 1.
Characteristics of subjects at time of enrollment into longitudinal study (mean ± sd)
| Variable | Longitudinal study
|
Cross-sectional study
|
||||
|---|---|---|---|---|---|---|
| Males (n = 102) | Females (n = 121) | P value | Males (n = 49) | Females (n = 57) | P value | |
| Age (yr) | 14.15 ± 2.59 | 14.03 ± 2.38 | 0.54 | 22.80 ± 4.05 | 24.12 ± 5.00 | 0.14 |
| BMI (kg/m2) | 23.42 ± 9.19 | 24.80 ± 8.44 | 0.10 | 26.88 ± 5.62 | 30.92 ± 8.71 | 0.006 |
| SBP (mm Hg) | 109.64 ± 12.15 | 105.86 ± 11.13 | 0.006 | 123.82 ± 12.33 | 113.01 ± 9.14 | <0.001 |
| DBP (mm Hg) | 63.97 ± 10.32 | 63.82 ± 9.46 | 0.89 | 68.27 ± 6.78 | 64.36 ± 6.17 | 0.003 |
| uCa (mg/h) | 15.27 ± 6.43 | 11.45 ± 5.98 | 0.002 | |||
| uNa (mmol/h) | 15.67 ± 9.95 | 16.23 ± 6.05 | 0.52 | |||
DBP, Diastolic BP; SBP, systolic BP; uCa, urinary Ca excretion rate.
SNP genotyping and coverage
CASR spans 103 kb on chromosome 3q13 (Fig. 1). There was substantial pairwise LD between adjacent markers (0.95 ≤ D′ ≤1.0), except between rs937626 and rs3749207. To determine whether the SNPs adequately assessed the variation within CASR with known SNPs genotyped in HapMap, the program Tagger (25) was used to examine the coverage by the SNPs selected for genotyping in this study. There were data for 11 of our 15 genotyped SNPs in the YRI population of the HapMap database, and these captured 50% of the HapMap SNP information in the region (minor allele frequency ≥0.1) at r2 ≥0.8, with a mean r2 of 0.59. Because several SNPs genotyped (four SNPs) were not in the HapMap database, these results are likely to underestimate the actual coverage of the gene in African-Americans. Table 2 shows that all 15 SNPs were in Hardy-Weinberg equilibrium and had minor allele frequencies greater than 0.05 in unrelated African-Americans.
Table 2.
Associations of CASR with BP in longitudinal study (P values)
| SNP | Position | NT | HWE (P value) | MAF | Population-based analysis (n = 223)
|
Family-based analysis (n = 123 families)
|
||
|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | |||||
| rs6776158 | Promoter | A/G | 0.62 | 0.42 | 0.91 | 0.13 | 0.41 | 0.02 |
| rs1501900 | Intron 1 | A/T | 0.70 | 0.08 | 0.90 | 0.31 | 0.90 | 0.36 |
| Rs937627 | Intron 1 | G/T | 0.07 | 0.10 | 0.13 | 0.32 | 0.89 | 0.64 |
| rs6438712 | Intron 1 | A/G | 0.96 | 0.29 | 0.004A | 0.16 | 0.11 | 0.005A |
| rs4678172 | Intron 1 | G/T | 0.51 | 0.30 | 0.004T | 0.11 | 0.006T | 0.006T |
| rs9874845 | Intron 1 | A/T | 0.88 | 0.34 | 0.019A | 0.13 | 0.04 | 0.01 |
| rs4678059 | Intron 1 | A/T | 1.00 | 0.14 | 0.45 | 0.47 | 0.81 | 0.24 |
| rs1965357 | Intron 1 | C/T | 0.51 | 0.18 | 0.68 | 0.21 | 0.04 | 0.02 |
| rs937626 | Intron 1 | A/G | 0.95 | 0.35 | 0.003A | 0.16 | 0.04 | 0.01 |
| rs3749204 | Intron 3 | A/T | 0.54 | 0.42 | 0.55 | 0.19 | 0.02 | 0.21 |
| rs3749207 | Intron 3 | C/T | 0.30 | 0.31 | 0.19 | 0.77 | 0.75 | 0.40 |
| rs3804593 | Intron 4 | A/G | 0.98 | 0.26 | 0.98 | 0.38 | 0.07 | 0.06 |
| rs2279802 | Intron 5 | A/G | 0.33 | 0.06 | 0.62 | 0.69 | 0.82 | 0.59 |
| rs2270916 | Intron 6 | C/T | 1.00 | 0.06 | 0.32 | 0.30 | 0.20 | 0.94 |
| rs9740 | 3′-UTR | A/G | 0.49 | 0.17 | 0.42 | 0.82 | 0.81 | 0.86 |
The chromosomal position was obtained from build 36.2. Letters in superscript depict the nucleotides that are associated with a higher BP (A, Adenine; T, thymine). HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency; NT, nucleotide; UTR, untranslated region.
Association studies using single SNPs
The P values for association studies in the longitudinal cohorts are given in Table 2. Three SNPs in strong LD (Fig. 1), rs6438712, rs4678172, and rs937626, were significantly associated with systolic BP, and a fourth was nominally associated. There were no significant associations with diastolic BP. One of the same SNPs (rs4678172) was associated with systolic BP (and two of the other three were nominally associated) in the family-based analysis, and two were significantly associated (and the other two nominally associated) with diastolic BP (Table 2).
In the cross-sectional study, a set of six SNPs associated with urinary Ca excretion (Table 3). Population-based analyses showed that two significantly associated with SNPs (rs4678059 and rs1965357) plus four flanking SNPs that were nominally associated. In the family-based analysis of the cross-sectional data, five of the SNPs were significantly associated and the sixth nominally associated. The six SNPs that were associated with urinary Ca excretion in both population- and family-based association studies included the four SNPs associated with BP. We have confirmed that the alleles that associated with a higher BP are corresponding to a lower urinary Ca excretion. There was no association of genotype with Na excretion rates as might be anticipated because it is a cumulative balance of Na, which would not be reflected in a single excretion rate, that would be expected to influence BP.
Table 3.
Associations of CASR with urinary Ca excretion in a cross-sectional study (P values)
| SNP | MAF | HWE (P value) | Ca excretion rate, log uCa (mg/h)
|
|
|---|---|---|---|---|
| Population-based analysis (n = 106) | Family-based analysis (n = 88) | |||
| rs6776158 | 0.46 | 0.05 | 0.95 | 0.26 |
| rs1501900 | 0.09 | 0.88 | 0.12 | 0.62 |
| rs937627 | 0.07 | 0.12 | 0.96 | 0.56 |
| rs6438712 | 0.28 | 1.00 | 0.03A | 0.0002A |
| rs4678172 | 0.29 | 1.00 | 0.02T | 0.0007T |
| rs9874845 | 0.31 | 1.00 | 0.02A | 0.0028A |
| rs4678059 | 0.12 | 0.32 | <0.0001A | 0.0001A |
| rs1965357 | 0.15 | 0.87 | 0.0007C | 0.03C |
| rs937626 | 0.34 | 1.00 | 0.02G | 0.002G |
| rs3749204 | 0.43 | 0.90 | 0.98 | 0.96 |
| rs3749207 | 0.31 | 0.26 | 0.28 | 0.85 |
| rs3804593 | 0.23 | 0.24 | 0.95 | 0.69 |
| rs2279802 | 0.05 | 1.00 | 0.69 | 0.63 |
| rs2270916 | 0.05 | 1.00 | 0.64 | a |
| rs9740 | 0.20 | 0.82 | 0.74 | 0.39 |
The increased risk allele that associates with a lower urinary Ca is depicted as superscript when P ≤ 0.001 and MAF is higher than 0.10. Letters in superscript depict the nucleotides that are associated with a lower urinary excretion of Ca (A, Adenine; T, thymine). HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency; uCa, urinary Ca.
Estimable size effect due to MAF was less than 0.05 and lack of variability.
Effect size
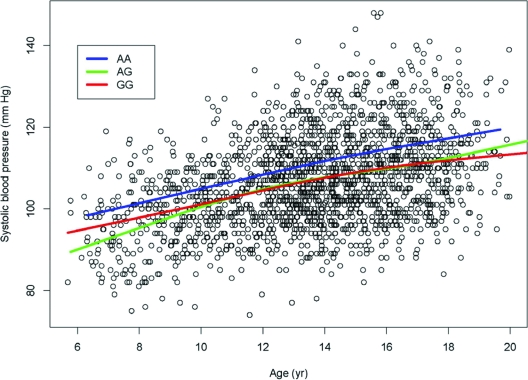
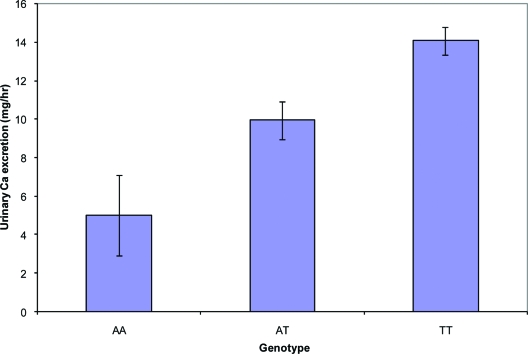
The significant SNPs were identified under the additive effect models, but the effect size of the significant SNPs in the population-based analysis was approximately 2 mm Hg between the homozygous group of two copies of the disease-associated allele and heterozygous and homozygous groups with non-disease-associated allele (Fig. 2). The effect size in the family-based analysis was 3 mm Hg for a copy of transmitted disease risk allele. For the secondary cross-sectional cohort, the power to detect an additive genetic effect of urinary Ca excretion was 60% at the 0.005 significance level, but the effect size for urinary Ca is 1.15 mg/h on average per copy of disease risk allele in the population-based analysis (Fig. 3) and 1.8 mg/h for family-based analysis.
Figure 2.
Effect size on systolic BP (mm Hg) over age for rs6438712 in the population-based longitudinal study. AA, AG, and GG are genotype groups. The other significantly associated SNPs (rs937626 and rs4678172) had very similar patterns as rs6438712. BP was approximately 2 mm Hg higher in the homozygous group where there were two copies of the increased risk allele than in the heterozygous or homozygous group with the non-risk-associated allele.
Figure 3.
Effect size on urinary calcium excretion of rs4678059 in the population-based cross-sectional study (± se). Urinary Ca increased on average by 1.15 mg/h per copy of the increased risk allele in the population-based analysis and 1.8 mg/h for family-based analysis.
Discussion
In the present study of African-Americans, common variations in CASR showed significant associations with BP and with urinary excretion rates of Ca in both population-based and family-based analyses. A set of six adjacent SNPs was significantly associated or showed marginal association with both phenotypes. To the extent that Ca excretion is determined by CASR effects on NKCC2 activity, the findings suggest that individual differences in BP in normotensive African-Americans could be related to CASR-mediated influences on Na uptake in TAL. None of the significantly associated SNPs were in coding regions, and therefore they may affect expression or be in LD with other sequence variations that create differences in function.
A loss-of-function variation in CASR expressed in kidney would be expected to increase activity of NKCC2 and potentially lead to an increase in BP and a reduction in Ca excretion. Ca excretion is lower in African-Americans when comparisons are made with European-Americans (26) even when dietary intake of Ca is known to be same (27). Urine concentrations are typically higher and urine volumes lower in African-Americans (13,28,29), which could result from enhanced water reabsorption in the collecting duct due to an increased reno-medullary concentration gradient resulting from greater Na reabsorption in TAL. Ethnic differences in excretion of certain electrolytes in response to furosemide, an inhibitor of NKCC2, also suggest greater NKCC2 activity in African-Americans (e.g. the lower urinary excretion rates of potassium and Ca in African-Americans in comparison with European-Americans were proportionately reduced after furosemide administration) (13). A preponderance of loss-of-function variations in CASR could contribute to the greater Na retention and the increased susceptibility to hypertension in African-Americans (30). On the other hand, individuals with loss-of-function mutations in CASR that result in familial hypocalciuric hypercalcemia (FHH) (31) have not been recognized as having hypertension more often. It is possible that the effects of severe mutations characteristic of those in FHH are compensated through changes in another system. Alternatively, to our knowledge, descriptions of FHH have been limited to European-Americans, and it may be the propensity of African-Americans to retain Na that sensitizes BP to variations in CASR.
A CASR molecular variant was shown previously to be significantly associated with urinary Ca excretion rates (32), and certain CASR variants together with birth weight were shown to be predictors of bone mineral density in women (33). In the current study, urinary Ca excretion overnight (beginning at 0700 h on the day subjects were admitted to the GCRC) showed nominally significant associations with rs4678059 and rs1965357, with P values of 0.01 and 0.02, respectively. The overnight urinary excretion of Ca may be less representative of CASR activity than the Ca excretion rate measured, albeit over a shorter time period, under conditions where environmental variations were minimized. Serum Ca levels showed no association with any of the BP-associated SNPs from the longitudinal study, nor was there a significant association of BP with any of the SNPs in the cross-sectional study.
Potential limiting factors in the present study were the relatively small sample sizes. At the same time, however, the phenotypes studied were carefully characterized. In the case of BP, it was measured repeatedly (as often as every 6 months for on average 7 yr), providing a highly accurate estimate of an individual’s BP. By virtue of the subjects’ young age, we avoided some of the confounding factors observed in older subjects with or without hypertension. Ca excretion rates were measured in an inpatient setting under controlled conditions. That studies were carried out in African-Americans may also have increased the likelihood for detecting an influence of CASR. African-Americans on average retain more Na than European-Americans (11), even at an early age before there is any hypertension (34), leaving BP more sensitive to any variation in Na reabsorption. This salt sensitivity of BP probably enhanced our ability to observe an association with CASR; indeed, when we performed similar studies in a comparable group of European-Americans, no significant associations of CASR with BP were observed (data not shown).
Although our interpretation of the current results is based on the premise that CASR expressed in TAL is the principal participant, it should be noted that CASR could influence BP by more than a single mechanism. CASR is expressed to varying degrees in not only other nephron regions (2,35) but also in many cell types including endothelium (36), any of which is positioned to influence BP.
In summary, findings in young African-Americans suggest the existence of functional heterogeneity in CASR that affects level of BP. Differences in Na uptake by NKCC2 in TAL could mediate the effect on BP. The present findings, if confirmed, could eventually lead to a recommendation that African-American young people supplement the intake of Ca to reduce risk of hypertension.
Acknowledgments
We are grateful to Beth Deem and Mary Anne Wagner for coordinating studies.
Footnotes
Support was provided by National Institutes of Health Grants RO1-HL35795 and MO1-RR00750 and by the Veterans Administration. Genotyping used the facilities of the Center for Medical Genomics at Indiana University School of Medicine, which is supported in part by the Indiana Genomics Initiative (INGEN), which in turn is supported in part by the Lilly Endowment, Inc.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 9, 2008
Abbreviations: BMI, Body mass index; BP, blood pressure; CASR, calcium-sensing receptor; FHH, familial hypocalciuric hypercalcemia; GCRC, General Clinical Research Center; LD, linkage disequilibrium; NKCC2, Na,K,2Cl cotransporter; SNP, single-nucleotide polymorphism; TAL, thick ascending limb.
References
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC 1993 Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366:575–580 [DOI] [PubMed] [Google Scholar]
- Riccardi D, Park J, Lee WS, Gamba G, Brown EM, Hebert SC 1995 Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci USA 92:131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC 1998 Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol 274:F611–F622 [DOI] [PubMed] [Google Scholar]
- Hebert SC, Brown EM, Harris HW 1997 Role of the Ca2+-sensing receptor in divalent mineral ion homeostasis. J Exp Biol 200:295–302 [DOI] [PubMed] [Google Scholar]
- Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T 2002 Association between activating mutations of calcium-sensing receptor and Bartter’s syndrome. Lancet 360:692–694 [DOI] [PubMed] [Google Scholar]
- Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaitre X, Paillard M, Planelles G, Dechaux M, Miller RT, Antignac C 2002 Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol 13:2259–2266 [DOI] [PubMed] [Google Scholar]
- El Hajj FG, Seifter J, Scott J, Brown EM 1998 Calcium-regulated renal calcium handling in healthy men: relationship to sodium handling. J Clin Endocrinol Metab 83:2366–2372 [DOI] [PubMed] [Google Scholar]
- McCarron DA, Morris CD, Henry HJ, Stanton JL 1984 Blood pressure and nutrient intake in the United States. Science 224:1392–1398 [DOI] [PubMed] [Google Scholar]
- Bucher HC, Cook RJ, Guyatt GH, Lang JD, Cook DJ, Hatala R, Hunt DL 1996 Effects of dietary calcium supplementation on blood pressure. A meta-analysis of randomized controlled trials. JAMA 275:1016–1022 [DOI] [PubMed] [Google Scholar]
- Helmer OM, Judson WE 1968 Metabolic studies on hypertensive patients with suppressed plasma renin activity not due to hyperaldosteronism. Circulation 38:965–976 [DOI] [PubMed] [Google Scholar]
- Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS 1986 Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 8(Suppl II):II127–II134 [DOI] [PubMed] [Google Scholar]
- Manatunga AK, Jones JJ, Pratt JH 1993 Longitudinal assessment of blood pressures in black and white children. Hypertension 22:84–89 [DOI] [PubMed] [Google Scholar]
- Chun TY, Bankir L, Eckert GJ, Bichet DG, Saha C, Zaidi SA, Wagner MA, Pratt JH 2008 Ethnic differences in renal responses to furosemide. Hypertension 52:241–248 [DOI] [PubMed] [Google Scholar]
- McPeek MS, Sun L 2000 Statistical tests for detection of misspecified relationships by use of genome-screen data. Am J Hum Genet 66:1076–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein MP, Duren WL, Boehnke M 2000 Improved inference of relationship for pairs of individuals. Am J Hum Genet 67:1219–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ 2005 Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265 [DOI] [PubMed] [Google Scholar]
- Ruppert D, Wand MP, Carroll RJ 2003 Semiparametric Regression. Cambridge, UK: Cambridge University Press [Google Scholar]
- Durban M, Harezlak J, Wand MP, Carroll RJ 2005 Simple fitting of subject-specific curves for longitudinal data. Stat Med 24:1153–1167 [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Geagert P, Liang KY, Zeger SL 2002 Analysis of Longitudinal Data. New York: Oxford University Press [Google Scholar]
- Falconer DS, Mackay TFC 1996 Introduction to quantitative genetics. London: Longman [Google Scholar]
- Fan R, Spinka C, Jin L, Jung J 2005 Pedigree linkage disequilibrium mapping of quantitative trait loci. Eur J Hum Genet 13:216–231 [DOI] [PubMed] [Google Scholar]
- Allison DB 1997 Transmission-disequilibrium tests for quantitative traits. Am J Hum Genet [Erratum (1997) 60:1571] 60:676–690 [PMC free article] [PubMed] [Google Scholar]
- Fan RZ, Jung J 2003 Association studies of QTL for multi-allele markers by mixed models. Hum Hered 54:132–150 [DOI] [PubMed] [Google Scholar]
- Nyholt DR 2004 A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 74:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D 2005 Efficiency and power in genetic association studies. Nat Genet 37:1217–1223 [DOI] [PubMed] [Google Scholar]
- Pratt JH, Manatunga AK, Peacock M 1996 A comparison of the urinary excretion of bone resorptive products in white and black children. J Lab Clin Med 127:67–70 [DOI] [PubMed] [Google Scholar]
- Wigertz K, Palacios C, Jackman LA, Martin BR, McCabe LD, McCabe GP, Peacock M, Pratt JH, Weaver CM 2005 Racial differences in calcium retention in response to dietary salt in adolescent girls. Am J Clin Nutr 81:845–850 [DOI] [PubMed] [Google Scholar]
- Cowley Jr AW, Skelton MM, Velasquez MT 1985 Sex differences in the endocrine predictors of essential hypertension. Vasopressin versus renin. Hypertension 7:I151–I160 [DOI] [PubMed] [Google Scholar]
- Bankir L, Perucca J, Weinberger MH 2007 Ethnic differences in urine concentration: possible relationship to blood pressure. Clin J Am Soc Nephrol 2:304–312 [DOI] [PubMed] [Google Scholar]
- Hajjar I, Kotchen TA 2003 Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA 290:199–206 [DOI] [PubMed] [Google Scholar]
- Pollak MR, Brown EM, Chou YH, Hebert SC, Marx SJ, Steinmann B, Levi T, Seidman CE, Seidman JG 1993 Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 75:1237–1239 [DOI] [PubMed] [Google Scholar]
- Vezzoli G, Tanini A, Ferrucci L, Soldati L, Bianchin C, Franceschelli F, Malentacchi C, Porfirio B, Adamo D, Terranegra A, Falchetti A, Cusi D, Bianchi G, Brandi ML 2002 Influence of calcium-sensing receptor gene on urinary calcium excretion in stone-forming patients. J Am Soc Nephrol 13:2517–2523 [DOI] [PubMed] [Google Scholar]
- Lips MA, Syddall HE, Gaunt TR, Rodriguez S, Day IN, Cooper C, Dennison EM; Southampton Genetic Epidemiology Research Group 2007 Interaction between birthweight and polymorphism in the calcium-sensing receptor gene in determination of adult bone mass: the Hertfordshire cohort study. J Rheumatol 34:769–775 [PMC free article] [PubMed] [Google Scholar]
- Pratt JH, Jones JJ, Miller JZ, Wagner MA, Fineberg NS 1989 Racial differences in aldosterone excretion and plasma aldosterone concentrations in children. N Engl J Med 321:1152–1157 [DOI] [PubMed] [Google Scholar]
- Huang C, Miller RT 2007 Regulation of renal ion transport by the calcium-sensing receptor: an update. Curr Opin Nephrol Hypertens 16:437–443 [DOI] [PubMed] [Google Scholar]
- Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, Dauban P, Petrel C, Ruat M, Edwards G 2005 Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: studies with calindol and Calhex 231. Circ Res 97:391–398 [DOI] [PubMed] [Google Scholar]