Abstract
Lubiprostone, a putative ClC-2 chloride channel opener, has been investigated for its effects on airway epithelia (tracheas). Lubiprostone is shown to increase submucosal gland secretion in pigs, sheep, and humans and to increase short-circuit current (SCC) in the surface epithelium of pigs and sheep. Use of appropriate blocking agents and ion-substitution experiments shows anion secretion is the driving force for fluid formation in both glands and surface epithelium. From SCC concentration-response relations, it is shown that for apical lubiprostone Kd = 10.5 nM with a Hill slope of 1.08, suggesting a single type of binding site and, from the speed of the response, close to the apical surface, confirmed the rapid blockade by Cd ions. Responses to lubiprostone were reversible and repeatable, responses being significantly larger with ventral compared with dorsal epithelium. Submucosal gland secretion rates following basolateral lubiprostone were, respectively, 0.2, 0.5, and 0.8 nl gl−1 min−1 in humans, sheep, and pigs. These rates dwarf any contribution surface secretion adds to the accumulation of surface liquid under the influence of lubiprostone. Lubiprostone stimulated gland secretion in two out of four human cystic fibrosis (CF) tissues and in two of three disease controls, chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis (COPD/IPF), but in neither type of tissue was the increase significant. Lubiprostone was able to increase gland secretion rates in normal human tissue in the continuing presence of a high forskolin concentration. Lubiprostone had no spasmogenic activity on trachealis muscle, making it a potential agent for increasing airway secretion that may have therapeutic utility.
Keywords: airway surface epithelium; airway submucosal glands; sheep, pig, and human tracheas; cystic fibrosis
airway secretions in human lungs are vital for the maintenance of mucociliary clearance. Generation of airway surface liquid arises by secretion from the surface epithelium plus fluid generated by the submucosal glands. Submucosal gland dysfunction is considered to be a primary defect in cystic fibrosis (CF) (37, 42). The submucosal glands are responsible for generation of most of the airway fluid, as shown by the small effect on mucus secretion of denuding bronchioles of surface epithelium (3). The CF transmembrane conductance regulator (CFTR), essential for maintenance of secretion, is found in both glands and the surface epithelium. Its relative abundance between these two sites is apparently dependent on the antibodies used for detection (11, 21). The CFTR-dependent secretion from the glands derives entirely from the serous acini (43), although its composition may be altered during passage along the ducts. Some consider that hyposecretion is the basic deficit in CF (17), whereas others consider hyperabsorption, due to unopposed deregulated sodium absorption in the surface epithelium, possibly the glands, too, is a key trigger (10). Both mechanisms concentrate the mucus. This might lead to local mucus stasis and set the scene for bacterial colonization. In this disease, CFTR is either absent or fails to function because of one of the many mutations that can affect this protein. Attempts to find a treatment for CF are aimed at restoring some secretory function to the airways, as well as other of the affected hollow organs, such as the alimentary canal and the pancreatic duct. Broadly, three approaches have been taken: to replace the faulty gene (gene therapy), to persuade the faulty protein to act normally (e.g., promoting trafficking of ΔF508 CFTR to the plasma membrane or enhancing its gating characteristics), or by making use of alternate chloride channels, such as calcium-activated chloride channels, CaCC (40). Indeed, aerosolization of P2Y2 receptor agonists has been shown to increase mucociliary clearance in the sheep, presumably acting via CaCC (36). There have also been suggestions that ClC-2-like channels may also be targets since they are widely distributed and often present in epithelia (27, 28). ClC-2 channels can be activated by a number of physical methods such as hyperpolarization, hypotonicity, and low pH (12, 13, 39). Lubiprostone is the first agent shown to activate ClC-2 channels in a number of epithelial cell lines, for example in T84 cells (8) and human embryonic kidney (HEK) cells expressing ClC-2 and amphibian A6 cells (4, 8) In another study, Calu-3 cells, derived from the serous cells of human airway submucosal gland cells, secreted anions in response to lubiprostone (24) and are known to express mRNA for ClC-2 (30). Responses to lubiprostone in Calu-3 epithelial monolayers were not diminished when CFTR was inhibited by either RNA interference knockdown by 95% or inhibition of CFTR chloride channels or both of these together. However, cell lines often have radically altered karyotypes and may behave differently from real epithelia. An obvious next step therefore is to examine the effects of lubiprostone on actual airway epithelia. In this study, the effect of lubiprostone is examined on the tracheal epithelium sheep, pigs, and humans. Sheep and pigs were chosen as there is a body of evidence to suggest that anatomically, functionally, electrophysiologically, and genetically, airways of these species resemble those of humans (16, 34, 35). Essentially, we have used two complimentary approaches, namely electrical methods to measure short-circuit current (SCC) responses from surface epithelium and an optical method to measure secretion from individual glands. The latter gives direct information about secretion volume, but, as the apical surface is covered with oil, experimental maneuvers are restricted, a limitation that does not apply to SCC measurement. However, SCC methods give no direct indication of secreted volume that can only be adduced indirectly, making certain assumptions. A further reason for using both methodologies is that it is unlikely that more than the initial ciliated part of the glands can be voltage-clamped because of the inevitable short space constant of the ducts.
Our aim is mainly to establish whether lubiprostone causes secretion in airway epithelia and then to describe some of the fundamental characteristics of its actions. Finally, and in the light of our new evidence, we will weigh previous findings to examine the prospect that lubiprostone may be useful in airway disease.
MATERIALS AND METHODS
Airway Tissues
All the experiments reported here have been made with epithelia from the tracheas (and bronchi of diseased human lungs) of sheep, pigs, and humans. Sheep and pig tracheas were generally obtained from an abattoir (University of Cambridge) or after acute experiments unrelated to the present study (Stanford University). In a few instances, tracheas of fetal lambs (having completed 95% of the normal gestation period of 145 days) and from pregnant ewes were collected following experimental work, unconnected with this study, and after they had been killed with a lethal dose of pentobarbitone (University of Cambridge). No animals were killed specifically for our experimental purposes. All protocols for handling sheep and pig tissues at Stanford University were approved by Administrative Panel on Laboratory Animal Care (the Institutional Animal Care and Use Committee of Stanford University), and in University of Cambridge they complied with the United Kingdom Animals (Scientific Procedures) Act 1986. Human airways were harvested at Stanford Hospital after lung transplants, the study being approved by the Institutional Review Board of Stanford University.
Pig and sheep tissues were collected in cooled oxygenated Krebs-Henseleit solution (KHS) or Krebs-Ringer bicarbonate (KRB) containing indomethacin, 3 μM (1 μM in KRB), to prevent prostaglandin formation. Tissues were used immediately or left overnight at 4°C and used on the second day.
SCC Recording
To prepare epithelial tissues for recording SCC or gland secretion, a variety of methods were used depending on the type of tissue. For fetal lambs, the tracheas were opened along the dorsal groove, and tissues were mounted whole in the Ussing chambers (0.2 cm2) to record currents from the ventral epithelium. In this way, four to five experimental samples could be obtained from a single trachea. For sheep and pigs, epithelial sheets were finely dissected under microscopic control from the underlying tissue to yield areas of 1–1.5 cm2. Generally, tissues from the ventral surface of the trachea were used, but dorsal samples were used in some instances for SCC measurements. Epithelia were mounted either in Ussing chambers (window area = 0.64 cm2) or used to measure gland secretion. All measurements of gland secretion were made with tissue from the ventral tracheal aspect. Preparations of epithelium were stretched as close as possible to their original size during mounting. In principle, human airway tissue was similarly managed as for sheep and pigs. However, samples were variable in size and condition so efforts were made to maximize the amount of information obtainable.
Short-circuit recording was made using the following protocol. Nonpolarizable voltage sensing electrodes were introduced into ports in each Ussing half-chamber as close as possible to the epithelial surfaces. Similar current-passing electrodes were introduced into ports into each half-chamber as far as possible from the tissue. Both sides of the tissues were bathed in 5 ml of KHS that was continuously warmed at 37°C and circulated using a gas lift of 95% O2-5% CO2. Once the transepithelial potential became steady, tissues were voltage-clamped at zero potential using a WPI Dual Voltage Clamp-1000. Fluid resistance compensation was used throughout. SCC were continuously recorded using an ADInstruments PowerLab/8SP and displayed on screen.
Optical Measurements
To measure secretion from individual submucosal glands, the optical method we (19) described previously was followed. Briefly, epithelia were mounted in a small chamber so that the basolateral side was bathed in KRB at 37°C while the apical surface, after cleaning and drying, was covered with a thin layer of water-saturated mineral oil. The chamber was superfused with 95% O2-5% CO2. Gland secretions formed spherical bubbles that were digitally imaged, stored, and later analyzed to give secretion rates. Data from a selection of large, medium, and small bubbles were averaged to find the mean secretion rates. Addition and removal of agents was achieved by changing the solution bathing the basolateral side of the mucosa.
Force Measurements in Trachealis Muscle
Experiments were made to examine whether lubiprostone had spasmogenic effects. Trachealis muscles attached to one tracheal cartilage ring were isolated and mounted in 5-ml organ baths and attached to a force transducer. Muscles were bathed in KHS at 37°C and bubbled with 95% O2-5% CO2. After equilibration, concentration-response curves to either lubiprostone or carbachol were obtained.
Materials
KHS had the following composition (in mM): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 10 glucose. In chloride-free solution, NaCl was replaced by sodium gluconate, KCl by K2SO4, and CaCl2 by CaNO3. The Ca2+ concentration was increased to 5 mM because of chelation by gluconate. These solutions had a pH of 7.4 when bubbled with 95% O2-5% CO2.
The composition of KRB was (in mM): 115 NaCl, 1.2 CaCl2, 1.2 MgCl2, 2.4 KH2PO4, 25 NaHCO3, and 10 glucose. Osmolarity was measured with a Wescor Vapro Vapor Pressure Osmometer and adjusted to ∼290 mosM. In bicarbonate-free KRB, NaHCO3 was replaced by HEPES, gassed with O2 and adjusted to pH 7.4.
Acetazolamide, amiloride, furosemide, bumetanide, and carbachol were all obtained from Sigma, forskolin and GlyH-101 from Calbiochem, and lubiprostone (Amitiza) from Takeda Pharmaceuticals America. When statistical comparisons were made, a standard Student's t-test was used throughout. P < 0.05 was considered to be significant.
RESULTS
In this study, we have used two separate and distinct approaches. First, we used conventional SCC measurements to examine secretion from the surface of epithelia under voltage-clamp conditions; second, we have measured secretion from individual submucosal glands and combined the values for many glands to obtain overall secretion rates. Most of the SCC measurements have been performed with sheep tracheal mucosae with confirmatory observations in the pig.
Secretion from individual submucosal glands has been made using tissues from sheep, pigs, and humans. Included with the observations using human tissues, we have examined tissues from CF patients and a number of disease controls (DC).
Lubiprostone Effects on Sheep and Pig Tracheal Mucosae
Basal transport parameters of ovine tracheal epithelia.
The results given in Table 1 summarize the basic electrical parameters of ventral and dorsal tracheal epithelia obtained from sheep. Data are given for the transepithelial potential and SCC on days 1 and 2 following culling. Both of these parameters were significantly lower on the second day. Of particular interest is the finding that SCC in ventral epithelia are significantly greater than those of dorsal epithelia on both days.
Table 1.
Comparison of the values of transepithelial potential (PD) and short-circuit current (SCC) in ovine dorsal and ventral tracheal epithelia on days 1 and 2 after overnight storage
| Dorsal | Ventral | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 1 | Day 2 | ||||||||||
| PD, mV | SCC, μA/cm2 | PD, mV | SCC, μA/cm2 | PD, mV | SCC, μA/cm2 | PD, mV | SCC, μA/cm2 | ||||||
| 16.4±1.4 | 63.7±3.6 | 9.5±1.7 | 37.0±4.1 | 12.0±1.1 | 86.2±4.5 | 7.4±1.2 | 59.3±5.8 | ||||||
| (n = 39) | (n = 39) | (n = 18) | (n = 18) | (n = 34) | (n = 34) | (n = 21) | (n = 21) | ||||||
| Statistical comparisons. A standard Student's t-test was used. | |||||||||||||
| PD (day 1 vs. day 2 dorsal) P < 0.005 | SCC (day 1 vs. day 2 dorsal) P < 0.001 | ||||||||||||
| PD (day 1 vs. day 2 ventral) P < 0.01 | SCC (day 1 vs. day 2 ventral) P < 0.006 | ||||||||||||
| PD (day 1 ventral vs. dorsal) P < 0.02 | SCC (day 1 ventral vs. dorsal) P < 0.002 | ||||||||||||
| PD (day 2 ventral vs. dorsal) NS | SCC (day 2 ventral vs. dorsal) P < 0.005 | ||||||||||||
Values given are the means ± SE, and n is the number of observations. NS, not significant.
Characteristics of responses to lubiprostone.
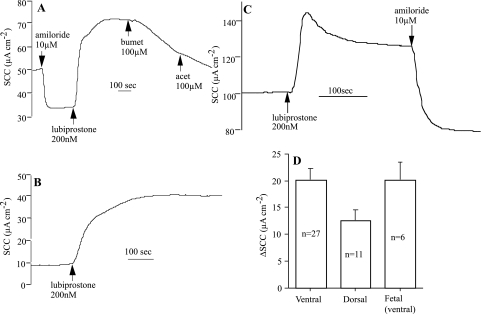
Figure 1 gives typical examples of responses to a maximally effective concentration (see later discussion) of lubiprostone (200 nM applied apically). The accompanying histogram shows that there is no difference in the responses to lubiprostone in ventral tracheal epithelia from fetal and adult sheep. Note that in Fig. 1C, the response shows an initial peak followed by relaxation to a plateau. This feature was not uncommon, and further examples can be seen in later figures. Fetal epithelia showed little or no response to amiloride (data not shown), since electrogenic sodium absorption develops to coincide with parturition (32). In adult tissues, lubiprostone was not inhibited by prior application of amiloride, suggesting the prostone has no effect on sodium transport and, from the direction of the current response, more likely indicates an effect on anion secretion indicated by sensitivity to bumetanide. However, further evidence (see later discussion) is needed to show that bumetanide affects the lubiprostone-generated current increase and that it is not due to an inhibition of the basal current. The data show that the responses of dorsal tracheal epithelia are significantly less than those of ventral tissue, although the data are on the margin of significance (P < 0.0501).
Fig. 1.
Examples of lubiprostone responses in ovine tracheal epithelium. Shown are responses from a ventral tracheal epithelium of a pregnant ewe (A) and from one of its twin fetuses after 138 days gestation (B). C shows the response of sheep ventral tracheal epithelium without prior addition of amiloride. Lubiprostone and amiloride were added to the apical bathing solution, whereas bumetanide (bumet) and acetazolamide (acet) were added basolaterally. D: mean responses to lubiprostone (200 nM) in ventral and dorsal epithelia from sheep (P < 0.0501) and for ventral fetal epithelia. ΔSCC, change in short-circuit current.
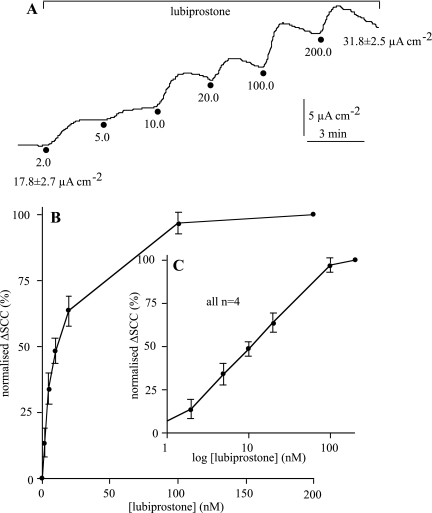
To determine the apparent Kd for lubiprostone, the agent was added cumulatively to the apical surface of ventral sheep tracheal epithelia. An example is given in Fig. 2A. The data from four identical experiments are combined in Fig. 2, B and C, where the relationship is shown on both arithmetic and logarithmic scales. Maximal responses are achieved at ∼200 nM, and note the overall current increase is 79% of the basal current. Analysis shows that the EC50 is 10.5 nM (95% confidence limits 8.0–13.6 nM) and that the Hill slope (nH) was 1.08 (95% confidence limits 0.72–1.44), suggesting a simple bimolecular interaction between the agent and its target receptor.
Fig. 2.
Relation between lubiprostone concentration and tracheal SCC responses in the sheep. The effect of the cumulative addition of lubiprostone from 2 to 200 nM on SCC is shown in A. The values at the beginning and end of the trace show the mean values of SCC for 4 ventral epithelia. Normalized values ± SE for the change in SCC with lubiprostone concentration are shown on an arithmetic scale in B and a logarithmic scale in C.
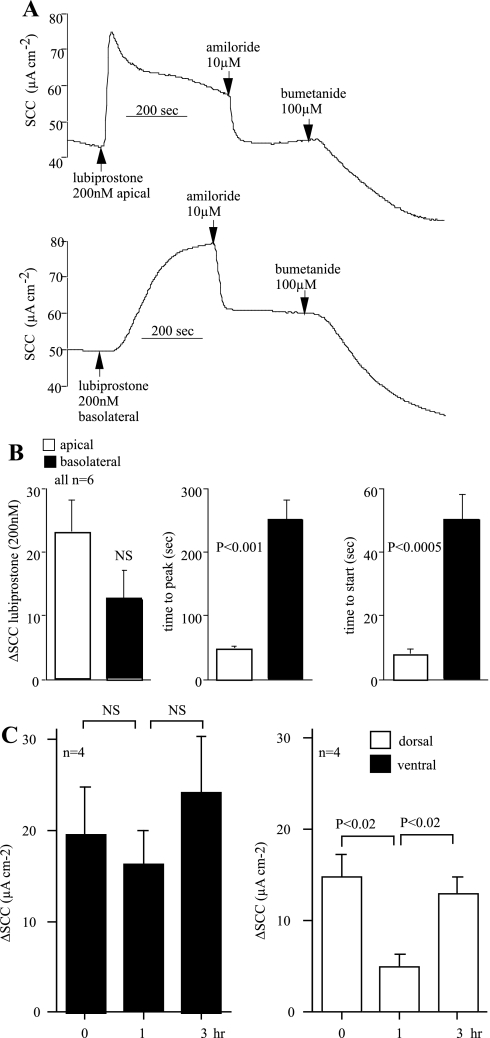
We compared the responses of apically applied lubiprostone with those obtained after basolateral application using adjacent pieces from the same trachea. In all, six pairs were examined, applying the prostone to the apical side in one and the basolateral surface in the other. Three measures were made; they were the peak value of the change in SCC, the time to achieve the peak response, and, finally, the time taken for the appearance of the response. The data are illustrated in Fig. 3. Basolateral application generally produced smaller responses than when the prostone was added apically, although in one exceptional instance they were equal (Fig. 3A), whereas in another example, basolateral addition gave a barely perceptible response. However, with such variable responses from basolateral application, the mean difference was not significant. Apical responses appeared within 10 s and peaked at ∼50 s, significantly faster than addition to the basal side (Fig. 3B). It is informative to compare apical addition of lubiprostone with that of amiloride, known to affect epithelial Na+ (ENaC) channels of surface epithelial cells. Amiloride gave values of 1.05 ± 0.05 s (n = 4) for onset and 48.3 ± 7.0 s (n = 4) for time to peak for amiloride, 10 μM added apically. Thus the time to onset of amiloride action was significantly less (P < 0.02) than that for lubiprostone, applied apically, whereas the time to peak was not different. Similar responses were obtained using the pig ventral tracheal epithelium; responses to basolateral application were slow to develop and reached approximately half of the value of responses to apical addition.
Fig. 3.
Lubiprostone induces a chloride SCC more efficiently if applied to the apical surface than to the basolateral surface of sheep tracheas. An example of 1 matched pair is given in A. Six matched pairs of ventral epithelia were exposed to 200 nM lubiprostone on either the apical or basolateral side. Amiloride was added apically and bumetanide basolaterally. B shows the means ± SE for the peak changes in SCC (ΔμA cm−2), the time taken to reach the peak value (seconds), and the time for the responses to begin (seconds). The repeatability and reversibility of lubiprostone action was investigated as shown in C. Here are shown results for repeated application of 200 nM lubiprostone (apically) at 0, 1, and 3 h with extensive washing between each addition. Data for both dorsal and ventral epithelia of sheep are shown. NS, not significant.
To determine whether responses to lubiprostone were reversible and repeatable, dorsal and ventral mucosae were prepared and allowed to equilibrate for 2 h. Lubiprostone was added apically at the maximally effective concentration followed by thorough washing. When the SCC had returned to the basal value, the same concentration was reapplied 1 h after the first exposure and again after washing, following a 2-h interval. There was no significant difference in the responses of ventral epithelia after all three exposures. In dorsal epithelia, the response after a 1-h interval was significantly less than that of the first exposure, but the initial effect was restored after a further 2-h interval (Fig. 3C).
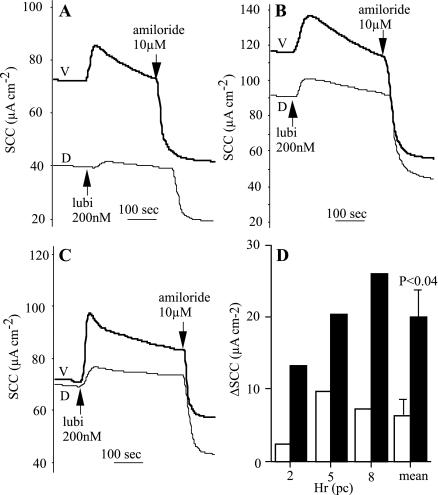
Differences in the transport parameters between dorsal and ventral tracheal epithelia are noted in Table 1. Furthermore, as shown in Fig. 1, it appears that a similar difference exists in the response to lubiprostone in tissues from the two locations. However, this difference only verged on significance. A more stringent test is to use matched pairs of mucosae. An experiment was designed using three matched pairs of mucosae all taken from the same trachea. Each pair consisted of a piece of ventral epithelium plus the diametrically opposite piece of dorsal epithelium. In all three pairs, the response of the ventral epithelium exceeded that of the comparable dorsal tissue (Fig. 4). Thus the basic differences in basal SCC between dorsal and ventral epithelia are also reflected in the response to lubiprostone.
Fig. 4.
Lubiprostone (lubi) responses are greater in ventral (V) than dorsal (D) sheep tracheal epithelia. Shown is a comparison of lubiprostone responses in matched pairs of dorsal and ventral epithelia from sheep trachea. In each tissue, responses to 200 nM lubiprostone and 10 μM amiloride, both applied apically, are shown. Lubiprostone responses in all 6 tissues are summarized in D, where open columns represent dorsal and filled columns ventral epithelia for measurements made at 2 h (A), 5 h (B), and 8 h (C) postculling (pc). The final pair of columns gives the mean values and standard errors for epithelia from both locations.
Nature of the transported ion.
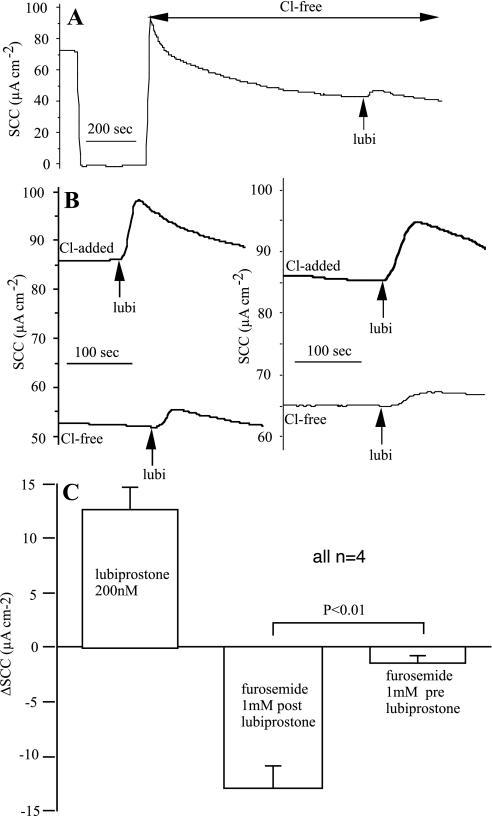
Although the direction of the current change following lubiprostone and its persistence in the presence of amiloride makes it likely that anion secretion forms the basis of the lubiprostone response, further definitive evidence is required. No effect of acetazolamide was found in a number of observations, suggesting that secretion of bicarbonate was minimally involved. Transferring epithelia to a totally chloride-free solution on both sides of the membrane caused a slow fall in SCC to a new steady state. Even then the response to lubiprostone, after 20 min, was not eliminated (Fig. 5A). Whether this was due to residual chloride ions remaining in cells, leak of Cl− ions from electrodes, or to the transport of another ion was not determined. Using this protocol, responses to lubiprostone were recorded following a 20-min exposure to Cl−-free solution. After extensive washing and restoring the normal bathing solution, the responses to lubiprostone were repeated when increased responses were found (Fig. 5B).
Fig. 5.
Cl− removal prevents responses to lubiprostone in sheep trachea. A shows the response to 200 nM lubiprostone, applied apically, after 20 min bathed in chloride-free solution. The 250-s gap at the beginning of the trace is when the clamp was switched off to replace Krebs-Henseleit solution (KHS) with Cl−-free KHS. B shows responses in 2 ventral tracheal preparations to 200 nM lubiprostone after 20-min exposure to chloride-free solution and again after washing and recovery in KHS. The effectiveness of furosemide to inhibit SCC when added before or after lubiprostone is shown in C. Data were from 4 pairs of preparations in which the effects of furosemide were measured when added before or after a response to lubiprostone (200 nM).
The basis of the basal SCC in sheep trachea is equivocal. Olver and Robinson (32) found that the sum of the net sodium and net chloride fluxes in adult sheep tracheas was not significantly different from the simultaneously recorded SCC. They also showed that 1 mM furosemide, to block the NaK2Cl triporter, inhibited SCC by an amount approximating to the chloride current measured isotopically. However, Cotton et al. (7) were unable to detect any basal active chloride secretion in sheep trachea. Our experience is similar to the latter in that neither bumetanide or furosemide alone had much effect on basal SCC. Using seven tracheal preparations exposed to 10 μM amiloride and 100 μM bumetanide (applied basolaterally for 30 min), the total basal SCC inhibition only reached 58.4 ± 3.5% (n = 7), the bumetanide contribution being only 12.6 ± 3.0%, i.e., just one-eighth of the basal current. Nevertheless, in the two experiments illustrated in Fig. 3A, and following lubiprostone, 100 μM bumetanide, in the presence of amiloride, produced rapid current reductions of 23.0 and 27.7 μA/cm2, corresponding to 52.3 and 55.4% reduction in the value of the basal SCC, much larger than the 12.6% anticipated from above. Furthermore, the effects of bumetanide were rapid, in contrast to the effect on basal SCC, and approximated to the SCC increase caused by lubiprostone. Formulating the hypothesis that responses to lubiprostone are blocked by furosemide, whereas basal SCC is largely unaffected, four pairs of ventral epithelia were subjected to a protocol where 1 mM furosemide (as used in Ref. 32) was added before or after lubiprostone. It is seen that the hypothesis is fulfilled, that is the mean responses to lubiprostone were identical to the SCC subsequently inhibited by furosemide, whereas furosemide alone had only a minor effect on basal SCC (Fig. 5C).
Target for lubiprostone.
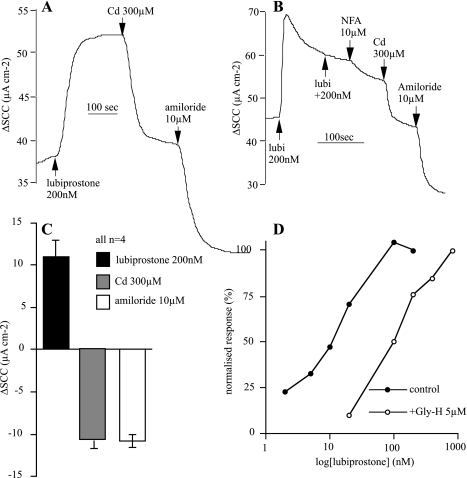
The effects of lubiprostone on tracheal epithelium were rapidly reversed by the apical application of cadmium ions (300 μM) that are known to block ClC-2 channels (Ref. 12; Fig. 6, A and C). However, specificity in anion channel blockers is notoriously difficult to achieve, and with this in mind we specifically chose an alternative agent, namely niflumic acid, a putative CaCC channel blocker (14). This agent produced a reduction in the lubiprostone-induced current that was completed by the subsequent addition of Cd2+ (Fig. 6B).
Fig. 6.
Inhibition of lubiprostone effects. A shows effects of Cd2+ ions, added apically on the response to lubiprostone, whereas B shows an effect of niflumic acid (NFA) also applied apically. C shows the cumulative data from 4 experiments conducted as in A. D shows normalized concentration-response curves to lubiprostone in sheep tracheas in the presence (open symbols) and absence (closed symbols) of 5 μM GlyH-101 applied apically.
GlyH-101 is an anion channel blocker originally considered to be specific for CFTR (29). Here, we found that 5 μM GlyH-101 caused a rightward shift in the concentration-response relationship to lubiprostone (Fig. 6D), but we are unable to distinguish whether this is due to CFTR blockade or the possibility that the compound can block other anion channels.
Lubiprostone Effects on Single Submucosal Glands
Earlier we showed that basolateral application of lubiprostone produced slowly developing SCC responses that usually failed to be as effective as addition to the apical side. In measuring gland secretion, lubiprostone could only be added basolaterally and depended on diffusion from an unstirred bath to reach the target. The expectation was that effects would appear slowly compared with those from current recording. Consequently, we have used 1 μM lubiprostone as a standard concentration as a compromise to achieve responses within a reasonable time frame.
Submucosal gland secretion in sheep, pigs, and humans.
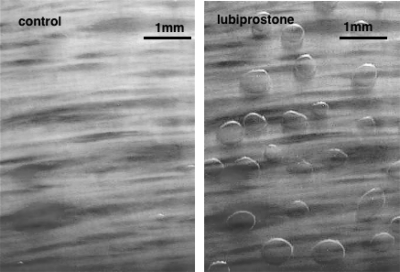
We show that lubiprostone causes secretion from submucosal glands in all three species examined. Figure 7 shows representative images of a sheep trachea before and 30 min after application of 1 μM lubiprostone basolaterally. Images were collected every 5 or 10 min throughout experiments and provided the opportunity to refresh the bath every 15–20 min.
Fig. 7.
Shown is the appearance of 11 mm2 of sheep trachea suspended at an air-Krebs interface at the end of a 30-min control period (left) and after a further 30 min exposed to 1 μM lubiprostone applied basolaterally (right). In both instances, the surface is covered with water-saturated mineral oil, and after lubiprostone is applied mucus secretions have collected as spherical droplets at the mouths of glands stimulated to secrete.
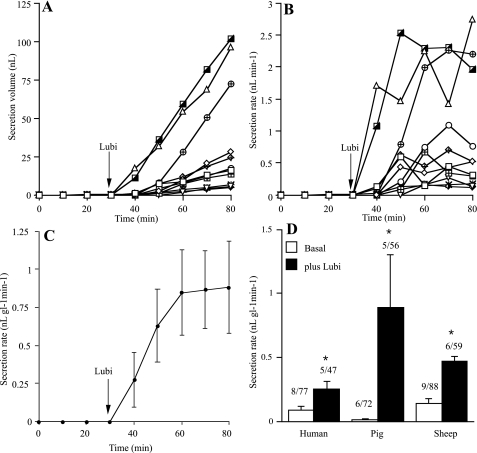
Results from the analysis of the accumulated images were undertaken off-line and are illustrated in Fig. 8, in this instance for an experiment with pig trachea. Figure 8A shows the accumulated volume of secretion from 11 glands during 50-min exposure to the drug, whereas Fig. 8B shows the secretion rates, in nanoliters per minute, for the same 11 glands. The mean secretion rate is shown in Fig. 8C, in nl gl−1 min−1, where it is seen to increase from virtually 0 to ∼0.8. It is to be noted that the secretion rate of individual glands is very varied, that may depend on gland size. The response is slow as predicted and reaches a new maintained steady state usually within 15 min. Similar experiments were carried out with tissues from sheep and humans, and the cumulative data are summarized in Fig. 8D representing tissue from 5 pigs, 6 sheep, and 5 humans in which the effects of lubiprostone were monitored on 162 glands. Lubiprostone caused a significant increase in secretion rate in all 3 species, secretion rate being greatest in pigs followed by sheep and then humans.
Fig. 8.
Lubiprostone induced gland secretion. A–C show results from a typical experiment, in this instance made with pig trachea. A shows the accumulation of secreted volume (in nanoliters) for 11 glands following addition of lubiprostone, 1 μM, basolateral, whereas B shows the same data expressed as secretion rate (nanoliters per minute). C shows secretion rates of 11 glands converted to a mean value (in nl gl−1 min−1). D shows cumulative data from similar experiments in humans, sheep, and pigs. *Significant increases in secretion rate by lubiprostone in all 3 species (P < 0.05). The numbers above each column indicates the number of subjects and the total number of glands examined (e.g., 6/72 indicates a total of 72 glands were measured in 6 subjects).
Anion dependence of submucosal gland secretion.
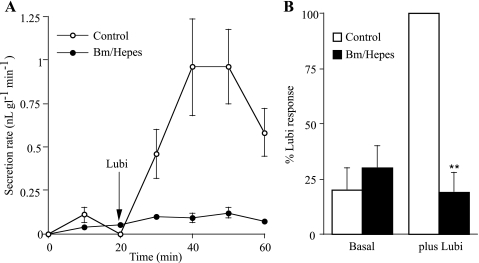
It was important to clarify whether lubiprostone-induced secretion was anion-dependent, thus providing a mechanism for osmotic drag and allowing mucus fluid to be secreted from the glands. To test this hypothesis, HCO3− was replaced by HEPES buffer, and the tissue was superfused with pure O2 to which bumetanide was added, the latter to prevent chloride entry into the basolateral aspects of the cells by inhibiting the NaK2Cl triporter, a technique previously used to inhibit anion-dependent secretion (18). We showed that this procedure virtually prevented the lubiprostone response. Figure 9A shows an example in pig trachea. Cumulative data are given in Fig. 9B representing results from two sheep and one pig (hence results are given as percentages of the lubiprostone responses in the control).
Fig. 9.
Anion removal prevents lubiprostone-induced gland secretion. Bumetanide (Bm; 100 μM) and replacement of HCO3− by HEPES buffer were used to effectively remove anions from the basolateral face of epithelia suspended at an air-Krebs interface. Responses to 1 μM lubiprostone, applied basolaterally, in normal and ion-substituted tissues were compared. A shows secretion rates in a pair of pig preparations. B shows a summary of bumetanide-HEPES inhibition of lubiprostone responses in 2 sheep and 1 pig, stated as a percentage of the normal lubiprostone response. **Significant difference (P < 0.01).
Target for lubiprostone.
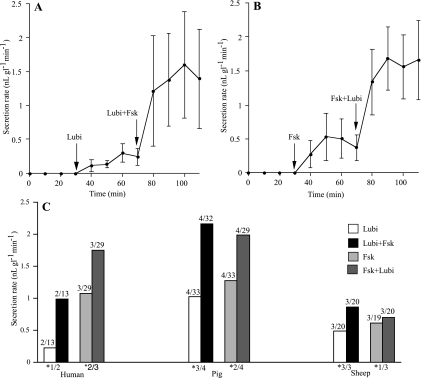
As it is not possible to add agents to the mucosal side while measuring submucosal gland secretion, we considered other ways to define the target, in particular whether the responses to lubiprostone are independent of CFTR. One approach is to determine whether in the presence of a maximally effective concentration of forskolin a further increase in secretion rate was caused by lubiprostone. If so, then these 2 agents are acting independently, although possibly on the same molecule. Results from 2 experiments using pig trachea are given in Fig. 10, A and B. It is shown that in the continued presence of forskolin (10 μM), lubiprostone (1 μM, basolaterally) caused a further significant increase in the mean secretion rate (Fig. 10B). Similarly, in the continued presence of lubiprostone (1 μM), forskolin (10 μM) caused a further increase in the mean secretion rate, which in this instance did not achieve significance (Fig. 10A). The large standard errors arise partly from the selection of bubbles of various sizes to obtain mean secretion rates. Processing the same data by the Mann-Whitney U test, a nonparametric rank test, the difference between lubiprostone and lubiprostone with forskolin was significant at P < 0.01. Nevertheless, we have retained the Student's t-test for all the data presented in this study. The data from 19 experiments are summarized in Fig. 10C. It is seen that both types of experiment (i.e., lubiprostone vs. lubiprostone + forskolin and forskolin vs. forskolin + lubiprostone) in all 3 species (sheep, pigs, and humans) resulted in increased secretion rates in the presence of 2 agonists. The details are summarized in Fig. 10C, and shown is the number of experiments where the increase in secretion rate was greater in the presence of both agonists compared with 1. Significant increases were recorded in 12/19 experiments, with at least 1 significant experiment in all 6 categories.
Fig. 10.
Effects on gland secretion of lubiprostone-forskolin (Lubi+Fsk) interactions. A and B show data from 2 pig tracheal preparations. In one, 1 μM lubiprostone was added and, 40 min later, followed by 10 μM Fsk in the continued presence of lubiprostone. In the other preparation, the order of agonist addition was reversed. Mean secretion rates for lubiprostone vs. Fsk plus lubiprostone were not significantly different (A; P < 0.12), whereas mean secretion rates for Fsk vs. lubiprostone plus Fsk were significantly different (B; P < 0.04). C details the data from 19 experiments in which Fsk and lubiprostone were used. The numbers above each column show the number of subjects and the total number of glands sampled (e.g., 4/32 means data from 32 glands from 4 subjects were recorded). Error bars are not shown as none of the differences are significant, however, numbers at the base of each pair of columns indicate the number of subjects where the second agonist caused a significant increase (P < 0.05) in secretion rate over that with the first agonist (e.g., *3/4 means a significant difference was obtained in 3 out of 4 subjects).
Human CF Submucosal Glands
Responses of normal human submucosal glands have already been reported in Fig. 8D.
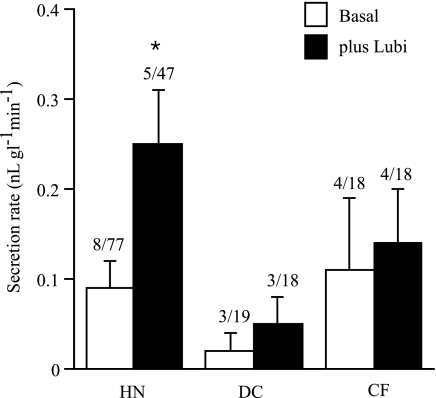
Figure 11 shows data from 4 CF patients and 3 DC [chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis (COPD/IPF)]. In neither group did lubiprostone cause a significant increase in secretion rate. It is worth examining in more detail the results from these experiments. Only two of the four CF tissues showed a response to lubiprostone. In one, basal secretion rate increased from 0.17 ± 0.05 to 0.23 ± 0.01 nl gl−1 min−1 in six glands with only one gland responding. In the other, the basal secretion rate was 0.01 ± 0.01 nl gl−1 min−1 that increased to 0.02 ± 0.01 nl gl−1 min−1 in seven glands with two responding, following lubiprostone. In general, few CF glands showed responses, whereas the majority showed no response at all.
Fig. 11.
Effects of lubiprostone (1 μM) on gland secretion in disease control (DC) and cystic fibrosis (CF) human tissues compared with normal human glands (HN). Numbers above each bar indicate the number of subjects investigated and the numbers of glands measured. *Significant difference.
In the DC, the basal rate of 0.02 ± 0.02 nl gl−1 min−1 increased to 0.05 ± 0.03 nl gl−1 min−1 after lubiprostone. In three DC tissues, one (IPF) showed no response to lubiprostone, whereas seven of nine glands of one COPD tissue and two of three glands in another IPF tissue did respond.
Miscellaneous Observations
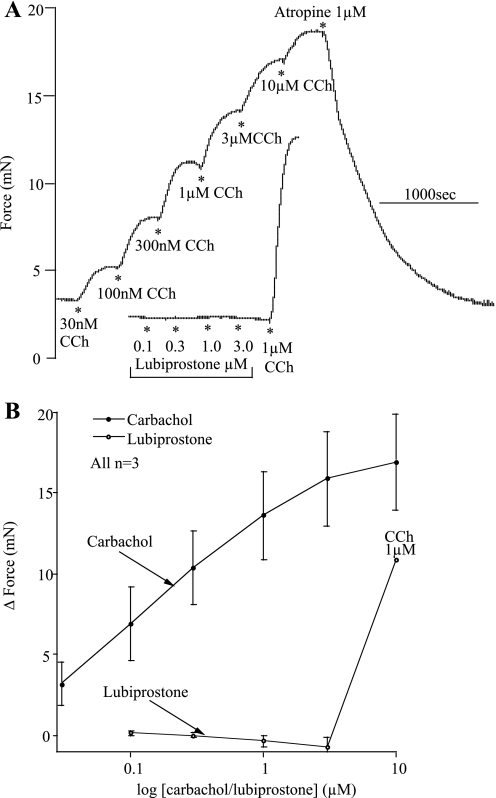
Using the trachealis muscles from sheep, we showed that lubiprostone neither caused contraction nor prevented carbachol-induced contractions, as shown in Fig. 12. Effects of carbachol were entirely due to an action on muscarinic receptors as they were completely abolished by atropine. Similar data were also obtained in the pig.
Fig. 12.
Carbachol (CCh), but not lubiprostone, contracts sheep trachealis muscles. A shows specimen traces of responses to lubiprostone or CCh in trachealis muscles. B shows concentration-response relationships for CCh and lubiprostone on trachealis muscles.
DISCUSSION
Our primary aim to discover whether lubiprostone caused airway secretion has been answered positively for sheep, pigs, and human airway mucosae. In the sheep and the pig, we have shown that lubiprostone increases both SCC responses in the surface epithelium as well as mucus secretion from submucosal glands. It is a simple matter to show that gland secretion contributes more fluid than is achieved with surface secretion. For example, the sheep response to a maximally effective concentration of lubiprostone causes SCC responses of ∼20 μA/cm2. Assuming that under open circuit conditions an equivalent amount of isotonic solute transport occurs, this would account for ∼2% of the secretion volume due to glands, at a density of 1 mm−2 and a secretion rate of 0.3 nl gl−1 min−1. Others, too, have argued that most of airway mucus arises from the glands (3, 33).
This is the first report of the effects of lubiprostone on isolated airway epithelia. Lubiprostone caused increased secretion, measured as SCC, in tracheal epithelia from lambs, pregnant ewes, and fetal sheep and in pig tracheas. There have been few reports of the effects of lubiprostone on ion transport in any native epithelia. One study describes the restoration of transepithelial resistance in isolated ischemic porcine ileal and colonic epithelia together with its effects on chloride secretion (25). Another recent report shows that high concentrations of lubiprostone were able to restore chloride secretion, when applied topically to nasal epithelia of CF mice, as judged by changes in nasal potential (23).
In our hands, apical lubiprostone was extremely potent, the maximally effective concentration being 200 nM and with a Kd of 10 nM in the sheep, close to the value of 17 nM found by others in T84 cells (8). The nH of the concentration-response relationship was close to 1.0, indicating a likely 1:1 stoichiometry between the prostone and its target, again as found in the earlier study (8).
Most agents affecting epithelial anion secretion in epithelia act on targets on the basolateral side of the tissue, examples being vasoactive intestinal polypeptide, substance P, neuropeptide Y, 5-hydroxytryptamine, and cholinergic agonists. Lubiprostone application to the apical face of tracheal epithelia of sheep and pigs produced a rapid response, whereas the response to basolateral addition was much slower, and the final effect on current was generally smaller. However, in the porcine ileum, basolaterally added lubiprostone only partially mimicked the action of the prostone added apically (25). Apparently, lubiprostone cannot be detected in the blood stream after oral administration to humans (1), and it may be that similar barriers may be present in airways. The speed of onset of effect to apically added lubiprostone suggests a superficial site for the target. However, the onset was slower than for amiloride, known to act on surface cell ENaC channels. The rate of formation of a drug receptor complex is given by v = k1[D], where k1 is the forward velocity constant and [D] the drug concentration. k1 is likely to be diffusion limited in the stationary layers covering the surface epithelium. As the concentration of amiloride used was 50 times greater than that of lubiprostone, and since the molecular weight of amiloride is approximately half that of lubiprostone, it is expected that the former would have a faster onset time. Thus the data indicate that the target for lubiprostone is superficial, viewed from the apical side. The immediate block of lubiprostone effects by nonlipid soluble Cd2+ ions suggests that the final effector mechanism is also superficial, possibly a channel on the apical face of the surface epithelium. The effects of lubiprostone were maintained, but reversible on washing and repeatable, with a long recovery time between applications.
The larger responses to lubiprostone seen in sheep ventral epithelia compared with matched dorsal epithelia and the significantly larger basal SCC values found in ventral epithelia should be considered in relation to the anatomic structure of the trachea. There are more submucosal glands in the ventral epithelium compared with dorsal (41), at least in human tracheas. The average number of glands in the membranous wall (dorsal) of 12 human tracheas was 808 (0.8 glands per square millimeter), whereas the cartilaginous wall (ventral) contained an average of 3,123 glands (1.0 glands per square millimeter). Assuming a similar difference applies to the sheep, then one implication might be that the basal SCC is partially generated by submucosal glands, and that lubiprostone exerts part of its effects on the glands. However, it seems unlikely that the complex ramifications of the glands can be properly voltage-clamped when much less resistive pathways are available across the surface epithelium or collecting ducts of the glands. This applies particularly to the serous acini at the ends of the tubular manifold, yet we know that human serous cells (Calu-3) respond to lubiprostone by secreting anions (24). Others, too, have pointed to a correlation between the submucosal gland density and basal SCC when comparing bronchioles with small bronchi (2).
Here, it is shown tracheal epithelia from sheep and near-term fetuses responded equally well to lubiprostone, and although it has been reported that ClC-2 is highly expressed in the fetal lung and is downregulated at birth (31), others (39) found that ClC-2 mRNA is only reduced by half in the adult.
The SCC data presented on chloride removal, the effects of bumetanide and furosemide, the persistence in the presence of amiloride, and the direction of the current responses support the view that electrogenic chloride secretion is the basis of the lubiprostone SCC response. However, the data with blockers of NaK2Cl cotransporter leaves unanswered questions about the nature of the basal SCC in the sheep trachea. Effective removal of both chloride and bicarbonate from the bathing solution virtually prevented the secretion caused by lubiprostone from individual submucosal glands. This is perhaps not surprising as lubiprostone causes a bumetanide- and acetazolamide-sensitive inhibition of SCC in Calu-3 monolayers (24). Nevertheless, these results support the view that lubiprostone-induced anion-led electrogenic secretion is the driving force for fluid formation, measured here as spherical droplets at gland duct openings.
There is accumulating evidence that lubiprostone can activate ClC-2 channels. Lubiprostone has been shown to activate Cl− currents in ClC-2-transfected HEK-293 cells but not HEK-293 cells transfected with CFTR (8). More recently, Bao et al. (4) have shown activation by lubiprostone of channels in amphibian A6 cells with properties corresponding to ClC-2 channels by comparison with HEK cells expressing hClC-2. Xenopus oocytes expressing hClC-2 channels responded to lubiprostone, a property not shared with water-injected oocytes (M. Murthy and A. W. Cuthbert, unpublished observations). Other more circumstantial evidence suggests that ClC-2 activation by apically applied lubiprostone is responsible for the recovery of transepithelial resistance in ischemically damaged pig intestine, a property not blocked by the CFTR inhibitor Dasu-2 (25). Although a similar recovery in transepithelial resistance can be obtained with PGE2, the latter needs to be added basolaterally and is inhibited by CFTR blockade (26).
The ClC-2 channel is commonly found in epithelial tissues. In some, it is colocalized with ZO-1 and occludin at interepithelial tight junction complexes located near the apical surface (15). ClC-2 has also been localized to the basolateral membrane in duodenal and colonic surface cells, suggesting that here it may be concerned with fluid absorption (6). Apical membrane colocalization with CFTR has been seen in human ciliated airway cells (22) but was not found in glands. ClC-2 channels can be activated by hyperpolarization, hypotonicity, or low pH. Activation of the ClC-2 channel is thought to require the dissociation of the NH2-terminal region from a docking site between transmembrane segments D7 and D8 (20).
A major reason for this study was to examine ClC-2 channels as possible surrogates for CFTR by using the putative ClC-2 activator lubiprostone in the airways. It is known from the work of Schweibert et al. (38) that ClC-2 currents can be obtained in human CF airway cells when subjected to hyperpolarization or lowered external pH, both methods of activating ClC-2 channels, indicating that ClC-2 channels can be activated in the absence of CFTR in normally CFTR-containing cells.
Our findings in this study have shown that lubiprostone effects on SCC are completely eliminated by Cd2+ consistent with an action on ClC-2 channels. Unfortunately, methodological limitations do not allow the effects of Cd2+ to be investigated on gland secretion. However, even low concentrations of niflumic acid and GlyH-101, putative inhibitors of CaCC and CFTR channels, respectively, also have inhibitory effects on lubiprostone-mediated responses. This could mean that lubiprostone activates ClC-2, CaCC, and CFTR channels or alternatively one or more of the inhibitors is/are nonspecific. The inhibition by GlyH-101 is unlike those in other epithelia. For example, in Calu-3 cells, derived from human airway serous cells, GlyH-101 had no effect on lubiprostone responses, even at 50 μM. Similarly, Calu-3 cells in which CFTR had been permanently reduced by 95% using siRNA showed unaffected responses to lubiprostone, even in the presence of 50 μM GlyH-101 (24). Human colonic epithelium responded equally well to lubiprostone in the presence or absence of 50 μM GlyH-101 (A. Ropenga and A. W. Cuthbert, unpublished observations). It is possible that the target molecule in the sheep is different from that in humans or has an altered sensitivity to GlyH-101. More likely is that GlyH-101 is a nonspecific anion channel blocker as found by others (A. S. Verkman, personal communication).
Lubiprostone-induced mucus bubbles showed considerable variation in size (Fig. 7). Choosing equally from large, medium, and small bubbles for analysis avoided bias when mean secretion rates were measured. We showed mean secretion rates were, in order, greatest in pigs followed by sheep and then humans.
We have investigated the action of lubiprostone on gland secretion in the presence of a high concentration of forskolin, arguing it would be unlikely to stimulate the adenylate cyclase system further, yet in sheep, pig, and human tissues, lubiprostone produced an additional increase in secretion rate in the continuous presence of forskolin. These data could be interpreted to suggest lubiprostone has an action independent of CFTR. Alternatively, lubiprostone might affect CFTR channel kinetics, for example to increase channel open time, thus promoting secretion but without generating cAMP (see Ref. 4). In this sense, lubiprostone would be acting as a CFTR potentiator, yet clearly it is not necessary to prime CFTR with forskolin for lubiprostone to cause secretion. In this latter situation, lubiprostone would be acting as a channel opener. Yet, HEK cells expressing CFTR fail to be activated by lubiprostone (8). It has recently been shown for A6 cells that, whereas low concentrations of the prostone activate ClC-2 channels, high concentrations can activate CFTR directly without affecting cAMP formation (4). Unlike the findings with A6 cells, no second site of lubiprostone action has been found in either sheep airways or T84 cells (8). In both instances, the concentration-response curves were monotonic with no indication of a second site of action.
In view of the close similarity of chemical structure of lubiprostone with prostaglandins, there have been suggestions that the prostone activates prostaglandin EP receptors in the human gut smooth muscle (5) based on inhibition by EP receptor antagonists, whereas others have claimed that lubiprostone fails to mimic PGE1 or PGE2 on uterine muscle cells (9). From a clinical standpoint, the matter is somewhat academic, as lubiprostone, given orally, is not absorbed into the bloodstream (1). Whether it can be absorbed from the airways is unknown, nevertheless we have examined trachealis muscles and found no indication that it is spasmogenic. Furthermore, when isolated submucosal glands are viewed with Normarski optics and exposed to a pulse of lubiprostone, the acini are seen to secrete without effects on the myoepithelial cells (J. J. Wine, unpublished observations).
Although lubiprostone increased the mean secretion rate of CF and DC human airway glands, neither increase was significant. Nevertheless, DC glands showed a 1.5-fold increase in secretion rate over basal, exactly as with normal human glands. With CF tissues, the lubiprostone-induced secretion was only 25% greater than basal. This may mean that, in some way as yet unknown, CFTR is involved in the response to lubiprostone, therefore expected to affect CF but not DC tissues. Furthermore, CF and DC tissues are from patients at the end stages of a terminal illness. How this may affect penetration of lubiprostone from the basolateral side and why few individual CF glands respond, whereas the majority do not, remain important questions. We observed histologically that human CF tissues from lung transplants developed a substantial amount of musculature around the glands (N. S. Joo, unpublished observations). Devising a way to apply lubiprostone apically while measuring gland secretion remains an important goal. As lubiprostone produces some mucus secretion in CF, it is possible that more potent agents of this type can be beneficial. Our findings with normal human airway tissue are of interest as they show lubiprostone is a potential agent for increasing airway secretion without producing bronchoconstriction that may have therapeutic utility.
GRANTS
This work was supported by grants from the Cystic Fibrosis Trust, the Cystic Fibrosis Foundation, and the National Institutes of Health.
Acknowledgments
We are grateful to lung transplant doctors, including R. C. Robbins and David Weill, and operating room nurses. We thank Kim Tran and Jonathon Chen for harvesting tissues and Mauri Krouse and Jennifer Lyons for useful discussion. We are especially grateful to the patients and their families.
REFERENCES
- 1.Ambizas EM, Ginzburg R. Lubiprostone: a chloride channel activator for the treatment of chronic constipation. Ann Pharmacother 41: 957–964, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Ballard ST, Fountain JD, Inglis SK, Corboz MR, Taylor AE. Chloride secretion across distal airway epithelium: relationship to submucosal gland distribution. Am J Physiol Lung Cell Mol Physiol 268: L526–L531, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Ballard ST, Trout L, Bebok Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol Lung Cell Mol Physiol 277: L694–L699, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol 295: G234–G251, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangia R, Sung EZ, Lee K, Sanger GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol 154: 126–135, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalan M, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral ClC-2 chloride channels in surface colon epithelium: regulation by a direct effect of intracellular chloride. Gastroenterology 126: 1104–1114, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Cotton CU, Lawson EE, Boucher RC, Gatzy JT. Bioelectric properties an ion transport of airways excised from adult and fetal sheep. J Appl Physiol 55: 1542–1549, 1983. [DOI] [PubMed] [Google Scholar]
- 8.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 287: C1173–C1183, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cuppoletti J, Malinowska DH, Chakrabarti J, Ueno R. Effects of lubiprostone on human uterine smooth muscle cells. Prostaglandins Other Lipid Mediat 86: 56–60, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest 132: 1631–1636. [DOI] [PubMed]
- 11.Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Mucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 2: 240–248, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Fritsch J, Edelman A. Modulation of the hyperpolarization-activated Cl− current in human intestinal T84 epithelial cells by phosphorylation. J Physiol 490: 115–128, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritsch J, Edelman A. Osmosensitivity of the hyperpolarization-activated chloride current in human intestinal T84 cells. Am J Physiol Cell Physiol 272: C778–C786, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Gruber AD, Schreur KD, Hong-Long JI, Fuller CM, Pauli BU. Molecular cloning and transmembrane structure of hCLCA2 from human lung, trachea and mammary gland. Am J Physiol Cell Physiol 276: C1261–C1270, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Gyomorey K, Yeger H, Ackerley C, Garami E, Bear CE. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am J Physiol Cell Physiol 279: C1787–C1794, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Harris A Towards an ovine model of cystic fibrosis. Hum Mol Genet 6: 2191–2193, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Joo NS, Irokawa T, Robbins RC, Wine JJ. Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem 281: 7392–7398, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Joo NS, Saenz Y, Krouse ME, Wine JJ. Mucus secretion from single submucosal glands of pig. J Biol Chem 277: 28167–28175, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Joo NS, Wu JV, Krouse ME, Senz Y, Wine JJ. An optical method for quantifying rates of mucus secretion from single submucosal glands. Am J Physiol Lung Cell Mol Physiol 281: L458–L468, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Jordt SE, Jentsch TJ. Molecular dissection of gating in the ClC-2 chloride channel. EMBO J 16: 1582–1592, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, Boucher RC. Charaterization of wild-type and deltaF508 cystic fibrosis transmembrane conductance regulator in human respiratory epithelia. Mol Biol Cell 16: 2154–2167, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol 282: C805–C816, 2002. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald KD, McKenzie KR, Henderson MJ, Hawkins CE, Vij N, Zeitlin PL. Lubiprostone activates non-CFTR-dependent respiratory epithelial chloride secretion in cystic fibrosis mice. Am J Physiol Lung Cell Mol Physiol 295: L933–L940, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacVinish LJ, Cope G, Ropenga A, Cuthbert AW. Chloride transporting capability of Calu-3 epithelia following persistent knockdown of the cystic fibrosis transmembrane conductance regulator, CFTR. Br J Pharmacol 150: 1055–1065, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am J Physiol Gastrointest Liver Physiol 292: G647–G656, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Moeser AJ, Haskell MM, Shifflett DE, Little D, Schultz BD, Blikslager AT. ClC-2 chloride secretion mediates prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Gastroenterology 127: 802–815, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Mohammad-Panah R, Gyomorey K, Rommens J, Choudhury M, Li C, Wang Y, Bear CE. ClC-2 contributes to native chloride secretion by a human intestinal cell line, Caco-2. J Biol Chem 276: 8306–8313, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Mohammad-Panah R, Ackerley C, Rommens J, Choudhury M, Wang Y, Bear CE. The chloride channel ClC-4 colocalises with cystic fibrosis transmembrane conductance regulator and may mediate chloride flux across the apical membrane of intestinal epithelia. J Biol Chem 277: 566–574, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol 124: 125–137, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mummery JL, Killey J, Linsdell P. Expression of the chloride channel ClC-K in human epithelial airway cells. Can J Physiol Pharmacol 83: 1123–1128, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Murray CB, Morales MM, Flotte TR, McGrath-Morrow SA, Guggino WB, Zeitlin PL. ClC-2: a developmentally dependent chloride channel expressed in the fetal lung and downregulated after birth. Am J Respir Cell Mol Biol 12: 597–604, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Olver RE, Robinson EJ. Sodium and chloride trasport by the tracheal epithelium of fetal, new born and adult sheep. J Physiol 375: 377–390, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reid L Measurement of the bronchial mucous gland layer: a diagnostic yardstick in chronic bronchitis. Thorax 15: 132–141, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCay PB, McLennon G, Meyerholz DK, Namati E, Ostedgaard LS, Prather RS, Sabater JR, Stoltz DA, Zabner J, Welsh MJ. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 295: L240–L263, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321: 1837–1841, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabater JR, Mao YM, Shaffer C, James MK, O'Riordan TG, Abraham WM. Aerosolization of P2Y2-receptor agonists enhances mucociliary clearance in sheep. J Appl Physiol 87: 2191–2196, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Salinas D, Haggie PM, Thiagarajah JR, Song Y, Rosbe K, Finkbeiner WE, Nielson D, Verkman AS. Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J 19: 431–433, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Schweibert EM, Cid-Soto LP, Stafford D, Carter M, Blaisdell CJ, Zeitlin PL, Guggino WB, Cutting GR. Analysis of ClC-2 channels as an alternative pathway for chloride conduction in cystic fibrosis airway cells. Proc Nat Acad Sci USA 95: 3879–3884, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherry AM, Stroffekova K, Knapp LN, Kupert EY, Cuppoletti J, Malinowska DH. Characterization of the human pH- and PKA-activated ClC-2G (2a)Cl− channel. Am J Physiol Cell Physiol 273: C384–C393, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Stutts MJ, Chinet TC, Mason SJ, Fullton JM, Clarke LL, Boucher RC. Regulation of Cl− channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proc Natl Acad Sci USA 89: 1621–1625, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tos M Mucous glands of the trachea in man. Anat Anz 128: 136–149, 1971. [PubMed] [Google Scholar]
- 42.Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc 1: 47–53, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Wu JV, Krouse ME, Wine JJ. Acinar origin of CFTR-dependent airway submucosal gland fluid secretion. Am J Physiol Lung Cell Mol Physiol 292: L304–L311, 2007. [DOI] [PubMed] [Google Scholar]