Abstract
The Br/+ mutant mouse displays decreased embryological expression of the homeobox transcription factor Six2, resulting in hertitable renal hypoplasia. The purpose of this study was to characterize the renal physiological consequences of embryonic haploinsuffiency of Six2 by analyzing renal morphology and function in the adult Br heterozygous mutant. Adult Br/+ kidneys weighed 50% less than those from wild-type mice and displayed glomerulopathy. Stereological analysis of renal glomeruli showed that Br/+ kidneys had an average of 88% fewer glomeruli than +/+ kidneys, whereas individual glomeruli in Br/+ mice maintained an average volume increase of 180% compared with normal nephrons. Immunostaining revealed increased levels of endothelin-1 (ET-1), endothelin receptors A (ETA) and B (ETB), and Na-K-ATPase were present in the dilated renal tubules of mutant mice. Physiological features of chronic renal failure (CRF) including elevated mean arterial pressure, increased plasma creatinine, and dilute urine excretion were measured in Br/+ mutant mice. Electron microscopy of the Br/+ glomeruli revealed pathological alterations such as hypercellularity, extracellular matrix accumulation, and a thick irregular glomerular basement membrane. These results indicate that adult Br/+ mice suffer from CRF associated with reduced nephron number and renal hypoplasia, as well as glomerulopathy. Defects are associated with embryological deficiencies of Six2, suggesting that proper levels of this protein during nephrogenesis are critical for normal glomerular development and adult renal function.
Keywords: Br mutant mouse, glomerulus
chronic renal failure (CRF) is a disease affecting an estimated 4.5% of adults in the United States with a high associated mortality, largely due to the subsequent development of cardiovascular disease (1, 78, 81). A leading cause of CRF in childhood is bilateral renal hypoplasia, which is characterized by abnormally small and dysplastic kidneys resulting from abnormal morphogenesis (37, 80). Typically, the hypoplastic kidney is associated with CRF since the nephrons do not differentiate properly and the renal tubules progressively distend while the interstitial architecture becomes disrupted. The kidney eventually fails since it becomes unable to excrete wastes, retain electrolytes, and concentrate urine. During this process, alterations in sodium and water reabsorption can cause the formation of renal cysts with secondary effects on the cardiovascular system resulting in systemic hypertension. Although numerous molecules are involved in the development of CRF, specific cellular and genetic defects causing renal hypoplasia remain poorly understood.
Reduced renal mass models, usually produced by surgical removal of one kidney and two-thirds of the remaining kidney, have long been used for studying the consequences of CRF. Typically performed in rats due to their larger size, relatively few reduced renal mass mice models have been developed to analyze renal failure as a consequence of decreased numbers of nephrons (79, 92, 100). The identification of a mouse model with heritable renal hypoplasia and low numbers of nephrons would be useful to identify specific morphogenetic pathways responsible for the progression of CRF and the cardiovascular complications.
We described a mouse mutant, called Brachyrrhine (Br), which arose from X-ray irradiation of the 3H1 strain and carries a semidominant mutation that results in renal hypoplasia and frontonasal dysplasia (35, 59–63, 68). The homozygous mutation is embryonic lethal, whereas the heterozygote mutant survives to adulthood and shows small kidneys and severe midfacial retrognathia. Analysis of Br/Br developing kidneys demonstrated the successful induction of the ureteric bud and initial metanephric mesenchymal condensation at embryonic (E) day 11.5, but showed severe disruption of nephrogenesis shortly afterward, with generalized glomerulopathy, large renal cysts, and the absence of a discernable renal cortex (60). We successfully mapped the Br mutation to a critical region containing only one gene, the Six2 homeobox transcription factor (35). During embryonic development of the Br/Br mouse, Six2 expression was shown to be almost completely absent from where it is normally expressed (35), which is primarily in the mesenchymal cells of the kidney and midface (75). In the same developing tissues of the heterozygous mutant mouse (Br/+), Six2 expression was measured at ∼50% of the levels measured in wild-type tissues (35). In a previous study, we noted that embryonic Br/+ kidneys were significantly smaller than wild-type, with a smaller nephrogenic zone and abnormal surface arteries (62). In addition, we observed distended collecting tubules in neonatal Br/+ kidneys, indicating possible cyst development. Recently, a Six2 knockout mouse was created and demonstrated a similar phenotype, with the homozygous null kidney showing severely disrupted nephrogenesis due to excessive mesenchymal-to-epithelial differentiation and subsequent depletion of the metanephric mesenchymal progenitor cells (87); however, the heterozygous Six2-null mouse was not described.
In this study, we analyze the morphology and physiological functioning of the hypoplastic Br/+ adult kidney and test the hypothesis that adult Br/+ mice display physiological features consistent with CRF. We collected large numbers of adult kidneys to compare gross, histological, and ultrastructural characteristics of Br/+ mutant kidneys with normal kidneys, and also undertook a stereological approach to measure total kidney volumes and quantify the number of glomeruli. Cardiorenal and metabolic physiological parameters were measured, and immunostaining of proteins critical to renal function was performed on Br/+ and normal kidneys. Real-time quantitative PCR confirmed Six2 is not normally expressed in the adult kidney, but only in the kidney during fetal development. Data from this study support the hypothesis that Six2 plays a critical role in the development of the kidney and that renal function is greatly compromised in the adult mouse when Six2 expression is downregulated prenatally.
MATERIALS AND METHODS
Animal breeding.
All experiments were conducted using inbred 3H1 (C3H/He × 101/H) wild-type (+/+) and heterozygous mutant (Br/+) mice. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee in accordance with local and federal standards. Breeding of 3H1 Br/+ and +/+ mice was carried out in the Animal Laboratory Service facility at University of Hawaii. Adult mice were housed under standard conditions with 12-h light cycle and they were supplied with tap water and food pellets (Agway Prolab Feed, Waverly, NY) ad libitum.
Real-time quantitative PCR.
Kidneys from embryos at E13.5, newborn, and adult mice were dissected out, minced, and immediately submerged in RNA-later (Sigma, St. Louis, MO) and stored at 4°C for up to 1 wk before processing. Kidneys were collected from three mice of each age and genotype. Total RNA was extracted from the tissues with a RNeasy kit (Qiagen) using a mortar and pestle to disrupt the tissue. Total RNA was reverse transcribed with iScript cDNA synthesis kit (Bio-Rad, Hercules, CA), and 200 ng of the resulting cDNA samples were used as template for quantitative (q)PCR by using the iQ SYBR Green Supermix reaction procedure with the MyiQ iCycler thermal cycler and single-color real-time PCR detection system (Bio-Rad). All reactions were run in triplicate to minimize experimental error. Primer sets specific for Six2 (forward) 5′-GCC TGC GAG CAC CTC CAC AAG AAT-3′, (reverse) 5′-CAC CGA CTT GCC ACT GCC ATT GAG-3′ and actin (forward) 5′-CAA TGA GCT GCG TGT GG-3′, (reverse) 5′-CAA CAC AGC CTG GAT GG-3′ were used in qPCR reactions. The PCR condition consisted of an initial 5-min denaturation at 94°C, followed by reactions cycled through denaturation for 15 s at 94°C, annealing for 20 s at 60°C, and extension for 40 s at 72°C. After 50 cycles of amplification, the PCR products were resolved on an agarose gel to confirm that a single band of predicted size was amplified. The expression levels of Six2 in each kidney sample were calibrated against actin expression, with the average relative ratio set to 1, as described previously (2, 35, 66). The amount of Six2 mRNA from the newborn and adult kidneys was compared with the normalized amounts from the E13.5 kidneys, and Six2 mRNA levels from Br/+ mutant kidneys were compared with wild-type levels in E13.5 kidneys.
Verification of renal hypoplasia in adult Br/+ mice.
Mean renal mass was determined in three groups of randomly selected adult animals: 3H1 stock mice, 3H1 +/+ mice, and 3H1 Br/+ mutant mice. The 3H1 +/+ mice were siblings of Br/+ mice and raised in the same litters. Mice were identified based on morphological phenotype (62) and weighed using precision standard TS 1205 electronic top loading balance (Ohaus, Pinebrook, NJ). The mice were killed by cervical dislocation following anesthesia with isoflurane, and both kidneys were resected. After the surrounding connective tissue was excised, wet weights were recorded. Additionally, left kidneys were dried in a 55°C oven for 48 h and the dry weights were recorded.
Stereological analysis of adult kidneys.
A total of 29 kidneys was used in this study, 15 3H1 +/+ and 14 Br/+ adult mice of both genders. The specimens were fixed and embedded in paraffin blocks, sectioned in a transverse plane at a thickness of 10 μm, and stained with hematoxylin and eosin (H&E). The sections were viewed at ×200 magnification using a Olympus BX41 microscope and photographed. Initially, 17 kidneys were exhaustively sectioned and preliminary experiments were conducted to establish and validate the stereological sampling system. Subsequently, 9 specimens were represented by ∼20% of the kidney while ∼10% of the kidney was retained for the remaining 6 animals.
For stereological estimates, a systematic random sampling procedure was used (43). Specimen volume of the reference tissue (Vref) was determined using the Cavalieri method. Stereological estimation of the number of glomeruli per unit volume was obtained using the disector method (42, 70). The disector area was 800 μm2, while every first and third sections were used so that each disector measured 20 μm in height. Glomeruli disappearing from the reference plane when viewing the look-up plane (Q−) were counted, and glomeruli underlying two margins of the disector were excluded.
Volume density (Nv) of the glomeruli was estimated as Q−/disector volume, and the number of glomeruli was calculated as Nv × Vref (70). Surface density was estimated by counting glomerular intersections with a system of rolling cycloid test lines and point counting grid intersections with individual glomeruli (6). Mean glomerular volume (Vm) and mean glomerular surface area (SAm) were computed from these basic stereological estimators (42, 70). At least 50 glomeruli from each mouse were measured for volume and surface area, and the mean values from each mouse were used for analysis.
A C++ computer program was written to automate data collection. Initial experiments were conducted utilizing the exhaustively sectioned specimens (7 3H1 +/+ and 7 Br/+ specimens). Convergence of glomerular number was used as a primary indicator of accuracy and this was achieved with a disector size of 800 μm2 and a Q− of ∼50. System validation was achieved by generating stereological estimators for a square lattice of spheres 20 μm in diameter and comprising 33% of the test volume. The lattice was sectioned electronically generating 10 sample fields of circles. The system achieved a percentage error of 3.7% for average object volume and 7.1% for average object surface area.
Physiological measurements of cardiorenal function.
Mean arterial pressure (MAP) measurement, heart rate (HR) recording, and blood sampling were performed on conscious animals after carotid artery catheter implantation, based on the procedure we previously described for rats (15, 53). Briefly, mice were anesthetized with isoflurane (1–3% in oxygen) administered via a gas mask Plexiglas tube modified for mice. Surgery was performed under aseptic conditions and catheters were sterilized before implantation. The carotid artery was catheterized with polyethylene tubing (PE-10) that was threaded through a larger flared polyethylene tubing (PE-50) used to secure the catheter to the carotid artery with silk (6–0) ties. The catheter was threaded subcutaneously to exit at the back of the neck and the skin incision was closed. The catheter was connected to a physiological recorder (Gould Instruments, Valley View, OH). The animal was removed from isoflurane exposure and allowed to recover from anesthesia in a commerical plastic experimental chamber (Kent Scientific, Torrington, CT).
The animal was continuously monitored for 60 to 120 min after recovery from isoflurane, to obtain steady baseline blood pressure and HR. Blood (0.2 ml) was sampled from the catheter and plasma was separated for osmolality and protein assays using a CX3 Delta Chemistry Analyzer (Beckman, Brea, CA). The mouse was euthanized by cervical dislocation following anesthesia with isoflurane (inhalant) and additional blood was obtained via cardiac puncture. For urine analysis, mice were placed individually into metabolic cages and urine was collected under mineral oil and pooled for a 24-h period to assess kidney function. Urine volume was determined gravimetrically and samples were analyzed for osmolality, creatinine, protein, potassium, and sodium using a CX3 Delta Chemistry Analyzer.
Physiological measurements of cholesterol and glucose metabolism.
Animals were fasted overnight and blood samples were obtained as described above. Plasma levels of glucose, triglycerides, cholesterol, and high-density lipoproteins were determined on a clinical chemistry analyzer (VITROS DT60, Ortho-Chemical Diagnostics, Rochester, NY). Plasma insulin and leptin levels were determined by radioimmunoassay (Linco Research, St. Louis, MO).
Histological and immunohistochemical analysis.
Normal and mutant mice were identified according to phenotype (62), euthanized with an overdose of isoflurane, and a midtransverse section of the left kidney from each mouse was fixed for 72 h at 4°C in 10% neutral buffered formaldehyde. The kidneys were dehydrated in an ascending concentration of ethanol, cleared in xylene, embedded in paraffin wax, sectioned at 6 μm, and stained with H&E.
For immunohistochemistry, the kidneys were excised and fixed in 4% paraformaldehyde (0.1 M PBS, pH 7.4) at room temperature for 4 h. Kidneys were transferred to 30% sucrose and stored at 4°C (24 h), and then snap-frozen in liquid nitrogen and embedded in OCT (Miles, Naperville, IL). Cryostat sections (10 μm) were air-dried and stored at −70°C. For ET-1, rabbit anti-ET-1 serum (Peninsula Laboratories) was used as primary antibody at 1:400. The secondary antibody was goat anti-rabbit Ig conjugated with FITC (Sigma) at 1:400. For ETA, a sheep anti-ETA (Maine Biotechnology Services) was used at 10 μg/ml and for ETB, the sheep anti-ETB (Maine Biotechnology Services) was used at 20 μg/ml. The secondary antibody was rabbit anti-sheep conjugated with FITC at 1:200 dilution. For Na-K-ATPase immunostaining, mouse monoclonal antibodies (anti-Na-K-ATPase α-1 subunit, Upstate Biotechnology) were used at 5 μg/ml, and FITC-conjugated goat anti-mouse IgG was used for secondary antibodies (Sigma). For immunostaining, the sections were defrosted (5 min) at room temperature, blocked using normal goat or rabbit serum (30 min), and then incubated with primary antibodies overnight at 4°C. After being washed, sections were incubated with secondary antibodies (1 h) at room temperature. All incubations were performed in a moist chamber. Negative controls were performed by either omitting the primary antibodies or replacing them with the same nonimmune serum routinely used for the experimental tissues. Images were taken on an Olympus BX41 fluorescent microscope.
Ultrastructural analysis.
For light and transmission electron microscopy (TEM), three pairs of age-matched normal and mutant mice (20–24 wk old) were deeply anesthetized with intraperitoneal injection of 0.6 ml avertin (30 mg/ml; 4–6 mg/10 g), following which the thoracic cavity of each mouse was quickly opened for perfusion. A 20-gauge needle was inserted into the left ventricle, saline was infused, and the right atrium was immediately transected. Gravity perfusion of 50 ml of 0.9% saline washout solution continued until the fluid was clear and was followed by infusion of 120 ml of cold (4°C) PIPES fixative (1% paraformaldehyde with 1% gluteraldehyde and 5% dextran in 0.1 M piperazine-N-N′,bis 2-ethane sulphonic acid) buffered with NaOH (pH 7.6) (8). The peritoneal cavity was opened and kidneys were harvested, weighed, and placed into cold PIPES fixative. Renal capsules and associated perirenal fat were removed and transverse slices were made. The cortex was dissected away from each slice and further minced into ∼1-mm3 pieces which were placed into small glass vials containing cold PIPES fixative for 48 h (4°C) and then washed three times (30 min each) in PIPES buffer with 2% sucrose. Renal cortical tissue samples were postfixed in a solution of one part 2% osmium tetroxide (OsO4) and 3% potassium ferricyanide K3Fe(CN)6 in PIPES buffer, and one part 6% sodium iodate and 4% sucrose in PIPES buffer overnight at 4°C (8). Tissue samples were then dehydrated in graded ethanols and propylene oxide at room temperature. Embedding was carried out in a 1:1 Epon/Araldite as previously described (24). This mixture was replaced by pure Epon/Araldite overnight and cured at 55°C for ∼72 h.
Thick sections (250 nm) were cut from the epoxy blocks on a RMC MTX ultramicrotome equipped with a Diatome diamond knife and stained with 1% toluidine blue (in 1% sodium borate). These sections were examined by bright field microscopy for presence and location of renal glomeruli. Thin sections (∼70 nm in thickness and silver-gray interference color) of the desired block region were cut and mounted on 200-μm-mesh naked copper grids. These were stained with 2% uranyl acetate, rinsed vigorously, counterstained with lead citrate (95), and rinsed again. Thin sections were examined and photographed in a Hitachi model H-7500 TEM at an accelerating voltage of 80 kV.
Glomerular basement membrane measurement.
Morphometry of glomerular basement membrane (GBM) was carried out as previously described (23). The method was a modification (29) of the “orthogonal intercept” procedure of Jensen and co-workers (48). Twenty sets of five identical magnification (37,500 diameters) transmission electron micrographs of GBMs from at least three glomeruli from each of two control (+/+) and three mutant (Br/+) animals were used in this study. Randomized measurements of peripheral GBMs (mesangial and stalk GBM regions were excluded) were made with a measuring ruler with a logarithmic scale utilizing a digitizing tablet and appropriate software (Bioquant, R&M Biometrics). Approximately 40 measurements were made from each micrograph set. “True” GBM thickness in each set of micrographs was generated from the measurements according to the orthogonal intercept technique.
Statistical analysis.
One-way ANOVA was performed to compare body and kidney weights using Prism3 statistical software (Graphpad, San Diego, CA). Physiological and stereological values were expressed as means ± SE or means ± SD. Differences between means for any parameter measured in two groups of mice were evaluated using Student's t-test (P < 0.05) following a test of normality of data. A correlation analysis was performed comparing MAP and plasma creatinine (mg/dl) of individual mice.
RESULTS
Adult f mice have severe renal hypoplasia with distended tubules, which is associated with the reduced expression of Six2 during embryonic development.
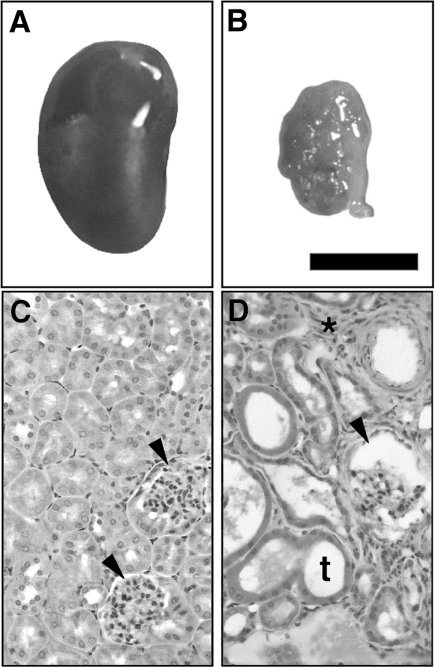
The newborn Br/+ heterozygote mutant mice have been previously described as displaying kidney hypoplasia (60). However, a detailed analysis of the microscopic morphology and physiological functioning of the adult Br/+ mutant kidney has not been previously undertaken. Comparison of the gross morphology of a kidney from a representative adult Br/+ mouse with one from its normal sibling littermate demonstrates that the Br/+ kidneys are much smaller in size with a rough external surface resulting from enlarged and distended renal tubules (Fig. 1, A and B). Histological sections confirmed that the adult Br/+ kidneys display distended tubules and renal cysts with increased interstitial tissue in contrast to normal kidney tissue (Fig. 1, C and D). The distended tubules in the mutant kidney varied in size and typically were lined by a single layer of epithelial cells surrounding an expanded fluid-filled lumen. In addition, some Br/+ mutant tubules contained casts while glomerular capsules appeared enlarged and filled with fluid, which suggested impaired renal function. Renal blood vessels also appeared distended with thicker vascular walls in the Br/+ mutants, and there was also an increase in cellularity between the tubules (Fig. 1D).
Fig. 1.
Photomicrographs of representative kidneys from sibling littermate +/+ (A, C) and Br/+ (B, D) mice. Note the larger size of the +/+ kidney compared with the smaller, cystic Br/+ kidney. Normal glomeruli (C, arrowheads) display cuboidal epithelia in +/+ animals compared with the 3H1 Br/+ kidney with enlarged glomeruli (D, arrowheads), enlarged tubules (t), and increased cellularity between nephrons (*). Bar = 3.0 mm in A and B; 200 μm in C and D.
To quantitatively compare characteristics such as average body weight, wet and dry kidney weight, and male vs. female kidney weight, we collected kidneys from a large number of 3H1 stock, 3H1 +/+, and 3H1 Br/+ mutant mice. The +/+ mice were siblings of Br/+ mice and raised in the same litters, so by comparing 3H1 stock and +/+, potential Br maternal/paternal effects could be identified. Overall, we collected 245 pairs of right and left adult kidneys for analysis: 15 from 3H1 stock males, 25 from 3H1 stock females, 34 from +/+ males, 48 from +/+ females, 66 from Br/+ male, and 57 from Br/+ females. Immediately after dissection, we weighed both the left and right kidneys (wet weight), and then fixed the right kidney for histology and oven-dried the left kidney to assess the dry weight. Since the mutant kidneys' tubules and glomeruli were distended with apparent higher proportional volumes of fluid, we measured the dry weight to compare the tissue mass. The collected data were subjected to one-way ANOVA, followed by a post hoc Tukey multiple comparison test to determine whether the differences between any two groups were statistically significant.
The results showed that adult male Br/+ mice weighed an average of 6.27 g less than their +/+ counterparts, and the adult female Br/+ mice weighed an average of 7.30 g less than +/+ females (Table 1). The wet weights of right and left kidneys from both male and female Br/+ mutant mice were significantly reduced, ranging from 43 to 52% less than normal (Table 1). There were no statistically significant differences between the 3H1 stock and 3H1 +/+ body weights or wet kidney weights. After drying, the left kidney weights also showed dramatic differences between +/+ and Br/+ mutant mice, decreased 51% in males and 53% in females. There was no significant difference between female 3H1 stock and 3H1 +/+ dry left kidney weights, but there was a difference between male 3H1 stock and 3H1 +/+ dry left kidney weights (P < 0.01). It is unclear whether this represents a real biological difference. In addition, the percentage of body weight represented by the total kidney weight was calculated and compared (Table 1). In Br/+ males, the kidneys made up 0.98% of body weight, while in +/+ males, the kidneys made up 1.49% of body weight. In Br/+ females, the kidneys made up 0.90% of body weight, while in +/+ females, the kidneys made up 1.20%. For both male and female mice, there was no significant difference between 3H1 stock and 3H1 +/+ total kidney weight of body weight percentage. Overall, these results statistically confirmed gross observations that in adult Br/+ mice, there was a greatly reduced renal mass, which was highly suggestive of defective renal physiologic function.
Table 1.
Comparison of gross renal characteristics between 3H1 +/+ and Br/+ mice
| 3H1 Stock | +/+ | Br/+ | P Value | |
|---|---|---|---|---|
| Mean BW, g | ||||
| Male | 28.11±7.13 (15) | 25.66±8.91 (34) | 19.39±5.86 (67) | <0.001 |
| Female | 26.36±6.12 (25) | 24.95±9.21 (48) | 17.65±5.81 (57) | <0.001 |
| Right kidney, g | ||||
| Male | 0.225±0.043 (15) | 0.198±0.061 (34) | 0.108±0.050 (66) | <0.001 |
| Female | 0.158±0.036 (25) | 0.148±0.042 (48) | 0.084±0.036 (57) | <0.001 |
| Left kidney, g | ||||
| Male | 0.207±0.038 (15) | 0.180±0.057 (34) | 0.087±0.042 (66) | <0.001 |
| Female | 0.147±0.029 (25) | 0.139±0.040 (48) | 0.073±0.032 (57) | <0.001 |
| Left kidney dry, g | ||||
| Male | 0.056±0.012 (15) | 0.043±0.017 (24) | 0.021±0.011 (65) | <0.001 |
| Female | 0.038±0.007 (25) | 0.034±0.011 (38) | 0.016±0.008 (57) | <0.001 |
| Total KW/BW, % | ||||
| Male | 1.563±0.157 (15) | 1.492±0.272 (34) | 0.983±0.217 (66) | <0.001 |
| Female | 1.167±0.116 (25) | 1.201±0.218 (48) | 0.898±0.229 (57) | <0.001 |
Values are means ± SD (n = mice). BW, body weight; KW, kidney weight. P values are for +/+ vs. Br/+ comparisons.
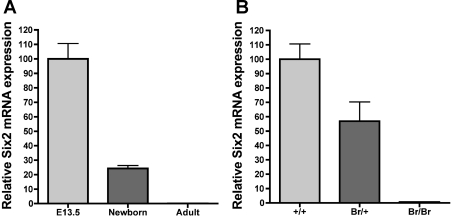
We previously showed that misexpression of the Six2 transcription factor during embryonic development is associated with Br/Br neonatal lethality and the Br/+ mutant phenotype (35). Six2 is hypothesized to inhibit the mesenchymal condensation and conversion to epithelial cells that is required for nephrogenesis, and thus maintain a mesenchymal progenitor cell population in the nephrogenic zone. Although hypothesized that Six2 is only expressed in the kidney during embryonic development, no studies have measured Six2 expression in adult mouse kidneys. Real-time qPCR performed on wild-type kidney samples showed that Six2 expression is high during embryonic development (E13.5), reduced 75.8% from E13.5 levels in newborns, and is undetectable in adult kidneys (Fig. 2A). In addition, we confirmed by qPCR that Six2 is decreased by 50% in isolated Br/+ E13.5 kidneys (Fig. 2B). Together, these data support the hypothesis that it is an embryonic Six2 deficiency that leads to the Br/+ mutant renal phenotype.
Fig. 2.
Real-time quantitative (q)PCR shows Six2 expression in the kidney is highest during embryonic (E) development, which is greatly decreased in Br mutant kidneys. Expression of Six2 in normal newborn and adult kidneys is shown relative to expression of Six2 in E day 13.5 (E13.5) kidneys after being normalized against the measured levels of actin (A). Expression of Six2 in Br/+ and Br/Br E13.5 kidneys is shown relative to expression of Six2 in +/+ E13.5 kidneys after being normalized against the measured levels of actin (B).
Kidneys of the Br/+ mice have a greatly reduced number and density of glomeruli, with an increased volume of individual glomeruli.
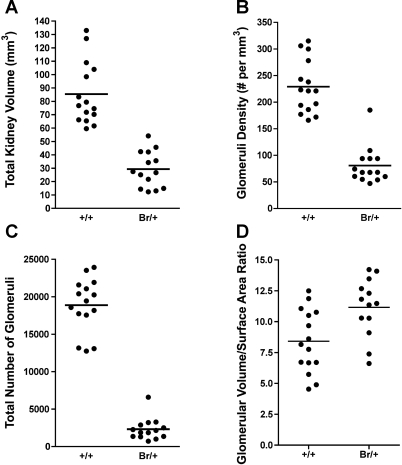
To determine whether the overall reduced renal mass in the Br/+ mice corresponded with a decreased number of nephrons, we undertook a stereological approach to accurately measure the number of glomeruli. A total of 15 kidneys from male and female adult +/+ mice and 14 kidneys for male and female Br/+ mice were fixed, stained with H&E, and subjected to this analysis. The mean total kidney volume of adult Br/+ mice was 29.31 ± 3.60 mm3, which was 64% less than the mean total kidney volume of 85.38 ± 6.06 mm3 for adult +/+ mice (P < 0.001; Fig. 3A). The mean glomeruli density in Br/+ kidneys was 80.7 ± 9.4 per mm3, which was 65% less than the mean glomeruli density of 229.0 ± 13.0 for +/+ kidneys (Fig. 3B).
Fig. 3.
Stereological studies comparing glomeruli in kidneys from adult Br/+ mice and adult +/+ mice. Data collected included total kidney volume (A), glomeruli density (B), total number of glomeruli per kidney (C), and mean glomeruli volume and mean glomeruli surface area (D). Shown are graphs for the 2 genotypes with the horizontal line representing the mean values.
The decreased total kidney volume combined with the decreased glomeruli density resulted in a very dramatically reduced number of glomeruli in Br/+ mutant kidneys. The mean total number of glomeruli for Br/+ kidneys was 2,311 ± 392, which was 88% lower than the mean total number of glomeruli of 18,880 ± 927 for +/+ kidneys (Fig. 3C). So although the gross weight of the Br/+ kidney is 43–52% of that of +/+ controls (Table 1), and the average volume of the Br/+ kidneys is 64% less than that of +/+ controls, the Br/+ kidneys only have ∼12% of the number of glomeruli measured in control +/+ kidneys.
The mean average glomeruli volume of adult Br/+ mice was 163,400 ± 17,820 μm3, which was 180% higher than the mean average glomeruli volume of 89,590 ± 4,800 μm3 for adult +/+ mice (P = 0.0002). This suggests that the reduced number of glomeruli in Br/+ kidneys has become hypertrophic or distended in an attempt to handle the increased physiologic renal burden, and it further verifies what has been observed in routine histological analysis of Br/+ kidneys. The mean average surface area of glomeruli in adult Br/+ was 14,600 ± 1,479 μm2, which was significantly higher than the mean average glomeruli surface area of 11,310 ± 788 μm2 for +/+ mice (P < 0.05). Expressing each mouse's Vm relative to its SAm confirms that there is a significant increase in the size of the mutant glomeruli (P < 0.01; Fig. 3D). In total, these stereological findings suggested that the Br/+ glomeruli are extremely overburdened, which indicated that Br/+ kidneys are unlikely to maintain an adequate glomerular filtration rate (GFR) or to concentrate urine as normal.
Br/+ mice have impaired renal function, hypertension, and low levels of metabolic indicators.
Since all morphological and stereological data suggested impaired renal physiology, we measured various aspects of cardiorenal physiological markers, as well as indicators of cholesterol and glucose metabolism. Plasma analysis revealed a small but statistically significant increase in plasma osmolality of Br/+ mutant mice compared with +/+ mice (Table 2). However, the average plasma creatinine concentration was greatly increased (233%; P < 0.01) in Br/+ mutant mice compared with normal, which indicated a steep decline in steady-state GFR. The average concentration of plasma proteins was not significantly different between Br/+ and +/+ mice (Table 2).
Table 2.
Comparison of cardiorenal physiological parameters between 3H1 +/+ and Br/+ mice
| +/+ | Br/+ | P Value | |
|---|---|---|---|
| POsm, mosmol/kgH2O | 333±3 (8) | 349±5 (9) | 0.02 |
| PCre, mg/dl | 0.18±0.02 (11) | 0.42±0.06 (11) | <0.01 |
| PPro, mg/ml | 45.2±2.0 (8) | 51.6±2.5 (9) | 0.07, ns |
| Urine volume, ml | 0.787±0.161 (12) | 1.310±0.280 (13) | <0.01 |
| UOsm, mosmol/kgH2O | 2,240±157 (13) | 693±142 (13) | <0.01 |
| UCre, mg/dl | 35.4±1.9 (13) | 9.3±1.8 (13) | <0.01 |
| UPro, mg/ml | 12.8±1.2 (8) | 12.2±1.8 (9) | 0.78, ns |
| UNa, mmol/l | 199.7±8.7 (7) | 112.9±18.0 (9) | <0.01 |
| UK, mmol/l | 271.55±19.55 (7) | 147.90±24.64 (9) | <0.01 |
| MAP, mmHg | 126±5 (12) | 156±8 (11) | <0.01 |
| HR, beats/min | 525±29 (12) | 621±34 (11) | 0.04 |
| Age, wk | 23.2±3.4 (13) | 17.8±2.4 (13) | 0.21, ns |
Values are means ± SE (n = mice). MAP, mean arterial pressure; HR, heart rate; POsm, plasma osmolality; PCre, plasma creatinine; PPro, plasma protein; UOsm, urine osmolality; UCre, urine creatinine; UPro, urine protein; UNa, urine sodium; UK, urine potassium; ns, not significant.
Urinalysis showed numerous features consistent with CRF in the Br/+ mutant mouse (Table 2). Polyuria was indicated by a significantly larger volume of urine output (over a 24-h period) for mutant mice compared with normal. In addition, the average urine osmolality of the Br/+ mutant mice was 69% lower than what was measured in +/+ mice, and the average urine creatinine concentration was 74% lower in mutant mice (Table 2). This decrease in urine osmolality and creatinine supports the elevated plasma creatinine as a strong indication of impaired creatinine clearance and depressed GFR. Accordingly, Na+ and K+ ions were present in significantly decreased concentrations in the urine of Br/+ animals compared with normal animals, but urinary protein concentrations were not significantly different between groups (Table 2).
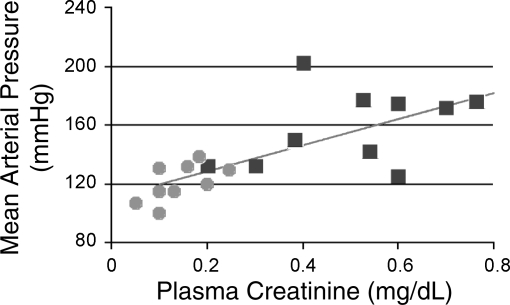
Hypertension is a common secondary effect of reduced numbers of glomeruli and CRF, both in animal models (7, 12, 13, 27, 30, 41, 79) and humans (25, 76, 78, 81, 86). The MAP of Br/+ mutant mice averaged 156 ± 8 mmHg, which was significantly higher than the average MAP of +/+ mice, 126 ± 5 mmHg (P < 0.01; Table 2). In addition, the average resting HR of Br/+ mice was 621 ± 34, which was significantly higher than the average resting HR of +/+ mice, 525 ± 29 (P < 0.05; Table 2). Furthermore, in 19 mice, the MAP and plasma creatinine concentrations were measured and showed a positive correlation (r = 0.59, P < 0.01; Fig. 4), suggesting that the impaired renal function in the Br/+ mutant mice likely contributed to the observed hypertension.
Fig. 4.
Scattergram showing a correlation between mean arterial pressure (mmHg) and plasma creatinine (mg/dl) as an indicator of reduced glomerular filtration rate (r = 0.59, P < 0.01). Circles represent values recorded for +/+ mice, and squares represent values recorded for Br/+ mice.
Physiological parameters of glucose and cholesterol metabolism were measured and compared between Br/+ and +/+ mice. The average plasma concentration of insulin was 58% lower in Br/+, and the average leptin and triglycerides concentrations were 70 and 42% lower, respectively (Table 3). The average plasma glucose level in Br/+ animals was lower than controls, but did not reach statistical significance. No statistically significant differences were observed in average plasma levels of cholesterol, HDL, or glucose (Table 3).
Table 3.
Comparison of cholesterol and glucose metabolism physiological parameters between 3H1 +/+ and Br/+ mice
| +/+ | Br/+ | P Value | |
|---|---|---|---|
| Glucose, mg/dl | 181±11 (16) | 152±11 (12) | 0.08, ns |
| Triglycerides, mg/dl | 93±4 (16) | 54±9 (12) | <0.01 |
| HDL, mg/dl | 111±6 (16) | 125±4 (12) | 0.12, ns |
| Insulin, ng/ml | 1.25±0.24 (14) | 0.53±0.07 (11) | 0.02 |
| Leptin, ng/ml | 11.05±1.38 (5) | 3.31±0.48 (7) | <0.01 |
| Cholesterol, mg/dl | 133±6 (16) | 140±6 (12) | 0.39, ns |
Values are means ± SE (n = mice).
Kidney tubules of the Br/+ mice have increased protein levels of ET-1, ETA, ETB, and Na-K-ATPase with altered subcellular localization.
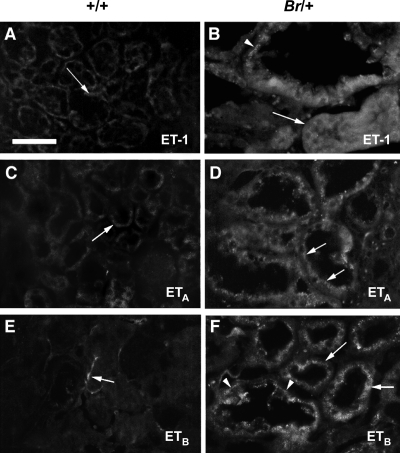
ET-1 is a multifunctional peptide that exerts potent effects on the cardiovascular and renal systems by signaling through endothelin receptors A and B (ETA and ETB) (65, 67). In the kidney, ET-1 acts in the renal vasculature to regulate renal hemodynamics and the GFR, while in nephron tubules, ET-1 regulates water and sodium reabsorption (38, 56). Immunofluorescent staining for ET-1 showed low-intensity staining in normal mouse kidney tubules, with staining confined to the basolateral surface of the renal tubules (Fig. 5A). However, enlarged Br mutant tubules showed intense staining throughout the epithelia, with staining also appearing along the apical surface (Fig. 5B). Immunostaining for ETA in the distended Br/+ mutant tubules was also much more intense than seen in normal tubules (Fig. 5, C and D). The subcellular localization of the ETA receptor also appeared altered in the Br/+ tubule epithelia, showing a greater apical localization than that seen in normal tubules. Immunostaining for the ETB receptor demonstrated a similar pattern, with faint basolateral staining in normal tubules (Fig. 5E), and highly elevated levels in the Br/+ enlarged tubules with greater apical localization (Fig. 5F). Although the mean plasma and urine concentrations of the ET-1 peptide were not significantly different between Br/+ and +/+ mice (Table 4), due to the increased average urine output of the Br/+ mice (Table 2), we can conclude that the overall urinary excretion of ET-1 is greater in the mutant mice. In addition, there was a much greater variability in urine levels of ET-1 in the Br/+ mice with a nearly fivefold higher standard deviation than in the +/+ mice.
Fig. 5.
Immunofluorescent staining of endothelin-1 (ET-1) in kidneys from +/+ (A, C, E) and Br/+ (B, D, F) adult mice. Renal tubules in +/+ mice showed low level background staining for ET-1 (A) compared with intense staining for ET-1 around enlarged mutant tubules (B, arrows). Staining for ETA (C, arrow) and ETB (E, arrow) revealed localization in the basolateral epithelia of normal tubules, but in the distended tubules of Br/+ mice, extensive staining was observed throughout the epithelium, particularly along the apical surfaces (D, F, respectively). Bar = 100 μm.
Table 4.
Plasma and urine concentration of ET-1 in 3H1 +/+ and Br/+ mice
| +/+ | Br/+ | P Value | |
|---|---|---|---|
| ET-1 plasma, pg/ml | 0.76±0.09 (7) | 0.74±0.05 (10) | 0.86,ns |
| ET-1 urine, pg/ml | 0.57±0.04 (8) | 0.89±0.15 (10) | 0.09,ns |
Values are means ± SE (n = mice).
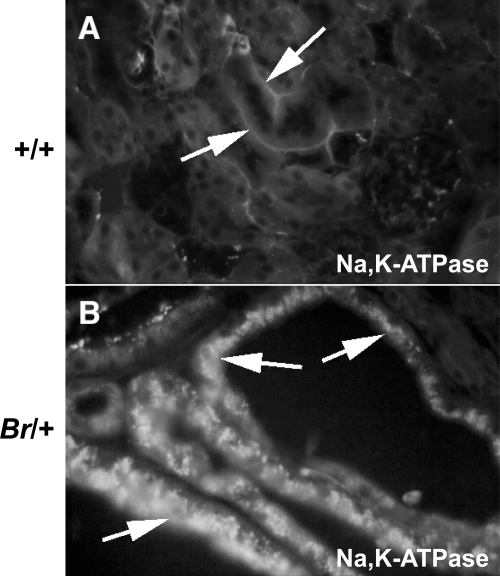
The sodium-potassium-activated adenosine triphosphatase (Na-K-ATPase) membrane transporter is critical to renal physiology by driving tubular reabsorption of sodium, and thus reabsorption of water (40, 91). In +/+ kidneys, immunostaining for Na-K-ATPase showed characteristic basolateral staining in normal renal tubules (Fig. 6A). However, in Br/+ mutant kidney tubules, dramatically increased levels of Na-K-ATPase immunostaining were detected, with much of the staining located on the apical surface of the tubule epithelial cells (Fig. 6B). It is unclear whether the Na-K-ATPase molecules observed in locations other than the basolateral surface are functional. Since the concentration of Na+ in the urine was significantly decreased in the Br/+ mice (Table 2) and yet the amount of excreted water was significantly increased, it is difficult to conclude the physiological consequences of this overexpression and disregulation of the Na-K-ATPase in the renal tubules.
Fig. 6.
Immunofluorescent staining of Na-K-ATPase in kidneys from +/+ (A) and Br/+ (B) adult mice. Na-K-ATPase localizes to the basolateral surface of the normal mouse renal tubule epithelial cells (A, arrows) but becomes highly overexpressed and apically mislocated in the distended renal tubules of Br/+ mice (B, arrows).
Ultrastructural analysis of Br/+ mutant glomeruli revealed hypercellularity, increased mesangial matrix, and thickened irregular GBMs.
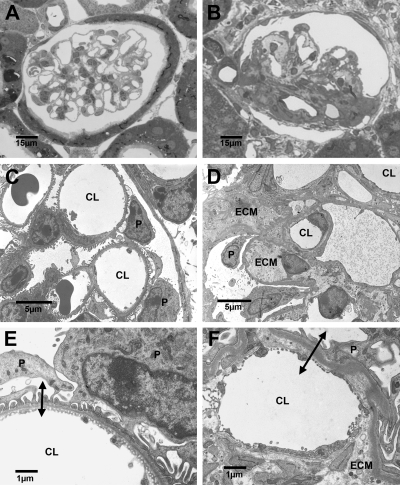
TEM analysis was carried out on renal tissues from three adult +/+ and Br/+ mice pairs primarily to provide qualitative ultrastructural data of renal glomeruli and to provide comparative views of the blood-urine barrier in both animal types. Light microscopic (LM) sections were made in an effort to identify glomerular position and relative number and to prepare the tissues for TEM thin sectioning. As seen in Fig. 7A, renal cortical tissues from control animals showed glomeruli with patent capillaries empty of blood cells and normal histoarchitectures. Contrariwise, many but not all mutants demonstrated substantial glomerulopathic alterations including hypercellularity and extracellular matrix overexpression (Fig. 7B).
Fig. 7.
Light (A, B) and transmission electron micrographs (TEM; C, D, E, F) of renal cortical tissues from age-matched adult +/+ (A, C, E) and Br/+ (B, D, E) mice. Control (+/+) mice (A) exhibit normal glomerular histoarchitectures, whereas massive increases in extracellular matrix (ECM) are seen in the mutants (B). TEM of normal (C) and mutant (D) tissues illustrate the increase in specific cellularity and ECM in the mutants. Relatively high-magnification TEM of normal (E) and mutant (F) mice show a substantial increase in the blood-urine barrier thickness (double-ended arrows) in the mutants. CL, capillary lumen; P, podoctye. A and B, ×600; C, ×3,000; D, ×2,800; E, ×9,200; F, ×9,100.
Ultrastructural studies of mutant/control renal tissues using TEM confirmed our LM observations. Figure 7C shows that glomeruli from +/+ mice appeared normal in all respects and demonstrated excellent perfusion fixation. Normal numbers and morphological features of podocytes, endothelial cells, and mesangial cells were found on the intervening GBM. At higher magnifications glomeruli from wild-type animals (Fig. 7E) exhibited classic ultrastructural features including a well-defined blood-urine barrier consisting of a highly fenestrated endothelium lining the capillary lumen, a relatively thin, moderately electron dense and regular GBM, and small podocytic foot processes connected by slit membranes.
As expected, glomeruli from Br/+ mice were more difficult to locate and characterize by TEM. As in the LM specimens, some but not all mutant glomeruli were indistinguishable from controls. Nevertheless, most showed clusters of mesangial cells with expanded areas of extracellular matrix (Fig. 7D), and many glomerular capillaries were highly occluded with nodular hypercellularity and areas of increased mesangial matrix. A dominant feature of mutant glomerulopathy was a thick irregular GBM, with substantial extracellular matrix accumulation.
At higher magnifications (Fig. 7F), the increased thickness of the blood-urine barrier in Br/+ mice was clearly evident. Capillary lumina were lined by poorly fenestrated endothelial cells subtended by a decidedly thickened and often highly irregular GBMs often associated with broad areas of extracellular matrix. Using an orthogonal intercept method, the thickness of GBMs was measured in age- and gender-matched +/+ and Br/+ mice and found to be significantly thicker in mutant kidneys (P < 0.0001; Table 5). In addition, many podocyte foot processes in the mutant mice were decidedly broadened and “club-like” (podocytic “effacement”).
Table 5.
GBM thickness in 3H1 +/+ and Br/+ mice measured from TEM
| +/+ | Br/+ | P Value | |
|---|---|---|---|
| GBM thickness, nm | 115.9±5.2 (8) | 150.4±4.3 (12) | < 0.0001 |
Values are means ± SE (n = glomeruli). For each glomerulus analyzed, 30–50 glomerular basement membrane (GBM) measurements were made and the mean was used as the data point for the t-test.
DISCUSSION
The Br mutant mice have been previously described as having semidominant hereditary kidney hypoplasia, along with frontonasal dysplasia (59, 60, 62, 68). In the present study, we characterize the kidney malformation and impaired renal functioning of the adult Br/+ heterozygote mutant mouse. Although the specific chromosomal aberration that causes the Br phenotype has not yet been identified, it appears likely to directly inhibit the transcription of the homeobox transcription factor Six2 during embryonic development (35). We mapped the Br mutation to the genomic region surrounding the Six2 gene, and Six2 mRNA and protein are nearly absent in Br/Br homozygote mutant embryos and ∼50% in the Br/+ heterozygote mutant embryos. This hypothesis is supported by the recent characterization of the Six2-null homozygote embryos, which appear very similar to Br/Br embryos, although analysis of the Six2-null heterozygotes was not performed (87). The Six2 transcription factor is expressed in the metanephric mesenchymal cells surrounding the branching uretic bud (20, 74, 75) and is believed to function as an inhibtor of nephrogenic differentiation in this cell population (35, 54, 87). When Six2 expression is lost in these cells, it appears that nephrogenesis proceeds too quickly and the mesenchymal progenitor cell population is lost, resulting in premature cessation of nephrogenesis (35, 54, 60, 87). The members of the DNA-binding Six family of proteins, including Six2, bind to the EYA family of transcriptional activators to initiate transcription of downstream targets (21, 50), although not much is known about which genes Six2 directly regulates.
Haploinsufficiency of critical transcription factors during mammalian embryonic development can often cause abnormal morphogenesis that may manifest with pathological consequences in adults. For example, haploinsufficiency of the transcription factor GATA3 during embryonic development causes human hypoparathyroidism, deafness, and renal syndrome, with hyperparathyroidism, deafness, and renal malformations (94). Embryonic gene dosage is also important for the forkhead transcription factor FOXC1 which, if missing one allele, causes defects of the anterior eye, leading to early glaucoma (71, 101). Having previously demonstrated haploinsufficiency of Six2 in the Br/+ embryos at E13.5 (35), we show here that Six2 expression in normal kidneys was greatly decreased by birth and was undetectable in adult kidneys. Therefore, the renal dysplasia and pathophysiology seen in the adult mice are likely solely due to the abnormal embryonic kidney development. We observed that the embryonic Br/Br kidney initially differentiates normally, with the ureteric bud forming at gestational day 11 and inducing condensation of the metanephric mesenchyme (60). However, we observed that at day 13, the nephrogenic zone remains poorly developed and Pax-2 expression is absent in the peripheral area of the kidney. Although some nephrons develop in the Br/Br and can be observed by gestational day 15, they appear greatly reduced in number (60). The decrease in glomerular number in the Br/+ adult kidney measured by stereology confirms that proper levels of Six2 during embryonic development are critical for the development of an adequate number of nephrons.
Gross observations of kidney hypoplasia in both male and female adult Br/+ mice, compared with their +/+ siblings, were confirmed by measuring large decreases in right and left kidneys weights, total body weights, and kidney weight as a percentage of body weight. The measured total kidney-to-body weight ratio of 3H1 and +/+ mice fit within the normal range for wild-type mice, whereas the ratios for Br/+ mice are below the reported values for any wild-type inbred strain (83). Stereological analysis of Br/+ mutant kidneys revealed that the observed renal hypoplasia is due to a significantly reduced number of nephrons, averaging only 12% of the number found in normal kidneys. The classic reduced renal mass model is the 5/6 nephrectomized rat, in where one kidney is surgically removed, along with two-thirds of the remaining kidney. This surgical reduction in the number of nephrons leads to pathological conditions including hypertension, CRF, cardiovascular disease, bone loss, and renal fibrosis and has provided a useful model for studying complications and treatments of these diseases (3, 7, 13, 14, 18, 26, 32, 41, 69, 89, 99). We hypothesize that the reduced renal mass in the Br/+ mouse, as in the nephrectomized rat, leads to an increased vascular resistance which may promote some these same pathologies. In this respect, the Br/+ mouse may serve as a useful new genetic model to study the pathological changes due to heritable reduced renal mass and reduced nephron number.
The glomerular changes in Br/+ kidneys revealed by ultrastructural analysis could represent a wide variety of hyperplastic and hypertrophic glomerulopathies. However, it was clear that the mutant glomeruli did not directly resemble the characteristic nodular glomerulosclerosis of chronic diabetic nephropathy (52). Rather they showed clusters of hypertrophic endothelial cells and mesangial cells surrounded by redundant extracellular (mesangial) matrix with evidence of hypovascularity. So, although low levels of insulin were measured in the Br/+ mice, it appears the primary kidney damage is not due to a diabetic condition. The thick irregular GBM and podocyte effacement observed in Br/+ glomeruli are typically associated with proteinuria, although the urinary concentration of protein in mutant mice was not significantly different from +/+ mice. However, given that the mutant mice showed roughly double the normal daily production of urine, the Br/+ mutant mice excreted roughly double the normal amount of protein. Further research needs to be done to determine whether the glomular filtration in these mutant mice allows increased amounts of serum proteins into the ultrafiltrate or whether an impaired reabsorption is the reason for this increase.
Histopathology confirmed gross observations of distended glomeruli and tubules with renal cysts in adult Br/+ kidneys, and stereology showed that average Br/+ glomeruli volume was nearly double compared with +/+ glomeruli. Shortly after birth, Br/+ kidneys become polycystic, presumably due to increased filtration demands (62). This cystic development appears secondary in Br/+ and is a characteristic feature of acquired cystic kidney disease and a common result of CRF (4, 5, 33, 58). The mechanism by which renal cysts form is not completely understood, but it is generally accepted that the process includes fluid accumulation and epithelial proliferation. Fluid accumulation occurs as a result of aqueous secretion throughout all segments of the nephron while mislocation of Na-K-ATPase to the apical epithelial surface causes secretion of sodium with concomitant water loss (39). It is well-documented that Na-K-ATPase polarity is affected during tubular distension in the genesis of renal cystic lesions (73, 96, 97). Immunolocalization of Na-K-ATPase in wild-type adult mice showed normal distribution along the tubular basolateral epithelium; however, distended tubules in Br/+ mutants showed intense staining throughout the epithelium. Therefore, the physiological defects in fluid reabsorption seen in Br/+ mutant mice were associated with a dramatic increase of Na-K-ATPase expression, as would be expected. Experimental evidence suggests that Na-K-ATPase activity is enhanced along nephronic segments in hypertensive rats displaying CRF (16, 34, 36). In Milan hypertensive rats, increased Na-K-ATPase activity is linked to a higher number of pump sites with a significant increase in pretranslational α and β isomer mRNA levels before the onset of hypertension (34). Thus, an increase in Na-K-ATPase activity in the Br/+ mice could lead to an increased pressor effect, contributing to the development of hypertension with progressively detrimental renal functional effects.
The Br/+ kidney tubule epithelia showed an increase in immunostaining intensity and apical distribution of ET-1, as well as ETA and ETB receptors. Br/+ mice also show an increased overall urinary excretion of ET-1, the secretion of which likely accounts for the apical distribution of ET-1 in the renal tubules. ET-1 is actively synthesized by renal tubular cells and appears to be upregulated during kidney disease (9, 11, 28, 57). It has been shown that renal ET-1 gene expression and urinary ET-1 excretion occur in a time-dependent fashion among rats with surgically reduced renal mass (10, 77), and it has also been shown that the synthesis of ET-1 and its receptors also increases in renal epithelial cells as cysts develop (45). In addition, CRF, renal cysts, and hypertension have all been observed in transgenic mice overexpressing ET-1 (44, 90, 93). ET-1 may contribute to the progression of CRF by promoting the renal inflammatory process (31) as well as the subsequent tissue repair processes (49, 55, 77, 82). As CRF progresses, production of vasoactive and inflammatory molecules correlates with increasing expression of ET-1 in renal tubular cells (22, 77). The findings in the Br/+ mice suggest that in individuals with renal hypoplasia and reduced nephron number, activated renal ET-1 may play an important role in the pathogenesis of CRF and systemic hypertension.
Rodents with CRF are characterized by an inability to concentrate urine and conserve fluid. Adult Br/+ mice showed polyuria, with almost double the daily output of urine as +/+ siblings, and the osmolality of urine in Br/+ mice was 31% of what was measured in +/+ mice. In addition, average urine concentrations of sodium and potassium were both significantly lower in Br/+ mice (43 and 46%, respectively). These urinalysis data indicate a decrease in the ability to reabsorb water in the nephron and collecting ducts. The overall diuresis in the Br/+ mice should presumably lead to loss of extracellular fluid volume, which was supported by our findings of a significant increase in plasma osmolality in Br/+ mice. Plasma creatinine values were 233% higher in Br/+ mice than in +/+, whereas urine creatinine was 74% lower, which together indicated a decreased GFR in the mutant mice. However, we hypothesize that an increased vascular resistance in Br/+ mice, due to fewer nephrons, will cause a compensatory rise in filtration rate of individual glomeruli, but since there are much less glomeruli, the overall GFR is decreased in these animals. The stereological data showed increased glomerular volume in the Br/+ kidneys, which is a morphological change likely resulting in increased filtration of individual glomeruli.
In addition to the decreased body weights, the Br/+ mutant mice were found to have significantly reduced levels plasma insulin, triglycerides, and leptin. It is possible that Br/+ mice exhibit signs of type I diabetes, which is characterized by an insulin deficiency due to the loss of pancreatic beta cells. Expression of mouse Six2 was briefly noted near the embryonic pancreas at E9.5 (75), and Northern blotting of human Six2 revealed expression in the adult pancreas (17), but the role in Six2 in pancreatic development or function has not been further investigated. It will be important to analyze the pancreas in the Br/+ mice to determine whether morphology or function is abnormal, as well as to ascertain the diabetic condition of these adult mice. The leptin and triglyceride levels may be lower in Br/+ mice due to the lower body weight, and thus less adipose tissue.
Hypertension typically develops with progression of CRF, and the Br/+ adult mice showed significantly higher MAP values compared with +/+ mice. Various hypertensive mouse models have been produced either by mutant screening or targeted gene disruptions. For example, Shesely et al. (88) described a mouse model with a disrupted endothelial nitric oxide synthase gene, which displayed blood pressures that were ∼20 mmHg higher than wild-type siblings. Also, Yang and colleagues (98) described a double human angiotensinogen transgenic mouse whose expression resulted in a MAP of ∼155 mmHg compared with controls with values of 111 mmHg. One inbred strain of mice was developed by Schlager (84, 85) and showed both hyper- and hypotensive mice with MAPs differing by 60 mmHg. Our study found an average MAP value of 126 mmHg in 3H1 +/+ mice, whereas the Br/+ mice displayed an average blood pressure value of 156 mmHg. This 30-mmHg MAP difference is consistent with other strains designed specifically to study hypertension, indicating that the Br mutation is associated with high blood pressure as well as CRF. Furthermore, correlation analysis showed that the Br/+ mice with high plasma creatinine levels were more likely to have a high MAP, suggesting that the hypertension seen in the Br/+ adult mice is associated with their deficient GFR. Future studies analyzing the cardiovascular health of the Br/+ mice will reveal whether these mice are similar to humans in developing cardiovascular disease subsequent to their CRF. It will also be important to characterize the progression of CRF and hypertension as the Br/+ mice age, as well as looking at the renin-angiotensin, erythropoietin, and vasopressin systems in these mice.
We conclude that the Br/+ mutant mouse, with its extensive disruption of nephrogenesis, is a novel reduced renal mass model which may be useful to study the morphological and functional changes associated with the progression of CRF due to a low nephron number. There are emerging data that variation in human kidney anatomy may be an important genetic risk factor for essential hypertension. In a landmark study, stereological analysis showed that the number of nephrons in the normal human kidney was highly variable, ranging from 300,000 to over 1 million (72), and subsequent studies showed even higher levels of variation in humans (46, 47). This finding provided evidence for a theory of “nephron endowment,” where the number of nephrons in an individual inversely correlates with their risk of developing essential hypertension and also CRF (19, 25, 64). Despite the difficulty in collecting such data, a recent study was able to show a high correlation between low nephron number and essential hypertension in humans by using stereological analysis to count glomeruli in kidneys at autopsy (51). Although many genes critical to kidney development have been identified, there are few which have shown to affect the number of nephrons in an adult kidney. From the data presented here, we hypothesize that haploinsufficiency of Six2 during embryonic development causes dramatically reduced nephron number and kidney hypoplasia, with the development of renal cysts. Gene dosage, and perhaps genetic polymorphisms which may influence expression or activity of Six2, should be investigated in human populations with familial kidney hypoplasia, reduced nephron numbers, or essential hypertension.
GRANTS
This work was supported by American Heart Association Hawaii Affiliate Grant 9951133Z and 1R01-DK-064752 (to S. Lozanoff), as well as by the North Dakota Lions Foundation (to E. C. Carlson).
Acknowledgments
M. Kuroyama, C. Burghardt, Dr. M. Himenes, and Dr. E. Margaryan provided assistance with renal and cardiovascular physiological experimentation while Dr. A. Theriault provided access to selected laboratory facilities necessary to complete the metabolic analysis. The authors thank D. I. Laturnus and J. L. Audette for superb help with preparation of tissues for electron microscopic observation.
REFERENCES
- 1.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002. [PubMed] [Google Scholar]
- 2.Alarcon VB, Marikawa Y. Molecular study of mouse peri-implantation development using the in vitro culture of aggregated inner cell mass. Mol Reprod Dev 67: 83–90, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest 76: 612–619, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreoli SP Acute renal failure in the newborn. Semin Perinatol 28: 112–123, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Avner ED Epithelial polarity and differentiation in polycystic kidney disease. J Cell Sci Suppl 17: 217–222, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc 142: 259–276, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin DS, Neugarten J. Hypertension and renal diseases. Am J Kidney Dis 10: 186–191, 1987. [DOI] [PubMed] [Google Scholar]
- 8.Baur PS, Stacey TR. The use of PIPES buffer in the fixation of mammalian and marine tissues for electron microscopy. J Microsc 109: 315–327, 1977. [DOI] [PubMed] [Google Scholar]
- 9.Benigni A Defining the role of endothelins in renal pathophysiology on the basis of selective and unselective endothelin receptor antagonist studies. Curr Opin Nephrol Hypertens 4: 349–353, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Benigni A, Perico N, Gaspari F, Zoja C, Bellizzi L, Gabanelli M, Remuzzi G. Increased renal endothelin production in rats with reduced renal mass. Am J Physiol Renal Fluid Electrolyte Physiol 260: F331–F339, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Benigni A, Perico N, Remuzzi G. Endothelin antagonists and renal protection. J Cardiovasc Pharmacol 35: S75–S78, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Bidani AK, Griffin KA. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hypertens 11: 73–80, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol Renal Fluid Electrolyte Physiol 265: F391–F398, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol Renal Fluid Electrolyte Physiol 252: F1003–F1010, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Bird DN, Sato AK, Knee DS, Uyehara CF, Person DA, Claybaugh JR. Effects of prenatal ethanol exposure and sex on the arginine vasopressin response to hemorrhage in the rat. Am J Physiol Regul Integr Comp Physiol 291: R77–R82, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bofill P, Goecke IA, Bonilla S, Alvo M, Marusic ET. Tissue-specific modulation of Na-K-ATPase alpha-subunit gene expression in uremic rats. Kidney Int 45: 672–678, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Boucher CA, Winchester CL, Hamilton GM, Winter AD, Johnson KJ, Bailey ME. Structure, mapping and expression of the human gene encoding the homeodomain protein, SIX2. Gene 247: 145–151, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Brenner BM Nephron adaptation to renal injury or ablation. Am J Physiol Renal Fluid Electrolyte Physiol 249: F324–F337, 1985. [DOI] [PubMed] [Google Scholar]
- 19.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Brodbeck S, Besenbeck B, Englert C. The transcription factor Six2 activates expression of the Gdnf gene as well as its own promoter. Mech Dev 121: 1211–1222, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Brodbeck S, Englert C. Genetic determination of nephrogenesis: the Pax/Eya/Six gene network. Pediatr Nephrol 19: 249–255, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Bruzzi I, Corna D, Zoja C, Orisio S, Schiffrin EL, Cavallotti D, Remuzzi G, Benigni A. Time course and localization of endothelin-1 gene expression in a model of renal disease progression. Am J Pathol 151: 1241–1247, 1997. [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson EC, Audette JL, Veitenheimer NJ, Risan JA, Laturnus DI, Epstein PN. Ultrastructural morphometry of capillary basement membrane thickness in normal and transgenic diabetic mice. Anat Rec A Discov Mol Cell Evol Biol 271: 332–341, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Carlson EC, Hinds D. Native banded collagen fibrils in the glomerular mesangial matrix of normal human and laboratory animals. J Ultrastruct Res 77: 241–247, 1981. [DOI] [PubMed] [Google Scholar]
- 25.Clark AT, Bertram JF. Molecular regulation of nephron endowment. Am J Physiol Renal Physiol 276: F485–F497, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Coimbra TM, Carvalho J, Fattori A, Da Silva CG, Lachat JJ. Transforming growth factor-beta production during the development of renal fibrosis in rats with subtotal renal ablation. Int J Exp Pathol 77: 167–173, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cullen-McEwen LA, Kett MM, Dowling J, Anderson WP, Bertram JF. Nephron number, renal function, and arterial pressure in aged GDNF heterozygous mice. Hypertension 41: 335–340, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Dhaun N, Goddard J, Webb DJ. The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol 17: 943–955, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Dische FE Measurement of glomerular basement membrane thickness and its application to the diagnosis of thin-membrane nephropathy. Arch Pathol Lab Med 116: 43–49, 1992. [PubMed] [Google Scholar]
- 30.Dominiczak AF, Clark JS, Jeffs B, Anderson NH, Negrin CD, Lee WK, Brosnan MJ. Genetics of experimental hypertension. J Hypertens 16: 1859–1869, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Espinosa G, Lopez Farre A, Cernadas MR, Manzarbeitia F, Tan D, Digiuni E, Mosquera JR, Monton M, Millas I, Hernando L, Casado S, Caramelo C. Role of endothelin in the pathophysiology of renal ischemia-reperfusion in normal rabbits. Kidney Int 50: 776–782, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Faraj AH, Morley AR. Remnant kidney pathology after five-sixth nephrectomy in rat. I. A biochemical and morphological study. APMIS 100: 1097–1105, 1992. [DOI] [PubMed] [Google Scholar]
- 33.Feiner HD, Katz LA, Gallo GR. Acquired cystic disease of kidney in chronic dialysis patients. Urology 17: 260–264, 1981. [DOI] [PubMed] [Google Scholar]
- 34.Ferrandi M, Tripodi G, Salardi S, Florio M, Modica R, Barassi P, Parenti P, Shainskaya A, Karlish S, Bianchi G, Ferrari P. Renal Na,K-ATPase in genetic hypertension. Hypertension 28: 1018–1025, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Fogelgren B, Kuroyama MC, McBratney-Owen B, Spence AA, Malahn LE, Anawati MK, Cabatbat C, Alarcon VB, Marikawa Y, Lozanoff S. Misexpression of Six2 is associated with heritable frontonasal dysplasia and renal hypoplasia in 3H1 Br mice. Dev Dyn 237: 1767–1779, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg LC, Narang N, McArdle S. Na-K-ATPase in nephron segments of rats developing spontaneous hypertension. Am J Physiol Renal Fluid Electrolyte Physiol 249: F863–F869, 1985. [DOI] [PubMed] [Google Scholar]
- 37.Glassberg KI, Stephens FD, Lebowitz RL, Braren V, Duckett JW, Jacobs EC, King LR, Perlmutter AD. Renal dysgenesis and cystic disease of the kidney: a report of the Committee on Terminology, Nomenclature and Classification, Section on Urology, American Academy of Pediatrics. J Urol 138: 1085–1092, 1987. [DOI] [PubMed] [Google Scholar]
- 38.Granger JP, Abram S, Stec D, Chandler D, LaMarca B. Endothelin, the kidney, and hypertension. Curr Hypertens Rep 8: 298–303, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Grantham JJ Polycystic kidney disease. I. Etiology and pathogenesis. Hosp Pract (Off Ed) 27: 51–59, 1992. [DOI] [PubMed] [Google Scholar]
- 40.Greger R Physiology of renal sodium transport. Am J Med Sci 319: 51–62, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Gretz NWR, Strauch M. The remnant kidney model. In: Experimental and Genetic Rat Models of Chronic Renal Failure, edited by Gretz NSM. Basel: Karger, 1993, p. 1–28.
- 42.Gundersen HJ Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R Thompson. J Microsc 143: 3–45, 1986. [PubMed] [Google Scholar]
- 43.Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology–reconsidered. J Microsc 193: 199–211, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Hocher B, Thone-Reineke C, Rohmeiss P, Schmager F, Slowinski T, Burst V, Siegmund F, Quertermous T, Bauer C, Neumayer HH, Schleuning WD, Theuring F. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J Clin Invest 99: 1380–1389, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hocher B, Zart R, Schwarz A, Vogt V, Braun C, Thone-Reineke C, Braun N, Neumayer HH, Koppenhagen K, Bauer C, Rohmeiss P. Renal endothelin system in polycystic kidney disease. J Am Soc Nephrol 9: 1169–1177, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl 83: S31–S37, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Hughson M, Farris AB 3rd, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 63: 2113–2122, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Jensen EB, Gundersen HJ, Osterby R. Determination of membrane thickness distribution from orthogonal intercepts. J Microsc 115: 19–33, 1979. [DOI] [PubMed] [Google Scholar]
- 49.Kaddoura S, Curzen NP, Evans TW, Firth JD, Poole-Wilson PA. Tissue expression of endothelin-1 mRNA in endotoxaemia. Biochem Biophys Res Commun 218: 641–647, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes–structure and function as transcription factors and their roles in development. Bioessays 22: 616–626, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Kimmelstein P, Wilson C. Intercapillary lesions in the glomeruli of the kidney. Am J Clin Pathol 12: 83–97, 1936. [PMC free article] [PubMed] [Google Scholar]
- 53.Knee DS, Sato AK, Uyehara CF, Claybaugh JR. Prenatal exposure to ethanol causes partial diabetes insipidus in adult rats. Am J Physiol Regul Integr Comp Physiol 287: R277–R283, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohan DE Endothelins in the kidney: physiology and pathophysiology. Am J Kidney Dis 22: 493–510, 1993. [DOI] [PubMed] [Google Scholar]
- 56.Kohan DE The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr Opin Nephrol Hypertens 15: 34–40, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Lariviere R, Lebel M. Endothelin-1 in chronic renal failure and hypertension. Can J Physiol Pharmacol 81: 607–621, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Levine E Acquired cystic kidney disease. Radiol Clin North Am 34: 947–964, 1996. [PubMed] [Google Scholar]
- 59.Lozanoff S Midfacial retrusion in adult brachyrrhine mice. Acta Anat (Basel) 147: 125–132, 1993. [DOI] [PubMed] [Google Scholar]
- 60.Lozanoff S, Johnston J, Ma W, Jourdan-Le Saux C. Immunohistochemical localization of Pax2 and associated proteins in the developing kidney of mice with renal hypoplasia. J Histochem Cytochem 49: 1081–1097, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Lozanoff S, Jureczek S, Feng T, Padwal R. Anterior cranial base morphology in mice with midfacial retrusion. Cleft Palate Craniofac J 31: 417–428, 1994. [DOI] [PubMed] [Google Scholar]
- 62.Ma W, Lozanoff S. External craniofacial features, body size, and renal morphology in prenatal brachyrrhine mice. Teratology 47: 321–332, 1993. [DOI] [PubMed] [Google Scholar]
- 63.Ma W, Lozanoff S. Morphological deficiency in the prenatal anterior cranial base of midfacially retrognathic mice. J Anat 188: 547–555, 1996. [PMC free article] [PubMed] [Google Scholar]
- 64.Mackenzie HS, Brenner BM. Fewer nephrons at birth: a missing link in the etiology of essential hypertension? Am J Kidney Dis 26: 91–98, 1995. [DOI] [PubMed] [Google Scholar]
- 65.Marasciulo FL, Montagnani M, Potenza MA. Endothelin-1: the yin and yang on vascular function. Curr Med Chem 13: 1655–1665, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Marikawa Y, Fujita TC, Alarcon VB. An enhancer-trap LacZ transgene reveals a distinct expression pattern of Kinesin family 26B in mouse embryos. Dev Genes Evol 214: 64–71, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Masaki T The endothelin family: an overview. J Cardiovasc Pharmacol 35: S3–S5, 2000. [DOI] [PubMed] [Google Scholar]
- 68.McBratney BM, Margaryan E, Ma W, Urban Z, Lozanoff S. Frontonasal dysplasia in 3H1 Br/Br mice. Anat Rec A Discov Mol Cell Evol Biol 271: 291–302, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Moscovici A, Bernheim J, Popovtzer MM, Rubinger D. Renal osteodystrophy in rats with reduced renal mass. Nephrol Dial Transplant 11, Suppl 3: 146–152, 1996. [DOI] [PubMed] [Google Scholar]
- 70.Mouton P Principles and Practices of Unbiased Stereology: An Introduction For Bioscientists. Baltimore, MD: Johns Hopkins University Press, 2002.
- 71.Nishimura DY, Searby CC, Alward WL, Walton D, Craig JE, Mackey DA, Kawase K, Kanis AB, Patil SR, Stone EM, Sheffield VC. A spectrum of FOXC1 mutations suggests gene dosage as a mechanism for developmental defects of the anterior chamber of the eye. Am J Hum Genet 68: 364–372, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992. [DOI] [PubMed] [Google Scholar]
- 73.Ogborn MR, Sareen S, Tomobe K, Takahashi H, Crocker JF. Renal tubule Na,K-ATPase polarity in different animal models of polycystic kidney disease. J Histochem Cytochem 43: 785–790, 1995. [DOI] [PubMed] [Google Scholar]
- 74.Ohto H, Takizawa T, Saito T, Kobayashi M, Ikeda K, Kawakami K. Tissue and developmental distribution of Six family gene products. Int J Dev Biol 42: 141–148, 1998. [PubMed] [Google Scholar]
- 75.Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BN, Hartenstein V, Zipursky SL, Gruss P. Homeobox genes and connective tissue patterning. Development 121: 693–705, 1995. [DOI] [PubMed] [Google Scholar]
- 76.Olson JL Hypertension: essential and secondary forms. In: Heptinstall's Pathology of the Kidney (5th ed.), edited by Jennette JC, Olson JL, Schwartz MM, and Silva FG. Philadelphia: Lippincott-Raven, 1998, p. 943–1002.
- 77.Orisio S, Benigni A, Bruzzi I, Corna D, Perico N, Zoja C, Benatti L, Remuzzi G. Renal endothelin gene expression is increased in remnant kidney and correlates with disease progression. Kidney Int 43: 354–358, 1993. [DOI] [PubMed] [Google Scholar]
- 78.Perazella MA, Khan S. Increased mortality in chronic kidney disease: a call to action. Am J Med Sci 331: 150–153, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Poladia DP, Kish K, Kutay B, Bauer J, Baum M, Bates CM. Link between reduced nephron number and hypertension: studies in a mutant mouse model. Pediatr Res 59: 489–493, 2006. [DOI] [PubMed] [Google Scholar]
- 80.Pope JCT, Brock JW 3rd, Adams MC, Stephens FD, Ichikawa I. How they begin and how they end: classic and new theories for the development and deterioration of congenital anomalies of the kidney and urinary tract, CAKUT. J Am Soc Nephrol 10: 2018–2028, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Schiffrin EL, Lariviere R, Li JS, Sventek P, Touyz RM. Deoxycorticosterone acetate plus salt induces overexpression of vascular endothelin-1 and severe vascular hypertrophy in spontaneously hypertensive rats. Hypertension 25: 769–773, 1995. [DOI] [PubMed] [Google Scholar]
- 83.Schlager G Kidney weight in mice: strain differences and genetic determination. J Hered 59: 171–174, 1968. [DOI] [PubMed] [Google Scholar]
- 84.Schlager G Selection for blood pressure levels in mice. Genetics 76: 537–549, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schlager G, Sides J. Characterization of hypertensive and hypotensive inbred strains of mice. Lab Anim Sci 47: 288–292, 1997. [PubMed] [Google Scholar]
- 86.Schrier RW Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol 47: 1–8, 2006. [DOI] [PubMed] [Google Scholar]
- 87.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25: 5214–5228, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA 93: 13176–13181, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shimamura T, Morrison AB. A progressive glomerulosclerosis occurring in partial five-sixths nephrectomized rats. Am J Pathol 79: 95–106, 1975. [PMC free article] [PubMed] [Google Scholar]
- 90.Shindo T, Kurihara H, Maemura K, Kurihara Y, Ueda O, Suzuki H, Kuwaki T, Ju KH, Wang Y, Ebihara A, Nishimatsu H, Moriyama N, Fukuda M, Akimoto Y, Hirano H, Morita H, Kumada M, Yazaki Y, Nagai R, Kimura K. Renal damage and salt-dependent hypertension in aged transgenic mice overexpressing endothelin-1. J Mol Med 80: 105–116, 2002. [DOI] [PubMed] [Google Scholar]
- 91.Skou JC, Esmann M. The Na,K-ATPase. J Bioenerg Biomembr 24: 249–261, 1992. [DOI] [PubMed] [Google Scholar]
- 92.Terzi F, Burtin M, Friedlander G. Using transgenic mice to analyze the mechanisms of progression of chronic renal failure. J Am Soc Nephrol 11, Suppl 16: S144–S148, 2000. [PubMed] [Google Scholar]
- 93.Theuring F, Thone-Reinecke C, Vogler H, Schmager F, Rohmeiss P, Slowinski T, Neumayer HH, Hocher B. Pathophysiology in endothelin-1 transgenic mice. J Cardiovasc Pharmacol 31, Suppl 1: S489–S491, 1998. [DOI] [PubMed] [Google Scholar]
- 94.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K. GATA3 haploinsufficiency causes human HDR syndrome. Nature 406: 419–422, 2000. [DOI] [PubMed] [Google Scholar]
- 95.Venable JH, Coggeshall R. A simplified lead citrate stain for use in electron microscopy. J Cell Biol 25: 407–408, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson PD Epithelial cell polarity and disease. Am J Physiol Renal Physiol 272: F434–F442, 1997. [DOI] [PubMed] [Google Scholar]
- 97.Wilson PD, Sherwood AC, Palla K, Du J, Watson R, Norman JT. Reversed polarity of Na+-K+-ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am J Physiol Renal Fluid Electrolyte Physiol 260: F420–F430, 1991. [DOI] [PubMed] [Google Scholar]
- 98.Yang G, Merrill DC, Thompson MW, Robillard JE, Sigmund CD. Functional expression of the human angiotensinogen gene in transgenic mice. J Biol Chem 269: 32497–32502, 1994. [PubMed] [Google Scholar]
- 99.Yoshioka T, Shiraga H, Yoshida Y, Fogo A, Glick AD, Deen WM, Hoyer JR, Ichikawa I. “Intact nephrons” as the primary origin of proteinuria in chronic renal disease. Study in the rat model of subtotal nephrectomy. J Clin Invest 82: 1614–1623, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zalups RK The Os/+ mouse: a genetic animal model of reduced renal mass. Am J Physiol Renal Fluid Electrolyte Physiol 264: F53–F60, 1993. [DOI] [PubMed] [Google Scholar]
- 101.Zhang HZ, Li P, Wang D, Huff S, Nimmakayalu M, Qumsiyeh M, Pober BR. FOXC1 gene deletion is associated with eye anomalies in ring chromosome 6. Am J Med Genet 124: 280–287, 2004. [DOI] [PubMed] [Google Scholar]