Abstract
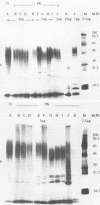
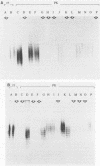
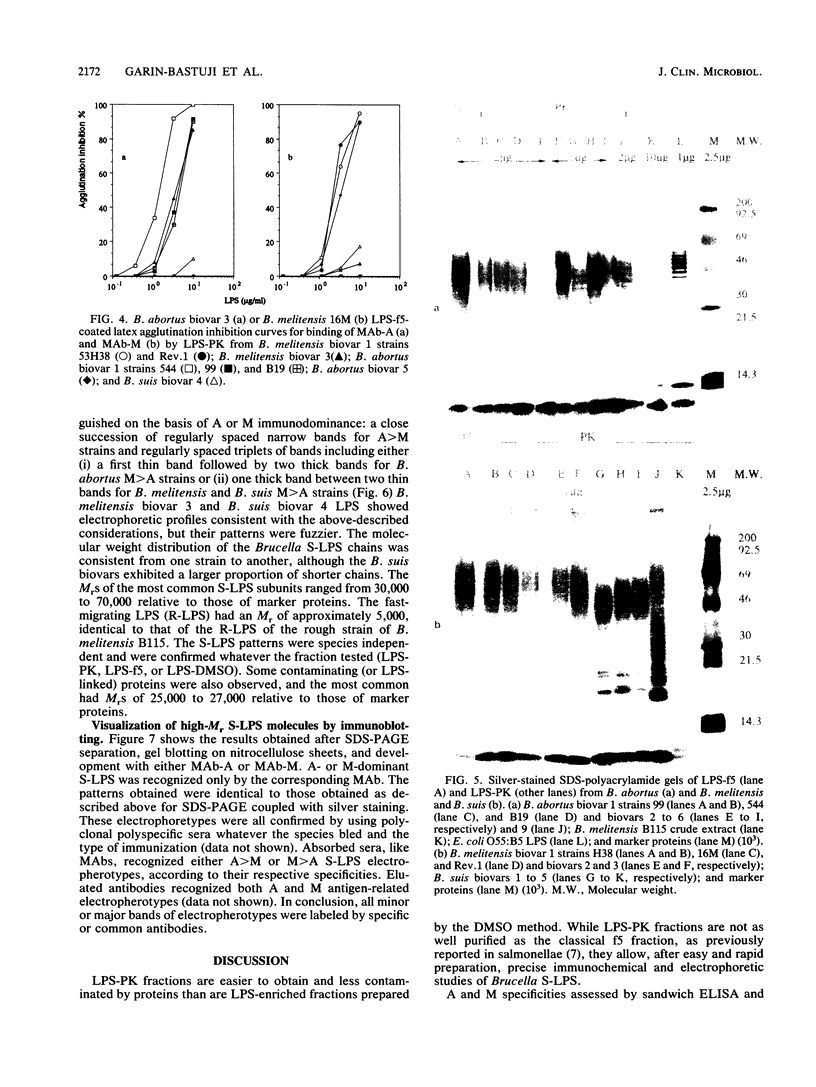
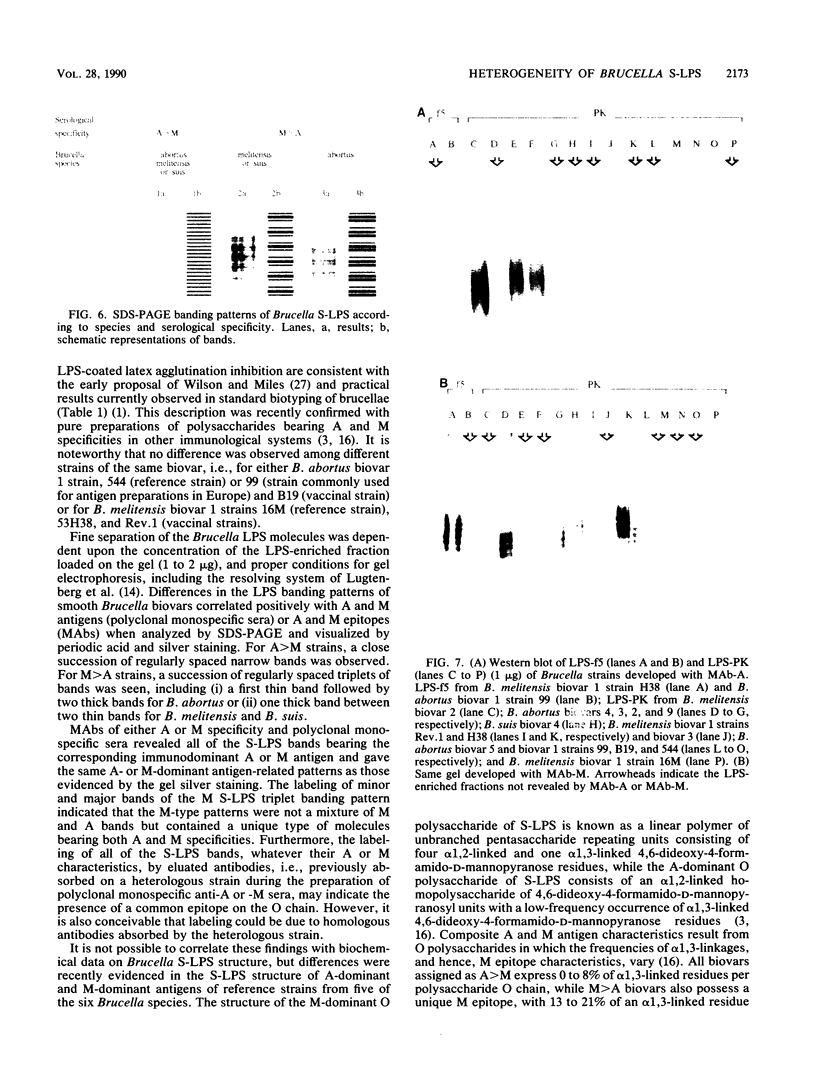
Smooth (S)-lipopolysaccharide (LPS) preparations from reference and field strains of several biovars of Brucella abortus, B. melitensis, and B. suis were prepared by (i) the hot phenol-water method, (ii) hot sodium dodecyl sulfate extraction and proteinase K digestion, or (iii) dimethyl sulfoxide extraction. These S-LPS-enriched fractions were further analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining after periodate oxidation. Immunoblots were developed by using either monoclonal antibodies specific for Brucella A or M antigens or polyclonal polyspecific or monospecific sera from rabbits, cattle, and goats. The specificity of monoclonal antibodies reactive with Brucella unique (A or M) epitopes was demonstrated by enzyme-linked immunosorbent assay, LPS latex agglutination, or agglutination inhibition. The most-represented subunits of S-LPS ranged in Mr from 30,000 to 70,000 relative to marker proteins. According to A or M immunodominance, two sodium dodecyl sulfate-polyacrylamide gel electrophoresis banding patterns were clearly distinguished among biovars, whatever the fraction tested: a close succession of regularly spaced narrow bands for A greater than M strains and regularly spaced triplets of bands including either (i) a first thin band followed by two thick bands for B. abortus M greater than A strains or (ii) one thick band between two thin bands for B. melitensis or B. suis M greater than A strains. Moreover, A and M specificities were reaffirmed by sandwich enzyme immunoassay and latex agglutination inhibition with monoclonal antibodies and polyclonal sera.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bundle D. R., Cherwonogrodzky J. W., Caroff M., Perry M. B. The lipopolysaccharides of Brucella abortus and B. melitensis. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):92–98. doi: 10.1016/0769-2609(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Bundle D. R., Cherwonogrodzky J. W., Gidney M. A., Meikle P. J., Perry M. B., Peters T. Definition of Brucella A and M epitopes by monoclonal typing reagents and synthetic oligosaccharides. Infect Immun. 1989 Sep;57(9):2829–2836. doi: 10.1128/iai.57.9.2829-2836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Limet J. Evidence of heterogeneity of lipopolysaccharides among Brucella biovars in relation to A and M specificities. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):27–37. doi: 10.1016/0769-2609(87)90051-2. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. M., Houle J. J. Purification of nonlipopolysaccharide antigen from Brucella abortus during preparation of antigen used for indirect hemolysis test. J Clin Microbiol. 1986 Nov;24(5):779–784. doi: 10.1128/jcm.24.5.779-784.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong D., Diaz R., Milner K., Rudbach J., Wilson J. B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970 Feb;1(2):174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limet J. N., Berbinschi A., Cloeckaert A., Cambiaso C. L., Masson P. L. Longitudinal study of brucellosis in mice by immunoassay of lipopolysaccharide-related antigens in blood and urine. J Med Microbiol. 1988 May;26(1):37–45. doi: 10.1099/00222615-26-1-37. [DOI] [PubMed] [Google Scholar]
- Limet J. N., Bosseray N., Garin-Bastuji B., Dubray G., Plommet M. Humoral immunity in mice mediated by monoclonal antibodies against the A and M antigens of Brucella. J Med Microbiol. 1989 Sep;30(1):37–43. doi: 10.1099/00222615-30-1-37. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Meikle P. J., Perry M. B., Cherwonogrodzky J. W., Bundle D. R. Fine structure of A and M antigens from Brucella biovars. Infect Immun. 1989 Sep;57(9):2820–2828. doi: 10.1128/iai.57.9.2820-2828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Borowiak D., Mayer H. Brucella lipopolysaccharides and polysaccharides. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):102–105. doi: 10.1016/0769-2609(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Moreno E., Pitt M. W., Jones L. M., Schurig G. G., Berman D. T. Purification and characterization of smooth and rough lipopolysaccharides from Brucella abortus. J Bacteriol. 1979 May;138(2):361–369. doi: 10.1128/jb.138.2.361-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. A., Douglas J. T. Analysis of Brucella lipopolysaccharide with specific and cross-reacting monoclonal antibodies. J Clin Microbiol. 1989 Oct;27(10):2331–2337. doi: 10.1128/jcm.27.10.2331-2337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M., Pugh G. W., Jr, Deyoe B. L. Chemical and protective properties of Brucella lipopolysaccharide obtained by butanol extraction. Am J Vet Res. 1989 Mar;50(3):311–317. [PubMed] [Google Scholar]
- Plommet M. Brucellosis and immunity: humoral and cellular components in mice. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):105–110. doi: 10.1016/0769-2609(87)90086-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Verger J. M., Grimont F., Grimont P. A., Grayon M. Taxonomy of the genus Brucella. Ann Inst Pasteur Microbiol. 1987 Mar-Apr;138(2):235–238. doi: 10.1016/0769-2609(87)90199-2. [DOI] [PubMed] [Google Scholar]