Abstract
Sodium homeostasis in terrestrial and freshwater vertebrates is controlled by the corticosteroid hormones, principally aldosterone, which stimulate electrogenic Na+ absorption in tight epithelia. Although aldosterone is known to increase apical membrane Na+ permeability in target cells through changes in gene transcription, the mechanistic basis of this effect remains poorly understood. The predominant early effect of aldosterone is to increase the activity of the epithelial sodium channel (ENaC), although ENaC mRNA and protein levels do not change initially. Rather, the open probability and/or number of channels in the apical membrane are greatly increased by unknown modulators. To identify hormone-stimulated gene products that modulate ENaC activity, a subtracted cDNA library was generated from A6 cells, a stable cell line of renal distal nephron origin, and the effect of candidates on ENaC activity was tested in a coexpression assay. We report here the identification of sgk (serum and glucocorticoid-regulated kinase), a member of the serine–threonine kinase family, as an aldosterone-induced regulator of ENaC activity. sgk mRNA and protein were strongly and rapidly hormone stimulated both in A6 cells and in rat kidney. Furthermore, sgk stimulated ENaC activity approximately 7-fold when they were coexpressed in Xenopus laevis oocytes. These data suggest that sgk plays a central role in aldosterone regulation of Na+ absorption and thus in the control of extracellular fluid volume, blood pressure, and sodium homeostasis.
Unlike marine animals, terrestrial and freshwater vertebrates must separately control salt and water excretion to maintain extracellular fluid volume, blood pressure, and ion concentrations (1). Adaptation to wide variations in dietary Na+ is controlled largely by the adrenal corticosteroid hormone aldosterone, which acts in tight epithelia such as the colon, parotid gland, and renal collecting duct (CD) to stimulate Na+ absorption (2). The principal early action of aldosterone (starting after a latent period of 45 min) is to increase apical membrane Na+ transport through the epithelial sodium channel (ENaC; for review, see ref. 3). Like all members of the nuclear receptor superfamily, the steroid receptors that mediate this effect modulate the rate of transcription of specific target genes. Interestingly, in spite of its genomic site of regulation, aldosterone appears to regulate primarily ENaC activity, not abundance (3–6). In renal CD and colon, for example, the bulk of increase in Na+ transport has already occurred before ENaC α-, β-, and γ-subunit mRNA levels begin to increase (7–10). Furthermore, in kidney, the late increase in ENaC subunit mRNAs is modest, although in colon, ultimately, there is a substantial increase in β- and γ-subunit mRNAs (9). Thus, although it is still uncertain whether aldosterone primarily increases ENaC open probability, number, or both (11), it is clear that the early transcriptional effects of aldosterone are not on ENaC gene transcription.
The above observations suggest that aldosterone rapidly induces the transcription of gene(s) encoding regulatory protein(s) that increase ENaC activity. We, therefore, wanted to identify aldosterone-induced genes whose products might stimulate ENaC activity. Toward this end, we used a PCR-based technique to generate a cDNA library representing rapidly induced genes in A6 cells, a CD-like cell line derived from Xenopus laevis kidney (12). We then characterized the effect of selected clones on ENaC activity in a coexpression assay. One of these, sgk (serum and glucocorticoid-regulated kinase), a member of the serine-threonine kinase family, was rapidly induced by dexamethasone in A6 cells and by aldosterone in rat distal nephron. Furthermore, sgk strongly stimulated ENaC activity in a coexpression assay. These observations suggest that sgk is an important mediator of the early aldosterone effect on sodium transport in distal nephron and possibly in other tight epithelia.
MATERIALS AND METHODS
A6 Cell Culture and Electrical Measurements.
A6 cells, originally obtained from the American Type Culture Collection, were maintained at 28°C in a humidified incubator with 1% CO2 in air in culture medium containing 5% fetal bovine serum, as described (13). For electrical measurements and RNA extraction, cells were cultured on transwell permeable filters (Costar). After epithelia had developed a stable electrical resistance, charcoal-stripped serum (13) was substituted for standard serum for 3 days, and then 10−7 M dexamethasone or vehicle was added. Potential difference and electrical resistance were measured with a Millicell-ERS (Millipore), and the ratio of the potential difference to the electrical resistance provided an indirect measurement of electrogenic Na+ transport (13). Dexamethasone was used to stimulate Na+ transport because it potently activates glucocorticoid receptor (GR; ref. 13): A6 cells contain markedly higher levels of GR than mineralocorticoid receptor (MR), and these two closely related receptors mediate indistinguishable effects on Na+ transport in CD cells (13, 14).
Construction of Up-Regulated cDNA Pools with Suppression-Subtractive Hybridization.
A6 cells were grown on filters as described above and incubated for 1 h with 10−7 M dexamethasone or vehicle (ethanol) before RNA extraction. RNA was extracted from both hormone-treated and untreated cells; double-stranded cDNA was synthesized and digested with RsaI. To identify up-regulated cDNAs, linkers were added to the hormone-treated group, and two rounds of hybridization followed by extension to fill in ends were performed in the presence of an excess of cDNA without linkers from the untreated cells, according to the manufacturer’s protocol (CLONTECH) (15). The excess cDNA without linkers forms both homoduplexes and heteroduplexes with the cDNA with linkers. Thus, the formation of homoduplexes in which both strands have linkers, and hence subsequent exponential PCR amplification, is suppressed. Under these conditions, cDNAs derived from induced mRNAs have an increased chance of forming homoduplexes, in which both strands have linkers, and thus escaping suppression. After these hybridization and extension steps, two rounds of PCR were performed with primers directed at the linker sequences. Reaction conditions in which linkers are added to the cDNAs derived from the hormone-treated cells are referred to as “forward” subtraction, and conditions in which linkers are added to the cDNAs from the the untreated cells (basal) are referred to as “reverse” subtraction. The final PCR products were cloned into pCR2.1. To screen for false positives, library cDNAs were amplified by PCR (with primers directed at internal linker sequences), and equal volumes of each were spotted on two membranes. 32P-labeled probes were prepared from forward and reverse subtracted DNAs. Dots were quantitated with a PhosphorImager (Molecular Dynamics), and clones showing signal ratios of more than 5:1 (forward subtracted vs. reverse subtracted probe) were further analyzed by Northern blot analysis and DNA sequencing.
Cloning of Full-Length Xenopus sgk.
Full-length Xenopus sgk (xsgk) was cloned by rapid amplification of cDNA ends with the Marathon DNA amplification kit (CLONTECH). Poly(A) RNA isolated from 1-h dexamethasone-treated A6 cells was used for reverse transcription followed by 5′ and 3′ rapid amplification of cDNA ends reactions, cloning into pCR2.1 vector (Invitrogen) and sequencing (University of California, San Francisco, Biomolecular Resource).
Northern Blot Analysis.
A6 cells were grown and treated with 10−7 M dexamethasone, and RNA was extracted as described above, at the times shown in Fig. 1. Formaldehyde gels were loaded with 12 μg per lane of RNA, transferred, and hybridized with 32P-labeled randomly primed A83 or Xenopus type 8 actin probes by using standard procedures. Where indicated, cycloheximide was added at a final concentration of 20 μg/ml 2 h before hormone addition (Fig. 1C). In experiments not shown, Northern blots were performed with poly(A)-selected RNA with indistinguishable results.
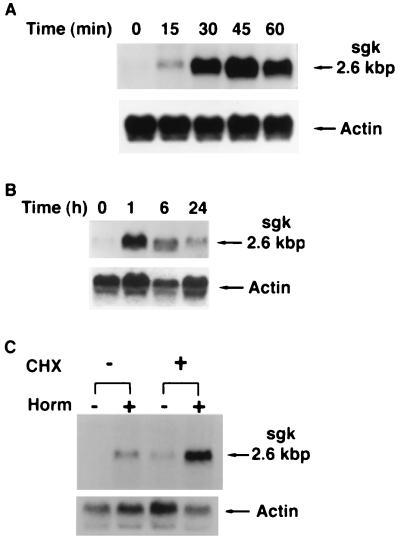
Figure 1.
sgk mRNA is rapidly and directly stimulated by dexamethasone in A6 cells. A6 cells were grown on permeable supports, and parallel cultures were treated with vehicle or 10−7 M dexamethasone for the times shown. Blots were hybridized with a probe for Xenopus sgk, stripped, and rehybridized with a probe for Xenopus type 8 actin. (A and B) Northern blots representing 1- and 24-h time courses of hormone treatment, respectively. In four separate experiments for each time course, fold activation (quantitated by PhosphorImager) varied by <20% at each time. sgk message constituted a single major band on Northern blot with a size of 2.6 kbp. (C) Hormone stimulation of sgk does not require new protein synthesis. A6 cells were incubated with cycloheximide (CHX) for 2 h and then cells were treated with vehicle or 10−7 M dexamethasone for 2 h additional, as shown. Cycloheximide blocked hormone induction of PD/R by 90% (not shown), as described (22). The blot is representative of three independent experiments with similar results.
Western Blot Analysis.
A6 cells were grown and treated as described above for Northern blots. After the times shown in Fig. 2, cells were incubated with 60 μl of lysis buffer per filter containing 50 mM Hepes (pH 7.4), 250 mM NaCl, 1.5% Nonidet P-40, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and 0.5 mM DTT supplemented with 1× protease inhibitors (Boehringer Mannheim) on a rocking platform at 4°C for 30 min, after which filters were cut out and cells were scraped and collected into an Eppendorf tube. After the sample was centrifuged at 4°C for 15 min at 10,000 rpm, the pellet was discarded and 105 μg of protein from each lysate were separated on a 10% SDS/PAGE gel and transferred electrophoretically to nitrocellulose membranes (Micron Separations, Westboro, MA). After the blots were blocked overnight with 5% dry milk in PBS/0.1% Tween 20, they were washed with PBS/0.1% Tween 20 and incubated with rabbit polyclonal antibody raised against rat sgk (1:1,000 dilution) in PBS/0.1% Tween 20 for 1 h at room temperature. Generation of anti-rat sgk polyclonal antibody was described (16). After the blots were washed with PBS/0.1% Tween 20, they were incubated with rabbit Ig and horseradish peroxidase-linked whole antibody (Amersham Life Sciences, Piscataway, NJ) at 1:5,000 dilution in 5% dry milk for 1 h at room temperature. After a final wash with PBS/0.1% Tween 20, bound antibody was detected by autoradiography of chemiluminescent signals with Hyperfilm (Amersham Life Sciences). In experiments not shown, in vitro expressed Xenopus sgk was shown to crossreact with anti-rat sgk antibody, giving a signal comparable to equal amounts of rat sgk.
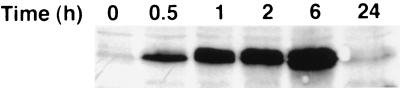
Figure 2.
sgk immunoreactive protein is rapidly stimulated by dexamethasone in A6 cells. A6 cells were grown on permeable supports and treated with 10−7 M dexamethasone for the times shown. Immunoblots were prepared and probed as described in Materials and Methods. The blot shown is representative of four different blots from two independent experiments performed on different days. A single major band of approximately 56 kDa was observed.
In Situ Hybridization.
Aldosterone (50 μg/100 g) or vehicle (dimethyl sulfoxide) were administered subcutaneously to adrenalectomized (adx)rats, and 4 h later they were killed and their kidneys were excised and frozen in OCT (Tissue Tek) in a dry ice-ethanol bath. Frozen tissue sections (15 μm) were cut on a freezing microtome, thaw mounted on Superfrost slides, fixed for 5 min in 4% paraformaldehyde, rinsed for 2 min in 0.1 M PBS, acetylated in 0.1 M triethanolamine (pH 8.0) containing 0.25% acetic anhydride, rinsed in 2× standard saline citrate (SSC; 1× = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7), taken through an ethanol series, rinsed for 5 min in chloroform, air dried, and stored at room temperature until further use, as described (17). A fragment encompassing nucleotides 314–985 of rat sgk was cloned by PCR from rat genomic DNA by using primers designed from the published sequence (16). The PCR fragment was cloned into pBluescript KS(−) (Stratagene), and antisense and [33P]UTP-labeled riboprobes were generated from 1 μg of linearized plasmid with T3 (antisense; linearized with BamHI) and T7 (sense; linearized with HindIII) RNA polymerase by using a standard in vitro transcription protocol (Promega). Probe was denatured at 65°C for 5 min and 2 × 106 cpm of probe was applied to each slide in a hybridization mix containing 10% dextran sulfate, 50% deionized formamide, 60 mM phosphate buffer, pH 7.0, 3× SSC, 1× Denhardt’s solution, 10 mM DTT, and 0.1 mg/ml yeast tRNA. Sections were hybridized under coverslips overnight at 55°C in a moist chamber. Coverslips were removed and sections rinsed in 2× SSC at room temperature, treated with RNase A for 30 min at 37°C (2 mg/100 ml in 0.5 M NaCl/0.1 M Tris⋅Cl, pH 8.0), and washed in 1× SSC for 30 min and 0.1× SSC at 60°C for 1 h. The sections were then passed through an alcohol series, air dried, and exposed to Hyperfilm (Amersham Life Sciences) for 3 days. The autoradiogram was scanned by using Adobe photoshop, and sections were quantitated by using the National Institutes of Health nih image program.
The sections were exposed to film, dipped in Kodak emulsion (diluted 1:1 with water), and exposed for 10 days. Slides were developed, fixed, and rinsed in water. Sections were then counterstained with hematoxylin and eosin.
Functional cRNA Expression in Xenopus laevis Oocytes.
The in vitro expression vector pSDeasy/xsgk was made by excising full-length xsgk from pCR2.1 as a BamHI–XbaI fragment and ligating into pSDeasy (18). Capped cRNAs were synthesized by SP6 RNA polymerase after linearization of pSDeasy vectors with BglII (for sgk and β ENaC) or AflIII (for α- and γ-ENaC subunits). Xenopus α-, β-, and γ-ENaC subunit cRNAs were coinjected (0.1–1.3 ng each) into stage V–VI oocytes together with sgk cRNA (0.5–5 ng) or water as control. After injection, the oocytes were incubated in a low-Na+ Barth’s solution, containing 10 mM NaCl, 86 mM N-methyl-d-glutamine⋅Cl (pH 7.4), 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM Hepes (pH 7.4). Electrophysiological measurements were performed at a clamp potential of −100 mV in a high-Na+ Barth’s solution, containing 96 mM NaCl (other ions as above), ≈20 h after RNA injection by using a laboratory-built two-electrode voltage clamp (19). Oocytes (six in each group) were continuously superfused in a 200-μl chamber at 6 μl/min. The macroscopic amiloride-sensitive current was defined as the difference between currents obtained in the presence (5 μM) and absence of amiloride.
Sgk coexpression with rat ROMK2 (a kind gift of J. Xu and S. Hebert, Yale Univ., New Haven, CT) was performed largely as above by using a pSPORT-ROMK2 expression vector (20). Capped cRNA was synthesized by T7 RNA polymerase after linearization of the vector with NotI. ROMK2 cRNA was coinjected (0.8 or 2.5 ng) into stage V–VI oocytes together with sgk cRNA (0.5 ng) or water as control. After injection, the oocytes were incubated for 20 h in Barth’s solution and electrophysiological measurements were performed at a clamp potential of −50 mV in the same solution. ROMK2-mediated current was defined as Ba2+-sensitive current (current inhibited by substitution of 1.8 mM Ca2+ with Ba2+ in the Barth’s solution).
RESULTS AND DISCUSSION
Suppression-subtractive hybridization (15) (see Materials and Methods) was used to generate a library of partial-length cDNAs representing rapidly induced mRNAs in A6 cells (12). After an initial screen for false positives (see Materials and Methods), selected library cDNAs were sequenced, and one of the clones, A83, had 92% amino acid identity (96% similarity) with rat sgk (serum and glucocorticoid-regulated kinase), a recently cloned member of the serine–threonine protein kinase family (16). In view of evidence suggesting that an unknown serine–threonine kinase phosphorylates ENaC and regulates its activity (3, 21), sgk was selected for further characterization.
As shown in Fig. 1A, sgk mRNA (a single band at 2.6 kbp on Northern blot) was rapidly induced by dexamethasone (more than 3-fold within 15 min) and by 45 min had reached maximal levels (more than 15-fold). When observed over 24 h, sgk mRNA reached a peak of 20-fold at 1 h and then declined to 1.6-fold above basal at 24 h (Fig. 1B). The response of sgk mRNA to dexamethasone was not inhibited by the protein synthesis inhibitor cycloheximide at levels that blocked more than 90% of Na+ transport (ref. 22 and S.-y.C., unpublished results), indicating direct regulation (Fig. 1C). In fact, both basal and hormone-induced levels of sgk were enhanced by cycloheximide, as has been described for immediate early genes (23). Importantly, sgk immunoreactive protein was also rapidly and strongly increased, as shown in Fig. 2. A doublet was visualized at approximately 56 kDa (somewhat larger than the predicted molecular mass of 49.1 kDa).
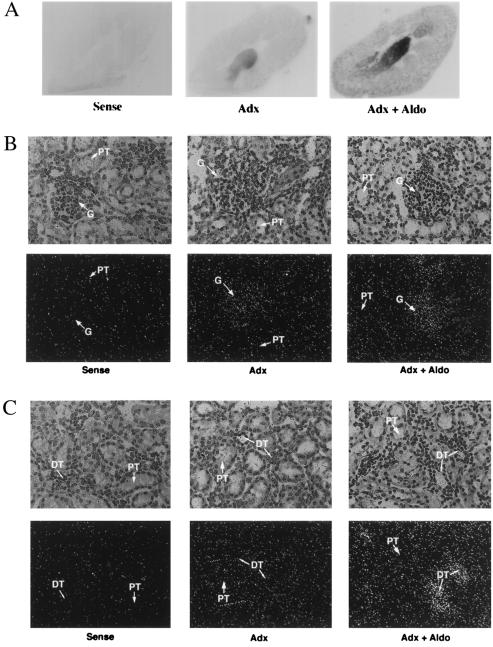
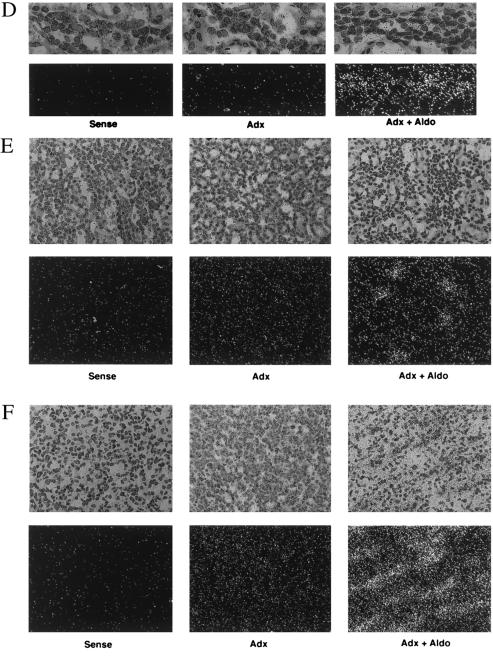
In A6 cells, MR is expressed at low levels, and corticosteroid regulation of Na+ transport is mediated by its close relative, GR (13, 24). To determine whether sgk was regulated by MR in mammalian kidney, we administered the physiological mineralocorticoid, aldosterone, to adx rats and performed in situ hybridizations to examine the localization of the sgk response (Fig. 3). In adx animals (Fig. 3A), sgk was expressed focally within the cortex, at low levels in outer medulla and moderate levels in inner medulla and papilla. Interestingly, examination of emulsion dipped sections counterstained with hematoxylin and eosin (Fig. 3 B–E) revealed that in adx animals sgk expression in cortex was primarily within glomeruli (Fig. 3B); very little was seen in tubules (Fig. 3 B–D). Basal expression in outer medulla was quite low, whereas expression levels in inner medulla and papilla were moderate and appeared to be in tubule cells (Figs. 3 E and F). As seen in the autoradiogram of whole kidney (Fig. 3A), sgk expression was strongly induced by aldosterone, focally in cortex and more generally in medulla, in a pattern suggestive of distal nephron. Inspection of emulsion-dipped sections revealed that glomerular expression was not stimulated by aldosterone (Fig. 3B), but, rather, sgk expression was strongly induced only in tubules (Fig. 3 C–F). In particular, in cortex, sgk expression was strongly induced in the smaller less eosinophilic tubule cells (consistent with distal nephron), whereas the larger more intensely eosinophilic proximal tubule cells did not express sgk in the presence or absence of aldosterone (color photomicrographs not shown and Fig. 3 C and D). In view of these observations and the increasingly uniform expression toward papilla (where CD cells represent the vast majority of the total), we conclude that aldosterone induces sgk expression predominantly in distal nephron and that it is expressed at low levels, if at all, in the proximal tubule. In addition, sgk is expressed at moderate levels, but is not aldosterone regulated, in glomeruli.
Figure 3.
In situ hybridization of sgk in rat kidney. Adx rats were treated for 4 h with vehicle (Adx, n = 5) or aldosterone (Adx + Aldo, n = 5). Longitudinal frozen sections (15 μm) were cut and hybridized with a rat sgk RNA probe (see Materials and Methods). (A) Autoradiagram of whole kidney. (B–F) Emulsion-dipped sections counterstained with hematoxylin and eosin at ×200 magnification (except D, which is ×400). A darkfield view is shown immediately beneath each light micrograph. Sense controls performed on sections from the adx group are shown for each kidney region. Sense controls for adx + aldo showed similar levels of signal (not shown). (B) Glomerulus (G). (C) Cortical tubules [proximal tubule (PT); distal tubule/collecting duct (DT)]. (D) ×400 magnification of cortex showing a tubule with morphology consistent with distal nephron. (E) Outer medulla. (F) Inner medulla/papilla.
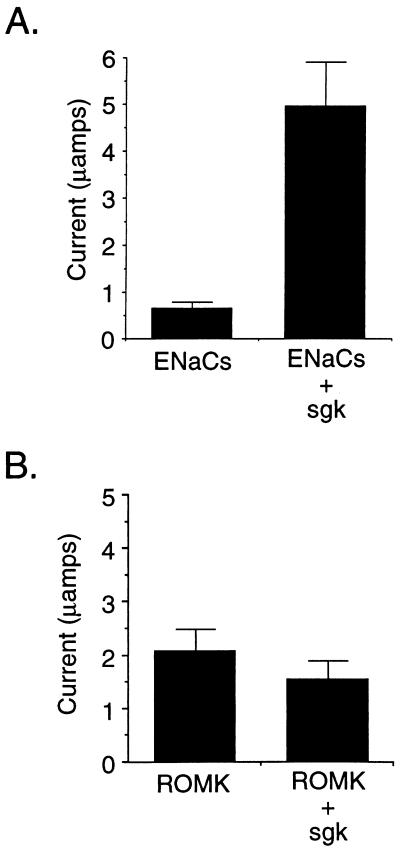
As noted above, ENaC constitutes the rate-limiting step for Na+ transport in aldosterone-responsive epithelia, and the principal early effect of aldosterone is to stimulate its activity (3, 11). Therefore, we directly tested whether sgk regulated ENaC activity in a coexpression assay in Xenopus oocytes, a widely used system for characterizing the function and regulation of a variety of transport proteins, including ENaC (25–27). As shown in Fig. 4A, coexpression of the three Xenopus ENaC subunits without sgk gave a significant Na+ current, consistent with previous reports (25, 28). This ENaC-mediated Na+ current was stimulated approximately 7-fold (6.9 ± 1.5) by coexpression of sgk (Fig. 4). In the absence of coexpressed ENaC subunits, amiloride-inhibitable Na+ current was very low (<0.1 μA). Importantly, sgk had no effect on this low level of current or on oocyte morphology in the absence of ENaCs (data not shown). Furthermore, as shown in Fig. 4B, sgk did not stimulate ROMK-mediated K+ current when the two were coexpressed in oocytes but, rather, tended to repress it (although the difference in current did not reach significance). We conclude that sgk stimulates ENaC activity and that this effect does not reflect a generalized stimulation of ion transport. Indeed, it is notable that the K+ channel ROMK2 was not activated by sgk, because it is likely the major apical K+-secreting channel in ENaC-expressing cortical distal nephron cells (29, 30). This observation is consistent with the idea that aldosterone does not exert a parallel regulatory action on ENaC and ROMK and that the major stimulus for increased K+ secretion during the early phase of the mineralocorticoid response is an increase in driving force because of the depolarizing effect of Na+ influx.
Figure 4.
(A) ENaC activity is stimulated by sgk when they are coexpressed in X. laevis oocytes. In vitro transcribed RNA for each of the three Xenopus ENaC subunits was injected into oocytes with or without sgk. Shown is amiloride-sensitive current from a representative experiment with six oocytes per condition (mean ± SE). The mean increase in amiloride-inhibitable sodium current was 6.9-fold ± 1.5 for 12 experiments (six oocytes per condition in each experiment) performed with oocytes from six different frogs. (B) Potassium channel ROMK2 is not stimulated by sgk. Experiments were performed in Xenopus oocytes as in A. Shown is a representative experiment with six oocytes per condition. In two independent experiments performed on different days (six oocytes per condition each), the average ROMK currents were: ROMK alone, 2.06 ± 0.41 μA; ROMK + sgk, 1.52 ± 0.35 μA (ratio ROMK + sgk/ROMK alone = 0.74). In the same set of experiments, ENaC controls were performed and currents were: ENaC alone, 0.442 ± 0.125 μA; ENaC + sgk, 3.2 ± 0.59 μA (ratio ENaC + sgk/ENaC alone = 7.3).
It has been known for almost 40 years that aldosterone regulates epithelial Na+ transport by increasing apical Na+ permeability through a transcriptional mechanism (31, 32). It also has been long suspected that aldosterone induces an ENaC modulator, but previous candidates have been weakly or slowly induced or have had a modest or equivocal effect on ENaC-mediated Na+ current (11, 33–35). One such candidate, K-Ras (35), had, in its activated form, a significant effect (≈4-fold) on the current mediated per surface-expressed ENaC in Xenopus oocytes, but it also induced oocyte maturation such that overall current was unchanged (36). The 7-fold stimulation of ENaC-mediated Na+ current that we have seen in Xenopus oocytes is substantially greater than that of any previously identified regulator or channel-activating mutation studied in Xenopus oocytes (27, 37) and is quantitatively sufficient to account for the early aldosterone effect in intact CD and other tight epithelia. Together with the observation that sgk mRNA is strongly and rapidly induced by dexamethasone in A6 cells and by aldosterone in distal nephron, these data suggest that sgk is an important mediator of aldosterone-induced epithelial sodium transport.
Based on the high homology of sgk to the serine–threonine kinase family (16) and functional (3) evidence that a serine–threonine kinase is implicated in modulating ENaC activity, it seems probable that sgk regulates Na+ transport through protein phosphorylation. Furthermore, although a kinase substrate for sgk has not been identified, recent evidence suggests that a serine–threonine kinase directly phosphorylates the β- and γ-ENaC subunits (21), resulting in either an increase in Po or increased apical targeting (reviewed in ref. 11). In this regard, it is interesting to note that protein kinase A-dependent phosphorylation of the water channel, aquaporin-2, has been implicated in its vasopressin-stimulated apical insertion (38). It is still possible, however, that another protein is the direct target of sgk, such as a methyltransferase (39), a channel-associated G protein (34), another kinase, or a component of the plasma membrane-trafficking machinery (40).
The overshoot induction of sgk resembles the response of other immediate-early genes (23) and may enhance the rapidity of the hormone-induced increase in Na+ current by providing a burst of sgk synthesis, followed by a lower steady state. Other components of the transepithelial Na+ transport system, such as the Na-K-ATPase are not initially rate limiting (11) and are modulated on a slower time scale, although they may be important for sustained transport (22) during the later phase of aldosterone’s effect. It is interesting to speculate that the sgk overshoot and rapid decline to near-basal levels are important for the dynamic response of the system: perhaps low levels of sgk (still sufficient to maintain phosphorylation in the face of ENaC turnover) prepare the animal for rapid down-regulation of channel activity in the face of a subsequent increase in sodium load.
A simple hormone response element was found in the rat sgk 5′-flanking region (16), consistent with direct regulation by either MR or GR, which both bind to and activate transcription from simple hormone response elements and have distinct activities only at composite or multimerized hormone response elements (41–43). Their similar transcriptional activities together with the similar effects they mediate on Na+ transport in cultured CD cells (13, 14) make it highly likely that MR and GR regulate sgk gene transcription by similar mechanisms. In this regard, it is important to note that hormonal specificity (as opposed to receptor specificity) in aldosterone-responsive epithelia is determined by an enzyme, 11-β-hydroxysteroid dehydrogenase, which inactivates glucocorticoids but not mineralocorticoids (44).
sgk was originally identified as a glucocorticoid-regulated mRNA in mammary epithelial cells (16) and has been found subsequently in most vertebrate tissues as well as in cultured cell lines, although at variable levels (16, 45). In adx rats it is expressed at moderate levels in glomeruli and inner medullary CD but not in proximal tubule (Fig. 3). The significance of glomerular sgk expression is uncertain at this time; it is plausible that it controls the activity of an ion channel, although it is unlikely to be ENaC because ENaC expression is low in glomeruli. In view of previous observations that sgk gene expression is induced by cell shrinkage in hepatocytes (45), it is interesting to speculate that its expression in inner medulla in adx animals is stimulated by the high osmolarity of this kidney region. Although the role of sgk in osmotic responses has not been determined, it is notable that amiloride-sensitive sodium channels have been implicated in the regulatory volume increase in hepatocytes (46). It is also notable that organisms as primitive as Caenorhabditis elegans express an sgk homolog (47), consistent with the idea that it predates vertebrate evolution. These observations suggest the interesting possibility that this ancient protein kinase modulates a class of related ion channels and transporters (perhaps originally involved in cell volume regulation). According to this view, sgk was adopted for the regulation of epithelial Na+ transport by early vertebrates as they made the transition from a marine into a freshwater environment (1).
Acknowledgments
M. Dallman and her laboratory are gratefully acknowledged for extensive help with performing in situ hybridizations. We are grateful to B. C. Rossier for providing XENaC cDNAs, J. Xu and S. Hebert for providing mouse ROMK2 cDNA, I. Forster for providing the setup and assistance with the electrophysiological experiments, and R. Pfeiffer for performing electrophysiological experiments. B. Yen is gratefully acknowledged for assistance with interpreting histological data. This work was supported by National Institutes of Health Grant R29-DK51151-03 and Swiss National Science Foundation Grant 31.49727.96. O.C.M. was supported by a TALENT stipend of the Dutch Organization for Scientific Research.
ABBREVIATIONS
- ENaC
epithelial sodium channel
- CD
collecting duct
- GR
glucocorticoid receptor
- sgk
serum and glucocorticoid-regulated kinase
- xsgk
Xenopus sgk
- MR
mineralocorticoid receptor
- adx
adrenalectomized
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Data deposition: The Xenopus laevis sgk (xsgk) cDNA sequence reported in this paper has been deposited in the GenBank database (accession no. AF057138).
References
- 1.Smith H W. From Fish to Philosopher. Garden City, NY: Doubleday; 1961. [Google Scholar]
- 2.Edelman I S, Fimognari G M. Recent Prog Horm Res. 1968;24:1–44. doi: 10.1016/b978-1-4831-9827-9.50007-1. [DOI] [PubMed] [Google Scholar]
- 3.Eaton D C, Becchetti A, Ma H, Ling B N. Kidney Int. 1995;48:941–949. doi: 10.1038/ki.1995.375. [DOI] [PubMed] [Google Scholar]
- 4.Kemendy A E, Kleyman T R, Eaton D C. Am J Physiol. 1992;263:C825–C837. doi: 10.1152/ajpcell.1992.263.4.C825. [DOI] [PubMed] [Google Scholar]
- 5.Kleyman T R, Coupaye G B, Ernst S A. J Biol Chem. 1992;267:9622–9628. [PubMed] [Google Scholar]
- 6.Ling B N, Zuckerman J B, Lin C, Harte B J, McNulty K A, Smith P R, Gomez L M, Worrell R T, Eaton D C, Kleyman T R. J Biol Chem. 1997;272:594–600. doi: 10.1074/jbc.272.1.594. [DOI] [PubMed] [Google Scholar]
- 7.Asher, C., Wald, H., Rossier, B. C. & Garty, H. (1996) Am. J. Physiol. C605–C611. [DOI] [PubMed]
- 8.Denault D L, Fejes-Toth G, Naray-Fejes-Toth A. Am J Physiol. 1996;271:C423–C428. doi: 10.1152/ajpcell.1996.271.1.C423. [DOI] [PubMed] [Google Scholar]
- 9.Escoubet B, Coureau C, Bonvalet J P, Farman N. Am J Physiol. 1997;272:C1482–C1491. doi: 10.1152/ajpcell.1997.272.5.C1482. [DOI] [PubMed] [Google Scholar]
- 10.May A, Puoti A, Gaeggeler H P, Horisberger J D, Rossier B C. J Am Soc Nephrol. 1997;8:1813–1822. doi: 10.1681/ASN.V8121813. [DOI] [PubMed] [Google Scholar]
- 11.Garty H, Palmer L G. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 12.Watlington C O, Perkins F M, Munson P J, Handler J S. Am J Physiol. 1982;242:F610–F619. doi: 10.1152/ajprenal.1982.242.6.F610. [DOI] [PubMed] [Google Scholar]
- 13.Chen S Y, Wang J, Liu W, Pearce D. Am J Physiol. 1998;274:C39–C46. doi: 10.1152/ajpcell.1998.274.1.C39. [DOI] [PubMed] [Google Scholar]
- 14.Naray-Fejes-Toth A, Fejes T G. Am J Physiol. 1990;259:F672–F678. doi: 10.1152/ajprenal.1990.259.4.F672. [DOI] [PubMed] [Google Scholar]
- 15.Diatchenko L, Lau Y F, Campbell A P, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov E D, Siebert P D. Proc Natl Acad Sci USA. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster M K, Goya L, Ge Y, Maiyar A C, Firestone G L. Mol Cell Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meijer O C, de Kloet E R. Eur J Pharmacol. 1994;266:255–261. doi: 10.1016/0922-4106(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 18.Puoti A, May A, Rossier B C, Horisberger J D. Proc Natl Acad Sci USA. 1997;94:5949–5954. doi: 10.1073/pnas.94.11.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forster I, Hernando N, Biber J, Murer H. J Gen Physiol. 1998;112:1–18. doi: 10.1085/jgp.112.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boim M A, Ho K, Shuck M E, Bienkowski M J, Block J H, Slightom J L, Yang Y, Brenner B M, Hebert S C. Am J Physiol. 1995;268:F1132–F1140. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- 21.Shimkets R A, Lifton R, Canessa C M. Proc Natl Acad Sci USA. 1998;95:3301–3305. doi: 10.1073/pnas.95.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verrey F, Kraehenbuhl J P, Rossier B C. Mol Endocrinol. 1989;3:1369–1376. doi: 10.1210/mend-3-9-1369. [DOI] [PubMed] [Google Scholar]
- 23.Lau L F, Nathans D. Proc Natl Acad Sci USA. 1987;84:1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt T J, Husted R F, Stokes J B. Am J Physiol. 1993;264:C875–C884. doi: 10.1152/ajpcell.1993.264.4.C875. [DOI] [PubMed] [Google Scholar]
- 25.Canessa C M, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J D, Rossier B C. Nature (London) 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 26.Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton R P, Rossier B C. EMBO J. 1996;15:2381–2387. [PMC free article] [PubMed] [Google Scholar]
- 27.Vallet V, Chraibi A, Gaeggeler H P, Horisberger J D, Rossier B C. Nature (London) 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- 28.Puoti A, May A, Canessa C M, Horisberger J D, Schild L, Rossier B C. Am J Physiol. 1995;269:C188–C197. doi: 10.1152/ajpcell.1995.269.1.C188. [DOI] [PubMed] [Google Scholar]
- 29.Mennitt P A, Wade J B, Ecelbarger C A, Palmer L G, Frindt G. J Am Soc Nephrol. 1997;8:1823–1830. doi: 10.1681/ASN.V8121823. [DOI] [PubMed] [Google Scholar]
- 30.Xu J Z, Hall A E, Peterson L N, Bienkowski M J, Eessalu T E, Hebert S C. Am J Physiol. 1997;273:F739–F748. doi: 10.1152/ajprenal.1997.273.5.F739. [DOI] [PubMed] [Google Scholar]
- 31.Crabbe J. Nature (London) 1963;200:787–788. doi: 10.1038/200787a0. [DOI] [PubMed] [Google Scholar]
- 32.Edelman I S, Bogoroch R, Porter G A. Proc Natl Acad Sci USA. 1963;50:1169–1176. doi: 10.1073/pnas.50.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verrey F. J Membr Biol. 1995;144:93–110. doi: 10.1007/BF00232796. [DOI] [PubMed] [Google Scholar]
- 34.Rokaw M D, Benos D J, Palevsky P M, Cunningham S A, West M E, Johnson J P. J Biol Chem. 1996;271:4491–4496. doi: 10.1074/jbc.271.8.4491. [DOI] [PubMed] [Google Scholar]
- 35.Spindler B, Mastroberardino L, Custer M, Verrey F. Pflügers Arch. 1997;434:323–331. doi: 10.1007/s004240050403. [DOI] [PubMed] [Google Scholar]
- 36.Mastroberardino L, Spindler B, Verrey F. Mol Biol Cell. 1998;9:3417–3427. doi: 10.1091/mbc.9.12.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schild L, Canessa C M, Shimkets R A, Gautschi I, Lifton R P, Rossier B C. Proc Natl Acad Sci USA. 1995;92:5699–5703. doi: 10.1073/pnas.92.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katsura, T., Gustafson, C. E., Ausiello, D. A. & Brown, D. (1997) Am. J. Physiol. F817–F822. [PubMed]
- 39.Sariban-Sohraby S, Burg M, Wiesmann W P, Chiang P K, Johnson J P. Science. 1984;225:745–746. doi: 10.1126/science.6463652. [DOI] [PubMed] [Google Scholar]
- 40.Shepherd P R, Withers D J, Siddle K. Biochem J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce D, Yamamoto K R. Science. 1993;259:1161–1165. doi: 10.1126/science.8382376. [DOI] [PubMed] [Google Scholar]
- 42.Rupprecht R, Arriza J L, Spengler D, Reul J M, Evans R M, Holsboer F, Damm K. Mol Endocrinol. 1993;7:597–603. doi: 10.1210/mend.7.4.8388999. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Wang J, Yu G, Pearce D. Mol Endocrinol. 1996;10:1399–1406. doi: 10.1210/mend.10.11.8923466. [DOI] [PubMed] [Google Scholar]
- 44.Funder J W, Pearce P T, Smith R, Smith A I. Science. 1988;242:583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- 45.Waldegger S, Barth P, Raber G, Lang F. Proc Natl Acad Sci USA. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wehner F, Tinel H. J Physiol (London) 1998;506:127–142. doi: 10.1111/j.1469-7793.1998.127bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson R, Ainscough R, Anderson K, Baynes C, Berks M, Bonfield J, Burton J, Connell M, Copsey T, Cooper J, et al. Nature (London) 1994;368:32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]