Abstract
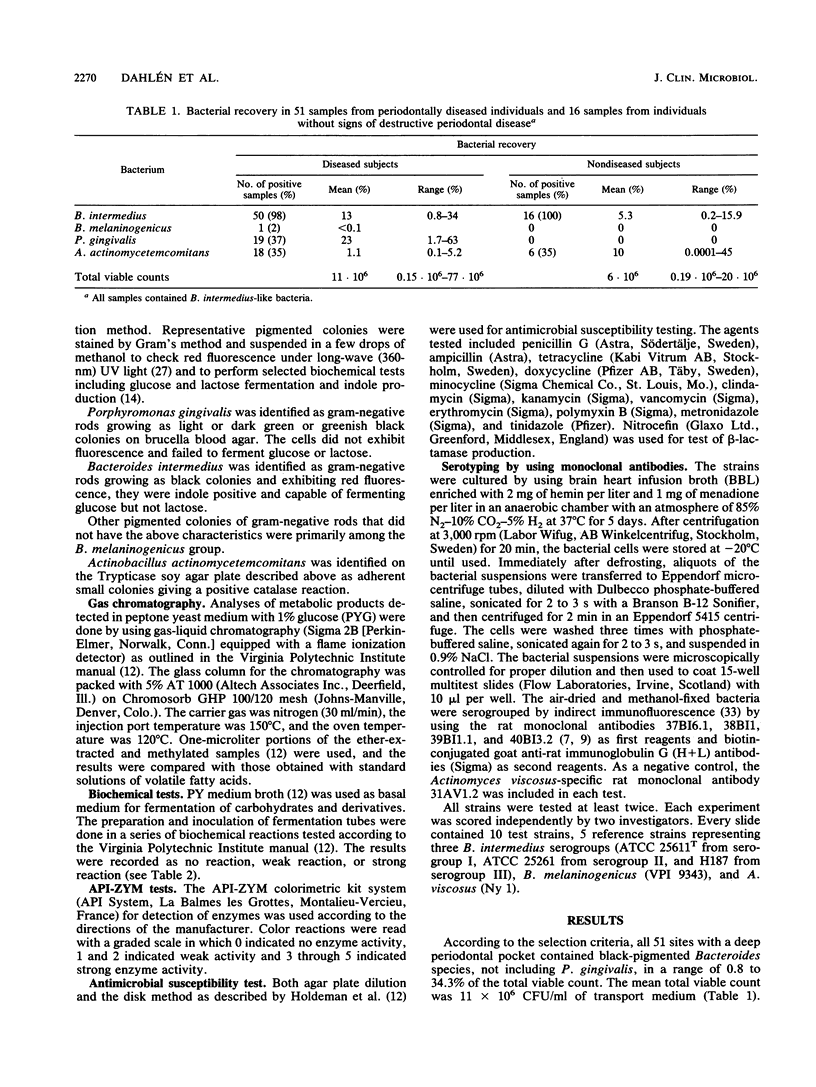
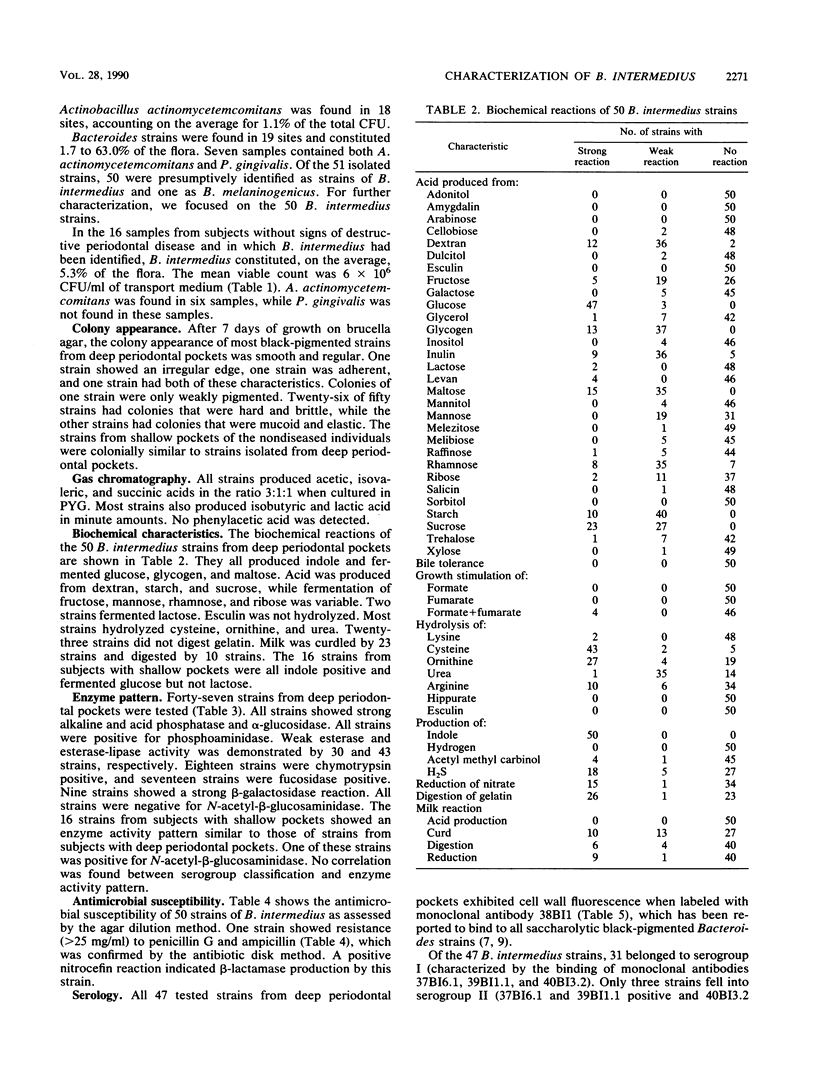
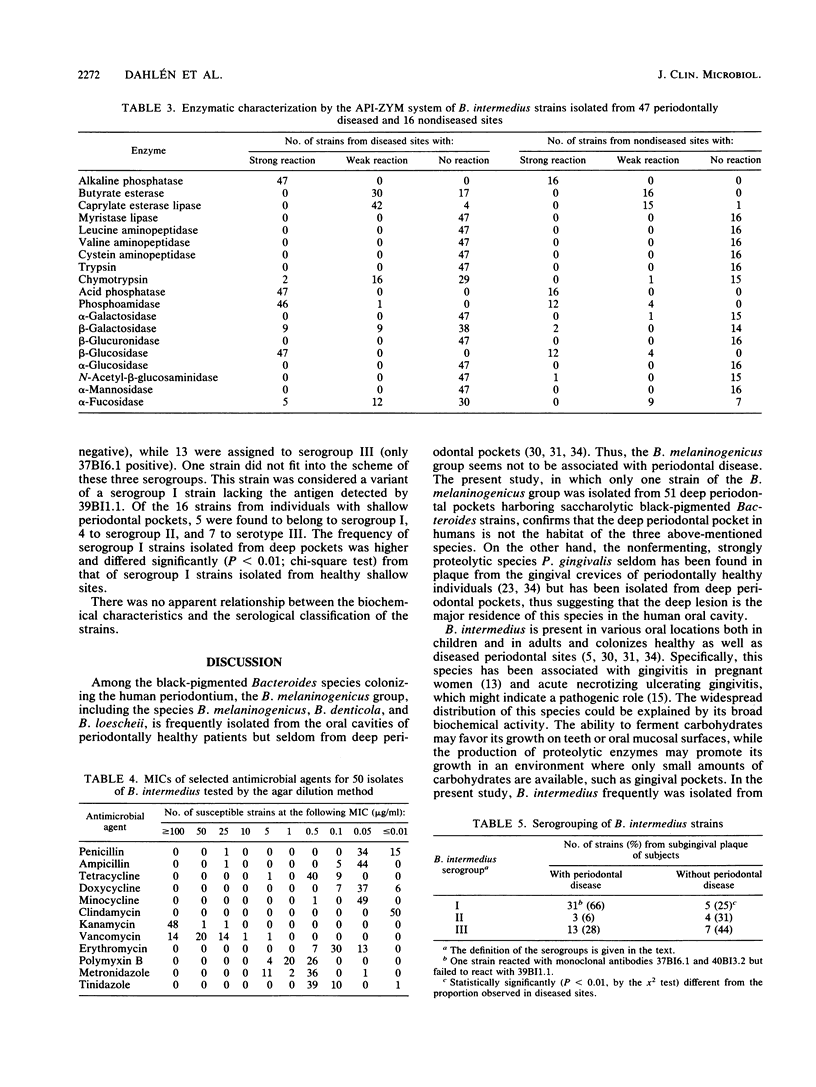
Fifty-one fluorescence-positive black-pigmented Bacteroides strains obtained from 51 patients with deep periodontal pockets (greater than 6 mm) were identified and characterized. Fifty of these strains were presumptively identified as Bacteroides intermedius according to the indole reaction. This was confirmed by further biochemical characterization. The 50 strains from diseased sites were then compared with 16 B. intermedius strains isolated from periodontally healthy individuals with no signs of destructive periodontal disease. Tests for antimicrobial susceptibility showed similar patterns for all 50 pocket-derived strains, except for one beta-lactamase-positive strain that was resistant to penicillin G and ampicillin. Forty-seven strains were tested for binding of three monoclonal antibodies defining three distinct serogroups of B. intermedius. Thirty-one strains belonged to serogroup I, three to serogroup II and thirteen to serogroup III. In comparison to the strains from the shallow periodontal pockets, serogroup I was significantly overrepresented in the patient group with periodontal disease. We conclude that saccharolytic black-pigmented Bacteroides species from deep periodontal pockets constituted, with very rare exceptions, a biochemically homogeneous but antigenically heterogeneous group of B. intermedius and that serogroup I is predominantly found in deep periodontal lesions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aicoforado G. A., McKay T. L., Slots J. Rapid method for detection of lactose fermenting oral microorganisms. Oral Microbiol Immunol. 1987 Mar;2(1):35–38. doi: 10.1111/j.1399-302x.1987.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Evans R. T., Slots J., Genco R. J. Susceptibility of human oral anaerobic bacteria to antibiotics suitable for topical use. J Clin Periodontol. 1985 Mar;12(3):201–208. doi: 10.1111/j.1600-051x.1985.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Brown W. J., Waatti P. E. Susceptibility testing of clinically isolated anaerobic bacteria by an agar dilution technique. Antimicrob Agents Chemother. 1980 Apr;17(4):629–635. doi: 10.1128/aac.17.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty S. L., Lopatin D. E., Syed S. A., Smith F. N. Humoral immune response to oral microorganisms in periodontitis. Infect Immun. 1982 Aug;37(2):499–505. doi: 10.1128/iai.37.2.499-505.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisken K. W., Tagg J. R., Laws A. J., Orr M. B. Suspected periodontopathic microorganisms and their oral habitats in young children. Oral Microbiol Immunol. 1987 Jun;2(2):60–64. doi: 10.1111/j.1399-302x.1987.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Gmür R., Guggenheim B. Antigenic heterogeneity of Bacteroides intermedius as recognized by monoclonal antibodies. Infect Immun. 1983 Nov;42(2):459–470. doi: 10.1128/iai.42.2.459-470.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmür R., Hrodek K., Saxer U. P., Guggenheim B. Double-blind analysis of the relation between adult periodontitis and systemic host response to suspected periodontal pathogens. Infect Immun. 1986 Jun;52(3):768–776. doi: 10.1128/iai.52.3.768-776.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmür R. Human serum antibodies against Bacteroides intermedius. Antigenic heterogeneity impairs the interpretation of the host response. J Periodontal Res. 1985 Sep;20(5):492–496. doi: 10.1111/j.1600-0765.1985.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Harding G. K., Sutter V. L., Finegold S. M., Bricknell K. S. Characterization of bacteroides melaninogenicus. J Clin Microbiol. 1976 Oct;4(4):354–359. doi: 10.1128/jcm.4.4.354-359.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstad T. Evaluation of the API ZYM system for identification of Bacteroides and Fusobacterium species. Med Microbiol Immunol. 1980;168(3):173–177. doi: 10.1007/BF02122851. [DOI] [PubMed] [Google Scholar]
- Kornman K. S., Loesche W. J. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980 Mar;15(2):111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Laughon B. E., Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol. 1982 Apr;53(4):223–230. doi: 10.1902/jop.1982.53.4.223. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Schmidt E., Morrison E. C. Bacterial profiles of subgingival plaques in periodontitis. J Periodontol. 1985 Aug;56(8):447–456. doi: 10.1902/jop.1985.56.8.447. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Cato E. P., Good I. J., Smith E. P., Ranney R. R., Palcanis K. G. Variation in periodontal floras. Infect Immun. 1984 Dec;46(3):720–726. doi: 10.1128/iai.46.3.720-726.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E. Microbiology of periodontal disease. J Periodontal Res. 1987 Sep;22(5):335–341. doi: 10.1111/j.1600-0765.1987.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Möller A. J. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol Tidskr. 1966 Dec 20;74(5 Suppl):1–380. [PubMed] [Google Scholar]
- Nakazawa F., Zambon J. J., Reynolds H. S., Genco R. J. Serological studies of oral Bacteroides intermedius. Infect Immun. 1988 Jun;56(6):1647–1651. doi: 10.1128/iai.56.6.1647-1651.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederau W., Höffler U., Pulverer G. Susceptibility of Bacteroides melaninogenicus to 45 antibiotics. Chemotherapy. 1980;26(2):121–127. doi: 10.1159/000237893. [DOI] [PubMed] [Google Scholar]
- Renvert S., Wikström M., Dahlén G., Slots J., Egelberg J. Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. J Clin Periodontol. 1990 Jul;17(6):345–350. doi: 10.1111/j.1600-051x.1990.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Bragd L., Wikström M., Dahlén G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontol. 1986 Jul;13(6):570–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Slots J. Enzymatic characterization of some oral and nonoral gram-negative bacteria with the API ZYM system. J Clin Microbiol. 1981 Sep;14(3):288–294. doi: 10.1128/jcm.14.3.288-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Reynolds H. S. Long-wave UV light fluorescence for identification of black-pigmented Bacteroides spp. J Clin Microbiol. 1982 Dec;16(6):1148–1151. doi: 10.1128/jcm.16.6.1148-1151.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Selective medium for isolation of Actinobacillus actinomycetemcomitans. J Clin Microbiol. 1982 Apr;15(4):606–609. doi: 10.1128/jcm.15.4.606-609.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979 Oct;6(5):351–382. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Socransky S. S., Goodson J. M. Microbiota of periodontal pockets losing crestal alveolar bone. J Periodontal Res. 1984 May;19(3):279–291. doi: 10.1111/j.1600-0765.1984.tb00819.x. [DOI] [PubMed] [Google Scholar]
- Van Winkelhoff A. J., Van der Velden U., Winkel E. G., de Graaff J. Black-pigmented Bacteroides and motile organisms on oral mucosal surfaces in individuals with and without periodontal breakdown. J Periodontal Res. 1986 Jul;21(4):434–439. doi: 10.1111/j.1600-0765.1986.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Van der Velden U., Van Winkelhoff A. J., Abbas F., De Graaff J. The habitat of periodontopathic micro-organisms. J Clin Periodontol. 1986 Mar;13(3):243–248. doi: 10.1111/j.1600-051x.1986.tb01467.x. [DOI] [PubMed] [Google Scholar]
- Wennström J. L., Dahlén G., Svensson J., Nyman S. Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius: predictors of attachment loss? Oral Microbiol Immunol. 1987 Dec;2(4):158–162. doi: 10.1111/j.1399-302x.1987.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Werner-Felmayer G., Guggenheim B., Gmür R. Production and characterization of monoclonal antibodies against Bacteroides forsythus and Wolinella recta. J Dent Res. 1988 Mar;67(3):548–553. doi: 10.1177/00220345880670030501. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., Reynolds H. S., Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect Immun. 1981 Apr;32(1):198–203. doi: 10.1128/iai.32.1.198-203.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


