Abstract
The aim of this study was to examine the dynamics of the development of resistance in fecal Escherichia coli populations during treatment with ampicillin for 7 days in pigs. Before treatment, only 6% of the isolates were ampicillin resistant, whereas more than 90% of the isolates were resistant after days 4 and 7 of treatment. Ampicillin-resistant E. coli isolates were mainly multiresistant, and 53% of the isolates from the treated pigs had one phenotype that included resistance to six antibiotics (ampicillin, chloramphenicol, sulfonamides, tetracycline, trimethoprim, and streptomycin) at day 7. Determination of the frequency of the four phylogenetic groups showed that there was a shift in the E. coli population in ampicillin-treated pigs; before treatment 75% of the isolates belonged to phylogroup B1, whereas at day 7 85% of the isolates belonged to phylogroup A. Pulsed-field gel electrophoresis (PFGE) typing revealed that ampicillin treatment selected ampicillin-resistant isolates with genotypes which were present before treatment. Comparison of antimicrobial phenotypes and PFGE genotypes showed that resistance traits were disseminated by vertical transmission through defined strains. One PFGE genotype, associated with the six-antibiotic-resistant phenotype and including a specific combination of resistance determinants, was predominant among the ampicillin-resistant strains before treatment and during treatment. These data indicate that ampicillin administration selected various ampicillin-resistant isolates that were present in the digestive tract before any treatment and that E. coli isolates belonging to one specific PFGE genotype encoding resistance to six antibiotics became the predominant strains as soon as ampicillin was present in the digestive tract.
Antimicrobial resistance in food animals deserves special attention, especially for pig farming, where worldwide consumption accounts for 60% of the antibiotics used in animals (13). The digestive tract of pigs can harbor antimicrobial- resistant bacteria in the commensal flora, which contain a reservoir of antibiotic resistance genes potentially transmissible to humans via the food chain and the environment (37, 39, 46). Escherichia coli is an indicator species for studies of the level of antibiotic resistance of the fecal flora and has commonly been used for this purpose in pigs (43). Epidemiological studies were performed with commensal and pathogenic E. coli strains to identify the genetic determinants of the resistance. These studies provided a descriptive and molecular epidemiology analysis of fecal antimicrobial-resistant bacteria from animals (4, 20, 25, 40). In addition, a relationship has been demonstrated between the use of antimicrobials in pig herds and the increased occurrence of resistant bacterial strains in the digestive tracts of pigs (3, 16, 46). However, the population processes underlying the emergence and spread of these antibiotic-resistant strains are little known, and how these strains are selected by antibiotic administration in the gut ecosystem remains unclear.
In an experimental setting, we previously showed that administration of ampicillin to pigs for 7 days led, whatever the mode of administration (intramuscular route or oral route under fed or fasting conditions), to a large increase in ampicillin resistance in the fecal E. coli population (2). Quantification of the blaTEM gene copies in swine feces by real-time PCR also indicated that there was increased excretion of these genes from treated animals and that there was a significant correlation between the quantities of blaTEM genes and the counts of ampicillin-resistant Enterobacteriaceae, confirming that these genes code for the most frequent mechanism of ampicillin resistance in Enterobacteriaceae (27). As blaTEM genes are plasmid mediated, two mechanisms could have contributed to the emergence of ampicillin resistance in fecal E. coli populations under ampicillin selection pressure: selection by ampicillin of resistant strains already present in the intestinal E. coli populations of the pigs and/or acquisition of blaTEM genes by previously susceptible strains by horizontal transfer. The aim of the present work was to explore these mechanisms by studying the dynamics of the development of resistance in the fecal populations of E. coli from pigs treated with ampicillin.
Our approach was to investigate the relatedness of ampicillin-resistant E. coli in order to determine whether resistance came from genetically related or unrelated strains. Pulsed-field gel electrophoresis (PFGE) allows identification of strains by their molecular genotypes and has great value in epidemiological analysis, in the differentiation of pathogenic strains, and in monitoring the spread of such strains between communities (14, 17, 24). This method can also be used to evaluate the potential clonal spread of resistant strains (1, 44). It has previously been used to investigate the dissemination and diversity of ampicillin-resistant commensal E. coli strains in cattle (22, 23) and dogs (32).
In the present study, fecal E. coli strains isolated from pigs were characterized by determining their antimicrobial resistance patterns, phylogenetic groups, and PFGE genotypes. Characterization performed before and during ampicillin treatment enabled us to investigate whether resistance traits were disseminated through specific strains or whether lateral spread of blaTEM genes to previously susceptible strains occurred.
MATERIALS AND METHODS
Study design and isolation of bacteria.
Eighteen 7-week-old, commercial healthy piglets that had never received antibiotics were used. These pigs were housed separately in individual pens during all experiments. Ampicillin was administered once a day using a dose of 20 mg/kg for 7 days by using three modes: the intramuscular route, the oral route in fasting pigs, and the oral route in fed pigs. The design was described in our previous study (2). Briefly, the study consisted of three temporally independent trials separated by 3-week intervals (in March, April, and May) involving six pigs per experiment, which were treated as follows: four pigs received ampicillin using two treatment modes (two pigs per mode), and two pigs were used as controls. Intramuscular injections of sodium ampicillin (Ampicilline Cadril; Laboratory Coophavet, Ancenis, France) were administered in the neck. For the oral route, a medicinal premix (Ampicilline 80 Porc Franvet; Laboratory Franvet, Segré, France) was dissolved in water and administered by gastric intubation. Ampicillin doses were based on approved doses for ampicillin in swine in France. For the oral route, the approved dose for the medicinal premix is 20 mg/kg per day, and the for intramuscular route, the approved dose range is 14 to 28 mg/kg per day. All pigs came from the same herd, for which 3-week batch farrowing management was used. The pigs used in a trial came from the same batch. All procedures involving animals were performed in accordance with the French legal requirements regarding the protection of laboratory animals and with authorization for animal experimentation 31-242 from the French Ministry of Agriculture.
Fecal samples were obtained from each pig before treatment and after 4 and 7 days of treatment. Samples were obtained from control pigs at the same times. Feces (5 g) from each pig and 45 ml of peptone water, including 30% glycerol, were placed in a BagFilter filter bag (Interscience, St. Nom, France). In the filter bag, the sample was blended, homogenized, and filtered using a BagMixer paddle blender (Interscience, St. Nom, France). Tenfold serial dilutions of the filtrate were prepared, and 100-μl samples of the dilutions were spread on MacConkey agar (AEB 151602; AES, Ker Lann, France) on days 0, 4, and 7. In order to select ampicillin-resistant strains before treatment (day 0 Ampr), fecal samples were also plated on MacConkey agar containing 128 μg/ml of ampicillin on day 0 (the breakpoint concentration proposed by the CLSI is 32 μg/ml [10]). We chose an ampicillin concentration of 128 μg/ml for selection of resistant isolates because we previously found that, as described by Livermore (28), the MICs for ampicillin-sensitive isolates were 2 to 8 μg/ml and that the MICs for (nearly) all ampicillin-resistant isolates were ≥256 μg/ml (2). For each pig, five colonies were picked at random from the MacConkey plates without ampicillin on days 0, 4, and 7 and from the ampicillin-containing plates on day 0. When lactose-negative colonies were present, they were also picked, and an attempt was made to conserve the proportion of the two phenotypes (lactose negative and lactose positive). All isolates were confirmed to be E. coli by using the API 20E Enterobacteriaceae identification system (bioMérieux, Marcy l'Etoile, France).
Antimicrobial susceptibility tests.
Antimicrobial susceptibility tests were performed by using a disk diffusion method according to the CLSI standards (9) on Mueller-Hinton agar (Bio-Rad Laboratories). E. coli ATCC 25922 was used as the control strain. The 14 antibiotic disks (Bio-Rad Laboratories) used in this study contained ampicillin (10 μg), amoxicillin (20 μg) plus clavulanic acid (10 μg), cephalothin (30 μg), ceftiofur (30 μg), streptomycin (10 μg), gentamicin (10 μg), kanamycin (30 μg), neomycin (30 μg), nalidixic acid (30 μg), ciprofloxacin (5 μg), tetracycline (30 μg), chloramphenicol (30 μg), trimethoprim (5 μg), and sulfonamides (300 μg). The susceptibility breakpoints for all antimicrobials were those recommended by CLSI (10, 34), except for neomycin, for which the breakpoints were provided by the manufacturer.
PFGE.
PFGE was performed using the method described by Gautom, with modifications (15). A bacterial suspension with an A600 of 1.3 was prepared using an overnight culture of E. coli isolates in Tris-EDTA buffer (100 mM Tris, 100 mM EDTA; pH 7.5). Two hundred microliters of the bacterial suspension was mixed with 10 μl of proteinase K (20 mg/ml; MP Biomedicals, Qbiogene, France) and incubated for 15 min at 37°C. Twenty-eight microliters of 10% sodium dodecyl sulfate and 200 μl of 1% Seakem Gold agarose (Lonza, Rockland, ME) prepared in SE buffer (25 mM EDTA, 75 mM NaCl; pH 7.5) were mixed with each bacterial suspension. The bacterium-agarose mixture was added immediately to plug molds (Bio-Rad Laboratories, Hercules, CA). Solidified plugs were transferred to 1.5 ml ES buffer (0.5 M EDTA, 1% sodium lauryl sarcosine; pH 9.0) with 40 μl of proteinase K (20 mg/ml) and incubated for 1 h at 55°C. The plugs were then washed once with sterile distilled water and three times with Tris-EDTA buffer (10 mM Tris, 1 mM EDTA; pH 7.5) (15 min for each wash) at 55°C. For restriction digestion, two 1-mm-wide plug slices were incubated at 37°C for 3 h with 30 U of XbaI enzyme (MP Biomedical, Qbiogene, France) in the appropriate buffer. Plug slices were loaded and electrophoresed in 1% SeaKem Gold agarose with 2 liters of 0.5× Tris-borate-EDTA. Electrophoresis was performed with the Gene Path system (Bio-Rad Laboratories) using the following conditions: initial switch time, 2.2 s; final switch time, 54.2 s; run time, 22 h; angle, 120°; gradient, 6 V/cm; temperature, 14°C; and ramping factor, linear. The system was manually shut down after 19 h of electrophoresis, and the gels were stained in 500 ml distilled water with ethidium bromide (Bio-Rad Laboratories) at a concentration of 0.5 μg/ml for 15 min, which was followed by a 1-h wash with distilled water. A lambda ladder standard (Bio-Rad Laboratories) was included in three lanes of every gel to allow comparison of fingerprints in different gels. Gel DNA bands patterns were analyzed using the Bio 1D++ software (version 99; Vilber Lourmat). The Dice coefficient of similarity was calculated, and the unweighted-pair group method was used for cluster analysis (1% position tolerance). Groups containing more than two indistinguishable isolates were designated by using letters. A control strain belonging to PFGE group A was included in each gel.
PCR amplification.
DNA was extracted from all of the E. coli isolates with a DNeasy blood and tissue kit (Qiagen, Hilden, Germany) and stored at −20°C until it was required. The PCR assays, in which Taq DNA polymerase (MP Biomedicals, Qbiogene, France) was used, were optimized and performed using a PTC-200 thermocycler (MJ Research, United States). The phylogenetic lineage was determined for all of the E. coli isolates based on methods adapted from the methods of Clermont et al. (8). Three separate PCRs were performed with primers targeting the chuA and yjaA genes and the TspE4.C2 anonymous DNA locus. Samples which did not produce any positive amplicons were amplified with primers targeting 16S rRNA genes from Enterobacteriaceae (6). Representative E. coli Reference Collection strains were used as template controls. The major resistance genes for beta-lactams (blaTEM), sulfonamides (sulI, sulII, and sulIII), phenicols (floR, cmlA, catI, catII, and catIII), streptomycin-spectinomycin (strA-strB and aadA1), and tetracycline [tet(A) and tet(B)] and the integrase genes intI1 and intI2 were detected using the primers and E. coli control strains listed in Table 1.
TABLE 1.
PCR conditions and control strains
| Gene | Primer sequences | Reference | Fragment size (bp) | Annealing temp (°C) | Positive control |
|---|---|---|---|---|---|
| blaTEM | TTCCTGTTTTTGCTCACCCAG | 2 | 112 | 60 | JS238(pOFX326)a |
| CTCAAGGATCTTACCGCTGTTG | |||||
| sulI | TGGTGACGGTGTTCGGCATTC | 38 | 789 | 62 | Se 131/AJ238350b |
| GCGAGGGTTTCCGAGAAGGTG | |||||
| sulII | CGGCATCGTCAACATAACC | 30 | 722 | 53 | Se 678/EF090911b |
| GTGTGCGGATGAAGTCAG | |||||
| sulIII | CATTCTAGAAAACAGTCGTAGTTCG | 36 | 990 | 53 | U39b |
| CATCTGCAGCTAACCTAGGGCTTTGGA | |||||
| floR | CACGTTGAGCCTCTATAT | 38 | 868 | 55 | BN10660c |
| ATGCAGAAGTAGAACGCG | |||||
| cmlA | TGTCATTTACGGCATACTCG | 38 | 455 | 57 | 1587 pcmlAc |
| ATCAGGCATCCCATTCCCAT | |||||
| catI | AGTTGCTCAATGTACCTATAACC | 30 | 547 | 53 | BM14 R55 IncCc |
| TTGTAATTCATTAAGCATTCTGCC | |||||
| catII | ACACTTTGCCCTTTATCGTC | 30 | 543 | 50 | BM21 Rsa IncWc |
| TGAAAGCCATCACATACTGC | |||||
| catIII | TTCGCCGTGAGCATTTTG | 30 | 286 | 59 | HB101 pUC CATIIIc |
| TCGGATGATTATGGGCAAC | |||||
| strA-strB | TATCTGCGATTGGACCCTTGG | 47 | 538 | 60 | Se 678/EF090911b |
| CATTGCTCATCATTTGATCGGCT | |||||
| aadA1 | GAGAACATAGCGTTGCCTTGG | 47 | 198 | 53 | Se 131/AJ238350b |
| TCGGCGCGATTTTGCCGGTTAC | |||||
| tet(A) | TTGTTCCTGAAGTGCCAGTAA | 49 | 370 | 50 | UA064 BM13/RP4d |
| GACGTCGTTCGAGTGAACCAGA | |||||
| tet(B) | CTCAGTATTCCAAGCCTTTG | 19 | 435 | 53 | UA228 BM13/pIP69d |
| CTAAGCACTTGTCTCCTGTT | |||||
| intI1 | GGGTCAAGGATCTGGATTTCG | 31 | 483 | 60 | Se 131/AJ238350b |
| ACATGGGTGTAAATCATCGTC | |||||
| intI2 | CACGGATATGCGACAAAAAGGT | 31 | 788 | 60 | pR67e |
| GTAGCAAACGAGTGACGAAATG |
RESULTS
As no major differences were observed between the three treatment groups for the distribution of antimicrobial resistance phenotypes, PFGE genotypes, and phylogenetic groups, the results for the ampicillin-treated pigs were pooled.
Increase in resistance during ampicillin treatment.
Antimicrobial susceptibility tests were performed for a total of 308 E. coli isolates. Table 2 shows the percentages of E. coli isolates resistant to ampicillin, cephalothin, chloramphenicol, tetracycline, trimethoprim, streptomycin, and sulfonamides for ampicillin-treated and control pigs at days 0, 4, and 7. None of the E. coli isolates was resistant to ceftiofur. Three isolates were resistant to amoxicillin-clavulanic acid, and two isolates were resistant to gentamicin, kanamycin, neomycin, ciprofloxacin, and nalidixic acid. Before treatment, only 23% of the E. coli isolates were susceptible to all the antibiotics tested. Most of the isolates were resistant to tetracycline (69%), and 46%, 45%, and 20% of them were resistant to sulfonamides, trimethoprim, and streptomycin, respectively. Only 6% of the isolates were resistant to ampicillin. For the ampicillin-treated group, the percentages of ampicillin-resistant E. coli isolates were 93% at day 4 and 96% at day 7, and the percentages for resistance to other antibiotics also increased. In contrast, for the control group the percentage of isolates resistant to ampicillin never exceeded 25%.
TABLE 2.
Percentages of resistant E. coli isolates in ampicillin-treated and control groups at days 0, 4, and 7a
| Antimicrobial | % of resistant isolates
|
||||
|---|---|---|---|---|---|
| Day 0 (n = 78) | Day 4 (n = 80)
|
Day 7 (n = 77)
|
|||
| Treated | Control | Treated | Control | ||
| Ampicillin | 6 | 93 | 25 | 96 | 25 |
| Cephalothin | 0 | 3 | 5 | 7 | 0 |
| Chloramphenicol | 3 | 32 | 0 | 56 | 0 |
| Tetracycline | 69 | 90 | 85 | 89 | 65 |
| Trimethoprim | 45 | 58 | 0 | 68 | 5 |
| Streptomycin | 20 | 82 | 25 | 86 | 20 |
| Sulfonamides | 46 | 63 | 0 | 77 | 10 |
The percentages of susceptible isolates were as follows: day 0, 23%; and control group at day 7, 4%. There were no susceptible isolates in the treated group at day 4 or in the control group at day 4 or day 7.
Ampicillin-resistant antimicrobial phenotypes.
Eighteen different ampicillin-resistant phenotypes were identified. Ampicillin-resistant isolates were mainly multiresistant (resistant to ≥4 of the antimicrobial agents tested). They frequently exhibited additional resistance to tetracycline, streptomycin, sulfonamides, and trimethoprim. Table 3 shows the percentages for the six main ampicillin-resistant phenotypes of E. coli isolates from ampicillin-treated pigs at days 0, 4, and 7. The main ampicillin-resistant phenotype included resistance to five additional antibiotics: chloramphenicol, sulfonamides, tetracycline, trimethoprim, and streptomycin. Before treatment (day 0), the baseline percentage for the six-antibiotic-resistant phenotype was 5%; after 7 days of treatment, 53% of all the isolates had this phenotype, and this phenotype was present in 10 of the 12 treated pigs at day 7. In addition, ampicillin-resistant phenotypes that appeared during treatment were also found for E. coli isolates picked from ampicillin-containing agar before treatment (day 0 Ampr). The phenotype that included resistance to six antibiotics was the most frequent phenotype in the ampicillin-resistant strains before treatment.
TABLE 3.
Percentages of antimicrobial phenotypes for E. coli isolates from ampicillin-treated pigs at days 0, 4, and 7a
| Antimicrobial phenotypeb | % of isolates
|
|||
|---|---|---|---|---|
| Day 0 (n = 58) | Day 4 (n = 60) | Day 7 (n = 57) | Day 0 Ampr (n = 56) | |
| Amp-susceptible phenotypes | 88 | 6 | 5 | 0 |
| Amp-resistant phenotypes | 12 | 94 | 95 | 100 |
| Ampr Ter | 7 | 5 | 2 | |
| Ampr Ter Strr | 8 | 9 | 11 | |
| Ampr Sulr Ter Strr | 15 | 9 | 2 | |
| Ampr Chlr Sulr Trimr Strr | 5 | 2 | 5 | 20 |
| Ampr Sulr Ter Trimr Strr | 18 | 4 | 4 | |
| Ampr Chlr Sulr Ter Trimr Strr | 5 | 32 | 53 | 48 |
| Others | 2 | 12 | 10 | 13 |
E. coli isolates were picked from ampicillin-free plates (day 0, day 4, and day 7) or from ampicillin-containing plates before treatment (day 0 Ampr).
Amp, ampicillin; Chl, chloramphenicol; Sul, sulfonamides; Te, tetracycline; Trim, trimethoprim; Str, streptomycin.
Genetic composition of E. coli populations.
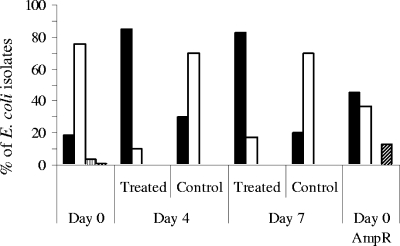
To investigate the effect of ampicillin administration on the genetic composition of E. coli populations, the phylogenetic groups of E. coli isolates were determined for ampicillin-treated and control pigs at days 0, 4, and 7 and for E. coli isolates picked from ampicillin-containing plates before treatment (day 0 Ampr) (Fig. 1). Ten of the 308 E. coli isolates typed were not classified. Before treatment (day 0), the four phylogenetic groups were represented in the E. coli isolates picked from ampicillin-free plates. The most abundant phylogenetic groups were groups B1 (75%) and A (18%), whereas groups B2 (4%) and D (1%) were rare. The percentage of isolates belonging to group B1 remained high in the control group at days 4 and 7, whereas the percentage fell to 10% in the treatment group. In E. coli strains isolated from ampicillin-treated pigs, group A became the most abundant phylogenetic group (85% of the isolates at day 4 and 82% of the isolates at day 7). Of the ampicillin-resistant isolates present before treatment (day 0 Ampr), 46% belonged to group A, 37% belonged to group B1, and 13% belonged to group D. In conclusion, at days 4 and 7, treatment with ampicillin led to a shift in the genetic composition of the fecal E. coli population, with selection for ampicillin-resistant isolates belonging mainly to group A.
FIG. 1.
Histogram plots for phylogenetic groups A (filled bars), B1 (open bars), B2 (bars with vertical stripes), and D (bars with diagonal stripes) of E. coli isolates from ampicillin-treated and control pigs at days 0, 4, and 7. E. coli isolates were picked from ampicillin-free plates (Day 0, Day 4, and Day 7) and also from ampicillin-containing plates before treatment (Day 0 AmpR).
Characteristics and distribution of the PFGE genotypes.
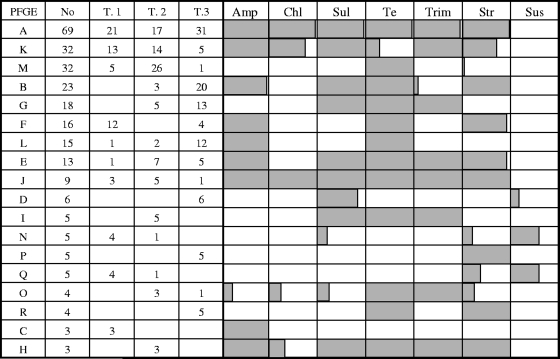
Among the 308 E. coli isolates subjected to PFGE typing, 46 genotypes were identified, and 2 isolates could not be typed by the PFGE method. Eighteen different PFGE groups containing more than two indistinguishable isolates were identified and designated PFGE groups A to R. Ten PFGE groups containing two indistinguishable isolates were also identified. The isolates belonging to these 10 groups were pooled in a group designated PFGE group Pr (n = 20). In addition, 18 unique fingerprints were identified, and they were pooled in a group designated PFGE group Uq (n = 18). Considerable variations in banding profiles were observed between the PFGE genotypes (data not shown). Each pig in the study harbored 3 to 11 different PFGE genotypes (mean, 6.8 PFGE genotypes). Figure 2 shows the distribution of the PFGE groups in the three trials in the experiment. The results indicated that pigs used in different trials (different batches) shared PFGE genotypes. Figure 2 also shows the relationship between the antimicrobial susceptibility phenotype and the PFGE group. For the main PFGE groups, a strong association between the genotype and the antimicrobial phenotype was observed, and ampicillin resistance was clearly associated with specific PFGE groups.
FIG. 2.
Distribution of PFGE groups in the three trials in the experiment and comparison of antimicrobial susceptibility phenotypes and PFGE groups. The percentage of isolates resistant to an antimicrobial is indicated by shading (all gray, 100%; all white, 0%). No, total number of isolates in the PGFE group; T.1, T.2, and T.3, number of isolates in the trial; Amp, ampicillin; Chl, chloramphenicol; Sul, sulfonamides; Te, tetracycline; Trim, trimethoprim; Str, streptomycin; Sus, susceptible.
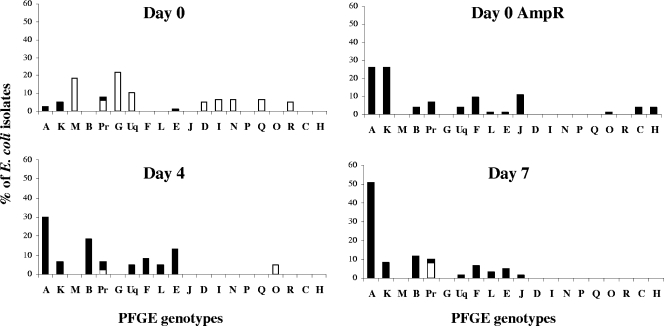
Figure 3 shows the percentage of E. coli isolates belonging to each PFGE type at days 0, 4, and 7 during ampicillin treatment. Figure 3 also shows the percentages of genotypes for E. coli isolates picked from ampicillin-containing agar before treatment (day 0 Ampr). Before ampicillin administration, the main PFGE group on ampicillin-free plates was PFGE group G (22% of the isolates), followed by PFGE group M (15%) (Fig. 3). Two main genotypes, genotypes A and K, encoded resistance to ampicillin. These two genotypes were the most abundant genotypes among the ampicillin-resistant isolates selected on ampicillin-containing plates before treatment (Fig. 3). At days 4 and 7, ampicillin treatment selected resistant E. coli isolates which were present in the digestive tract before treatment (Fig. 3). Moreover, ampicillin selected E. coli isolates belonging mainly to PFGE group A, as these isolates accounted for 53% of the isolates at day 7. PFGE genotypes associated with susceptibility to ampicillin before treatment were never associated with resistance to ampicillin during treatment.
FIG. 3.
Percentages of PFGE genotypes for E. coli isolates at days 0, 4, and 7 during ampicillin treatment. E. coli isolates were picked from ampicillin-free plates (Day 0, Day 4, and Day 7) or from ampicillin-containing plates before treatment (Day 0 AmpR). A to Q, PFGE groups with at least three indistinguishable isolates; Pr, pool of 10 groups containing two isolates each; Uq, pool of 18 unique isolates. The percentages of ampicillin-resistant isolates are indicated by the filled portions of bars, and the percentages of ampicillin-susceptible isolates are indicated by the open portions of bars.
PFGE genotypes and ampicillin-resistant phenotypes.
Comparison of phenotype profiles with PFGE genotypes revealed that 23 genotypes were associated with ampicillin-resistant phenotypes. Within- and between-group antimicrobial phenotype combinations were examined. Table 4 shows the percentages of PFGE genotypes associated with ampicillin-resistant phenotypes for E. coli isolates from ampicillin-treated pigs. Table 4 also shows the distribution of PFGE genotypes at days 0, 4, and 7 and the distribution for E. coli isolates picked from ampicillin-containing plates before treatment. The data show that one PFGE genotype could be associated with variable resistance patterns and that identical resistance patterns were observed for different PFGE genotypes. However, each resistance pattern was associated mainly with one PFGE group, and this association remained stable during treatment. PFGE group A was associated with the phenotype resistant to six antibiotics. This antibiotic-resistant phenotype was also associated with six other PFGE genotypes: groups J, K, H, and O and two unique fingerprints. However, 76% of the isolates which exhibited resistance to six antibiotics belonged to group A.
TABLE 4.
Relationship between PFGE genotypes and ampicillin-resistant phenotypes for E. coli isolates from ampicillin-treated pigs at days 0, 4, and 7a
| Antimicrobial phenotypeb | nc | PFGE genotyped | % of isolates
|
||||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 4 | Day 7 | Day 0 Ampr | Total | |||
| Ampr Ter | 9 | L | 34 | 22 | 11 | 67 | |
| Uq | 11 | 11 | |||||
| A | 11 | 11 | |||||
| F | 11 | 11 | |||||
| Ampr Ter Strr | 16 | F | 25 | 25 | 38 | 88 | |
| Pr | 6 | 6 | 12 | ||||
| Ampr Sulr Ter Strr | 20 | B | 50 | 25 | 15 | 90 | |
| Pr | 10 | 10 | |||||
| Ampr Chlr Sulr | 18 | K | 10 | 6 | 10 | 44 | 70 |
| Trimr Strr | A | 6 | 6 | 12 | |||
| Pr | 6 | 6 | 12 | ||||
| Uq | 6 | 6 | |||||
| Ampr Sulr Ter | 18 | E | 40 | 10 | 6 | 56 | |
| Trimr Strr | A | 6 | 6 | 12 | |||
| B | 10 | 10 | |||||
| H | 10 | 10 | |||||
| K | 6 | 6 | |||||
| Uq | 6 | 6 | |||||
| Ampr Chlr Sulr Ter | 83 | A | 2 | 20 | 34 | 20 | 76 |
| Trimr Strr | J | 1 | 10 | 11 | |||
| K | 1 | 1 | 7 | 9 | |||
| Uq | 2 | 2 | |||||
| H | 1 | 1 | |||||
| O | 1 | 1 | |||||
E. coli isolates were picked from ampicillin-free plates (day 0, day 4, and day 7) or from ampicillin-containing plates before treatment (day 0 Ampr).
Amp, ampicillin; Chl, chloramphenicol; Sul, sulfonamides; Te, tetracycline; Trim, trimethoprim, Str, streptomycin.
Number of E. coli isolates for each phenotype.
A, B, E, F, H, J, K, L, and O, PFGE groups with at least three indistinguishable isolates; Pr, pool of 10 groups containing two isolates each; Uq, pool of 18 unique isolates.
Resistance determinants and integrase genes.
Resistance determinants and integrase genes were amplified for the isolates associated with the phenotype resistant to six antibiotics. Table 5 shows that seven PFGE genotypes were associated with this phenotype and that isolates belonging to one PFGE type could belong to different phylogenetic groups (e.g., PFGE types A, J, and K). All in all, 11 different genotypes (combinations of PFGE type and phylogenetic group) were associated with this six-antibiotic-resistant phenotype. Screening of resistance determinants revealed that there were different combinations of resistance genes for the six-antibiotic-resistant phenotype. The strains belonging to PFGE group A (except for one isolate) harbored the following specific combination: blaTEM, catI, sulI, sulII, tet(B), strA-strB, and intI1. Another combination, blaTEM, cmlA, sulI, sulIII, tet(A), aadA1, and intI, was found for genotypes J and K. Four other different and unique combinations were also identified. Moreover, 49 of the 50 ampicillin-resistant isolates tested harbored blaTEM genes.
TABLE 5.
Distribution of antimicrobial resistance and integrase genes detected in the genotypes associated with the phenotype profile Ampr Chlr Sulr Ter Trimr Strra
| PFGE groupb | Phylogroup | No. of isolatesc | Resistance genes detected |
|---|---|---|---|
| A | A | 29 | blaTEM, catI, sulI, sulII, tet(B), strA-strB, intI1 |
| A | B1 | 1 | blaTEM, cmlA, sulI, sulIII, tet(A), aadA1, intI1 |
| J | B1 | 7 | blaTEM, cmlA, sulI, sulIII, tet(A), aadA1, intI1 |
| J | A | 1 | blaTEM, cmlA, sulI, sulIII, tet(A), aadA1, intI1 |
| J | A | 1 | blaTEM, catI, sulI, sulII, tet(B), strA-strB, intI1 |
| K | B1 | 6 | blaTEM, cmlA, sulI, sulIII, tet(A), aadA1, intI1 |
| K | A | 1 | tet(A), strA-strB, aadA1, intI2 |
| O | B1 | 1 | blaTEM, cmlA, sulI, sulIII, tet(A), aadA1, intI1 |
| H | D | 1 | blaTEM, sulI, sulII, tet(A), strA-strB, aadA1, intI1 |
| Uqa | B1 | 1 | blaTEM, catI, sulI, sulII, tet(A), strA-strB, aadA1, intI1 |
| Uqb | A | 1 | blaTEM, tet(A) |
Amp, ampicillin; Chl, chloramphenicol; Sul, sulfonamides; Te, tetracycline; Trim, trimethoprim, Str, streptomycin.
Groups are indicated by letters; unique PFGE genotypes are indicated by Uqa and Uqb.
For the genotypes, all the isolates with a genotype were screened, except for PFGE group A phylogroup A, for which 50% of the isolates were tested.
DISCUSSION
In this study we showed that ampicillin administration in swine led to clonal selection of ampicillin-resistant fecal E. coli strains belonging to phylogenetic group A. These strains were present at low levels before treatment in the digestive tract of the pigs. We also showed that the principal ampicillin-resistant phenotype selected by ampicillin administration included resistance to six antibiotics (ampicillin, chloramphenicol, sulfonamide, tetracycline, trimethoprim, and streptomycin). Moreover, this phenotype was linked mainly to one PFGE genotype which was characterized by a specific combination of resistance genes and which was the predominant PFGE genotype in the ampicillin-resistant strains harbored by the pig intestinal flora before any antibiotic treatment.
The animals involved in the present investigation were used in a previous study, during which we obtained counts for ampicillin-resistant Enterobacteriaceae in the same fecal samples (2). We previously observed that ampicillin-resistant Enterobacteriaceae isolates (mainly E. coli isolates) increased in number (2), which is in agreement with the observation in this study that ampicillin treatment was associated with an increase in the percentage of ampicillin-resistant E. coli isolates from 6% to 96%. Together, these results suggest that ampicillin treatment actually selected ampicillin-resistant E. coli strains in the digestive tract.
Before treatment, the main phylogenetic group among E. coli commensal strains was group B1, followed by group A. Groups B2 and D, which potentially include virulent extraintestinal strains (5, 29, 33), were rare. The predominance of group B1 has also been observed in fecal E. coli populations of bovine origin (21, 51). In swine, it was shown that most commensal strains belonged to groups A and B1 (7), with occasional predominance of phylogroup A (41, 52). In our study, ampicillin treatment led to a shift in the composition of the fecal E. coli population, with selection for ampicillin-resistant strains belonging to phylogroup A. This result can be related to the results of Walk et al. (51), who found an overabundance of resistant phylogroup A strains on conventional dairy farms compared with organic farms. They hypothesized that the difference in the genetic compositions of the resident E. coli flora might be linked to greater use of ampicillin on conventional farms.
Ampicillin-resistant strains that appeared during treatment had several antimicrobial phenotypes, mainly multiresistant phenotypes (resistance to at least four antimicrobial agents). These phenotypes were present in the digestive tract before treatment. The main ampicillin-resistant phenotype included resistance to six antibiotics and was the most abundant phenotype on ampicillin-containing plates before treatment. We used PFGE typing to assess the potential clonal spread of ampicillin-resistant strains. The distribution of PFGE genotypes of E. coli isolates at days 0, 4, and 7 during treatment and of E. coli isolates picked from ampicillin-containing plates before treatment showed that ampicillin administration selected ampicillin-resistant E. coli isolates which were present in the digestive tract before treatment. Comparison of antimicrobial phenotypes with PFGE groups revealed that the PFGE genotypes could be associated with different antimicrobial phenotypes and that antimicrobial phenotypes could be associated with different PFGE genotypes. These results suggest that horizontal transfer or enrichment of resistance determinants can occur in the intestinal E. coli population. As we have shown that ampicillin resistance was correlated mainly with the presence of blaTEM genes, which are plasmid mediated (27), the presence of many different PFGE genotypes in the ampicillin-resistant population is not surprising. However, each ampicillin-resistant phenotype was linked mainly to one PFGE genotype. This result suggests that resistance traits were disseminated by vertical transmission through defined strains. When Mentula et al. studied the impact of ampicillin administration on the fecal flora in dogs, they found considerable diversity in ampicillin-resistant strains using PFGE typing (32). Their results indicated that there was selection of genetically heterogeneous resistant E. coli strains rather than selection of a single clone or emergence of resistance in previously susceptible strains. In our study, we showed that, among the various ampicillin-resistant strains, E. coli isolates exhibiting resistance to six antibiotics were predominant and were associated mainly with one genotype. In addition, this six-antibiotic-resistant phenotype was the most abundant phenotype in the ampicillin-resistant E. coli population before treatment.
Comparison of PFGE genotypes with phylogenetic groups revealed that that a PFGE profile could be associated with different phylogenetic groups (Table 5). PFGE typing involves digesting DNA with restriction endonucleases that cleave infrequently (we used the XbaI enzyme in our study), and the DNA restriction patterns obtained depend on the number and location of the restriction sites for the enzyme (15). Different PFGE banding patterns reflect differences in the genome, but identical patterns are not synonymous with identical genomes (50). Identification of the phylogenetic group was based on screening of three chromosomal genes by PCR using the method described by Clermont et al. (8). Strains belonging to different phylogenetic groups have at least one difference in the genome, but the difference is not necessarily seen when the strains are typed by PFGE. This fact explains why we observed different phylogenetic groups that were associated with the same PFGE genotype.
In the present study, we used pigs obtained from the same herd but from distinct batches, which were temporally distinct, and from different sows. Our results indicated that these pigs shared E. coli isolates associated with the same genotypes. This result suggests that a reservoir of antimicrobial-resistant E. coli strains could be present in the pig farm environment, as demonstrated previously for cattle and broilers (40, 42). As mentioned by other workers, mechanisms unrelated to specific antimicrobial selection could be implicated in the wide distribution and maintenance of the antimicrobial resistance genes. These mechanisms include plasmid addiction, close linkage to other selectively advantageous genes (45), and a limited metabolic burden associated with carriage of antimicrobial resistance genes (12, 35), and in some instances, a secondary advantage can be provided by the antimicrobial resistance genes in the absence of specific antimicrobial drug selection (11). In our study, we showed that one of the seven PFGE genotypes associated with the six-antibiotic-resistant phenotype, PFGE genotype A, included a specific combination of antimicrobial resistance determinants. As suggested by Livermore (26), we hypothesize that this genotype encoding resistance to ampicillin is particularly well adapted because it may represent a combination of resistance mechanisms that impose a small fitness burden on a biologically fit host strain.
In conclusion, ampicillin administration in pigs selected ampicillin-resistant strains from a reservoir of strains that were present in the commensal flora before treatment. One strain with a genotype encoding resistance to six antibiotics persisted in the digestive tract at a low level, and this strain became the predominant strain as soon as ampicillin was present in the digestive tract. The accumulation of resistance in this strain might reflect the level of selection pressure and the fitness of the strain. In order to prevent the emergence and spread of resistance, the mechanisms by which the antimicrobial resistance genes are maintained in the digestive tract need to be identified.
Acknowledgments
This work was supported by grant 03011987 from the Conseil Régional de Midi-Pyrénées.
We thank N. Arpaillange and G. Faucon for technical support and A. Ferran for critical reading of the manuscript. We are grateful to B. Doublet, A. Cloeckaert, M. Grape, and M. Sunde for providing the control strains.
Footnotes
Published ahead of print on 6 March 2009.
REFERENCES
- 1.Aarts, H. J., K. S. Boumedine, X. Nesme, and A. Cloeckaert. 2001. Molecular tools for the characterisation of antibiotic-resistant bacteria. Vet. Res. 32:363-380. [DOI] [PubMed] [Google Scholar]
- 2.Bibbal, D., V. Dupouy, J. P. Ferre, P. L. Toutain, O. Fayet, M. F. Prere, and A. Bousquet-Melou. 2007. Impact of three ampicillin dosage regimens on selection of ampicillin resistance in Enterobacteriaceae and excretion of blaTEM genes in swine feces. Appl. Environ. Microbiol. 73:4785-4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake, D. P., R. W. Humphry, K. P. Scott, K. Hillman, D. R. Fenlon, and J. C. Low. 2003. Influence of tetracycline exposure on tetracycline resistance and the carriage of tetracycline resistance genes within commensal Escherichia coli populations. J. Appl. Microbiol. 94:1087-1097. [DOI] [PubMed] [Google Scholar]
- 4.Boerlin, P., R. Travis, C. L. Gyles, R. Reid-Smith, N. Janecko, H. Lim, V. Nicholson, S. A. McEwen, R. Friendship, and M. Archambault. 2005. Antimicrobial resistance and virulence genes of Escherichia coli isolates from swine in Ontario. Appl. Environ. Microbiol. 71:6753-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonacorsi, S., and E. Bingen. 2005. Molecular epidemiology of Escherichia coli causing neonatal meningitis. Int. J. Med. Microbiol. 295:373-381. [DOI] [PubMed] [Google Scholar]
- 6.Castillo, M., S. M. Martin-Orue, E. G. Manzanilla, I. Badiola, M. Martin, and J. Gasa. 2006. Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR. Vet. Microbiol. 114:165-170. [DOI] [PubMed] [Google Scholar]
- 7.Chapman, T. A., X. Y. Wu, I. Barchia, K. A. Bettelheim, S. Driesen, D. Trott, M. Wilson, and J. J. Chin. 2006. Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl. Environ. Microbiol. 72:4782-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CLSI. 2006. Performance standards for antimicobial disk susceptibility tests. Approved standard, 9th ed. Document M2-A9. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.CLSI. 2007. Performance standards for antimicrobial susceptibility testing, 17th informational supplement. Document M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 11.Enne, V. I., P. M. Bennett, D. M. Livermore, and L. M. Hall. 2004. Enhancement of host fitness by the sul2-coding plasmid p9123 in the absence of selective pressure. J. Antimicrob. Chemother. 53:958-963. [DOI] [PubMed] [Google Scholar]
- 12.Enne, V. I., A. A. Delsol, G. R. Davis, S. L. Hayward, J. M. Roe, and P. M. Bennett. 2005. Assessment of the fitness impacts on Escherichia coli of acquisition of antibiotic resistance genes encoded by different types of genetic element. J. Antimicrob. Chemother. 56:544-551. [DOI] [PubMed] [Google Scholar]
- 13.FEDESA. 2000. The microbial threat. Press release on the European Union conference. FEDESA, Copenhagen, Denmark.
- 14.Foley, S. L., S. Simjee, J. Meng, D. G. White, P. F. McDermott, and S. Zhao. 2004. Evaluation of molecular typing methods for Escherichia coli O157:H7 isolates from cattle, food, and humans. J. Food Prot. 67:651-657. [DOI] [PubMed] [Google Scholar]
- 15.Gautom, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gellin, G., B. E. Langlois, K. A. Dawson, and D. K. Aaron. 1989. Antibiotic resistance of gram-negative enteric bacteria from pigs in three herds with different histories of antibiotic exposure. Appl. Environ. Microbiol. 55:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerner-Smidt, P., K. Hise, J. Kincaid, S. Hunter, S. Rolando, E. Hyytia-Trees, E. M. Ribot, and B. Swaminathan. 2006. PulseNet USA: a five-year update. Foodborne Pathog. Dis. 3:9-19. [DOI] [PubMed] [Google Scholar]
- 18.Grape, M., A. Farra, G. Kronvall, and L. Sundstrom. 2005. Integrons and gene cassettes in clinical isolates of co-trimoxazole-resistant Gram-negative bacteria. Clin. Microbiol. Infect. 11:185-192. [DOI] [PubMed] [Google Scholar]
- 19.Guardabassi, L., L. Dijkshoorn, J. M. Collard, J. E. Olsen, and A. Dalsgaard. 2000. Distribution and in-vitro transfer of tetracycline resistance determinants in clinical and aquatic Acinetobacter strains. J. Med. Microbiol. 49:929-936. [DOI] [PubMed] [Google Scholar]
- 20.Guerra, B., E. Junker, A. Schroeter, R. Helmuth, B. E. Guth, and L. Beutin. 2006. Phenotypic and genotypic characterization of antimicrobial resistance in Escherichia coli O111 isolates. J. Antimicrob. Chemother. 57:1210-1214. [DOI] [PubMed] [Google Scholar]
- 21.Houser, B. A., S. C. Donaldson, R. Padte, A. A. Sawant, C. DebRoy, and B. M. Jayarao. 2008. Assessment of phenotypic and genotypic diversity of Escherichia coli shed by healthy lactating dairy cattle. Foodborne Pathog. Dis. 5:41-51. [DOI] [PubMed] [Google Scholar]
- 22.Hoyle, D. V., H. C. Davison, H. I. Knight, C. M. Yates, O. Dobay, G. J. Gunn, S. G. Amyes, and M. E. Woolhouse. 2006. Molecular characterisation of bovine faecal Escherichia coli shows persistence of defined ampicillin resistant strains and the presence of class 1 integrons on an organic beef farm. Vet. Microbiol. 115:250-257. [DOI] [PubMed] [Google Scholar]
- 23.Hoyle, D. V., C. M. Yates, M. E. Chase-Topping, E. J. Turner, S. E. Davies, J. C. Low, G. J. Gunn, M. E. Woolhouse, and S. G. Amyes. 2005. Molecular epidemiology of antimicrobial-resistant commensal Escherichia coli strains in a cohort of newborn calves. Appl. Environ. Microbiol. 71:6680-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyytia-Trees, E. K., K. Cooper, E. M. Ribot, and P. Gerner-Smidt. 2007. Recent developments and future prospects in subtyping of foodborne bacterial pathogens. Future Microbiol. 2:175-185. [DOI] [PubMed] [Google Scholar]
- 25.Lanz, R., P. Kuhnert, and P. Boerlin. 2003. Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Vet. Microbiol. 91:73-84. [DOI] [PubMed] [Google Scholar]
- 26.Livermore, D. 2007. The zeitgeist of resistance. J. Antimicrob. Chemother. 60(Suppl. 1):i59-i61. [DOI] [PubMed] [Google Scholar]
- 27.Livermore, D. M. 1998. Beta-lactamase-mediated resistance and opportunities for its control. J. Antimicrob. Chemother. 41(Suppl. D):25-41. [DOI] [PubMed] [Google Scholar]
- 28.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maynard, C., S. Bekal, F. Sanschagrin, R. C. Levesque, R. Brousseau, L. Masson, S. Lariviere, and J. Harel. 2004. Heterogeneity among virulence and antimicrobial resistance gene profiles of extraintestinal Escherichia coli isolates of animal and human origin. J. Clin. Microbiol. 42:5444-5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maynard, C., J. M. Fairbrother, S. Bekal, F. Sanschagrin, R. C. Levesque, R. Brousseau, L. Masson, S. Lariviere, and J. Harel. 2003. Antimicrobial resistance genes in enterotoxigenic Escherichia coli O149:K91 isolates obtained over a 23-year period from pigs. Antimicrob. Agents Chemother. 47:3214-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazel, D., B. Dychinco, V. A. Webb, and J. Davies. 2000. Antibiotic resistance in the ECOR collection: integrons and identification of a novel aad gene. Antimicrob. Agents Chemother. 44:1568-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mentula, S., T. Virtanen, A. Kanervo-Nordstrom, J. Harmoinen, E. Westermarck, M. Rautio, P. Huovinen, and E. Kononen. 2006. Relatedness of Escherichia coli strains with different susceptibility patterns isolated from beagle dogs during ampicillin treatment. Int. J. Antimicrob. Agents 27:46-50. [DOI] [PubMed] [Google Scholar]
- 33.Moulin-Schouleur, M., M. Reperant, S. Laurent, A. Bree, S. Mignon- Grasteau, P. Germon, D. Rasschaert, and C. Schouler. 2007. Extraintestinal pathogenic Escherichia coli strains of avian and human origin: link between phylogenetic relationships and common virulence patterns. J. Clin. Microbiol. 45:3366-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NCCLS/CLSI. 2004. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, informational supplement. Document M31-S1. NCCLS/CLSI, Wayne, PA.
- 35.Nguyen, T. N., Q. G. Phan, L. P. Duong, K. P. Bertrand, and R. E. Lenski. 1989. Effects of carriage and expression of the Tn10 tetracycline-resistance operon on the fitness of Escherichia coli K12. Mol. Biol. Evol. 6:213-225. [DOI] [PubMed] [Google Scholar]
- 36.Perreten, V., and P. Boerlin. 2003. A new sulfonamide resistance gene (sul3) in Escherichia coli is widespread in the pig population of Switzerland. Antimicrob. Agents Chemother. 47:1169-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips, I., M. Casewell, T. Cox, B. De Groot, C. Friis, R. Jones, C. Nightingale, R. Preston, and J. Waddell. 2004. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 53:28-52. [DOI] [PubMed] [Google Scholar]
- 38.Saenz, Y., L. Brinas, E. Dominguez, J. Ruiz, M. Zarazaga, J. Vila, and C. Torres. 2004. Mechanisms of resistance in multiple-antibiotic-resistant Escherichia coli strains of human, animal, and food origins. Antimicrob. Agents Chemother. 48:3996-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salyers, A. A., A. Gupta, and Y. Wang. 2004. Human intestinal bacteria as reservoirs for antibiotic resistance genes. Trends Microbiol. 12:412-416. [DOI] [PubMed] [Google Scholar]
- 40.Sawant, A. A., N. V. Hegde, B. A. Straley, S. C. Donaldson, B. C. Love, S. J. Knabel, and B. M. Jayarao. 2007. Antimicrobial-resistant enteric bacteria from dairy cattle. Appl. Environ. Microbiol. 73:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schierack, P., N. Walk, K. Reiter, K. D. Weyrauch, and L. H. Wieler. 2007. Composition of intestinal Enterobacteriaceae populations of healthy domestic pigs. Microbiology 153:3830-3837. [DOI] [PubMed] [Google Scholar]
- 42.Smith, J. L., D. J. Drum, Y. Dai, J. M. Kim, S. Sanchez, J. J. Maurer, C. L. Hofacre, and M. D. Lee. 2007. Impact of antimicrobial usage on antimicrobial resistance in commensal Escherichia coli strains colonizing broiler chickens. Appl. Environ. Microbiol. 73:1404-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorum, H., and M. Sunde. 2001. Resistance to antibiotics in the normal flora of animals. Vet. Res. 32:227-241. [DOI] [PubMed] [Google Scholar]
- 44.Stefani, S., and A. Agodi. 2000. Molecular epidemiology of antibiotic resistance. Int. J. Antimicrob. Agents 13:143-153. [DOI] [PubMed] [Google Scholar]
- 45.Summers, A. O. 2002. Generally overlooked fundamentals of bacterial genetics and ecology. Clin. Infect. Dis. 34(Suppl. 3):S85-S92. [DOI] [PubMed] [Google Scholar]
- 46.Sunde, M., K. Fossum, A. Solberg, and H. Sorum. 1998. Antibiotic resistance in Escherichia coli of the normal intestinal flora of swine. Microb. Drug Resist. 4:289-299. [DOI] [PubMed] [Google Scholar]
- 47.Sunde, M., and M. Norstrom. 2005. The genetic background for streptomycin resistance in Escherichia coli influences the distribution of MICs. J. Antimicrob. Chemother. 56:87-90. [DOI] [PubMed] [Google Scholar]
- 48.Sunde, M., and M. Norstrom. 2006. The prevalence of, associations between and conjugal transfer of antibiotic resistance genes in Escherichia coli isolated from Norwegian meat and meat products. J. Antimicrob. Chemother. 58:741-747. [DOI] [PubMed] [Google Scholar]
- 49.Sunde, M., and H. Sorum. 2001. Self-transmissible multidrug resistance plasmids in Escherichia coli of the normal intestinal flora of healthy swine. Microb. Drug Resist. 7:191-196. [DOI] [PubMed] [Google Scholar]
- 50.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walk, S. T., J. M. Mladonicky, J. A. Middleton, A. J. Heidt, J. R. Cunningham, P. Bartlett, K. Sato, and T. S. Whittam. 2007. Influence of antibiotic selection on genetic composition of Escherichia coli populations from conventional and organic dairy farms. Appl. Environ. Microbiol. 73:5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu, X. Y., T. Chapman, D. J. Trott, K. Bettelheim, T. N. Do, S. Driesen, M. J. Walker, and J. Chin. 2007. Comparative analysis of virulence genes, genetic diversity, and phylogeny of commensal and enterotoxigenic Escherichia coli isolates from weaned pigs. Appl. Environ. Microbiol. 73:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]