Abstract
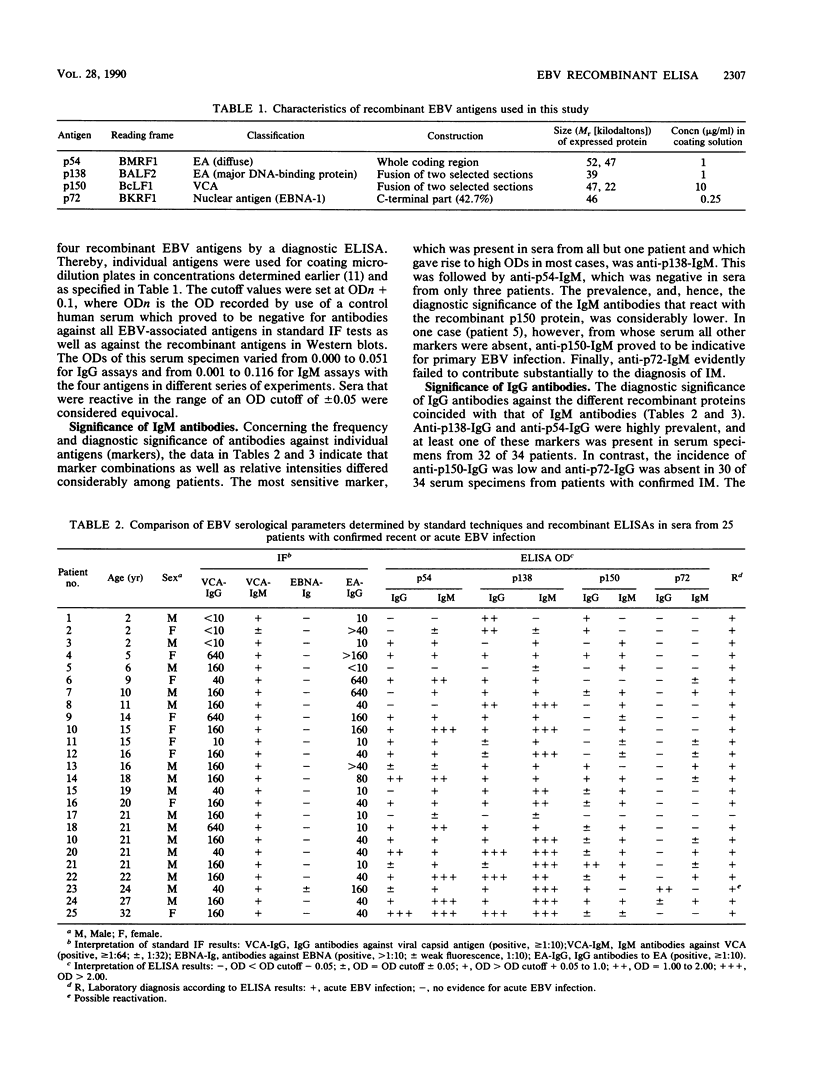
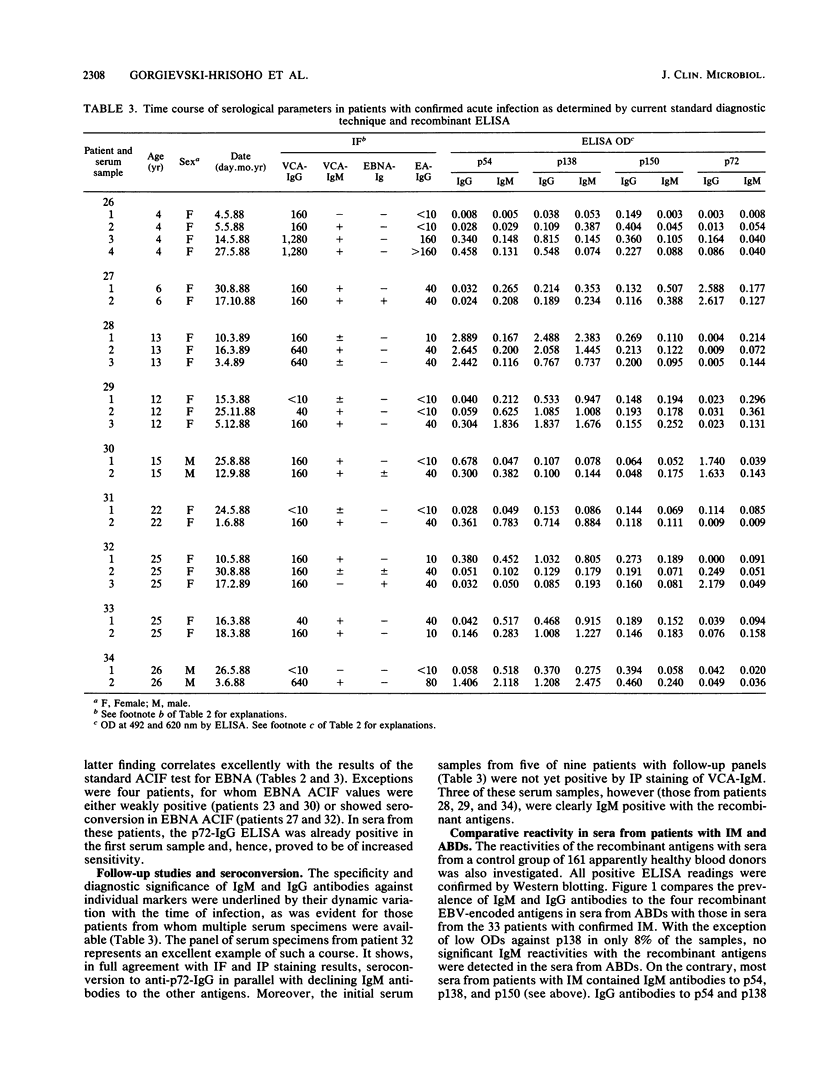
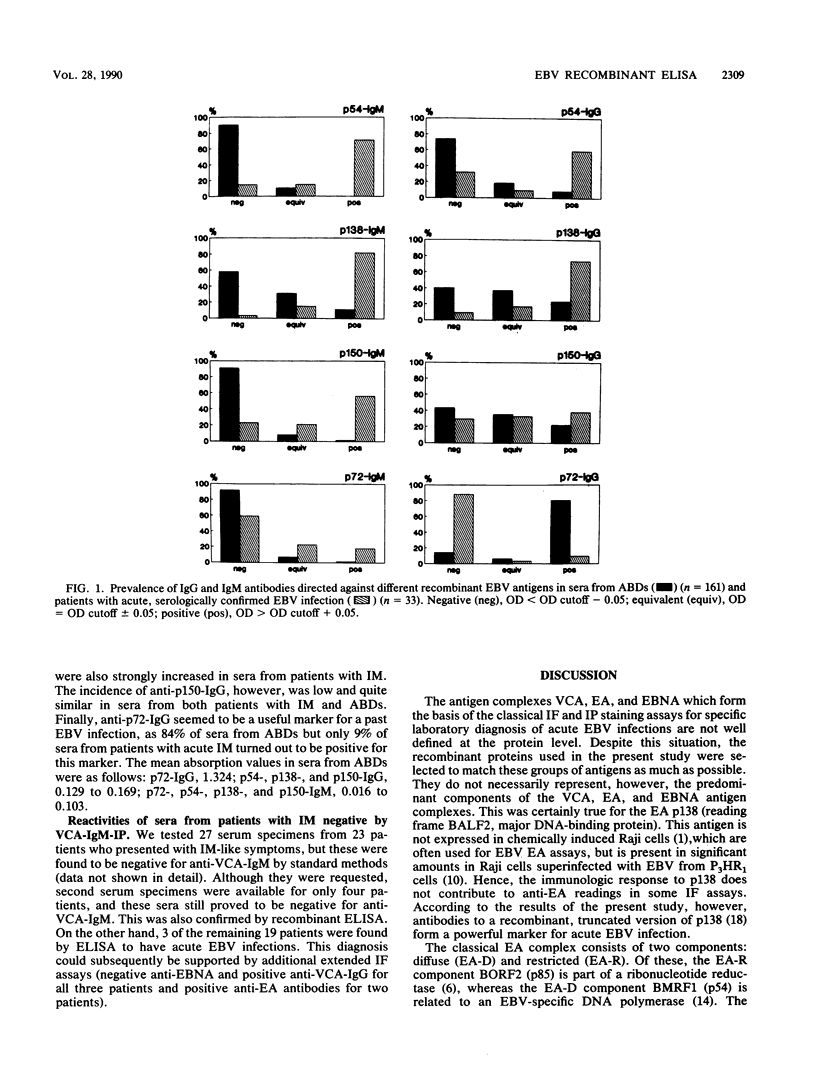
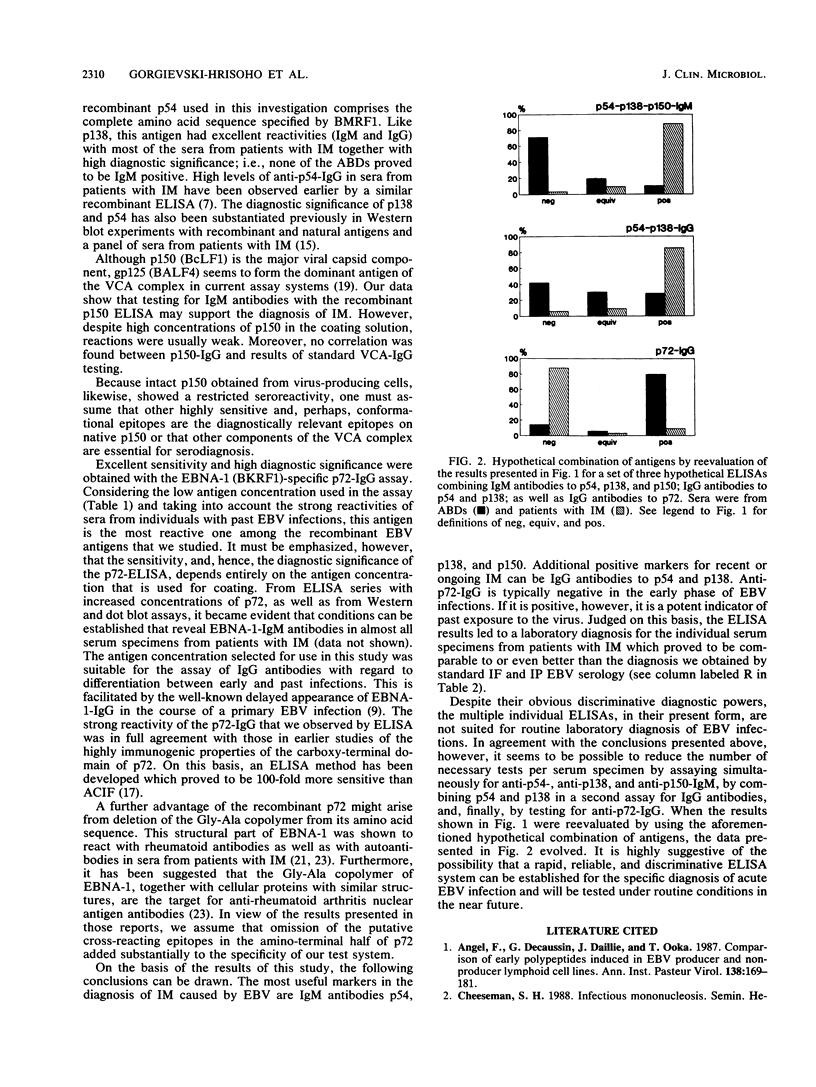
Four recombinant, diagnostically useful Epstein-Barr virus (EBV) proteins representative of the viral capsid antigen (p150), diffuse early antigen (p54), the major DNA-binding protein (p138), and the EBV nuclear antigen (p72) (W. Hinderer, H. Nebel-Schickel, H.H. Sonneborn, M. Motz, R. Kühbeck, and H. Wolf, J. Exp. Clin. Cancer Res. 7[Suppl.]:132, 1988) were used to set up individual enzyme-linked immunosorbent assays (ELISAs) for the qualitative and quantitative detection of immunoglobulin M (IgM) and IgG antibodies. In direct comparison with results obtained by standard immunofluorescence or immunoperoxidase assays, it was then shown that the recombinant EBV ELISAs provide the means for specific and sensitive serodiagnosis of infectious mononucleosis (IM) caused by EBV. The most useful markers in sera from such patients proved to be IgM antibodies against p54, p138, and p150. Additional positive markers for recent or ongoing IM apparently were IgG antibodies against p54 and p138. In contrast, anti-p72 IgG had a high preference for sera from healthy blood donors and, therefore, can be considered indicative of past exposure to the virus. Altogether, the individual ELISAs proved to be as specific and at least as sensitive for the diagnosis of IM as the currently available standard techniques are. Moreover, our findings suggest that, by combining individual test antigens, a workable ELISA system consisting of three assays (IgM against p54, p138, and p150; IgG against p54 and p138; and IgG against p72) can be established for the standardized rapid diagnosis of acute EBV infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans A. S., Niederman J. C., Cenabre L. C., West B., Richards V. A. A prospective evaluation of heterophile and Epstein-Barr virus-specific IgM antibody tests in clinical and subclinical infectious mononucleosis: Specificity and sensitivity of the tests and persistence of antibody. J Infect Dis. 1975 Nov;132(5):546–554. doi: 10.1093/infdis/132.5.546. [DOI] [PubMed] [Google Scholar]
- Goldschmidts W. L., Ginsburg M., Pearson G. R. Neutralization of Epstein-Barr virus-induced ribonucleotide reductase with antibody to the major restricted early antigen polypeptide. Virology. 1989 May;170(1):330–333. doi: 10.1016/0042-6822(89)90390-5. [DOI] [PubMed] [Google Scholar]
- Halprin J., Scott A. L., Jacobson L., Levine P. H., Ho J. H., Niederman J. C., Hayward S. D., Milman G. Enzyme-linked immunosorbent assay of antibodies to Epstein-Barr virus nuclear and early antigens in patients with infectious mononucleosis and nasopharyngeal carcinoma. Ann Intern Med. 1986 Mar;104(3):331–337. doi: 10.7326/0003-4819-104-3-331. [DOI] [PubMed] [Google Scholar]
- Henle G., Lennette E. T., Alspaugh M. A., Henle W. Rheumatoid factor as a cause of positive reactions in tests for Epstein-Barr virus-specific IgM antibodies. Clin Exp Immunol. 1979 Jun;36(3):415–422. [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G. E., Horwitz C. A. Epstein-Barr virus specific diagnostic tests in infectious mononucleosis. Hum Pathol. 1974 Sep;5(5):551–565. doi: 10.1016/s0046-8177(74)80006-7. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., Andersson J., Ernberg I., Klein G., Horwitz C. A., Marklund G., Rymo L., Wellinder C., Straus S. E. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc Natl Acad Sci U S A. 1987 Jan;84(2):570–574. doi: 10.1073/pnas.84.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li J. S., Zhou B. S., Dutschman G. E., Grill S. P., Tan R. S., Cheng Y. C. Association of Epstein-Barr virus early antigen diffuse component and virus-specified DNA polymerase activity. J Virol. 1987 Sep;61(9):2947–2949. doi: 10.1128/jvi.61.9.2947-2949.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp J. M., Herbrink P. Epstein-Barr virus specific marker molecules for early diagnosis of infectious mononucleosis. J Virol Methods. 1988 Sep;21(1-4):133–146. doi: 10.1016/0166-0934(88)90060-2. [DOI] [PubMed] [Google Scholar]
- Middeldorp J. M., Meloen R. H. Epitope-mapping on the Epstein-Barr virus major capsid protein using systematic synthesis of overlapping oligopeptides. J Virol Methods. 1988 Sep;21(1-4):147–159. doi: 10.1016/0166-0934(88)90061-4. [DOI] [PubMed] [Google Scholar]
- Milman G., Scott A. L., Cho M. S., Hartman S. C., Ades D. K., Hayward G. S., Ki P. F., August J. T., Hayward S. D. Carboxyl-terminal domain of the Epstein-Barr virus nuclear antigen is highly immunogenic in man. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6300–6304. doi: 10.1073/pnas.82.18.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motz M., Fan J., Seibl R., Jilg W., Wolf H. Expression of the Epstein-Barr virus 138-kDa early protein in Escherichia coli for the use as antigen in diagnostic tests. Gene. 1986;42(3):303–312. doi: 10.1016/0378-1119(86)90234-9. [DOI] [PubMed] [Google Scholar]
- Pearson G. R. ELISA tests and monoclonal antibodies for EBV. J Virol Methods. 1988 Sep;21(1-4):97–104. doi: 10.1016/0166-0934(88)90056-0. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Rhodes G., Rumpold H., Kurki P., Patrick K. M., Carson D. A., Vaughan J. H. Autoantibodies in infectious mononucleosis have specificity for the glycine-alanine repeating region of the Epstein-Barr virus nuclear antigen. J Exp Med. 1987 Apr 1;165(4):1026–1040. doi: 10.1084/jem.165.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumaya C. V., Ench Y. Epstein-Barr virus infectious mononucleosis in children. II. Heterophil antibody and viral-specific responses. Pediatrics. 1985 Jun;75(6):1011–1019. [PubMed] [Google Scholar]
- Venables P. J., Pawlowski T., Mumford P. A., Brown C., Crawford D. H., Maini R. N. Reaction of antibodies to rheumatoid arthritis nuclear antigen with a synthetic peptide corresponding to part of Epstein-Barr nuclear antigen 1. Ann Rheum Dis. 1988 Apr;47(4):270–279. doi: 10.1136/ard.47.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]


