Abstract
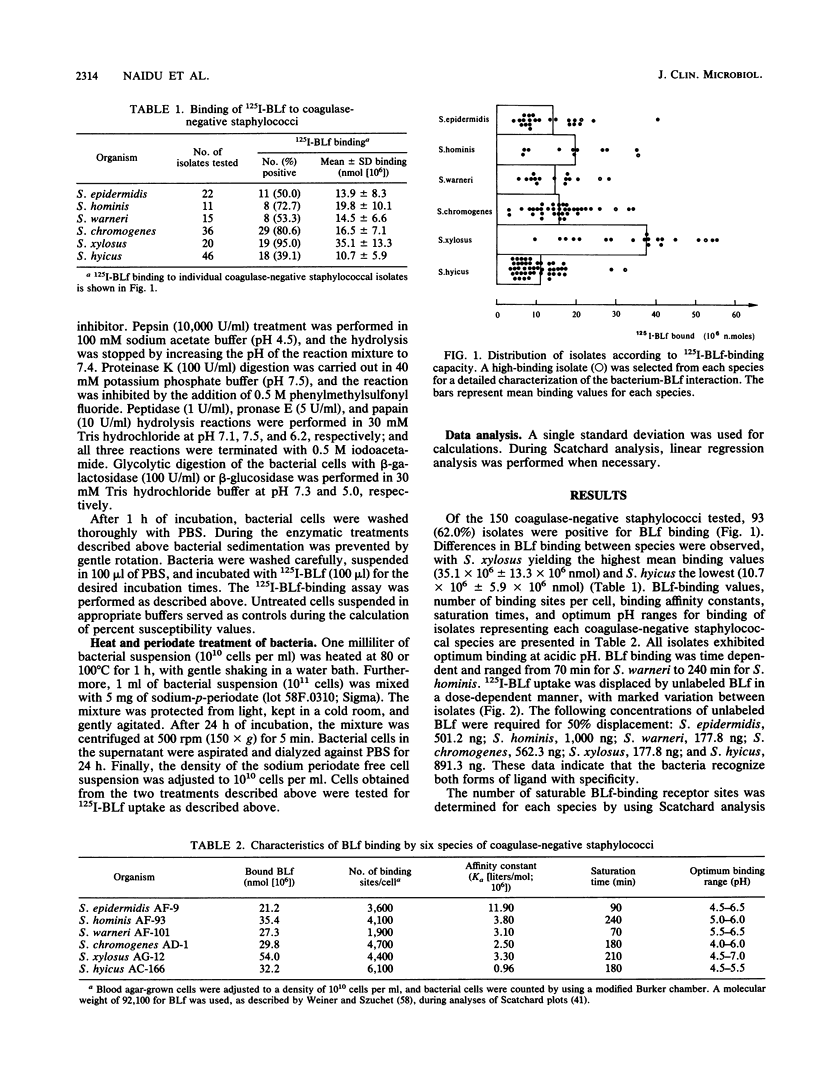
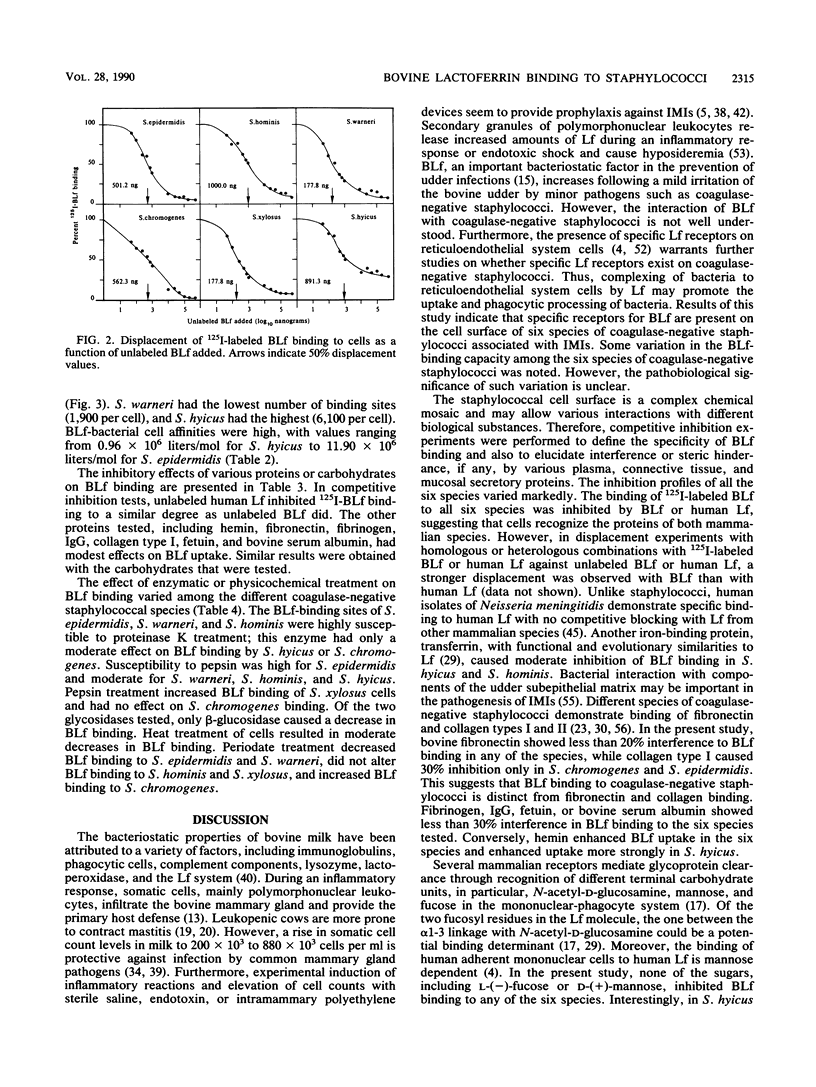
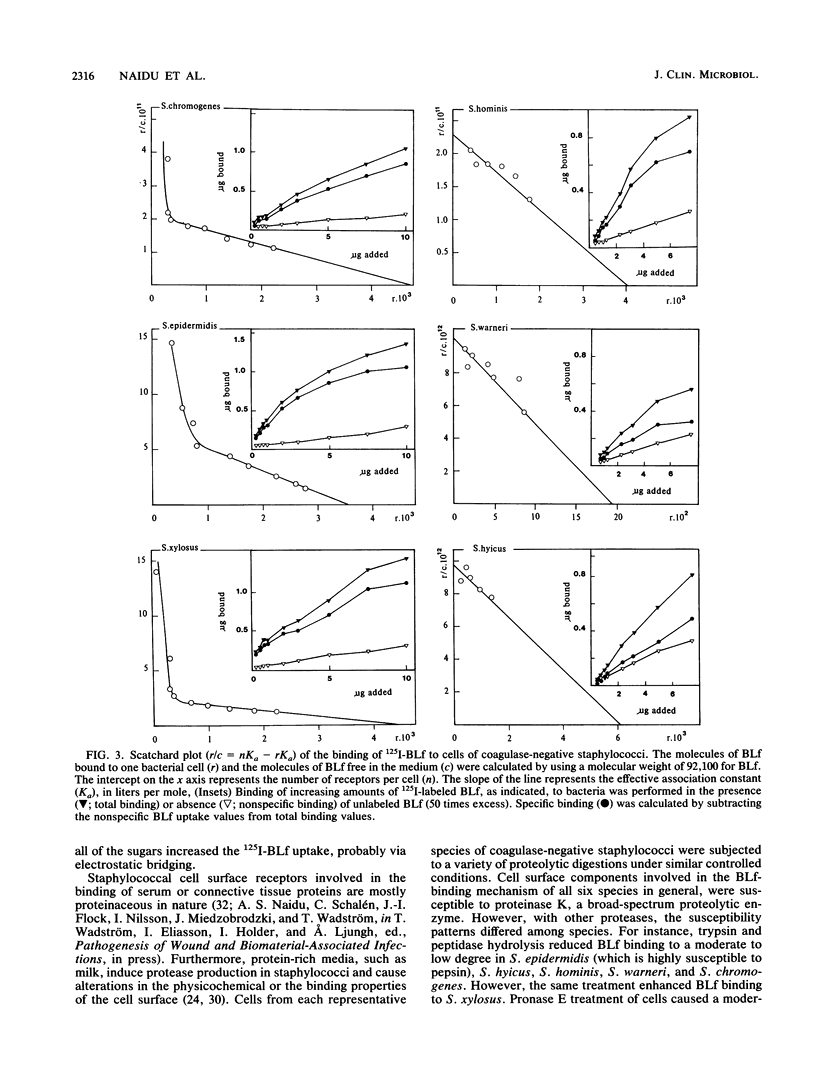
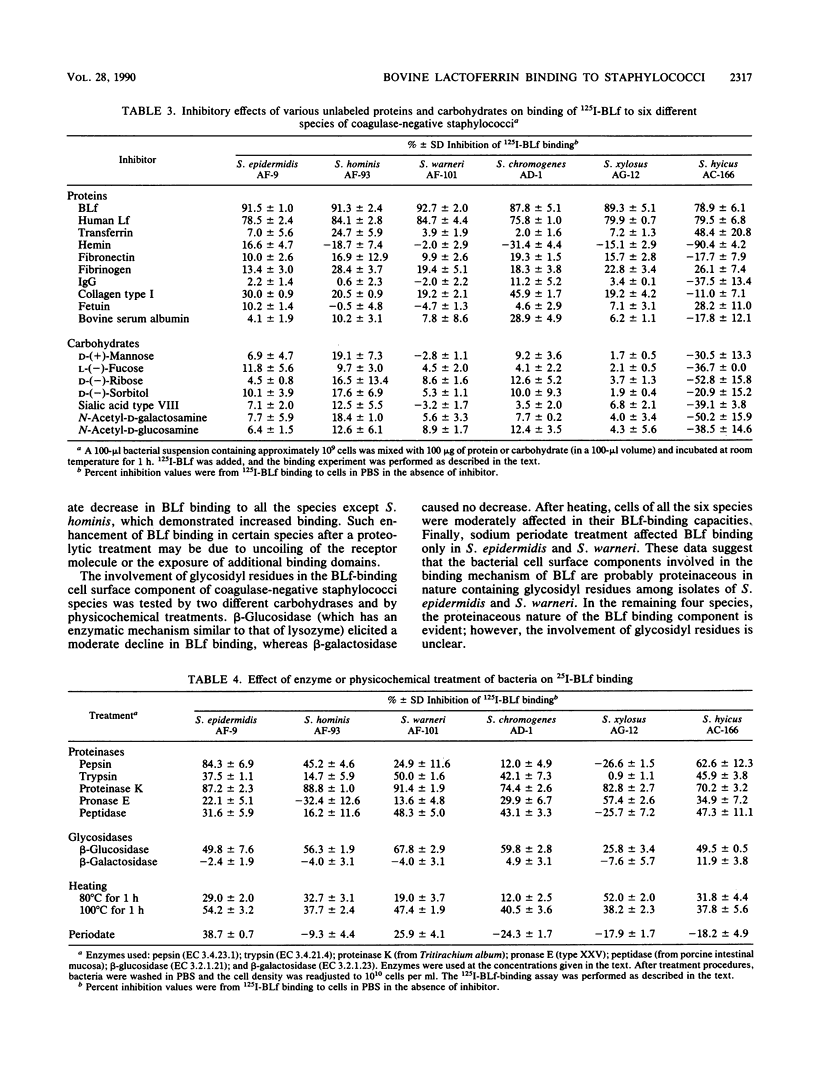
Bovine lactoferrin (BLf), an acute-phase iron-binding secretory protein present in secretions of the bovine udder, was demonstrated to bind to the following staphylococcal species associated with bovine intramammary infections: S. epidermidis, S. warneri, S. hominis, S. xylosus, S. hyicus, and S. chromogenes. The degree of 125I-labeled BLf uptake significantly varied among the blood agar-grown cells of all six species of coagulase-negative staphylococci tested. Isolates identified as S. xylosus demonstrated the highest (mean, 35.1 x 10(6) +/- 13.3 x 10(6) nmol) and S. hyicus the lowest (mean, 10.7 x 10(6) +/- 5.9 x 10(6) nmol) binding to 125I-BLf. BLf binding was optimum at an acidic pH, with time-dependent binding saturation ranging from 70 min for S. warneri to 240 min for S. hominis. The BLf-binding mechanism was specific, with affinity constants (Ka values) ranging between 0.96 x 10(6) and 11.90 x 10(6) liters/mol. The numbers of BLf-binding sites per cell, as determined by using Scatchard analysis, were as follows: S. epidermidis, 3,600; S. warneri, 1,900; S. hominis, 4,100; S. xylosus, 4,400; S. hyicus, 6,100; and S. chromogenes, 4,700. 125I-BLf binding to all species was inhibited by unlabled BLf and unlabeled human lactoferrin, whereas none of the various plasma, connective tissue, or mucosal secretory proteins or carbohydrates tested caused significant interference. BLf-binding receptors of the six coagulase-negative staphylococcal species demonstrated marked differences in patterns of susceptibility to proteolytic or glycolytic enzyme digestion and to heat or periodate treatment. These data suggest that the BLf-binding components in S. epidermidis and S. warneri are proteins containing glycosidyl residues. In the remaining four species, the proteinaceous nature of the BLf-binding component was evident, but the involvement of glycosidyl residues was not clear. Results of this study establish the presence of specific binding components for BLf on coagulase-negative staphylococci isolated from bovine intramammary infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- BLOBEL H., KATSUBE Y. EFFECTS OF EXPERIMENTALLY INDUCED LEUKOCYTOSIS IN BOVINE MAMMARY GLANDS UPON INFECTIONS WITH STAPHYLOCOCCUS AUREUS, STREPTOCOCCUS AGALACTIAE, AND AEROBACTER AEROGENES. Am J Vet Res. 1964 Jul;25:1085–1089. [PubMed] [Google Scholar]
- Baggiolini M., De Duve C., Masson P. L., Heremans J. F. Association of lactoferrin with specific granules in rabbit heterophil leukocytes. J Exp Med. 1970 Mar 1;131(3):559–570. doi: 10.1084/jem.131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. M., Davis J. Lactoferrin binding to human peripheral blood cells: an interaction with a B-enriched population of lymphocytes and a subpopulation of adherent mononuclear cells. J Immunol. 1981 Sep;127(3):1211–1216. [PubMed] [Google Scholar]
- Bramley A. J. The effect of subclinical Staphylococcus epidermidis infection of the lactating bovine udder on its susceptibility to infection with Streptococcus agalactiae or Escherichia coli. Br Vet J. 1978 Mar-Apr;134(2):146–151. doi: 10.1016/s0007-1935(17)33538-8. [DOI] [PubMed] [Google Scholar]
- Brown R. W. Intramammary infections produced by various strains of Staphylococcus epidermidis and Micrococcus. Cornell Vet. 1973 Oct;63(4):630–645. [PubMed] [Google Scholar]
- Broxmeyer H. E., Juliano L., Waheed A., Shadduck R. K. Release from mouse macrophages of acidic isoferritins that suppress hematopoietic progenitor cells is induced by purified L cell colony stimulating factor and suppressed by human lactoferrin. J Immunol. 1985 Nov;135(5):3224–3231. [PubMed] [Google Scholar]
- Broxmeyer H. E., Platzer E. Lactoferrin acts on I-A and I-E/C antigen+ subpopulations of mouse peritoneal macrophages in the absence of T lymphocytes and other cell types to inhibit production of granulocyte-macrophage colony stimulatory factors in vitro. J Immunol. 1984 Jul;133(1):306–314. [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon R. J., Schanbacher F. L., Ferguson L. C., Smith K. L. Changes in lactoferrin, immunoglobulin G, bovine serum albumin, and alpha-lactalbumin during acute experimental and natural coliform mastitis in cows. Infect Immun. 1976 Feb;13(2):533–542. doi: 10.1128/iai.13.2.533-542.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg O. Staphylococcus epidermidis isolated from bovine milk. Biochemical properties, phage sensitivity and pathogenicity for the udder. Acta Vet Scand Suppl. 1973:1–144. [PubMed] [Google Scholar]
- Imber M. J., Pizzo S. V. Clearance and binding of native and defucosylated lactoferrin. Biochem J. 1983 May 15;212(2):249–257. doi: 10.1042/bj2120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N. C. Common mammary pathogens and factors in infection and mastitis. J Dairy Sci. 1979 Jan;62(1):128–134. doi: 10.3168/jds.S0022-0302(79)83214-2. [DOI] [PubMed] [Google Scholar]
- Jain N. C., Lasmanis J., Schalm O. W. Influence of egg albumin-induced leukopenia on experimental Aerobacter aerogenes mastitis and on natural infection of mammary gland with coagulase-negative staphylococcus in a cow. Am J Vet Res. 1967 Sep;28(126):1243–1250. [PubMed] [Google Scholar]
- Jain N. C., Schalm O. W., Lasmanis J. Comparison in normal and leukopenic cows of experimental mastitis due to Aerobacter aerogenes or Escherichia coli endotoxin. Am J Vet Res. 1969 May;30(5):715–724. [PubMed] [Google Scholar]
- Law B. A., Reiter B. The isolation and bacteriostatic properties of lactoferrin from bovine milk whey. J Dairy Res. 1977 Oct;44(3):595–599. doi: 10.1017/s0022029900020550. [DOI] [PubMed] [Google Scholar]
- Linde C., Holmberg O., Aström G. Interference between staphylococcus epidermidis (Se) and staphylococcus aureus (Sa) in the bovine udder. Acta Vet Scand. 1975;16(1):146–148. doi: 10.1186/BF03546706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo W., Fröman G., Wadström T. Interaction of sub-epithelial connective tissue components with Staphylococcus aureus and coagulase-negative staphylococci from bovine mastitis. Vet Microbiol. 1988 Oct;18(2):163–176. doi: 10.1016/0378-1135(88)90062-4. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz-Boutigue M. H., Jollès J., Mazurier J., Schoentgen F., Legrand D., Spik G., Montreuil J., Jollès P. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984 Dec 17;145(3):659–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- Miedzobrodzki J., Naidu A. S., Watts J. L., Ciborowski P., Palm K., Wadström T. Effect of milk on fibronectin and collagen type I binding to Staphylococcus aureus and coagulase-negative staphylococci isolated from bovine mastitis. J Clin Microbiol. 1989 Mar;27(3):540–544. doi: 10.1128/jcm.27.3.540-544.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu A. S., Ekstrand J., Wadström T. Binding of type-I and type-II collagens to Staphylococcus aureus strains isolated from patients with toxic shock syndrome compared to other staphylococcal infections. FEMS Microbiol Immunol. 1989 Mar;1(4):219–227. doi: 10.1111/j.1574-6968.1989.tb02386.x. [DOI] [PubMed] [Google Scholar]
- Naidu A. S., Paulsson M., Wadström T. Particle agglutination assays for rapid detection of fibronectin, fibrinogen, and collagen receptors on Staphylococcus aureus. J Clin Microbiol. 1988 Aug;26(8):1549–1554. doi: 10.1128/jcm.26.8.1549-1554.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natzke R. P., Everett R. W., Guthrie R. S., Keown J. F., Meek A. M., Merrill W. G., Roberts S. J., Schmidt G. H. Mastitis control program: effect on milk production. J Dairy Sci. 1972 Sep;55(9):1256–1260. doi: 10.3168/jds.S0022-0302(72)85658-3. [DOI] [PubMed] [Google Scholar]
- Oliver S. P., Smith K. L. Milk yield and secretion composition following intramammary infusion of colchicine. J Dairy Sci. 1982 Feb;65(2):204–210. doi: 10.3168/jds.s0022-0302(82)82178-4. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim Biophys Acta. 1968 Dec 23;170(2):351–365. doi: 10.1016/0304-4165(68)90015-9. [DOI] [PubMed] [Google Scholar]
- Oseas R., Yang H. H., Baehner R. L., Boxer L. A. Lactoferrin: a promoter of polymorphonuclear leukocyte adhesiveness. Blood. 1981 May;57(5):939–945. [PubMed] [Google Scholar]
- Paape M. J., Schultze W. D., Guidry A. J., Kortum W. M., Weinland B. T. Effect of an intramammary polyethylene device on the concentration of leukocytes and immunoglobulins in milk and on the leukocyte response to Escherichia coli endotoxin and challenge exposure with Staphylococcus aureus. Am J Vet Res. 1981 May;42(5):774–783. [PubMed] [Google Scholar]
- Postle D. S., Roguinsky M., Poutrel B. Induced staphylococcal infections in the bovine mammary gland. Am J Vet Res. 1978 Jan;39(1):29–35. [PubMed] [Google Scholar]
- Reiter B. Review of the progress of dairy science: antimicrobial systems in milk. J Dairy Res. 1978 Feb;45(1):131–147. doi: 10.1017/s0022029900016290. [DOI] [PubMed] [Google Scholar]
- Schanbacher F. L., Smith K. L. Formation and role of unusual whey proteins and enzymes: relation to mammary function. J Dairy Sci. 1975 Jul;58(7):1048–1062. doi: 10.3168/jds.S0022-0302(75)84678-9. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988 May;56(5):1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. L., Conrad H. R., Porter R. M. Lactoferrin and IgG immunoglobulins from involuted bovine mammary glands. J Dairy Sci. 1971 Oct;54(10):1427–1435. doi: 10.3168/jds.S0022-0302(71)86043-5. [DOI] [PubMed] [Google Scholar]
- Smith K. L., Oliver S. P. Lactoferrin: a component of nonspecific defense of the involuting bovine mammary gland. Adv Exp Med Biol. 1981;137:535–554. [PubMed] [Google Scholar]
- Spik G., Cheron A., Montreuil J., Dolby J. M. Bacteriostasis of a milk-sensitive strain of Escherichia coli by immunoglobulins and iron-binding proteins in association. Immunology. 1978 Oct;35(4):663–671. [PMC free article] [PubMed] [Google Scholar]
- Stabenfeldt G. H., Spencer G. R. The lesions in bovine udders shedding nonhemolytic coagulase-negative staphylococci. Pathol Vet. 1966;3(1):27–39. doi: 10.1177/030098586600300102. [DOI] [PubMed] [Google Scholar]
- Symposium: bovine mastitis. J Dairy Sci. 1979 Jan;62(1):117–176. [PubMed] [Google Scholar]
- Timms L. L., Schultz L. H. Dynamics and significance of coagulase-negative staphylococcal intramammary infections. J Dairy Sci. 1987 Dec;70(12):2648–2657. doi: 10.3168/jds.S0022-0302(87)80335-1. [DOI] [PubMed] [Google Scholar]
- Timms L. L., Schultz L. H. Mastitis therapy for cows elevated somatic cell counts or clinical mastitis. J Dairy Sci. 1984 Feb;67(2):367–371. doi: 10.3168/jds.s0022-0302(84)81310-7. [DOI] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L., Heremans J. F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974 Oct 1;140(4):1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L. The binding of human lactoferrin to mouse peritoneal cells. J Exp Med. 1976 Dec 1;144(6):1568–1580. doi: 10.1084/jem.144.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadström T. Molecular aspects on pathogenesis of wound and foreign body infections due to staphylococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Aug;266(1-2):191–211. doi: 10.1016/s0176-6724(87)80032-9. [DOI] [PubMed] [Google Scholar]
- Watts J. L., Naidu A. S., Wadström T. Collagen binding, elastase production, and slime production associated with coagulase-negative staphylococci isolated from bovine intramammary infections. J Clin Microbiol. 1990 Mar;28(3):580–583. doi: 10.1128/jcm.28.3.580-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J. L., Nickerson S. C. A comparison of the STAPH-Ident and STAPH-Trac systems to conventional methods in the identification of staphylococci isolated from bovine udders. Vet Microbiol. 1986 Jul;12(2):179–187. doi: 10.1016/0378-1135(86)90079-9. [DOI] [PubMed] [Google Scholar]
- Weiner R. E., Szuchet S. The molecular weight of bovine lactoferrin. Biochim Biophys Acta. 1975 May 30;393(1):143–147. doi: 10.1016/0005-2795(75)90224-x. [DOI] [PubMed] [Google Scholar]
- Welty F. K., Smith K. L., Schanbacher F. L. Lactoferrin concentration during involution of the bovine mammary gland. J Dairy Sci. 1976 Feb;59(2):224–231. doi: 10.3168/jds.s0022-0302(76)84188-4. [DOI] [PubMed] [Google Scholar]
- de Vet B. J., ten Hoopen C. H. Lactoferrin in human neutrophilic polymorphonuclear leukocytes in relation to iron metabolism. Acta Med Scand. 1978;203(3):197–203. doi: 10.1111/j.0954-6820.1978.tb14856.x. [DOI] [PubMed] [Google Scholar]


