Abstract
Parenteral and respiratory vaccinations with the intracellular bacterium Francisella tularensis have been studied using the live vaccine strain (LVS) in a mouse model, and spleen cells from immune mice are often used for immunological studies. However, mechanisms of host immunological responses may be different in nonlymphoid organs that are important sites of infection, such as lung and liver. Using parenteral (intradermal) or respiratory (cloud aerosol) vaccination, here we examine the functions of resulting LVS-immune liver or lung cells, respectively. Surprisingly, LVS was considerably more virulent when administered by cloud aerosol than by intranasal instillation, suggesting method-dependent differences in initial localization and/or dissemination patterns. Only low doses were sublethal, and resolution of sublethal cloud aerosol infection was dependent on gamma interferon (IFN-γ), tumor necrosis factor alpha, and inducible nitric oxide synthase. Nonetheless, survival of cloud aerosol or parenteral infection resulted in the development of a protective immune response against lethal LVS intraperitoneal or aerosol challenge, reflecting development of systemic secondary immunity in both cases. Such immunity was further detected by directly examining the functions of LVS-immune lung or liver lymphocytes in vitro. Lung lymphocytes primed by respiratory infection, as well as liver lymphocytes primed by parenteral infection, clearly controlled in vitro intracellular bacterial growth primarily via mechanisms that were not dependent on IFN-γ activity. Thus, our results indicate functional similarities between immune T cells residing in spleens, livers, and lungs of LVS-immune mice.
Francisella tularensis is a small gram-negative intracellular pathogen that can infect a variety of mammalian and arthropod hosts and cause tularemia, a disease that can be rapidly fatal. Infection can be initiated by a variety of routes, including via wounds, ingestion of contaminated food or water, insect bites, or by inhalation exposure (17). Aerogenic infection with as few as 10 organisms of type A F. tularensis subsp. tularensis may cause a fulminant and even fatal pulmonary disease. The traits of low infectious dose, airborne transmission, and high mortality rates have led to categorization of F. tularensis as a potential biowarfare agent (10).
An attenuated live vaccine strain (LVS4), derived from virulent type B F. tularensis subsp. holarctica in Russia in the 1940s, appears to provide some protection against tularemia in humans (10, 38). Moreover, LVS has been used as a convenient and safe experimental model, since it exhibits attenuated virulence in humans but can cause a fatal disease in mice that is similar to human type A infection (15). In mice, the outcome of LVS infection is critically dependent on the route of inoculation. For BALB/cByJ male mice, the intrapertioneal (i.p.) 50% lethal dose (LD50) approaches 1 single bacterium, whereas the intradermal (i.d.) LD50 is approximately 106 bacteria (15). The LD50 of respiratory LVS infection via intranasal (i.n.) instillation or nose-only aerosol apparatus has been reported to be intermediate, about 1,000 to 3,000 CFU (7, 42).
The spectrum of effector functions provided by T cells during adaptive immune responses to intracellular pathogens, which clearly include T-cell production of gamma interferon (IFN-γ) and induction of nitric oxide, is only partially understood. For Francisella, the majority of the studies to date have focused on mechanisms of protection following parenteral LVS vaccination. Similar to other intracellular bacteria, a strong innate immune response to LVS develops in mice; this response involves the production of early IFN-γ and tumor necrosis factor alpha (TNF-α) (15). During adaptive immunity generated by infection with this live attenuated strain, T cells are absolutely required for host survival of LVS infection, whereas B cells appear to play a minor role (15). In murine spleens, both CD4+ and CD8+ as well as an unusual population of CD4− CD8− T cells appear to be important and effect control of intracellular bacterial growth in vitro that depends only partially on IFN-γ but more heavily on TNF-α and ultimately production of nitric oxide (8, 9, 15).
However, for Francisella infections as well as many other intracellular infections, knowledge of T-cell mechanisms has been mostly derived from studies of splenic T cells, which are readily available in numbers sufficient for detailed study. Little is known about immunological effector mechanisms that control infection in nonlymphoid organs of liver and lung, the other major sites of Francisella infection. Further, little is known about the quality of T-cell effector mechanisms engendered by vaccination via parenteral compared to respiratory routes. Different methods of experimental aerosol infection, which include intranasal administration, intratracheal or intrabronchial instillation, nose-only inhalation exposure, or whole-body aerosol exposure, may also qualitatively influence immune mechanisms. Notably, i.d. LVS immunization protects BALB/c mice against i.d. challenge with virulent type A F. tularensis but not against respiratory challenge; respiratory LVS vaccination, in contrast, provides protection against virulent respiratory challenge (7, 42). Thus, the responding cell populations, frequencies, or immune effector functions may be different when elicited by parenteral or respiratory vaccination. Here, we used i.d. or respiratory vaccination via a whole-body inhalation exposure system to examine T-cell function. We show that, similar to LVS-immune splenic T cells, LVS-immune lung and liver lymphocytes both controlled the intramacrophage growth of Francisella LVS during secondary immune responses by largely IFN-γ-independent mechanisms.
MATERIALS AND METHODS
Experimental animals.
Wild-type (WT) BALB/cByJ and C57BL/6J control male and female mice, as well as mice deficient in IFN-γ, IFN-γ receptor, TNF-α, or inducible nitric oxide synthase (iNOS) (all on a C57BL/6J background), were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were housed in a facility at the Center for Biologics Evaluation and Research (CBER), fed autoclaved food and water ad libitum, and used between 8 and 12 weeks of age. All experiments were performed under protocols approved by FDA CBER or University of New Mexico Institutional Animal Care and Use Committees.
Bacteria, growth conditions, and preparation of aerosol infection stocks.
F. tularensis LVS (for the Elkins lab, ATCC 29684 from the American Type Culture Collection [Rockville, MD]; for the Lyons Lab, lot 4, USAMRIID [Frederick, MD]) was cultured on modified Mueller-Hinton (MH) agar plates or in modified MH broth (Difco Laboratories, Detroit, MI) supplemented with ferric pyrophosphate and IsoVitaleX (Becton Dickinson, Cockeysville, MD) as described previously (20) with some modifications, including adjustment of the pH of the MH broth to 7.2 to minimize changes in the bacterial population to avirulent colony variants (12, 13). Aliquots of bacteria were frozen in broth alone at −70°C and thawed for individual experiments. Viable bacteria were quantified by plating serial dilutions on MH agar plates that were incubated for 2 to 3 days at 37°C in 5% CO2. All aerosol stocks were confirmed as fully virulent by determining that the i.p. LD50 in BALB/cByJ male mice was <3 CFU (15).
F. tularensis LVS parenteral and aerosol infections.
Mice were infected with LVS i.d. by administering the indicated numbers of bacteria in 100 μl at the base of the tail or i.p. in 500 μl diluted in sterile phosphate-buffered saline (PBS; Lonza, Walkersville, MD) containing <0.01 ng/ml of endotoxin. Actual bacterial doses were determined by simultaneous plate count. Whole-body aerosol exposures were performed at CBER FDA as follows. Mice were placed in the chamber of an inhalation exposure system (Glas-Col, Terre Haute, IN), and the nebulizer was filled with a 10-ml suspension of F. tularensis LVS at 107 to 109 bacteria/ml diluted in PBS-2% fetal calf serum (FCS). The air pressure setting was adjusted to approximately 17 lb/in2, and vacuum pressure was adjusted to approximately 30 lb/in2 after initiation of the nebulizing cycle. Total numbers of aerosolized bacteria were estimated by determining CFU/ml and the volume of the remaining liquid in the nebulizer at the end of the 30-min cycle. For each group (see Table 1, below), the average actual estimated aerosolized CFU of bacteria was as follows: 109 group, 7.5 × 108; 108 group, 8.1 × 107; 107 group, 5.8 × 106. Ten to 600 bacteria were delivered into the lungs (see Table 1). Actual delivery dosages were confirmed by sacrificing three to five mice immediately after infection and homogenizing lungs (see below). Intranasal LVS infections were performed at the University of New Mexico using isoflurane anesthesia following by instillation of bacteria in 50 μl into the external nares, as previously described (42).
TABLE 1.
F. tularensis LVS cloud aerosol infection parameters
| CFU in nebulizera | No. of experimentsb | Day 0 uptakec | Survival (%) | MTDd (n) |
|---|---|---|---|---|
| 109 | 3 | 5.7 × 102 ± 9.7 × 101 | 1/15 (7) | 9 ± 3 (14) |
| 108 | 4 | 1.5 × 102 ± 3.5 × 101 | 4/28 (14) | 13 ± 2 (24) |
| 107 | 6 | 1.0 × 101 ± 2 × 100 | 30/35 (86) | 12 ± 2 (5) |
Total number of viable bacteria placed in the nebulizer in a 10-ml volume.
Total number of separate aerosol infection experiments included in the calculations shown here; only experiments using male C57BL/6J mice are included in this table.
Average ± SEM of CFU of bacteria delivered to the lungs following aerosol exposure, based on plate count of either dilutions or the entire homogenate of both lungs from individual mice, as needed. For the 107 dose, 5 of 26 mice(19%) sacrificed on day zero had no detectable colonies in the total lung homogenate; for these samples, no value was included in the uptake calculation shown for this dose.
MTD, mean time to death, with the day of infection designated as day zero; data are the average day of death ± SEM. Only mice that died were included in the calculation. n is the number of mice included in the mean time to death calculation(see survival column).
Determination of F. tularensis LVS bacterial loads in infected mice.
Numbers of CFU in the organs of infected mice were determined by removing spleens, livers, and lungs aseptically into sterile PBS and homogenizing organs using a stomacher (Tekmar, Cincinnati, OH). Appropriate dilutions were plated on MH agar plates, CFU determined, and bacterial loads calculated. Lung homogenates were plated on MH agar plates containing 7.5 mg/liter colistin sulfate salt, 0.5 mg/liter lincomycin hydrochloride, 4 mg/liter trimethoprim, and 10 mg/liter ampicillin (all purchased from Sigma-Aldrich, St. Louis, MO), similar to previously described methods for isolating Francisella from clinical specimens (28).
Lavage of nasal passages.
Nasal cavities of infected mice were lavaged by inserting a 25-gauge needle assembled on a 3-ml syringe into the posterior opening of the nasopharynx and injecting 0.5 ml of PBS through to the external nares. CFU were enumerated by plating the entire lavage fluid volume recovered on MH agar plates.
Infection of bone marrow-derived macrophages with F. tularensis LVS, coculture with lymphocytes, and statistical analyses.
Cocultures were performed in 24- or 48-well plates as described previously (4, 8). Briefly, bone marrow macrophages (BMMφ) were cultured in complete Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated FCS (HyClone, Logan, UT), 10% L-929-conditioned medium, 0.2 mM l-glutamine, 10 mM HEPES buffer, 0.075% sodium bicarbonate, and 0.1 mM nonessential amino acids in 24- or 48-well plates. Confluent macrophage monolayers were infected for 2 h with F. tularensis LVS at a multiplicity of infection (MOI) of 1:20 to 1:40 (bacterium-BMMφ ratio), washed, treated for 45 min with 50 μg/ml gentamicin, and washed with antibiotic-free medium. Single-cell suspensions of splenic, pulmonary, or liver lymphocytes (0.5 × 106 to 5 × 106/well; see below) were added to LVS-infected macrophages. In experiments using BMMφ derived from knockout mice, adherent cells were removed from added lymphocyte populations by plastic adherence (2 h at 37°C in 5% CO2) to minimize any influence of WT macrophages in the cocultures. At the indicated time points, supernatants from cocultures were harvested, and LVS replication was determined by lysing infected macrophages and plating. When no bacterial growth was detected, a CFU value equal to half the limit of detection was assigned, and means (± standard deviations [SD]) were calculated. Where indicated, the significance of differences was analyzed using a two-tailed Student's t test, with P values of <0.05 chosen as reflecting a significant difference.
Preparation of splenic, lung, and liver leukocytes.
Disrupted spleens were used to prepare single-cell suspensions; erythrocytes were lysed with ammonium chloride (ACK lysing buffer; Lonza) and cells washed, and viability was assessed by exclusion of trypan blue. For liver leukocyte preparations, emulsified livers were washed three times with room temperature PBS. Cells were collected and suspended in 2.5 ml/liver of 40% Percoll (Sigma, St. Louis, MO). After centrifugation at 1,400 rpm for 20 min at room temperature, the top layer was removed, the pellet was collected, and remaining cells were resuspended in PBS-2% FBS (19). Erythrocytes were lysed, cells were washed, and viability was assessed. Lung leukocytes were prepared as described previously (35) with some modifications. Briefly, lungs were excised and gently homogenized in a stomacher in PBS-2% FCS containing 150 U/ml collagenase and then treated with collagenase for 90 min at 37°C in 5% CO2. Tissue fragments were removed using a Filtra bag (VWR International, Bridgeport, NJ) and cell strainers (BD Falcon, Franklin Lakes, NJ), erythrocytes were lysed, and cells were washed. Where indicated, a magnetic-activated cell sorter Midi system and magnetic beads (Miltenyi Biotec, Auburn, CA) were used to enrich cell subpopulations in which single-cell suspensions were treated with the appropriate amount of magnetic beads according the manufacturer's instructions. T cells were prepared from whole liver lymphocyte preparations by in vitro depletion of NK cells and B cells by using anti-DX5 and anti-CD19 beads, followed by enrichment using anti-Thy1.2 beads. Conversely, NK cells were prepared by in vitro depletion of CD4+, CD8+, and CD19+ cells with the corresponding beads, followed by enrichment using anti-DX5 beads. The composition and relative purity of the resulting cells were assessed by multiparameter flow cytometry (see below). Liver T-cell preparations were 90 to 93% CD3+ TCRβ+, with the balance being myeloid cells including macrophages and dendritic cells; NK cells were ∼90% NK1.1+ DX5+.
Flow cytometry analyses of lymphocyte populations.
Single-cell suspensions were prepared and stained for a panel of murine cell surface markers and analyzed using a Becton-Dickinson LSR II flow cytometer (San Jose, CA) and FACS Diva or FlowJo software essentially as previously described (43). Clones used included 30-F11 (anti-CD45), RM4-5 (anti-CD4), 53-6.7 (anti-CD8a), GL3 (anti-γ/δ T-cell receptor [TCR]), NK1.1 (anti-NK1.1), 30-H12 or 53-2.1 (anti-Thy1.2), H57-597 (anti-TCRβ chain), 17A2 (anti-CD3), M1/70 (anti-CD11b), RA3-6B2 (anti-CD45/B220), NK1.1 (anti-NK1.1), and DX5 (anti-CD49b). All antibodies listed above and FcBlock were obtained from BD Pharmingen (San Diego, CA), and optimal concentrations were determined in separate experiments for use in six- to eight-color staining protocols, using appropriate fluorochrome-labeled isotype control antibodies. Naïve lung cells were routinely about 50% CD45+, while cells from infected mice were 80 to 90% CD45+. Naïve and immune liver cells were routinely about 70 to 90% CD45+. Only results from CD45+ populations are shown here.
Cytokine blockade and cytokine and nitrite measurements.
Low-endotoxin, azide-free neutralizing antibodies to murine IFN-γ (clone R4-6A2; rat immunoglobulin G1 [IgG1]) were added to cocultures as indicated at 25 μg/ml to block IFN-γ activity; blockade was confirmed by enzyme-linked immunosorbent assay (ELISA). An isotype-matched antibody to interleukin-4 (IL-4; clone 11B11; rat IgG1) was used as a control/comparator. Both were purchased from BD Pharmingen. Murine IFN-γ, IL-12 p40, and TNF-α were measured using standard sandwich ELISAs. To determine cytokine levels in the sera, blood was collected from mice at the time of sacrifice and the resulting serum tested in the assays using appropriate dilutions. Antibody pairs and standards were purchased from BD Pharmingen (San Diego, CA), and the assays were performed according to the instructions of the manufacturer. Cytokines were measured by comparison to recombinant standard proteins using the four-parameter fit regression in the SoftMax Pro ELISA analysis software (Molecular Devices, Sunnyvale, CA). NO was detected in culture supernatants using the Griess reaction (21). Samples of supernatants were incubated with an equal volume of commercial Griess reagent (Sigma-Aldrich, St. Louis, MO) and absorbance was measured at 490 nm. Nitrite (NO2) was measured by comparison to serially diluted NaNO2 as a standard using four-parameter fit regression as described above.
Measurement of antibodies to F. tularensis LVS.
Serum antibodies to LVS were assessed using a solid-phase ELISA as previously described (32). Briefly, Immulon 1 plates were coated with whole bacteria and blocked, serial dilutions of normal or test sera were added, and antibodies were detected using peroxidase-labeled goat anti-mouse IgM or IgG antibodies and 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) substrate.
RESULTS
Development and characterization of a murine model of F. tularensis LVS cloud aerosol infection.
In order to mimic a field aerogenic transmission of Francisella, we first established a whole-body cloud aerosol infection model by delivering different doses of F. tularensis LVS into an inhalation exposure chamber. As shown in Table 1, delivery of approximately 150 or more CFU of LVS into the lungs of C57BL/6J mice resulted in a lethal infection within 7 to 15 days; time to death decreased with increasing dose. When approximately 10 CFU were delivered to the lungs by whole-body cloud aerosol exposure, the majority of the animals survived for over 30 days, cleared bacteria, and mounted readily detectable anti-Francisella antibody responses (serum anti-LVS IgG antibody titers of 1:1,000 or greater) (data not shown). A 10-fold-lower initial dose (resulting in delivery of one to two bacteria) resulted in variable infections; about 75 to 80% of the animals did not appear to be productively infected, as determined by lung bacterial counts, seroconversion, and/or resistance to secondary lethal LVS challenge (data not shown). Of note, these outcomes also suggested that the lethality of apparently low numbers of LVS was not attributable to failure to detect LVS CFU in lung homogenates. Collectively, a dose of 10 CFU was considered suitable for reliable subsequent sublethal aerosol LVS infection.
Although it is difficult to precisely calculate the LD50 of cloud aerosol LVS infection because of the limitations in controlling administered doses and doses that differ by 1 log10, we estimated the cloud aerosol LVS LD50 using all available data to be approximately 30 to 50 CFU (Table 1). Even though imprecise, this estimate is 1 to 2 orders of magnitude lower than the previously estimated LD50 of 1 × 103 to 3 × 103 CFU when LVS was administered i.n. (42) or by nose-only apparatus (7). We therefore explored a variety of explanations for these differences. We first examined the nasal passages of mice exposed to the sublethal dose (∼10 CFU) of LVS. Only a few colonies (5 ± 3 CFU for five mice) were detected in washes recovered from the nasal cavities. Further, since previous studies had shown that the i.d. and i.p. LD50s of LVS are somewhat higher in female C57BL/6J mice than in male C57BL/6J mice (15) and since most previous aerosol studies employed female mice, we determined the susceptibility of C57BL/6 males and females. Despite slightly higher initial lung bacterial burdens in females compared to males, bacterial loads in both genders were nearly identical by day 3 after aerosol LVS infection, and male and female mice displayed identical survival patterns (data not shown). Finally, we examined whether stress associated with containment increased the apparent susceptibility of mice to LVS infection. Naïve C57BL/6J male mice were exposed to diluent (PBS-FCS) only in the aerosol chamber, and exposed mice and unexposed control mice were immediately infected with high i.d. doses of LVS that approached lethal doses. Few deaths ensued, and there were no apparent differences in visible health status or weights between groups (data not shown).
We further considered the possibility that differences in LVS sources and/or bacterial growth methods might contribute to the apparent difference. To perform a direct comparison, infection stocks of LVS from two different sources, prepared independently in two different laboratories, were exchanged and used with different exposure methods. Adult male and female BALB/cByJ and C57BL/6J mice were infected i.n. (Lyons lab) or by cloud aerosol (Elkins lab), using a range of doses for each. For example, using male C57BL/6J mice, survival patterns were similar for three different i.n. doses, without regard to bacterial stocks (Fig. 1A; data not shown for groups exposed to 50 CFU of each stock, in which all mice survived). Conversely, mice administered Lyons LVS stock by cloud aerosol succumbed to infection (Fig. 1B), similar to the pattern with Elkins stock (Table 1).
FIG. 1.
Mice exhibit greater susceptibility to cloud aerosol F. tularensis LVS infection than to intranasal LVS infection. Age-matched, C57BL/6J adult male mice were infected with stocks of LVS prepared by two different laboratories (Lyons LVS and Elkins LVS) by intranasal instillation under anesthesia at the University of New Mexico (A) or by whole-body inhalation exposure at FDA CBER (B), and survival of groups of eight mice was monitored for 30 days. Actual numbers of CFU deposited in lungs immediately after infection, determined by sacrifice of groups of three to five mice and plating of lung homogenates, are shown in the figure legend. The figure depicts results using C57BL/6J male mice; other experiments of similar design utilized C57BL/6J female mice, BALB/cByJ male mice, and BALB/cByJ female mice, all with similar outcomes.
Because LVS virulence was clearly related to the method of administration, the time course of sublethal cloud aerosol LVS infection using a dose of approximately 10 CFU was then characterized in detail. Lung bacterial burdens were substantial and peaked between days 7 and 10 (Fig. 2A). Dissemination to both spleens and livers was detected by day 4, and high bacterial loads reflecting replication in all organs were seen by day 7. By day 28, mice cleared the bacteria in all organs (Fig. 2A). Serum cytokine levels assessed during the same time course were related to bacterial dissemination (Fig. 2B). Thus, robust serum IFN-γ responses were detected only after day 7 and declined by day 10, whereas moderate serum levels of TNF-α were detected between days 7 and 10 that decreased to undetectable levels by day 21. Small and variable serum levels of IL-12 p40 were detected throughout active infection (data not shown).
FIG. 2.
Time course of bacterial burdens and cytokine production following sublethal cloud aerosol LVS infection. Male C57BL/6J mice were infected by whole-body inhalation exposure as described in Materials and Methods; at the indicated times after infection, groups of three mice were sacrificed and total numbers of CFU ± SD in lungs, livers, and spleens were determined by homogenization and plating (A). The limits of detection were 50 CFU for spleen and lung and 100 CFU for liver. Sera were obtained from all mice at the time of sacrifice and assessed by ELISA for concentrations ± SEM of IFN-γ (B), TNF-α (B), or IL-12 p40 (not shown). The limits of detection were approximately 50 pg/ml for IFN-γ and TNF-α. The figure depicts one representative experiment of three of similar design and outcome.
Roles of IFN-γ, TNF-α, and iNOS in primary F. tularensis LVS aerosol infection.
To investigate the roles of mediators known to be important, such as IFN-γ, TNF-α, and nitric oxide, we characterized sublethal cloud aerosol LVS infection in the respective knockout mice. Numbers of LVS bacteria were similar initially but higher in lungs of IFN-γ knockout mice (Fig. 3A) or IFN-γ receptor knockout mice (data not shown) compared to WT mice within 2 days after aerosol infection (Fig. 3A) and notably higher in spleens (Fig. 3A) and in liver (data not shown) upon dissemination. Aerosol-infected IFN-γ knockout mice died within 7 to 8 days after infection (Fig. 3A). In contrast, aerosol-infected TNF-α knockout mice exhibited similar bacterial burdens in lungs compared to those from infected WT mice until at least day 3 after infection, but burdens were increased compared to WT mice by day 7 (Fig. 3B), with similar patterns in bacterial burdens in spleens (Fig. 3B) and livers (data not shown) upon dissemination. TNF-α-deficient mice ultimately succumbed 7 to 10 days after aerosol LVS infection (Fig. 3B). In contrast, aerosol LVS-infected iNOS knockout mice appeared healthy during the first week following exposure, and bacterial burdens and dissemination patterns were comparable to WT mice through at least day 7 for all organs (Fig. 3C). Aerosol-infected iNOS knockout mice exhibited heterogeneity in time to death, ranging from 7 to 17 days after infection (Fig. 3C), with much higher bacterial organ burdens in available knockout mice that survived through day 13 (Fig. 3C).
FIG. 3.
IFN-γ knockout (GKO) mice and TNF-α knockout mice die within 1 week, but iNOS knockout mice succumb within 2 weeks, following low-dose aerosol LVS infection. Age-matched C57BL/6J WT and GKO mice (A), TNF-α knockout mice (B), or iNOS knockout mice (C) were simultaneously exposed to a low-dose cloud aerosol LVS infection (Table 1). At the indicated times after infection, groups of three mice were sacrificed and total numbers of CFU ± SD in lungs (left panels), spleens (middle panels), and livers (not shown) were determined by homogenization and plating The limits of detection were 50 CFU for spleen and lung and 100 CFU for liver. In the experiments depicted here, initial bacterial burdens in the lungs (day zero) were determined only for wild-type mice; in other experiments initial bacterial burdens were comparable between WT and knockout mice. Other mice were monitored for survival for 45 days (right panels). CFU results in panel A are from one representative experiment of two of similar design using GKO mice and two of similar design using IFN-γ receptor knockout mice (not shown), while survival studies are combined from two experiments using GKO mice (n = 8 total mice; 4 mice/individual experiment); not shown are results from two experiments using IFN-γ receptor knockout mice, in which 11 of 11 knockout mice died with a mean time to death of 8.0 ± 0.6 days. CFU results in panel B are from one representative experiment of two of similar design, while survival studies are combined from three experiments (n = 15 total mice; 5, 4, and 6 mice per individual experiment). CFU results in panel C are from one representative experiment of three of similar design, while survival studies are combined from four experiments (n = 21 total mice; 5, 4, 6, and 6 mice per individual experiment).
Development of protective immunity following sublethal F. tularensis LVS cloud aerosol infection.
The observation that innate immune responses only marginally controlled respiratory LVS infection administered by cloud aerosol raised the question as to whether successful adaptive immunity was actually engendered. However, as noted above, such infection of WT mice resulted in development of high titers of serum anti-LVS antibodies (data not shown). To determine whether any protection was elicited, lethal challenge with LVS was used as a convenient option that did not necessitate biosafety level 3 work. Here, 19 of 20 mice (95%) given sublethal primary infections of 101 or more CFU by the aerosol route and then challenged 1 to 2 months later using a lethal i.p. LVS dose (106 CFU; ∼105 LD50s) survived, while 18 of 18 survived lethal aerosol LVS challenge (2 × 102 CFU; ∼10 LD50s).
Immune lung lymphocytes from aerosol F. tularensis LVS-primed mice control intracellular bacterial infection via IFN-γ-independent mechanisms.
Because protection after aerosol infection was demonstrable, we examined the composition and function of cells in lungs of naïve and aerosol LVS-infected mice, compared to naïve or LVS-immune spleens, for reference (Table 2). Naïve lung cells were comprised of about 25% α/β+ T cells, 30% B cells, 20% macrophages, and 10% dendritic cells, and the balance neutrophils and NK cells. In contrast, cells obtained from lungs during active aerosol LVS infection exhibited a dramatic increase in total numbers that was almost entirely attributable to an increase in α/β+ T cells, particularly CD4+ T cells.
TABLE 2.
Compositions of cell preparations obtained from tissues of naive and LVS-infected mice
| Cell type and marker(s)a | % of cells with indicated marker(s) in:
|
|||||
|---|---|---|---|---|---|---|
| Spleen
|
Lung
|
Liver
|
||||
| Naive (7 × 107)b | i.d. LVS vaccinated (2.1 × 108)b | Naïve (1.0 × 106)b | Aerosol LVS infected (3.7 × 107)b | Naïve (8.5 × 105)b | i.d. LVS vaccinated (3.2 × 106)b | |
| T cells | ||||||
| Total α/β TCR+ | 37.8 | 34.0 | 27.7 | 72.2 | 16.8 | 27.2 |
| CD3+ CD4+ | 20.1 | 17.1 | 12.8 | 53.5 | 9.1 | 9.6 |
| CD3+ CD8+ | 13.3 | 11.5 | 6.4 | 15.5 | 6.1 | 8.3 |
| CD3+ CD4− CD8− | 3.6 | 4.5 | 3.5 | 4.1 | 1.1 | 8.6 |
| γ/δ TCR+ | 1.1 | 0.8 | 0.4 | 0.4 | NT | NT |
| NK cells | ||||||
| NK1.1+ TCRβ− | 2.7 | 1.2 | 2.2 | 2.5 | 9.9 | 6.7 |
| NK1.1+ TCRβ+ | 1.4 | 1.0 | 2.7 | 0.7 | 19.9 | 7.5 |
| B cells, CD19+ B220+ | 51.0 | 48.1 | 31.1 | 8.5 | 15.8 | 16.7 |
| Macrophages, CD11b+ CD11c− Gr-1− | 3.5 | 2.8 | 21.4 | 9.2 | 13.9 | 7.9 |
| Dendritic cells, CD11c+ | 2.7 | 6.3 | 8.4 | 2.5 | 12.1 | 7.8 |
| Neutrophils, Gr-1hi CD11bhi | 1.1 | 3.1 | 4.1 | 4.5 | 11.3 | 22.3 |
Cells were obtained from either 2 naïve mice or 15 mice infected with ∼10 CFU of LVS by cloud aerosol 18 days earlier (infected lung cells) or 3 naive mice and 5 mice infected with 104 CFU of LVS i.d. 21 days earlier (vaccinated spleen and vaccinated liver cells). The “infected” lung cell population, also used in Fig. 4, contained 136 total CFU of bacteria, as determined by plate count, while the spleen and liver cell populations contained no detectable CFU bacteria. Cells were lysed, stained with a panel of anti-cell surface receptor antibodies in a seven-color protocol, and analyzed using an LSR II flow cytometer with appropriate isotype control antibodies and single-color compensation controls. The indicated cell surface markers were used to define various subpopulations. All T-cell subpopulations were first gated on either TCRβ+ or TCRδ+ T cells. NK1.1+ cells were also DX5+, but the latter marker was not used in these analyses. CD11c+ cells included both myeloid-like dendritic cells and plasmacytoid-like dendritic cells, i.e., both CD11b+ and CD11b− cells were CD8a+/− Gr-1+/− and expressed variable amounts of F4/80. Neutrophils were identified by distinct, high-level expression of Gr-1 and CD11b as well as having the property of large side scatter. Percentages of cells were derived from the flow cytometry analyses. NT, not tested in this example, but in similar experiments γ/δ TCR+ cells were typically ≤1.5% of the total.
The average yield of total nucleated, viable cells obtained per mouse is shown in parentheses.
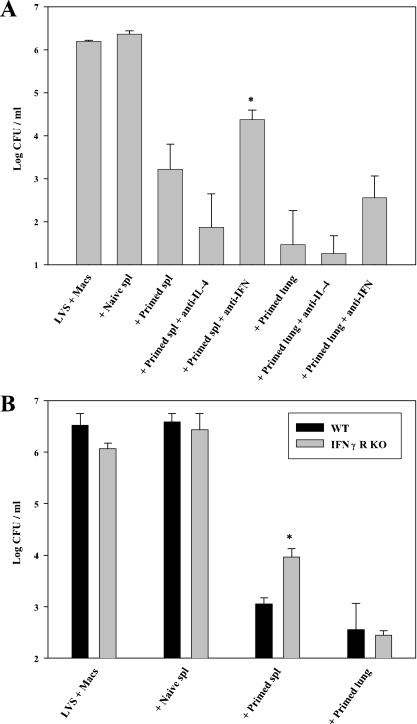
To begin to explore mechanisms of protection in lungs provided by responding T cells in more detail than is readily feasible in vivo, the activity of LVS-immune lung lymphocytes obtained from mice that survived primary cloud aerosol LVS infection was compared to that of splenic lymphocytes obtained from the same mice in an in vitro coculture model that measures T-cell control of LVS intramacrophage growth (4, 8, 9). After complete clearance of LVS infection by about 3 weeks, cell numbers in lungs returned to approximately the same numbers as exhibited by naïve mice (data not shown), necessitating the use of cells from recently infected mice for these functional studies. Because of these practical considerations, whole populations from recently infected mice with a high proportion of T cells were used without further enrichment. BMMφ were infected with LVS and cocultured with naïve or LVS-immune lymphocytes, and after 3 days the resulting CFU were assessed. Bacterial numbers were greatly reduced in cocultures containing either LVS-immune splenic or lung lymphocytes, compared to those with naïve cells or without added lymphocytes (Fig. 4A). In repeated experiments, lung lymphocytes consistently exhibited somewhat more control than the matched numbers of splenic lymphocytes but were otherwise comparable. Similar to previous studies using LVS-immune splenocytes from i.d.-vaccinated mice (8), antibodies to IFN-γ but not control isotype-matched antibodies to IL-4 (Fig. 4A) or use of macrophages derived from IFN-γ receptor knockout mice (Fig. 4B) in cocultures containing splenic lymphocytes partially, but not completely, reversed the control of bacterial growth. Most notably, antibodies to IFN-γ (Fig. 4A) or use of macrophages derived from IFN-γ receptor knockout mice (Fig. 4B) in cocultures containing lung lymphocytes had minimal impact. Thus, it appears that there is only a minor contribution of IFN-γ to the ability of LVS-primed lung lymphocyte populations to control intramacrophage growth.
FIG. 4.
LVS-immune lung lymphocytes control intramacrophage Francisella growth by IFN-γ-independent mechanisms. Confluent macrophages derived from bone marrow of either WT C57BL/6J (A and B) or IFN-γ receptor knockout (B) mice, as indicated, were infected at an MOI of 1:40 with LVS and cocultured with the indicated LVS-immune populations. Naïve splenic lymphocytes were obtained from WT C57BL/6 mice, and primed lymphocytes (both lung and spleen) were obtained from C57BL/6J mice given a sublethal cloud aerosol LVS dose 18 days earlier (100 to 300 total CFU remaining of LVS bacteria in either tissue, which had no appreciable impact on numbers of bacteria recovered from cocultures). Lung and spleen cells used in this experiment were those described in Table 2. Experiments in panels A and B used 1 × 106 added lymphocytes for all 48-well cocultures. Neutralizing amounts (25 μg/ml) of anti-IFN-γ or isotype-matched anti-IL-4 monoclonal antibodies were added to the indicated cultures (A) at the start of coculture. Numbers of CFU ± the standard error of the mean (SEM) were determined on day 3 from cultures containing only LVS-infected macrophages (LVS + Macs) or containing LVS-infected macrophages cocultured with the indicated cell populations and antibodies. *, P < 0.02 compared to the corresponding primed spleen cell cultures without antibodies (A) or cocultured with WT macrophages (B). The figure depicts one representative experiment of six (A) or two (B) of similar design and outcome. Similar results were obtained in experiments using lung lymphocytes obtained from recently infected mice, as shown here, and in those using lung lymphocytes obtained from mice that were aerosol-infected 1 to 3 months earlier (data not shown).
Immune liver T lymphocytes from intradermally F. tularensis LVS-primed mice control intracellular bacterial infection via IFN-γ-independent mechanisms.
We similarly examined the ability of immune lymphocytes obtained from LVS-immune livers to control intramacrophage Francisella growth. In this case, considerably larger numbers of leukocytes were obtained from i.d.-infected mice compared to aerosol-infected mice, and thus liver leukocytes from mice given LVS i.d. 3 to 12 weeks earlier were used and compared to splenic leukocytes from the same mice. Naive liver lymphocyte populations prepared contained roughly equal proportions of B cells (∼15%), macrophages (10 to 15%), dendritic cells (10 to 15%), and neutrophils (10 to 20%) and, unlike lung cells, a substantial number of NK and NK T cells (10 to 20%) (Table 2). However, while LVS-immune liver lymphocytes contained somewhat increased proportions and a fourfold increase in total number of T cells compared to naive liver lymphocytes, T cells still comprised a relatively low proportion of the total, typically 25 to 30% (Table 2). Although previous results indicated that B cells and myeloid cells do not contribute substantially to in vitro control of intramacrophage bacterial growth (4), other results indicate that CpG-DNA-primed NK cells can do so (K. Elkins et al., submitted for publication), and NK cells are a prominent source of IFN-γ that may confound these experiments. We therefore prepared enriched total T cells and non-T cells to examine activities directly. As seen in Fig. 5A, LVS-immune total liver leukocytes and liver T cells, but not naïve total cells or naïve T cells, strongly controlled intramacrophage LVS growth. As noted previously for splenic T cells (4, 8, 9), this control was accompanied by the production of IFN-γ (Fig. 5B), TNF-α (Fig. 5C), and nitric oxide (Fig. 5D). In contrast, LVS-immune cells remaining after T-cell enrichment or enriched directly for DX5+ NK cells (∼60% NK1.1+) did not exhibit significant control; the degree of control was comparable to that of naïve cells (data not shown). Thus, immune liver T cells, and only T cells, are responsible for the ability of LVS-primed liver lymphocyte populations to control intramacrophage LVS growth; to maximize the available number of cells and minimize any impact of cell manipulations, total cell preparations were used in subsequent experiments.
FIG. 5.
LVS-immune liver T lymphocytes control intramacrophage Francisella growth. Confluent macrophages derived from bone marrow of WT C57BL/6J were infected at an MOI of 1:40 with LVS and cocultured with the indicated naïve or LVS-immune total or T-cell-enriched populations. Naïve splenic or liver lymphocytes were obtained from WT C57BL/6 mice, and primed lymphocytes (both liver and spleen) were obtained from C57BL/6J mice given 105 LVS i.d. 1.5 months earlier, using 1 × 106 added lymphocytes for all 48-well cocultures. In this experiment, enriched T-cell populations were 92% CD3+ TCRβ+, 4% CD11b+ CD11c−, 3.5% CD11b+ CD11c+, 0.7% NK1.1+ DX5+, and <0.5% CD19+. Numbers of CFU ± SEM were determined on day 3 from cultures containing only LVS-infected macrophages (LVS + Macs) or containing LVS-infected macrophages cocultured with the indicated cell populations and antibodies. Supernatants from the corresponding cultures were obtained prior to macrophage lysis and assessed for quantities of IFN-γ (B), TNF-α (C), or nitric oxide (D). *, P < 0.05 compared to the corresponding cultures of LVS-infected macrophages without added lymphocytes. The figure depicts one representative experiment of four total experiments of similar design and outcome.
Similar to trends found using lung lymphocytes primed by aerosol, titration of matched numbers of cells added to LVS-infected macrophages indicated that liver lymphocytes were more consistently potent in controlling intramacrophage bacterial growth at the two highest cell numbers studied (Fig. 6A), despite liver leukocyte preparations having a lower proportion of T cells (Table 2). Antibodies to IFN-γ but not to IL-4 (Fig. 6B), or use of macrophages derived from IFN-γ receptor knockout mice (Fig. 6C), in cocultures containing liver lymphocytes partially, but not completely, reversed the control of bacterial growth. This was to a similar degree as that seen using splenic lymphocytes. Thus, the activity of IFN-γ appears to contribute only partially to the ability of LVS-primed liver lymphocyte populations to control intramacrophage LVS growth.
FIG. 6.
LVS-immune liver lymphocytes control intramacrophage Francisella growth by IFN-γ-independent mechanisms. Confluent macrophages derived from bone marrow of either WT C57BL/6J (A, B, and C) or IFN-γ receptor knockout (C) mice, as indicated, were infected at an MOI of 1:40 with LVS and cocultured with the indicated naïve or LVS-immune populations. Naïve splenic or liver lymphocytes were obtained from WT C57BL/6 mice, and primed lymphocytes (both liver and spleen) were obtained from C57BL/6J mice given 105 LVS i.d. 1 month earlier. Experiments in panel A used the indicated numbers of added lymphocytes, and experiments in panels B and C used 2 × 106 total added lymphocytes for all 48-well cocultures. Neutralizing amounts (25 μg/ml) of anti-IFN-γ or isotype-matched anti-IL-4 monoclonal antibodies were added to the indicated cultures (B) at the start of coculture. Numbers of CFU ± SEM were determined on day 3 from cultures containing only LVS-infected macrophages (LVS + Macs) or containing LVS-infected macrophages cocultured with the indicated cell populations and antibodies. *, P < 0.03 for primed liver cells compared to the corresponding primed spleen cell cultures at the indicated cell numbers. The figure depicts one representative experiment of three (A), seven (B), or three (C) experiments of similar design and outcome. Similar results were obtained in experiments using liver lymphocytes obtained from i.d.-primed mice, as shown here, compared to those using liver lymphocytes obtained from mice that were aerosol-infected 18 days earlier (not shown).
DISCUSSION
Immunization using parenteral and mucosal routes clearly results in quantitatively and qualitatively different immune responses, but effector mechanisms of protective immunity against respiratory infections, as well as tissue-specific responses, remain incompletely understood. Here, we found that priming of mice via cloud aerosol vaccination with F. tularensis LVS provided the means to obtain sufficient lung lymphocytes for detailed studies; similarly, parenteral priming engendered sufficient LVS-immune liver lymphocytes for analyses. In establishing feasibility, we found that cloud aerosol LVS exposure readily resulted in establishment of lung infection that disseminated to other organs of the reticuloendothelial system (Table 1; Fig. 2), with a resulting stimulation of cytokine production (Fig. 2), antibody production (see text), effector T-cell priming (Fig. 4 to 6), and development of systemic and respiratory protective immunity against lethal LVS challenge (see text). The initial survival after primary aerosol LVS vaccination was clearly dependent on IFN-γ, TNF-α, and iNOS (Fig. 3), likely products of both innate and adaptive immune responses.
Most importantly, adaptive immune responses by LVS-immune primed lung T lymphocytes that controlled the intracellular replication of bacteria in vitro were readily demonstrable. Similarly, liver T cells primed by intradermal vaccination with LVS clearly have the capacity to control the intracellular replication of bacteria in vitro. It is likely that specific antibodies produced after aerosol LVS vaccination contribute to at least part of the in vivo protection against LVS observed (15, 16); here, however, T-cell function was studied in vitro independently of antibody function, which is not measured by this coculture system (4, 8, 9, 14, 15; S. C. Cowley and K. L. Elkins, unpublished data). On a per-cell basis, the activities of LVS-immune lung and liver lymphocytes were comparable, if not superior, to the activity of splenic T cells primed by the homologous route (Fig. 4 to 6). Although challenging given the quantities of cells required, the present studies establish the feasibility of future studies designed to compare lymphocytes from each tissue obtained after parenteral and respiratory vaccination. At present, we nonetheless conclude that the ability of aerosol-primed, LVS-immune lung cells and parenterally primed, liver T cells to control intramacrophage Francisella growth appears qualitatively similar to that of splenic T cells: growth control activities of cells from nonlymphoid organs, albeit primed by different routes of vaccination, were at most only partially dependent on the activity of IFN-γ (Fig. 4 to 6). This result is consistent with the concept that antigen-specific T cells primed in lymphoid sites migrate to nonlymphoid sites and are maintained in tissues as memory cells (24).
A variety of methods have been used to initiate respiratory infections in experimental animals, including direct instillation of bacteria in liquid diluents into nasal passages or trachea, the use of a nose-only apparatus that forces animals to breath measured amounts of a nebulized bacterial suspension, and use of an apparatus that allows whole-body inhalation exposure (1). The former two methods, already described for use in Francisella infections, have the advantage of facilitating relatively precise control of the quantity and placement of dose in the airways, but at the expense of requiring anesthesia and/or animal confinement. The alternative approach of whole-body inhalation exposure, often used for studies of Mycobacterium tuberculosis, may better mimic field conditions but has the disadvantage of having less precise control of inhaled dose, as well as the potential for nonrespiratory sites of entry, such as eyes or skin. Surprisingly, we find that by this method the apparent virulence of the attenuated LVS strain is quite high, resulting in significant lung pathology (data not shown) and lethality at doses nearly 2 orders of magnitude lower compared to intranasal (42) or nose-only (7) infection (Fig. 1). A variety of explanations for these differences were explored but without clear-cut explanations emerging. The two bacterial stocks used here were obtained originally from different sources and prepared using similar, but not identical, media and growth conditions. Because direct comparisons that demonstrated that the apparent virulence of LVS tracked with the method of infection, and not with bacterial stock or type of mice (Fig. 1), it is unlikely that the magnitude of differences in apparent virulence between i.n. and cloud aerosol infection observed here can be attributed to differences in the bacteria or mice. We found no evidence for entry via other portals, since very few bacteria were found in nasal passages, and symptoms of ocular or gastrointestinal infection were never observed. Further studies will be necessary to explore the possibility that the different methods result in different initial localization of LVS bacteria within lung tissue, e.g., within the upper or lower lung; attempts to study this point using green fluorescent protein-LVS coupled with several imaging amplification approaches were unfortunately limited by sensitivity and numbers of infecting bacteria (data not shown). Regardless, these results emphasize the need to completely characterize respiratory infection models and critically consider the impact of different experimental methodologies in interpreting results.
Like all other intracellular pathogens, survival of parenteral LVS infection of mice is clearly dependent on IFN-γ, TNF-α, and iNOS (15). However, previous studies using a lethal 104 CFU dose of LVS administered by nose-only infection found no obvious differences in CFU in lungs after 4 days of infection between mice treated with anti-IFN-γ and control mice (6). Further, 40 to 50% of GKO mice and TNF receptor knockout mice and 100% of iNOS knockout mice infected with a 102 sublethal LVS dose by aerosol survived (5). These studies were interpreted as implying that different host defenses were operative in Francisella infections in different tissues or after different routes of exposure. In clear contrast, here we showed that murine survival of a sublethal dose of LVS administered by cloud aerosol was clearly dependent on IFN-γ, TNF-α, and iNOS (Fig. 3), the same mediators that are critical following parenteral infection (15). Although differences due to different LVS strains or different bacterial growth methods cannot yet be ruled out, the qualitatively different results appear more likely due to different infection methodologies. Here, studies comparing the time course of dissemination and bacterial burdens in the various knockout mice indicated a clear temporal relationship between these mediators: impacts on the course of LVS infection in the absence of IFN-γ were apparent within only 2 days after infection (Fig. 3A), TNF-α only after 3 days (Fig. 3B and data not shown), and iNOS only after 7 days (Fig. 3C and data not shown). Of note, it is likely that both IFN-γ and TNF-α are initially derived from multiple types of myeloid cells of the innate immune system (11), including NK cells (26, 40). Notably, the slower time course of death in iNOS knockout mice seen here (Fig. 3C) and previously after parenteral LVS infection (25) is most consistent with a role primarily during the later adaptive phase of immunity. This interpretation may be consistent with the previous conclusion that alveolar macrophages, while having the capacity to respond to IFN-γ to control the intracellular replication of LVS, do so in a largely iNOS-independent fashion (29). Thus, iNOS may be of lesser importance in lung responses than in splenic or liver responses to LVS infection.
Studies using both humans (22, 33, 34) and nonhuman primates (39) have suggested that aerosol LVS vaccination is superior to parenteral vaccination in reducing disease symptoms, bacterial burdens, and/or dissemination upon aerosol challenge with fully virulent type A Francisella. Similarly, a larger proportion of BALB/c mice vaccinated with LVS i.n. survived lethal type A Francisella challenge than did those vaccinated s.c. (7, 42), indicating than these properties can be modeled using mice. On the other hand, the demonstration of protection against death following either aerosol or i.d. type A challenge reportedly required the use of BALB/c mice and was not evident in C57BL/6J mice (7, 42); these observations are not yet understood. The ability to detect effector T-cell activities, including control of the intramacrophage growth of Francisella, using LVS-immune lung and liver lymphocytes will no doubt aid in determining the basis for the differences between mouse strains and thus in further evaluating the utility of the mouse model. Here, we demonstrated a clear increase in myeloid cells and especially T cells in lungs and livers of vaccinated mice; these numbers may increase by proliferation, recruitment, or both. Of note, the increased cell numbers were maintained in LVS-immune livers primed by intradermal vaccination but not in LVS-immune lungs primed by aerosol infection. This raises the question as to whether Francisella effector memory T-cell populations are not maintained appropriately in lungs via continual recruitment, such as is the case in respiratory Sendai virus infections (18). Future studies to compare the interactions of LVS-immune T cells with bone marrow-derived macrophages, used here and previously because of the relative ease of obtaining larger quantities, to those with primary splenic, alveolar, and liver macrophages can now be performed. An equally important point of future focus will be the detailed architecture which allows Francisella-immune T cells to interact with and effect control of intracellular bacterial growth within tissues in vivo. In livers, for instance, Francisella replicates in macrophages, hepatocytes, and possibly endothelial cells (36). However, it has been argued that hepatocytes contact sinus endothelial cells and stellate cells but are unlikely to interact directly with T cells in a liver sinusoid (23).
Similar to activities in the lung, the effector activities of lymphocytes from liver have received considerably less attention than those from the professional lymphoid organs (including spleen and lymph nodes). Comparisons may be further confounded by differing compositions of liver leukocyte preparations with different methodologies; for instance, the liver leukocyte preparations studied here appear to be markedly different from those analyzed in another recent study (31). Despite such differences, recent studies using LVS vaccination have demonstrated the capacity of immune lung T cells (26) and liver lymphocytes (2, 40) to produce intracellular IFN-γ. Further, important differences between the intracellular cytokines detected in lung T cells after i.n. vaccination (IL-17A+, Th-17 like) compared to those after i.d. vaccination (IFN-γ+, Th-1 like) that are regulated at least in part by prostaglandin E2 have been uncovered; indeed, the environment in the lung may be considerably more immunosuppressive than that in other organs (3, 41). However, as emphasized here (Fig. 4 to 6) and in previous studies (8, 9, 15), IFN-γ production alone by any cell type is highly unlikely to predict successful vaccination. Thus, the capacity to evaluate relevant T-cell effector functions beyond simple production of cytokines is critical to progress in understanding tissue-specific protective mechanisms. The in vitro approach used here does not focus on cytokine production or T-cell proliferation, two traditional measures of T-cell function, but instead on the ability of immune T cell populations to directly impact the replication of bacteria. Importantly, LVS-immune lung and liver lymphocytes have readily detectable and qualitatively similar functions to those of LVS-immune splenic lymphocytes by this criterion, although the impact of vaccination by different routes of exposure remains to be comprehensively compared. In all cases, neutralization of IFN-γ or use of infected macrophages that could not respond to IFN-γ had at best a modest impact on bacterial growth control. Thus, the important roles of IFN-γ often observed in vivo during secondary Francisella challenge (7, 27, 30, 32, 37) are not likely to be at the level of activation of infected macrophages. Future studies will therefore seek to exploit the in vitro approach used here, coupled with in vivo studies, to determine the full range of effector mechanisms operable by these different sources of T cells beyond IFN-γ and derive functional correlates of protection for this and other intracellular bacteria.
Acknowledgments
We are grateful to our CBER colleagues, Siobhán Cowley and Sheldon Morris, for thoughtful and careful review of the manuscript.
This work was supported in part by an interagency agreement with the National Institute of Allergy and Infectious Diseases (Y1-AI-6153-01/224-06-1322) and NIAID Public Health Service Grant PO1 AI056295, NIH, Bethesda, MD.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 23 February 2009.
REFERENCES
- 1.Bakker-Woudenberg, I. A. 2003. Experimental models of pulmonary infection. J. Microbiol. Methods 54295-313. [DOI] [PubMed] [Google Scholar]
- 2.Bokhari, S. M., K. J. Kim, D. M. Pinson, J. Slusser, H. W. Yeh, and M. J. Parmely. 2008. NK cells and gamma interferon coordinate the formation and function of hepatic granulomas in mice infected with the Francisella tularensis live vaccine strain. Infect. Immun. 761379-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosio, C. M., and S. W. Dow. 2005. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 1756792-6801. [DOI] [PubMed] [Google Scholar]
- 4.Bosio, C. M., and K. L. Elkins. 2001. Susceptibility to secondary Francisella tularensis LVS infection in B-cell-deficient mice is associated with neutrophilia but not with defects in specific T-cell-mediated immunity. Infect. Immun. 69194-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, W., R. KuoLee, H. Shen, and J. W. Conlan. 2004. Susceptibility of immunodeficient mice to aerosol and systemic infection with virulent strains of Francisella tularensis. Microb. Pathog. 36311-318. [DOI] [PubMed] [Google Scholar]
- 6.Conlan, J. W., R. KuoLee, H. Shen, and A. Webb. 2002. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb. Pathog. 32127-134. [DOI] [PubMed] [Google Scholar]
- 7.Conlan, J. W., H. Shen, R. Kuolee, X. Zhao, and W. Chen. 2005. Aerosol-, but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma-dependent mechanism. Vaccine 232477-2485. [DOI] [PubMed] [Google Scholar]
- 8.Cowley, S. C., and K. L. Elkins. 2003. Multiple T cell subsets control Francisella tularensis LVS intracellular growth without stimulation through macrophage interferon gamma receptors. J. Exp. Med. 198379-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley, S. C., E. Hamilton, J. A. Frelinger, J. Su, J. Forman, and K. L. Elkins. 2005. CD4− CD8− T cells control intracellular bacterial infections both in vitro and in vivo. J. Exp. Med. 202309-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 2852763-2773. [DOI] [PubMed] [Google Scholar]
- 11.De Pascalis, R., B. C. Taylor, and K. L. Elkins. 2008. Diverse myeloid and lymphoid cell subpopulations produce gamma interferon during early innate immune responses to Francisella tularensis live vaccine strain. Infect. Immun. 764311-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eigelsbach, H. T., W. Braun, and R. D. Herring. 1951. Studies on the variation of Bacterium tularense. J. Bacteriol. 61557-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eigelsbach, H. T., and C. M. Downs. 1961. Prophylactic effectiveness of live and killed tularemia vaccines. J. Immunol. 87415-425. [PubMed] [Google Scholar]
- 14.Elkins, K. L., A. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 701936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2003. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 5132-142. [DOI] [PubMed] [Google Scholar]
- 16.Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2007. Innate and adaptive immunity to Francisella. Ann. N. Y. Acad. Sci. 1105284-324. [DOI] [PubMed] [Google Scholar]
- 17.Ellis, J., P. C. Oyston, M. Green, and R. W. Titball. 2002. Tularemia. Clin. Microbiol. Rev. 15631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ely, K. H., T. Cookenham, A. D. Roberts, and D. L. Woodland. 2006. Memory T cell populations in the lung airways are maintained by continual recruitment. J. Immunol. 176537-543. [DOI] [PubMed] [Google Scholar]
- 19.Fogler, W. E., K. Volker, M. Watanabe, J. M. Wigginton, P. Roessler, M. J. Brunda, J. R. Ortaldo, and R. H. Wiltrout. 1998. Recruitment of hepatic NK cells by IL-12 is dependent on IFN-gamma and VCAM-1 and is rapidly down-regulated by a mechanism involving T cells and expression of Fas. J. Immunol. 1616014-6021. [PubMed] [Google Scholar]
- 20.Fortier, A. H., M. V. Slayter, R. Ziemba, M. S. Meltzer, and C. A. Nacy. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 592922-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green, L. C., A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126131-138. [DOI] [PubMed] [Google Scholar]
- 22.Hornick, R. B., and H. T. Eigelsbach. 1966. Aerogenic immunization of man with live tularemia vaccine. Bacteriol. Rev. 3049-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knolle, P. A., and G. Gerken. 2000. Local control of the immune response in the liver. Immunol. Rev. 17421-34. [DOI] [PubMed] [Google Scholar]
- 24.Lefrancois, L. 2006. Development, trafficking, and function of memory T-cell subsets. Immunol. Rev. 21193-103. [DOI] [PubMed] [Google Scholar]
- 25.Lindgren, H., S. Stenmark, W. Chen, A. Tarnvik, and A. Sjostedt. 2004. Distinct roles of reactive nitrogen and oxygen species to control infection with the facultative intracellular bacterium Francisella tularensis. Infect. Immun. 727172-7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez, M. C., N. S. Duckett, S. D. Baron, and D. W. Metzger. 2004. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell. Immunol. 23275-85. [DOI] [PubMed] [Google Scholar]
- 27.Pammit, M. A., E. K. Raulie, C. M. Lauriano, K. E. Klose, and B. P. Arulanandam. 2006. Intranasal vaccination with a defined attenuated Francisella novicida strain induces gamma interferon-dependent antibody-mediated protection against tularemia. Infect. Immun. 742063-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen, J. M., M. E. Schriefer, K. L. Gage, J. A. Montenieri, L. G. Carter, M. Stanley, and M. C. Chu. 2004. Methods for enhanced culture recovery of Francisella tularensis. Appl. Environ. Microbiol. 703733-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polsinelli, T., M. S. Meltzer, and A. H. Fortier. 1994. Nitric oxide-independent killing of Francisella tularensis by IFN-γ-stimulated murine alveolar macrophages. J. Immunol. 1531238-1245. [PubMed] [Google Scholar]
- 30.Powell, H. J., Y. Cong, J. J. Yu, M. N. Guentzel, M. T. Berton, K. E. Klose, A. K. Murthy, and B. P. Arulanandam. 2008. CD4+ T cells are required during priming but not the effector phase of antibody-mediated IFN-gamma-dependent protective immunity against pulmonary Francisella novicida infection. Immunol. Cell Biol. 86515-522. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen, J. W., J. Cello, H. Gil, C. A. Forestal, M. B. Furie, D. G. Thanassi, and J. L. Benach. 2006. Mac-1+ cells are the predominant subset in the early hepatic lesions of mice infected with Francisella tularensis. Infect. Immun. 746590-6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhinehart-Jones, T. R., A. H. Fortier, and K. L. Elkins. 1994. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect. Immun. 623129-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saslaw, S., H. T. Eigelsbach, J. A. Prior, H. E. Wilson, and S. Carhart. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107134-146. [DOI] [PubMed] [Google Scholar]
- 34.Saslaw, S., H. T. Eigelsbach, H. E. Wilson, J. Prior, and S. Carhart. 1961. Tularemia vaccine study. I. Intracutaneous challenge. Arch. Intern. Med. 107121-133. [DOI] [PubMed] [Google Scholar]
- 35.Saunders, B. M., and C. Cheers. 1995. Inflammatory response following intranasal infection with Mycobacterium avium complex: role of T-cell subsets and gamma interferon. Infect. Immun. 632282-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sjöstedt, A. 2006. Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect. 8561-567. [DOI] [PubMed] [Google Scholar]
- 37.Sjöstedt, A., R. J. North, and J. W. Conlan. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 1421369-1374. [DOI] [PubMed] [Google Scholar]
- 38.Tigertt, W. D. 1962. Soviet viable Pasteurella tularensis vaccines. A review of selected articles. Bacteriol. Rev. 26354-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tulis, J. J., H. T. Eigelsbach, and R. W. Kerpsack. 1970. Host-parasite relationship in monkeys administered live tularemia vaccine. Am. J. Pathol. 58329-336. [PMC free article] [PubMed] [Google Scholar]
- 40.Wickstrum, J. R., K. J. Hong, S. Bokhari, N. Reed, N. McWilliams, R. T. Horvat, and M. J. Parmely. 2007. Coactivating signals for the hepatic lymphocyte gamma interferon response to Francisella tularensis. Infect. Immun. 751335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woolard, M. D., L. L. Hensley, T. H. Kawula, and J. A. Frelinger. 2008. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect. Immun. 762651-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, T. H., J. A. Hutt, K. A. Garrison, L. S. Berliba, Y. Zhou, and C. R. Lyons. 2005. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect. Immun. 732644-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yee, D., T. R. Rhinehart-Jones, and K. L. Elkins. 1996. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of protective immunity to an intracellular pathogen, Francisella tularensis strain LVS. J. Immunol. 1575042-5048. [PubMed] [Google Scholar]