Abstract
The tumor necrosis factor receptor family molecule 4-1BB (CD137) has diverse roles in adaptive and innate immune responses. However, little is known of its role in bacterial infections. Previously, we showed that 4-1BB-deficient mice have enhanced susceptibility to Listeria monocytogenes infection, and mice pretreated with agonistic anti-4-1BB antibody (3E1) were much more resistant to L. monocytogenes infection than mice treated with control antibody. In this study, we report that stimulating 4-1BB by administering 3E1 in the early phase of L. monocytogenes infection is critical for promoting the survival of mice by inducing rapid infiltration of neutrophils and monocytes into L. monocytogenes-infected livers. The levels of tumor necrosis factor alpha, interleukin 6, and monocyte chemoattractant protein 1 in the livers of 3E1-treated mice increased as early as 30 min postinfection and peaked by 1 to 2 h, while those in mice treated with control antibody started to increase only at 16 h postinfection. Monocytes and neutrophils from the 3E1-treated mice had higher levels of activation markers, phagocytic activity, and reactive oxygen species than those from control mice. In vitro stimulation of 4-1BB induced the production of the inflammatory cytokines/chemokines of neutrophils, but not those of monocytes. These results suggest that 4-1BB stimulation of neutrophils in the early phase of L. monocytogenes infection causes rapid production of inflammatory cytokines/chemokines and that the subsequent infiltration of neutrophils and monocytes is crucial for eliminating the infecting L. monocytogenes.
Listeria monocytogenes is a gram-positive intracellular pathogen responsible for listeriosis, a life-threatening infection in immunocompromised patients, newborns, or elderly people. The murine model of listeriosis has been used to investigate immune responses to bacterial infection (11). L. monocytogenes infects both phagocytic and nonphagocytic cells, escapes from intracellular vacuoles into the cytosol by secreting listeriolysin, replicates, and spreads to neighboring cells by actin-based motility. Intravenous (i.v.) bacteria are rapidly cleared from the bloodstream; most of them are taken up by the liver and spleen within 10 min of infection. Although T-cell-dependent adaptive immune responses are required to clear L. monocytogenes infection, they take several days to develop. Therefore, early control of the infection is critical for the survival of mice and primarily depends on innate immunity (5); indeed, it has even been shown that lymphocytes are detrimental during the early innate immune response to L. monocytogenes (4). Neutrophils and monocytes/macrophages are thought to be the main cells responsible for killing L. monocytogenes during the innate immune response. Thus, depletion of neutrophils from mice by using anti-Gr-1 antibodies greatly enhances their susceptibility to infection with L. monocytogenes (35), and the increased number of neutrophils resulting from deficiency in LFA-1 in mice confers resistance to listeriosis (30). Recruitment of monocytes is also essential to eradicate L. monocytogenes from infected mice, as indicated by reports that blocking complement receptor 3 of monocytes exacerbates listeriosis (38), and CC chemokine receptor 2-deficient mice have defects in the emigration of monocytes from the bone marrow and are highly susceptible to L. monocytogenes infection (22, 40). Although recruitment of neutrophils/monocytes is critical for eradication of L. monocytogenes during the early phase of infection, the molecular mechanisms of bacterial killing and the receptor molecules responsible for activation of neutrophils/monocytes against the bacteria are not clearly defined.
The 4-1BB (CD137) receptor, a member of the tumor necrosis factor receptor superfamily (TNFRSF 9), is expressed on activated T cells (43), and the in vivo effects of 4-1BB activation on T-cell-dependent immune responses, such as eradication of established tumors (29), antiviral responses (1), and enhancement of the memory pool of antigen-specific CD8+ T cells (34), have been well defined. However, recent findings indicate that 4-1BB activation also plays an important role in other immune cells. 4-1BB is constitutively expressed on innate immune cells, including neutrophils (24), dendritic cells (10), natural killer (NK) cells (28), mast cells (31), and eosinophils (9). Its activation results in proliferation, gamma interferon secretion, and tumor rejection by NK cells (28); the production of cytokines by dendritic cells and the expression of costimulatory molecules on these cells (10); proliferation, survival, and cytokine production in human monocytes (21); and abrogation of the granulocyte-macrophage colony-stimulating factor-mediated antiapoptotic functions of human neutrophils (17). Previously, we reported that 4-1BB-deficient (4-1BB−/−) mice are very susceptible to L. monocytogenes infection because the antibacterial activity of their neutrophils is defective (24). Furthermore, pretreatment of agonistic anti-4-1BB monoclonal antibody (MAb) markedly increased the survival of L. monocytogenes-infected 4-1BB+/+ mice. In this study, we further characterized the mechanism of 4-1BB-mediated protection of L. monocytogenes-infected mice. We found that activation of 4-1BB in the early phase of L. monocytogenes infection rapidly stimulated the induction of proinflammatory cytokines/chemokines and the subsequent recruitment and activation of neutrophils and monocytes into the bacterium-infected livers.
MATERIALS AND METHODS
Animals.
Female BALB/c mice 8 to 10 weeks of age were purchased from Orient Bio-Charles River (Seoul, Korea). All mice were maintained under specific-pathogen-free conditions in the animal facility of the Immunomodulation Research Centre, University of Ulsan, and used following the Experimental Animal Guidelines of the University of Ulsan.
Antibodies and reagents.
Hybridoma cells (3E1 and 3H3) were a kind gift of R. Mittler (Emory University, Atlanta, GA). 3E1, 3H3, and RB6-8C5 cells were purified from ascites fluid, and control rat immunoglobulin G (IgG) was purified from rat serum using a protein G column (Sigma-Aldrich, St. Louis, MO). Anti-4-1BB MAb (3E1) was conjugated with fluorescein isothiocyanate (FITC) for flow cytometry. The following antibodies were purchased from BD PharMingen (San Diego, CA) unless otherwise stated: FITC-conjugated rat IgG2a, purified anti-CD16/CD32 (FcγIII/IIR), FITC-conjugated anti-Ly6G (1A8), FITC-conjugated anti-CD62L (MEL-14), biotin-conjugated anti-Ly6C (AL-21), phycoerythrin (PE)-conjugated anti-neutrophil (7/4; Serotec, United Kingdom), PE-conjugated anti-F4/80 (BM8; eBioscience, San Diego, CA), PE-conjugated anti-major histocompatibility complex (MHC) class II (SF1-1.1), PE-conjugated anti-CD4 (L3T4), PE-conjugated anti-CD8 (53-6.7), PE-conjugated anti-DX5α, PE-conjugated anti-CD11c (HL3), PE-conjugated anti-TREM-1(174031; R&D Systems, Minneapolis, MN), PE-conjugated anti-CXCR2 (242216; R&D Systems, Minneapolis, MN), and PE-conjugated anti-CD11b (M170) MAbs. To produce heat-inactivated 3E1 (HI-3E1), 3E1 was incubated for 20 min at 80°C.
Bacteria and infection of mice.
L. monocytogenes was obtained from the Korean Type Culture Collection (ATCC 19111). The bacteria were grown in brain heart infusion broth (Difco Laboratories, Detroit, MI) at 37°C for 18 h, and aliquots were frozen at −80°C. In each experiment, the viability of the frozen aliquots, which was always more than 90%, was confirmed by plating them on brain heart infusion agar (Difco). Mice were i.v. infected via the left tail vein with L. monocytogenes suspended in 200 μl of PBS, and 100 μg of rat IgG or 3E1 in 200 μl of phosphate-buffered saline (PBS) was i.v. injected into the mice via the right tail vein on the indicated days. For depletion of neutrophils, anti-Gr-1 MAb RB6-8C5 (500 μg/mouse) was intraperitoneally injected 2 days before infection.
Isolation of HILs.
Hepatic infiltrated leukocytes (HILs) were prepared as described previously (24). In brief, a liver was perfused with Ca2+ Mg2+-free Hanks balanced salt solution (HBSS) and pressed through a stainless steel mesh, and the leukocytes were purified on 40% to 70% Percoll gradients (Amersham Bioscience, Uppsala, Sweden). The cells were washed with HBSS three times and used for fluorescence-activated cell sorter (FACS) analysis and for measuring phagocytic activity and reactive oxygen species (RO) generation. For FACS analysis, cells were washed with FACS buffer (PBS containing 1% bovine serum albumin and 0.1% sodium azide), incubated with Fc Block (2.4G2) for 10 min on ice, and stained with antibodies for specific markers. All samples were subsequently analyzed by FACSCalibur (BD Biosciences, San Jose, CA). To obtain highly pure neutrophils and monocytes, the isolated hepatic leukocytes were sorted to CD11b+ Gr-1int or CD11b+ Gr-1hi cells using a FACSvantage SE (BD Biosciences, San Jose, CA).
Isolation of cells from bone marrow.
Neutrophils were isolated from bone marrow according to the method of Lowell and Berton (26) with minor modifications. Long bones from mice (humerus, femur, and tibia) were removed, the ends were clipped, and then they were flushed using a 27-gauge needle and ice-cold Ca2+ Mg2+-free HBSS. Clumps of marrow were broken up by repeated pipetting. Cells from the marrow were centrifuged at 500 × g at 4°C for 5 min and resuspended in 4 ml of HBSS. They were placed in a 15-ml polypropylene tube and layered over a four-step gradient (52%, 65%, 70%, and 75% Percoll diluted with HBSS). The tube was centrifuged at 1,500 × g for 30 min at 4°C in a swinging-bucket rotor using the slow brake to prevent disruption of the layers during deceleration. The cells were then removed from the polymorphonuclear-leukocyte-enriched fraction at the interface of the 70% and 75% layers, diluted with 10 ml of HBSS, and sedimented for 7 min at 1,500 × g. Monocytes were isolated from bone marrow by immunomagnetic-bead depletion with streptavidin microbeads after incubation with biotin-conjugated CD4, CD8α, MHC class II, B220, CD43, and CD24. After incubation with antibodies, the negatively fractionated cells were obtained with a magnetically activated cell sorter (Miltenyi). The cells were resuspended in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and antibiotics and counted. Routinely, purities as determined by flow cytometric analysis were >85% for neutrophils and >90% for monocytes.
Determination of viable L. monocytogenes in liver and spleen.
L. monocytogenes-infected mice were anesthetized with ketamine, and their livers were perfused with sterile RPMI 1640 medium containing 10% FBS to wash bacteria out of their blood vessels. The numbers of viable bacteria (CFU) in livers and spleens were determined by plating serial dilutions of organ homogenates in PBS containing 0.05% Triton X-100 and counting the colonies.
Histology and immunostaining.
Livers were excised on the indicated days postinfection (p.i.), fixed in 4% paraformaldehyde, and embedded in paraffin. Deparaffinized sections were stained with hematoxylin and eosin (Sigma-Aldrich). For in situ detection of cells infiltrated into the liver, frozen 6-μm-thick liver sections were prepared at 12 h p.i. and stained with FITC-conjugated anti-CD11b MAb and PE-conjugated anti-Gr-1 MAb or FITC-conjugated anti-CD11b MAb and PE-conjugated F4/80 MAb. Slides were mounted in GVA mounting solution (Zymed, San Francisco, CA) and examined using a laser scanning confocal microscope (FV500; Olympus, London, United Kingdom).
Measurement of cytokines by the CBA method.
Mice were killed; blood and whole liver tissues were collected, weighed, and homogenized in an equal volume of PBS containing protease inhibitors; and the supernatants were collected by centrifugation. The cytokines in the supernatants were quantified using a cytometric bead array (CBA) kit (BD Biosciences) on a FACSCaliber cytometer equipped with CellQuest Pro and CBA software (Becton Dickinson). For measuring the production of cytokines from cultured neutrophils or monocytes, isolated cells were seeded at 4 × 105 cells/well in a 96-well culture plate in RPMI 1640 medium (containing 10% FBS and antibiotics) and cultured with combinations of rat IgG, 3E1 (5 μg/ml), HI-3E1 (5 μg/ml), and heat-killed L. monocytogenes (4 × 106 bacteria/well). After 24 and 48 h, culture supernatants were collected, and cytokines in the supernatants were quantified by the CBA method, as described above.
RT-PCR and RPA.
Total RNA was isolated from liver tissues using TRIzol (Invitrogen Life Technologies, Carlsbad, CA), and levels of specific cytokine/chemokine mRNAs were determined by reverse transcription (RT)-PCR and the RNase protection assay (RPA) method, according to the manufacturer's instructions (Riboquant; BD Pharmingen). For RT-PCR, 2 μg of RNA was reverse transcribed using primers specific for MIP-2 (forward, 5′-CCACTCTCAAGGGCGGTCAA; reverse, 3′-CCCCTTATCCCCAGTCTCTTTCAC), interleukin 1β (IL-1β) (forward, 5′-TGAAATGCCACCTTTTGACA; reverse, 3′-AGCTCATATGGGTCCGACAG), IL-12p35 (forward, 5′-GATGACATGGTGAAGACGGCC; reverse, 3′-GGAGGTTTCTGGCGCAGAGT), IL-12p40(forward, 5′-CTGGCCAGTACACCTGCCAC; reverse, 3′-GTGCTTCCAACGCCAGTTCA), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (forward, 5′-ATCATCTCCGCCCCTTCTGC; reverse, 3′-CCACCACCCTGTTGCTGTAG). The PCR products were stained with ethidium bromide after electrophoresis on 1% agarose gels. For RPA, 5 μg of total RNA was hybridized with [32P]UTP-labeled riboprobes (mCK-2b and mCK-5c; BD Pharmingen) overnight at 56°C. After hybridization, unhybridized single-stranded RNA was digested with RNase. The protected RNA was then purified by phenol-chloroform extraction and ethanol precipitation. The samples were subjected to electrophoresis on a 6% polyacrylamide/7 M urea gel. The gel was dried and subjected to autoradiographic analysis.
Determination of phagocytosis and ROS.
For determination of phagocytosis, HILs were prepared, stained with PE-conjugated anti-CD11b and Cy5-conjugated anti-Gr-1 MAb, and incubated with FITC-labeled dextran (Molecular Probes, OR) at a final concentration of 0.5 mg/ml. After 0, 10, 20, or 60 min of incubation, the cells were cooled to 4°C, washed four times with cold PBS, and analyzed by FACS. For measuring ROS generation, HILs were stained with PE-conjugated anti-CD11b MAb and Cy5-conjugated anti-Gr-1 MAb. The oxidation-sensitive dye 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA) (Molecular Probes, St. Louis, MO) (2 μM) was added and incubated at 37°C for 25 min. Samples were washed and analyzed by FACS for fluorescent signals within the CD11b+ Gr-1int or CD11b+ Gr-1hi populations (see below).
Statistical analysis.
Statistical evaluation of differences between experimental groups was performed with the log rank test for mouse survival curves and with Student's t test.
RESULTS
Administration of anti-4-1BB MAb in the early phase of infection increases survival of Listeria-infected mice.
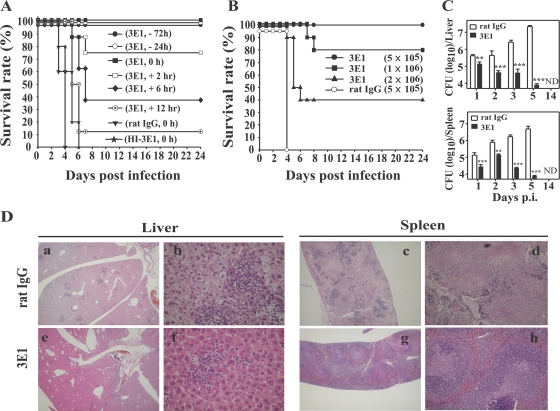
We found that anti-4-1BB MAb administration was effective in protecting mice from L. monocytogenes infection only when it was injected at early times p.i. All mice i.v. infected with 2 × 105 L. monocytogenes bacteria (100% lethal dose [LD100]) and coinjected with control rat IgG died between days 4 and 8 p.i. However, a single injection of 100 μg 3E1 at −72, −24, or 0 h p.i. completely rescued the mice from death (Fig. 1A). These protective effects of 3E1 on mouse survival were progressively reduced if 3E1 was injected at later time points. Administration of 3E1 at 2, 6, 12, or 24 h p.i. resulted in 75, 40, 10, or 0% survival, respectively. Remarkably, 3E1 was also effective against extremely high doses of L. monocytogenes infection. Infection of mice with 5 LD100 or 10 LD100 L. monocytogenes and coinjection of 3E1 produced 60% or 40% survival, respectively (Fig. 1B). All other experiments in this study were carried out using a regimen of 3E1 administration at 0 h p.i. Another clone of agonistic anti-4-1BB MAb, 3H3, had the same protective effect, and the survival of L. monocytogenes-infected C57BL/6 mice was also enhanced as much as that of L. monocytogenes-infected BALB/c mice by 3E1 administration, indicating that the 4-1BB effects on L. monocytogenes-infected mice were not antibody isotype- or mouse strain-specific (data not shown). In addition, HI-3E1 had no effect on the survival of L. monocytogenes-infected mice, indicating that the therapeutic effect of 3E1 was caused by specific triggering of 4-1BB.
FIG. 1.
Anti-4-1BB MAb administration rescues L. monocytogenes-infected mice. (A) Groups of eight mice were infected with 2 × 105 L. monocytogenes bacteria/mouse at 0 h and injected with 3E1 (100 μg) at the indicated times. Survival was monitored daily up to 30 days after infection. (B) Anti-4-1BB MAb administration is effective at up to 10 LD100. Groups of 10 mice were infected with 5 × 105, 1 × 106, or 2 × 106 L. monocytogenes bacteria/mouse and injected with 100 μg of 3E1 or rat IgG. (C) Mice were infected with 2 × 105 L. monocytogenes bacteria, and the numbers of CFU in the spleen and liver were determined on days 1, 2, 3, 5, and 14 p.i. The data are from one representative of three independent experiments and are expressed as means plus standard errors. **, P < 0.01; ***, P < 0.001. ND, not detected. (D) Livers and spleens from the mice in panel C were collected on day 4 p.i., paraffin sectioned, and stained with hematoxylin and eosin. Magnification: a, c, e, and g, ×20; b and f, ×400; and d and h, ×100.
The liver and spleen are two major organs in which systemically infected L. monocytogenes grows (32). We found that the numbers of CFU in the livers and spleens of 3E1-treated mice were lower than those in control antibody-treated mice on all p.i. days investigated. It is noteworthy that as time passed the numbers of CFU in the 3E1-treated mice decreased, while those in the control mice increased (Fig. 1C). It is clear that the control mice succumbed to death because of outgrowth of L. monocytogenes, while the 3E1-treated mice controlled L. monocytogenes at an early phase of infection and resulted in complete eradication of the bacteria. Histological data also support the view that 3E1-helped mice control the outgrowth of L. monocytogenes in infected organs. Granulomatous lesions in the liver, which are histological characteristics of systemic Listeria infection (20), were smaller and fewer in the 3E1-treated mice than in control antibody-treated mice. As with the results in the liver, the 3E1-treated mice displayed less disruption of the white and red pulp architecture of the spleen than the control mice (Fig. 1D).
Anti-4-1BB MAb induces rapid recruitment of CD11b+ Gr-1int and CD11b+ Gr-1hi cells into the liver.
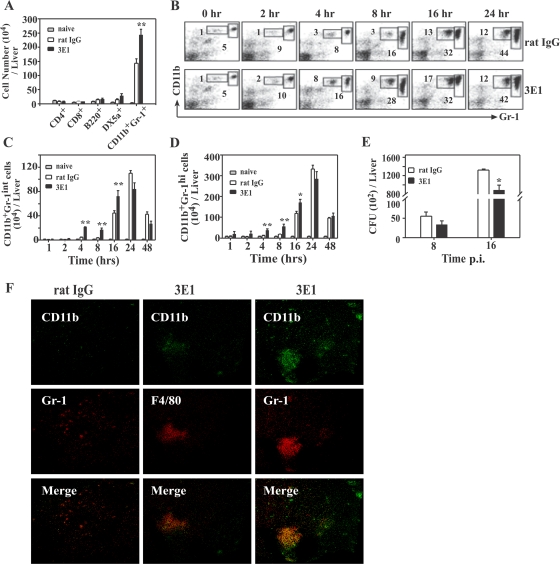
To identify the cellular/molecular mechanisms underlying the remarkable effects of 3E1 on L. monocytogenes infection, we characterized the cells that infiltrated the livers of infected mice. For this, we infected mice with a sublethal dose of 2 × 104 L. monocytogenes bacteria (0.1 LD100). HILs were isolated from the L. monocytogenes-infected mice, and leukocyte subsets were analyzed by flow cytometry. As shown in Fig. 2A, at 16 h p.i. the numbers of CD4+, CD8+, B220+ (B cells), or DX5α+ (NK) cells in HILs were little increased by L. monocytogenes infection and were comparable to those in the 3E1- and control antibody-treated mice. In contrast, the number of CD11b+ Gr-1+ cells was greatly increased by L. monocytogenes infection in both the control and 3E1-treated mice. However, the 3E1-treated mice had much higher numbers of these cells than the controls. Detailed kinetic analysis revealed that the CD11b+ Gr-1+ cells were divided into two subsets, CD11b+ Gr-1int and CD11b+ Gr-1hi, whose numbers depended on the level of Gr-1 expression; there was a higher percentage of these two cell populations in the HILs of 3E1-treated mice than in those of control mice at 4 and 8 h p.i. (Fig. 2B). The total number of these two cell populations in the liver was also higher in 3E1 mice than control mice between 4 and 16 h p.i. (Fig. 2C and D). However, at 24 h p.i. and thereafter, the numbers and percentages of the two types of cell were comparable in the 3E1-treated and control mice. In parallel with the increased number of CD11b+ Gr-1int and CD11b+ Gr-1hi cells, 3E1-treated mice had lower numbers of L. monocytogenes bacteria than control mice at 8 and 16 h p.i. (Fig. 2E). Furthermore, in agreement with the flow cytometric results (Fig. 2B), immunohistochemical microscopic observation revealed more CD11b+ Gr-1+ and CD11b+ F4/80+ cells in liver sections of the 3E1-treated mice than in those of the controls (Fig. 2F). F4/80 is a surface marker for monocytes/macrophages.
FIG. 2.
Anti-4-BB MAb treatment rapidly recruits CD11b+ Gr-1int and CD11b+ Gr-1hi cells to the livers of L. monocytogenes-infected mice with a concurrent decrease in the number of L. monocytogenes bacteria in the liver. (A to D) Mice were infected with 2 × 104 L. monocytogenes bacteria and treated with 100 μg of 3E1 or rat IgG. At the indicated time points, they were anesthetized, the livers were perfused with PBS, and HILs were isolated as described in Materials and Methods. (A) Numbers of CD4+, CD8+, B220+, DX5α+, and CD11b+ Gr-1+ cells in the livers of L. monocytogenes-infected mice. HILs isolated from mice at 16 h p.i. were stained with PE-conjugated anti-CD4, -CD8, -B220, -DX5α, or -CD11b or FITC-conjugated anti-Gr-1 antibody and analyzed by FACS. Each point represents the mean plus standard error (SE) of five mice per group. (B) Time-dependent increase of CD11b+ Gr-1int and CD11b+ Gr-1hi cells. HILs from L. monocytogenes-infected mice were isolated at the indicated times, stained with PE-conjugated anti-CD11b and FITC-conjugated anti-Gr-1 antibody, and analyzed by FACS. (C and D) Total numbers of CD11b+ Gr-1int (C) and CD11b+ Gr-1hi cells (D) were calculated from the number of HILs per liver, and the proportion of each subset was obtained by FACS analysis. (E) Mice were infected with 2 × 104 L. monocytogenes bacteria and injected with 100 μg of 3E1 or rat IgG. At the indicated times p.i., mice were killed, livers were isolated, and the number of CFU/liver was determined. The results are the means plus SE of five mice per group. *, P < 0.05; **, P < 0.01. (F) Immunohistochemical identification of CD11b+ F4/80+ and CD11b+ Gr-1+ cells in livers of L. monocytogenes-infected and 3E1-treated mice. Mice were infected with 2 × 104 CFU L. monocytogenes and injected with 100 μg of 3E1 or rat IgG. At 8 h p.i., frozen liver sections were prepared and stained for CD11b (green) and Gr-1 (red) or CD11b (green) and F4/80 (red). The sections were viewed and photographed using a laser scanning confocal fluorescence microscope system (magnification, ×200).
The CD11b+ Gr-1int cells are monocytes, and the CD11b+ Gr-1hi cells are neutrophils.
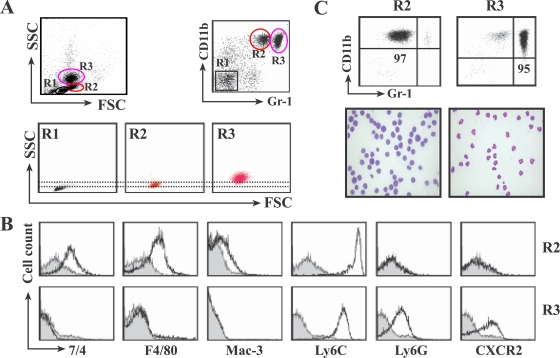
We characterized the CD11b+ Gr-1int and CD11b+ Gr-1hi cells recruited into the livers of L. monocytogenes-infected mice. Both peripheral neutrophils and monocytes express CD11b and Gr-1 on their surfaces, but they can be distinguished by several other properties, such as the number of granules and the level of Gr-1 expression, myeloid and granulocyte marker expression profiles, and the shapes of nuclei (23). FACS analysis confirmed that CD11b+ Gr-1hi cells had high-intensity side scatter characteristics (SSC), and the CD11b+ Gr-1int cells had low-intensity SSC (Fig. 3A). Analysis of surface marker expression revealed that the CD11b+ Gr-1int cells were 7/4+, F4/80+, Mac-3+, Ly6Chi, Ly6G−, and CXCR2−, while the CD11b+ Gr-1hi cells were 7/4−, F4/80−, Mac-3−, Ly6Cint, Ly6G+, and CXCR2+ (Fig. 3B). These profiles indicate that the CD11b+ Gr-1int cells are monocytes and the CD11b+ Gr-1hi cells are neutrophils, as previously reported by other investigators (18, 23, 25). Finally, microscopic observation of the sorted cells showed that the CD11b+ Gr-1int cells were large cells with the crescent-shaped nuclei and pale-blue cytoplasm characteristic of monocytes, while the CD11b+ Gr-1hi cells were primarily composed of smaller cells with a multilobed ring-shaped nuclear morphology indicative of neutrophils (Fig. 3C).
FIG. 3.
In the livers of L. monocytogenes-infected mice, the CD11b+ Gr-1int cells are monocytes and CD11b+ Gr-1hi cells are neutrophils. Mice were infected with 2 × 104 L. monocytogenes bacteria and injected with 100 μg of 3E1. At 16 h p.i., HILs were isolated, stained with antibodies, and analyzed by FACS. (A) Cells were double stained with PE-conjugated anti-CD11b and FITC-conjugated anti-Gr-1 antibodies and gated on CD11b+ Gr-1int (R2) and CD11b+ Gr-1hi (R3). R2 and R3 cells were further analyzed by SSC and forward scatter (FSC) parameters. (B) Expression of surface markers on CD11b+ Gr-1int cells and CD11b+ Gr-1hi cells. HILs were triple stained with antibodies for CD11b, Gr-1, and each surface marker. After being gated on CD11b+ Gr-1int (R2) and CD11b+ Gr-1hi (R3) as in panel A, R2 and R3 cells were further analyzed for the expression of 7/4, F4/80, Mac-3, Ly6C, Ly6G, or CXCR2. (C) Nuclear morphology reveals that CD11b+ Gr-1int cells are monocytes and CD11b+ Gr-1hi cells are neutrophils. The R2 and R3 cells in panel A were sorted by FACS, cytospun, and stained with Diff-Quik. The top row shows the purity of the sorted cells, and bottom row shows photomicrographs (magnification, ×1,000). The data are representative of experiments repeated three times with four mice per group.
Anti-4-1BB MAb administration rapidly upregulates levels of proinflammatory cytokines/chemokines in L. monocytogenes-infected liver.
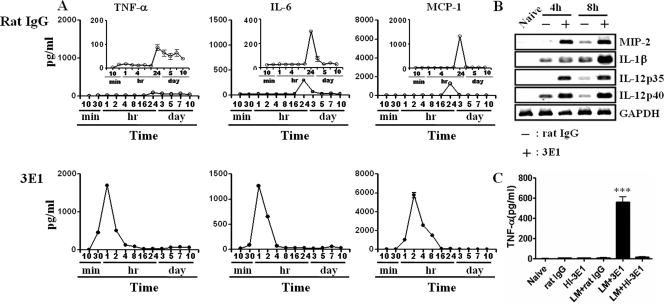
Cytokines/chemokines and their receptors play critical roles in early control of L. monocytogenes infection, as demonstrated by knockout mice. Thus, mice deficient for tumor necrosis factor alpha (TNF-α) and its receptor (8, 15), IL-6 (7), monocyte chemoattractant protein 1 (MCP-1) and its receptor CCR2 (22, 40, 41), IL-12 (39), or gamma interferon (16) are much more susceptible to L. monocytogenes infection. We determined cytokine/chemokine levels in the liver at early times of infection. As shown in Fig. 4A, levels of TNF-α, IL-6, and MCP-1 in control antibody-treated mice did not change up to 16 h p.i. In contrast, the levels of those cytokines in the 3E1-treated mice started to increase as early as 30 min p.i. and were significantly higher than those of control mice. In 3E1-treated mice, TNF-α and IL-6 peaked at 1 h p.i. and MCP-1 at 2 h p.i. Thereafter, cytokine levels gradually decreased to basal levels at 16 h p.i. The induction profiles of cytokines in sera were the same as in the liver (data not shown). An increase in the transcript levels of MIP-2, IL-1β, and IL-12 was also observed (Fig. 4B). HI-3E1 had no effect on the levels of TNF-α, suggesting there was no contamination with endotoxin in our system (Fig. 4C).
FIG. 4.
Anti-4-1BB MAb administration to L. monocytogenes-infected mice rapidly enhances levels of TNF-α, IL-6, and MCP-1 in the liver. (A and B) Mice were infected with 2 × 104 L. monocytogenes bacteria and injected with 100 μg of 3E1 or rat IgG. At the indicated times p.i., the livers were perfused with PBS. The concentrations of cytokines in liver homogenates were determined using a CBA kit (A), and message levels by RT-PCR (B). (C) Naïve or L. monocytogenes-infected mice were i.v. injected with PBS, rat IgG, 3E1, or HI-3E1. After 2 h, the levels of TNF-α in liver homogenates were determined. The data are representative of experiments repeated three times with three mice per group. Data are means ± standard errors. ***, P < 0.001.
Anti-4-1BB administration to L. monocytogenes-infected mice induces activation and maturation of monocytes and neutrophils in HILs.
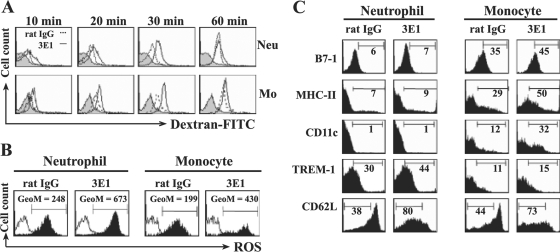
Monocytes and neutrophils eliminate bacteria by phagocytosis and by killing them with ROS, and mice that lack ROS-generating enzymes are more susceptible to L. monocytogenes infection (8). We determined whether 3E1 administration enhanced phagocytic activities and ROS generation. To measure phagocytic activities, mice were infected with L. monocytogenes and injected with 3E1 or rat IgG at 0 h p.i.; HILs were then isolated at 16 h p.i. and incubated with FITC-labeled dextran beads. After the indicated times of incubation, the cells were gated for monocytes or neutrophils, and the percent FITC-positive cells was analyzed. As shown in Fig. 5A, monocytes and neutrophils isolated from 3E1-treated mice had higher phagocytic activities than those isolated from control antibody-treated mice. Moreover, monocytes and neutrophils isolated from 3E1-treated mice also produced more ROS (Fig. 5B).
FIG. 5.
Anti-4-1BB MAb administration to L. monocytogenes-infected mice induces activation and maturation of monocytes (Mo) and neutrophils (Neu) in HILs. Mice were infected with 2 × 104 L. monocytogenes bacteria and injected with 100 μg of 3E1 or rat IgG. At 16 h p.i., HILs were isolated and stained with PE-conjugated anti-Gr-1 and Cy-conjugated anti-CD11b MAbs. (A) Anti-4-1BB MAb-induced phagocytic activity. Cells were incubated with FITC-dextran for the indicated times, washed, and analyzed for FITC-positive cells among the CD11b+ Gr-1int or CD11b+ Gr-1hi cells by FACS. Gray histogram, cells kept on ice; dotted lines, cells from rat IgG-treated mice; solid lines, cells from 3E1-treated mice. (B) Anti-4-1BB MAb induces ROS generation. HILs were stained with 2 μM DCF-DA for 30 min and analyzed for DCF-DA-positive cells among CD11b+ Gr-1int or CD11b+ Gr-1hi gated cells by FACS. Open histogram, unstained cells; filled histogram, cells stained with DCF-DA. GeoM, geometric mean. (C) Anti-4-1BB MAb enhances the expression of maturation/activation surface markers on the monocytes and neutrophils of L. monocytogenes-infected mice. Mice were infected with 2 × 104 L. monocytogenes bacteria and injected with 100 μg of 3E1 or rat IgG. At 16 h p.i., HILs were isolated, stained with Cy-conjugated anti-CD11b, PE-conjugated anti-Gr-1, and FITC-conjugated surface marker antibodies. The cells were first gated on CD11b+ Gr-1int or CD11b+ Gr-1hi and analyzed for each expression marker. One experiment representative of three is shown.
In addition to enhanced phagocytic activity and ROS generation, the liver-infiltrated monocytes and neutrophils from 3E1-treated mice had more activation/maturation phenotypic surface markers than cells from control antibody-treated mice (Fig. 5C). Monocytes from 3E1-treated mice expressed higher levels of B7-1, MHC class II (I-Ad), CD11c, and TREM-1 and lower levels of CD62L, and neutrophils from 3E1-treated mice expressed higher levels of TREM-1 and lower levels of CD62L on their surfaces. TREM-1 is an activating receptor of the Ig superfamily expressed on myeloid cells (2). These results show that 4-1BB stimulation by 3E1 induces activation and maturation of recruited neutrophils and monocytes in L. monocytogenes-infected mice, as well as infiltration of those cells into infection sites.
In vitro stimulation of neutrophils by anti-4-1BB MAb causes production of IL-6 and TNF-α and upregulation of chemokine mRNAs.
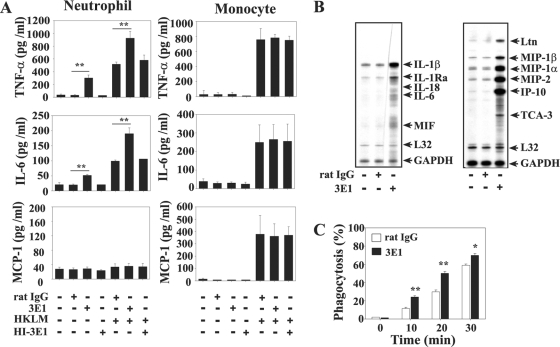
Although 3E1 administration to L. monocytogenes-infected mice induced activation and infiltration of neutrophils and monocytes into the bacterium-infected livers, it was not clear whether 3E1 exerted its effects directly on those cells or whether the cells were secondarily activated by 3E1. Murine neutrophils constitutively express 4-1BB (24), but murine monocytes do not, unless they are induced to differentiate into dendritic cells (our unpublished data). Consistent with the status of 4-1BB expression on the surface of each cell type, we found that 3E1 could directly stimulate the expression of cytokine genes and proteins in neutrophils, but not in monocytes. As shown in Fig. 6A, 3E1 induced the production of IL-6 and TNF-α in cultures of neutrophils. 3E1 and heat-killed L. monocytogenes induced neutrophils to produce these cytokines synergistically, suggesting the existence of signaling cross talk between 4-1BB and pattern recognition receptors. MCP-1 was not produced in response to 3E1 and/or heat-killed L. monocytogenes in neutrophils. As expected, 3E1 did not induce monocytes to produce these cytokines, although heat-killed L. monocytogenes strongly induced IL-6, TNF-α, and MCP-1 production by the monocytes (Fig. 6A). 3E1 also strongly increased levels of chemokine/cytokine transcripts, such as those for IL-1β, IL-1Rα, IL-6, MIF, Ltn, MIP-1α, MIP-1β, IP-10, and TCA-3 in neutrophils, as determined by the RPA method (Fig. 6B). In addition, we found that 3E1 significantly enhanced the phagocytic activities of neutrophils in vitro (Fig. 6C). These results suggest that 3E1 may directly activate neutrophils to be efficient bacterium-killing cells in L. monocytogenes-infected mice and that monocyte activation and recruitment into bacterium-infected livers may be secondary effects of 4-1BB stimulation of other cells.
FIG. 6.
In vitro addition of anti-4-1BB MAb induces production of cytokines/chemokines in neutrophils, but not monocytes. (A) Neutrophils and monocytes were purified from the bone marrow of naïve mice. The cells were cultured in 96-well culture plates with combinations of rat IgG (5 μg/ml), 3E1 (5 μg/ml), HI-3E1 (5 μg/ml), and heat-killed L. monocytogenes (HKLM) (1 × 104 bacteria/well). After 24 h, the concentrations of each cytokine/chemokine in the culture supernatants were measured with a CBA kit. (B) Total RNA was isolated from neutrophils cultured with control rat IgG or 3E1 for 12 h using TRIzol reagent. Cytokine and chemokine mRNA levels were quantified by an RNase protection assay as described in Materials and Methods. (C) Increase of neutrophil phagocytic activity by 4-1BB stimulation. Neutrophils purified from bone marrow were incubated with FITC-dextran (1 mg/ml) and rat IgG or 3EI (1 μg/ml) for the indicated times, washed, and analyzed by FACS for FITC-positive cells. The results are from one representative of three independent experiments and are expressed as means plus standard deviations. *, P < 0.05; **, P < 0.01.
DISCUSSION
Upon infection with L. monocytogenes, neutrophils and monocytes are the first to migrate to infected tissues. Chemokines and their receptors play key roles in recruiting neutrophils and monocytes to infection sites. Murine neutrophils express CXC chemokine receptors and are recruited in response to CXC ELR+ chemokines, such as KC and MIP-2 (3), while monocytes primarily express CC chemokine receptors that respond to MCPs (27). In this study, we found that anti-4-1BB MAb administration to L. monocytogenes-infected mice rapidly induced TNF-α, MCP-1, and IL-6 in sera and livers as early as 30 min p.i., while levels of these proteins were little changed in L. monocytogenes-infected control mice up to 16 h p.i. (Fig. 4). Message levels of IL-1, IL-12, and MIP-2 were also highly increased by anti-4-1BB MAb administration. Some of these cytokines/chemokines in the L. monocytogenes-infected mice were possibly produced by 4-1BB-activated neutrophils, because direct addition of 3E1 to neutrophils increased proteins and message levels for these chemokines/cytokines, except for MCP-1 (Fig. 6). Because monocytes do not express 4-1BB and neutrophils do not produce MCP-1 in response to 4-1BB stimulation, the presence of MCP-1 in sera of 3E1-treated mice is probably not caused by direct action of 3E1 on neutrophils or monocytes. It may be induced by cells activated by bacterial products or by the IL-6 produced by the 4-1BB-stimulated neutrophils (Fig. 6). It has been shown that IL-6 binds to soluble IL-6 receptors, forming a complex that induces the endothelial cells, mesothelial cells, or fibroblasts at inflammatory sites to release MCP-1 (19, 37, 42).
In parallel with the rapid induction of chemokine/cytokine levels, recruitment of neutrophils/monocytes into the livers of 3E1-treated mice was faster than that for control antibody-treated mice (Fig. 2B, C, and D). The liver plays a crucial role in eradicating blood-infected L. monocytogenes. It has been shown that most of the L. monocytogenes bacteria introduced via the i.v. route are trapped in the liver, bound to the surfaces of Kupffer cells (liver-resident macrophages), and are subsequently killed by infiltrated neutrophils (13). Depleting neutrophils or blocking mobilization of neutrophils into the liver in L. monocytogenes-infected mice results in a marked increase in the number of L. monocytogenes bacteria in the liver (6, 35), and the mice die of liver failure (36). Therefore, the rapid infiltration of neutrophils into livers is critical for early defense against L. monocytogenes infection, and this may be one mechanism of 4-1BB-mediated protection of L. monocytogenes-infected mice.
It is also conceivable that 4-1BB signaling on infiltrated neutrophils results in their activation to form efficient bacterium-killing cells. 4-1BB-4-1BB ligand (4-1BBL) interaction may well occur normally during bacterial infection. 4-1BBL is predominantly expressed on murine antigen-presenting cells (APCs), such as dendritic cells, monocytes/macrophages, and activated B cells (12, 33). Although expression of 4-1BBL on Kupffer cells has not been examined, there is a good possibility that it is also expressed on the surfaces of these cells, one of the liver-resident APCs. In addition, Kupffer cells bind neutrophils via CD11b/CD18-CD54 interaction in L. monocytogenes-infected mice (14). Thus, during Kupffer cell-neutrophil binding or some other kind of APC-neutrophil binding in the livers of L. monocytogenes-infected mice, 4-1BB-4-1BBL interaction may occur; the neutrophils may be activated by the 4-1BB signaling induced by 4-1BBL in APCs or by an administered anti-4-1BB MAb, and this could be synergized by bacterial products present on the surfaces of the APCs (Fig. 6).
In summary, our data show that 4-1BB stimulation in the early phase of L. monocytogenes infection contributes to the effective control of bacteria by rapid production of inflammatory cytokines/chemokines and subsequent infiltration of neutrophils and monocytes. A strategy for achieving 4-1BB activation using antibody or 4-1BBL molecules may be useful in the treatment of listeriosis.
Acknowledgments
We thank Byoung S. Kwon (Korea National Cancer Center) and R. Mittler (Emory University, Atlanta, GA) for their kind gifts of antibodies and for helpful comments and discussion.
This work was supported by the 2008 Research Fund of the University of Ulsan. S. A. Ju, J. D. Kim, and S. C. Lee were supported by a Korea Research Foundation Grant funded by the Korean Government (KRF-2007-412-J00302), and S. M. Park, Q. T. Nguyen, and B. H. Seong were supported by the Second Project of BK21, the Ministry of Education and Human Resources Development.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 23 February 2009.
REFERENCES
- 1.Bertram, E. M., W. Dawicki, and T. H. Watts. 2004. Role of T cell costimulation in anti-viral immunity. Semin. Immunol. 16185-196. [DOI] [PubMed] [Google Scholar]
- 2.Bouchon, A., J. Dietrich, and M. Colonna. 2000. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 1644991-4995. [DOI] [PubMed] [Google Scholar]
- 3.Cacalano, G., J. Lee, K. Kikly, A. M. Ryan, S. Pitts-Meek, B. Hultgren, W. I. Wood, and M. W. Moore. 1994. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science 265682-684. [DOI] [PubMed] [Google Scholar]
- 4.Carrero, J. A., B. Calderon, and E. R. Unanue. 2006. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J. Exp. Med. 203933-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cousens, L. P., and E. J. Wing. 2000. Innate defenses in the liver during Listeria infection. Immunol. Rev. 174150-159. [DOI] [PubMed] [Google Scholar]
- 6.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 1521836-1846. [PubMed] [Google Scholar]
- 7.Dalrymple, S. A., L. A. Lucian, R. Slattery, T. McNeil, D. M. Aud, S. Fuchino, F. Lee, and R. Murray. 1995. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect. Immun. 632262-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endres, R., A. Luz, H. Schulze, H. Neubauer, A. Futterer, S. M. Holland, H. Wagner, and K. Pfeffer. 1997. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity 7419-432. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima, A., T. Yamaguchi, W. Ishida, K. Fukata, R. S. Mittler, H. Yagita, and H. Ueno. 2005. Engagement of 4-1BB inhibits the development of experimental allergic conjunctivitis in mice. J. Immunol. 1754897-4903. [DOI] [PubMed] [Google Scholar]
- 10.Futagawa, T., H. Akiba, T. Kodama, K. Takeda, Y. Hosoda, H. Yagita, and K. Okumura. 2002. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 14275-286. [DOI] [PubMed] [Google Scholar]
- 11.Garifulin, O., and V. Boyartchuk. 2005. Listeria monocytogenes as a probe of immune function. Brief Funct. Genomic Proteomic 4258-269. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin, R. G., W. S. Din, T. Davis-Smith, D. M. Anderson, S. D. Gimpel, T. A. Sato, C. R. Maliszewski, C. I. Brannan, N. G. Copeland, N. A. Jenkins, et al. 1993. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur. J. Immunol. 232631-2641. [DOI] [PubMed] [Google Scholar]
- 13.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1996. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J. Immunol. 1572514-2520. [PubMed] [Google Scholar]
- 14.Gregory, S. H., and E. J. Wing. 2002. Neutrophil-Kupffer cell interaction: a critical component of host defenses to systemic bacterial infections. J. Leukoc. Biol. 72239-248. [PubMed] [Google Scholar]
- 15.Grivennikov, S. I., A. V. Tumanov, D. J. Liepinsh, A. A. Kruglov, B. I. Marakusha, A. N. Shakhov, T. Murakami, L. N. Drutskaya, I. Forster, B. E. Clausen, L. Tessarollo, B. Ryffel, D. V. Kuprash, and S. A. Nedospasov. 2005. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity 2293-104. [DOI] [PubMed] [Google Scholar]
- 16.Harty, J. T., and M. J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity 3109-117. [DOI] [PubMed] [Google Scholar]
- 17.Heinisch, I. V., I. Daigle, B. Knopfli, and H. U. Simon. 2000. CD137 activation abrogates granulocyte-macrophage colony-stimulating factor-mediated anti-apoptosis in neutrophils. Eur. J. Immunol. 303441-3446. [DOI] [PubMed] [Google Scholar]
- 18.Henderson, R. B., J. A. Hobbs, M. Mathies, and N. Hogg. 1998. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood 102328-335. [DOI] [PubMed] [Google Scholar]
- 19.Hurst, S. M., T. S. Wilkinson, R. M. McLoughlin, S. Jones, S. Horiuchi, N. Yamamoto, S. Rose-John, G. M. Fuller, N. Topley, and S. A. Jones. 2001. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 14705-714. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann, S. H. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11129-163. [DOI] [PubMed] [Google Scholar]
- 21.Kienzle, G., and J. von Kempis. 2000. CD137 (ILA/4-1BB), expressed by primary human monocytes, induces monocyte activation and apoptosis of B lymphocytes. Int. Immunol. 1273-82. [DOI] [PubMed] [Google Scholar]
- 22.Kurihara, T., G. Warr, J. Loy, and R. Bravo. 1997. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 1861757-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagasse, E., and I. L. Weissman. 1996. Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods 197139-150. [DOI] [PubMed] [Google Scholar]
- 24.Lee, S. C., S. A. Ju, H. N. Pack, S. K. Heo, J. H. Suh, S. M. Park, B. K. Choi, B. S. Kwon, and B. S. Kim. 2005. 4-1BB (CD137) is required for rapid clearance of Listeria monocytogenes infection. Infect. Immun. 735144-5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leenen, P. J., M. F. de Bruijn, J. S. Voerman, P. A. Campbell, and W. van Ewijk. 1994. Markers of mouse macrophage development detected by monoclonal antibodies. J. Immunol. Methods 1745-19. [DOI] [PubMed] [Google Scholar]
- 26.Lowell, C. A., and G. Berton. 1998. Resistance to endotoxic shock and reduced neutrophil migration in mice deficient for the Src-family kinases Hck and Fgr. Proc. Natl. Acad. Sci. USA 957580-7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu, B., B. J. Rutledge, L. Gu, J. Fiorillo, N. W. Lukacs, S. L. Kunkel, R. North, C. Gerard, and B. J. Rollins. 1998. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 187601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melero, I., J. V. Johnston, W. W. Shufford, R. S. Mittler, and L. Chen. 1998. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 190167-172. [DOI] [PubMed] [Google Scholar]
- 29.Melero, I., W. W. Shuford, S. A. Newby, A. Aruffo, J. A. Ledbetter, K. E. Hellstrom, R. S. Mittler, and L. Chen. 1997. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 3682-685. [DOI] [PubMed] [Google Scholar]
- 30.Miyamoto, M., M. Emoto, Y. Emoto, V. Brinkmann, I. Yoshizawa, P. Seiler, P. Aichele, E. Kita, and S. H. Kaufmann. 2003. Neutrophilia in LFA-1-deficient mice confers resistance to listeriosis: possible contribution of granulocyte-colony-stimulating factor and IL-17. J. Immunol. 1705228-5234. [DOI] [PubMed] [Google Scholar]
- 31.Nishimoto, H., S. W. Lee, H. Hong, K. G. Potter, M. Maeda-Yamamoto, T. Kinoshita, Y. Kawakami, R. S. Mittler, B. S. Kwon, C. F. Ware, M. Croft, and T. Kawakami. 2005. Costimulation of mast cells by 4-1BB, a member of the tumor necrosis factor receptor superfamily, with the high-affinity IgE receptor. Blood 1064241-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pamer, E. G. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4812-823. [DOI] [PubMed] [Google Scholar]
- 33.Pollok, K. E., Y. J. Kim, J. Hurtado, Z. Zhou, K. K. Kim, and B. S. Kwon. 1994. 4-1BB T-cell antigen binds to mature B cells and macrophages, and costimulates anti-mu-primed splenic B cells. Eur. J. Immunol. 24367-374. [DOI] [PubMed] [Google Scholar]
- 34.Pulle, G., M. Vidric, and T. H. Watts. 2006. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J. Immunol. 1762739-2748. [DOI] [PubMed] [Google Scholar]
- 35.Rogers, H. W., and E. R. Unanue. 1993. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect. Immun. 615090-5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers, H. W., M. P. Callery, B. Deck, and E. R. Unanue. 1996. Listeria monocytogenes induces apoptosis of infected hepatocytes. J. Immunol. 156679-684. [PubMed] [Google Scholar]
- 37.Romano, M., M. Sironi, C. Toniatti, N. Polentarutti, P. Fruscella, P. Ghezzi, R. Faggioni, W. Luini, V. van Hinsbergh, S. Sozzani, F. Bussolino, V. Poli, G. Ciliberto, and A. Mantovani. 1997. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6315-325. [DOI] [PubMed] [Google Scholar]
- 38.Rosen, H., S. Gordon, and R. J. North. 1989. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J. Exp. Med. 17027-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 1693863-3868. [DOI] [PubMed] [Google Scholar]
- 40.Serbina, N. V., and E. G. Pamer. 2006. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7311-317. [DOI] [PubMed] [Google Scholar]
- 41.Serbina, N. V., T. P. Salazar-Mather, C. A. Biron, W. A. Kuziel, and E. G. Pamer. 2003. TNF/iNOS-producing dendritic cells mediate innate immune defence against bacterial infection. Immunity 1959-70. [DOI] [PubMed] [Google Scholar]
- 42.Sporri, B., K. M. Muller, U. Wiesmann, and M. Bickel. 1999. Soluble IL-6 receptor induces calcium flux and selectively modulates chemokine expression in human dermal fibroblasts. Int. Immunol. 111053-1058. [DOI] [PubMed] [Google Scholar]
- 43.Vinay, D. S., K. Cha, and B. S. Kwon. 2006. Dual immunoregulatory pathways of 4-1BB signaling. J. Mol. Med. 84726-736. [DOI] [PubMed] [Google Scholar]