Abstract
Currently available Neisseria meningitidis serogroup B (MenB) vaccines are based on outer membrane vesicles (OMVs) that are obtained from wild-type strains. They are purified with the aim of decreasing the lipooligosaccharide (LOS) content and hence reduce the reactogenicity of the vaccine even though LOS is a potential protective antigen. In <2-year-old children, these MenB vaccines confer protection only against strains expressing homologous PorA, a major and variable outer membrane protein. Our objective was to develop a safe LOS-based vaccine against MenB. To this end, we used modified porA knockout strains expressing genetically detoxified (msbB gene-deleted) L2 and L3,7 LOSs, allowing the production of LOS-enriched OMVs. The vaccine-induced antibodies were found to be bactericidal against nearly all invasive strains, irrespective of capsular serogroup. In addition, we have also demonstrated that LOS lacking the terminal galactose (with a lgtB mutation; truncated L3 LOS), but not LOS produced without the galE gene, induced a bactericidal antibody response in mice similar to that seen for LOS containing the full lacto-N-neotetraose (L3,7 LOS). In conclusion, a bivalent detoxified LOS OMV-based vaccine demonstrated the potential to afford a broad cross-protection against meningococcal disease.
The gram-negative Neisseria meningitidis serogroup B (MenB) is a major cause of bacterial meningitis in younger populations. The disease is associated with high morbidity and mortality rates, despite the availability of optimized treatments. Therefore, disease prevention by vaccination is considered a better approach than treatment. Currently available MenB vaccines are based on outer membrane vesicles (OMVs) that are obtained from wild-type strains and purified with the aim of decreasing the endotoxin lipooligosaccharide (LOS) content, hence reducing the reactogenicity of the vaccine. However, in <2-year-old children, these MenB vaccines confer protection only against strains expressing the homologous PorA, a major and variable outer membrane protein (OMP) present on the vaccine OMVs (25, 34, 43). Other vaccine approaches able to afford a larger cross-protection, among which the development of a safe LOS-based vaccine might be a valuable option, seem necessary. Indeed, antibodies to LOS have been shown to be bactericidal, both in humans (4) and in monkeys (45), and natural bacterial clearance in humans appears to be linked with anti-LOS activity (5). These two observations point out LOS as a potential vaccine candidate.
LOS is composed of a lipid A anchor to the bacterial outer membrane and a variable glycan moiety. Although LOS has endotoxin properties, it behaves like an exotoxin due to the capacity of N. meningitidis to secrete large amounts of its outer membrane in the form of blebs or OMVs. Moreover, the fulminant cases of meningococcemia relate to a great extent to the amount of circulating LOS (2).
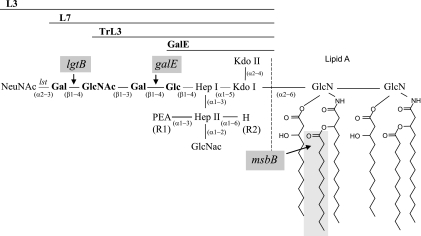
Original immunotyping allowed the determination of 11 distinct LOS types among meningococcal strains, designated L1 to L11 (33). Every LOS contains a conserved inner core consisting of two l-glycero-d-manno-heptose residues (HepI and HepII) and N-acetylglucosamine (GlcNAc) with variable glycan extensions on HepI and HepII. The LOS types are determined by the length and composition of the α- and β-chain extensions of HepI as well as the presence or absence of a γ-chain extension and of phosphoethanolamine (PEA) residues on HepII (see Fig. 1) (3).
FIG. 1.
LOS structures used in this study. The LNnT tetrasaccharide is indicated in bold. The L2, L3v, and L4 LOS are similar to the L3 LOS except for the R1 and R2 residues. For L2, R1 is Glc and R2 is PEA; for L3v, R1 is PEA and R2 is PEA; and for L4, R1 is H and R2 is PEA. galE, lgtB, or msbB mutations of LOS are also indicated (gray boxes). Kdo, 3-deoxy-d-manno-2-octulosonic acid.
Meningococcal LOS is the target of the C4b component of the complement system (32), and because of its adhesin and toxic properties, it can be considered a major virulence factor of MenB (38). The L3,7 immunotype (combined expression of L3 and L7) dominates in case isolates, representing up to 70% of disease cases, whereas another immunotype, L1,8, is most prevalent among carrier isolates (18, 29, 33). Differences in prevalence may be related to the composition of the different LOS immunotypes, which afford different adhesion/invasion and complement resistance properties (24, 32, 40). The L3,7 LOS has been demonstrated to resist covalent binding by C4b (32). The lacto-N-neotetraose (LNnT) α chain of the L7 immunotype can be sialylated (resulting in an L3 LOS), which further prevents the activation and deposition of complement on the bacterial surface (6). These observations might explain the L3,7 dominance among case isolates. The L2 immunotype is the second most prevalent immunotype, representing 20 to 30% of cases. This remote second position might be due to the fact that it does not resist binding by C4b.
In the context of developing an LOS-based vaccine, immunotypes found in carrier isolates are considered less adequate. On the contrary, the two major immunotypes involved in disease, namely, L3,7 and L2, appear to be suitable vaccine candidates. Previous attempts to develop LOS as a vaccine antigen were undertaken, but it appeared difficult to maintain the appropriate antigenic conformation (23, 39). In this study, we describe preclinical immunological responses induced by genetically detoxified bivalent LOS in a formulation of OMVs. This approach combines the advantage of an appropriate LOS structure with reduced reactogenicity.
MATERIALS AND METHODS
N. meningitidis transformation.
Cells of N. meningitidis were incubated overnight on chocolate base (GC; Difco) or Mueller-Hinton (MH; Difco) plates in the presence of 5% CO2. Cells were collected in 2 ml of liquid GC or MH medium containing 10 mM MgCl2 and diluted to an optical density of 0.1 at 550 nm. Two micrograms of DNA was added to the cell suspension before a 6-h incubation period at 37°C (with shaking). Next, 100 μl of culture, either undiluted or at 1/10, 1/100, and 1/1,000 dilutions, were spread on GC or MH plates containing the corresponding antibiotic (see below). Recombinant colonies appeared after 48 h of incubation at 37°C in the presence of 5% CO2.
Construction of H44/76 lacking capsular polysaccharides (ΔsiaD).
Homologous recombination at the sia locus of wild-type H44/76 was achieved using the pIP10 plasmid (16, 40). Erythromycin (10 μg/ml)-resistant, capsule-deficient strains were selected by colony blotting using the anti-group B polysaccharide (B-PS) 735 monoclonal antibody (Boerhinger Mannheim, Germany). The binding of the monoclonal antibody was visualized with a biotinylated anti-mouse immunoglobulin (Ig) (1/1,000; Amersham).
Construction of H44/76 lacking capsular polysaccharides (Δcps) and expressing truncated ΔgalE LOS.
Plasmid pMF121 (10) was used to construct an H44/76 strain lacking the capsular polysaccharide. This plasmid contains the flanking regions of the gene locus coding for members of the biosynthesis pathway of the B-PS, as well as the erythromycin resistance gene. Deletion of the B-PS locus resulted in a loss of expression of the group B cps and loss of the active copy of the galE gene, leading to galactose-deficient LOS (ΔgalE LOS). Selection of erythromycin (10 μg/ml)-resistant, capsule-deficient strains was performed by colony blotting using the anti-B PS 735 monoclonal antibody (Boerhinger Mannheim, Germany). The binding of the monoclonal antibody was visualized with a biotinylated anti-mouse Ig (1/1,000; Amersham).
Construction of H44/76 expressing truncated L3 LOS (with lgtB mutation).
A strain expressing truncated L3 LOS was obtained by insertion of a kanamycin resistance gene in the coding region of the lgtB gene. Homologous recombination was achieved at the lgtB locus by using the pMJ1b11-EcoNI plasmid (15). Circular PMJ1b11-EcoNI DNA was used to transform wild-type H44/76. Transformants were selected on agar plates containing 200 μg/ml of kanamycin and selection of a strain presenting a clean truncated L3 profile was performed based on the LOS electrophoresis profile on 16% Tricine gels.
Construction of an H44/76 PorA knockout strain.
Plasmid pCMK (42) was used to construct a wild-type or uncapsulated H44/76 strain lacking expression of PorA. Kanamycin-resistant colonies were screened for the deletion of the porA gene by PCR on boiled bacterial lysate using primers PPA1 and PPA2 (Table 1). The absence of PorA expression was further confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis.
TABLE 1.
Primers used in this study
| Primer | Sequence |
|---|---|
| PPA1 | 5′-GCGGCCGTTGCCGATGTCAGCC-3′ |
| PPA2 | 5′-GGCATAGCTGATGCGTGGAACTGC-3′ |
| mb-amo-5′ | 5′-AAAACTGCAGCAACATGGGCTGTCCCG-3′ |
| mb-650-3 | 5′-GTAGTCTAGATTCAGACGGCGCGTCCGAAAT-3′ |
| circmbATG | 5′-AGGAGATCTCGATACACATTTTCTTTTCAG-3′ |
| circmb290 | 5′-AGGAGATCTGCATTATTTGGACGACGCGCTG-3′ |
| mb-scr-amo | 5′-GAAGCGATCCGCAGCAG-3′ |
| mb-scr-650 | 5′-CAATGCGGCTCAATCCG-3′ |
Plasmid pCMT was used to construct a truncated H44/76 strain deficient in PorA expression. This plasmid is similar to pCMK except that a tetracycline resistance gene was used instead of a kanamycin resistance gene. The tetracycline resistance cassette (tetM gene from pAM120; accession number U49939) was excised from pTC07 (27) as a 2.7-kb SpeI/NcoI fragment, blunted, and introduced into a pCM vector (PstI restricted and blunted) between the porA 5′ recombinogenic region and the porA-lacO promoter region. The tetM gene was cloned in the same orientation as the porA promoter.
Plasmid pCMC was used to construct a strain expressing truncated L2 LOS but deficient in PorA expression. This plasmid is similar to pCMK except that the chloramphenicol resistance gene was used instead of a kanamycin resistance gene.
Construction of Neisseria recombinant strains expressing detoxified LOS.
Recombinant Neisseria strains with a detoxified lipid A moiety were obtained by inactivation of the msbB gene with plasmid pRIT15418 or pRIT15419. These vectors contain a 1,584-bp 5′ recombinogenic sequence and a 286-bp 3′ recombinogenic sequence; a spectinomycin resistance gene was cloned instead of the section corresponding to amino acids 1 to 95 of the corresponding MsbB protein.
Primers mb-amo-5′ and mb-650-3 (Table 1) were used to amplify a 2,152-bp fragment corresponding to 1,582 bp of the H44/76 msbB upstream sequence and the 5′ part of the msbB open reading frame (570 bp). This PCR fragment was cloned in the pGEM-T vector (Promega) and then submitted to circle PCR mutagenesis (17) in order to delete nucleotides +3 to +284 of the msbB coding sequence and to insert a BglII site allowing the insertion of an antibiotic resistance gene. The circle PCR was performed using oligonucleotide primers cirmcbATG and circmb290 (Table 1), and the PCR fragment was restricted with BglII.
A spectinomycin resistance cassette (spc) with strong transcriptional and translational terminators on either side was isolated as a BamHI fragment from plasmid pHP 45Ω (received from C. Tinsley, Necker Hospital) (30) and cloned in place of the deleted part of the msbB open reading frame to obtain plasmid pRIT15418 (spc in clockwise orientation) and pRIT15419 (spc in counterclockwise orientation).
Circular DNA was used to transform wild-type H44/76 or L2 strains. Neisseria transformants were selected on GC agar plates supplemented with spectinomycin (100 μg/ml) and screened by PCR for double crossing-over events with primers mb-scr-amo and mb-scr-650 (Table 1).
Δcps LOS knockout H44/76 strain.
The Δcps LOS knockout H44/76 strain was kindly provided by Martine Bos (Utrecht University, The Netherlands) (1).
Culture and preparation of OMVs.
A vial of frozen N. meningitidis was thawed, and 0.1 ml of bacterial suspension was streaked onto agar plate. The plate was incubated at 37°C for 18 h. Grown bacteria were resuspended in 8 ml of a liquid medium containing the adequate antibiotic, and 2 ml of the resuspended solid preculture was added to a 2-liter flask containing 400 ml of liquid medium supplemented with the adequate antibiotic. The flask was placed on a shaking table (200 rpm) and incubated at 37°C for 16 h. The cells were separated from the culture broth by centrifugation at 5,000 × g at 4°C for 15 min.
The cell pellet was resuspended in 5 volumes of extraction buffer (0.1 M Tris-Cl, 1 mM EDTA, 0.1% or 0.5% Na deoxycholate, pH 8.6) and incubated under shaking for 30 min at room temperature followed by 30 min at 30°C. The treatment inactivates the cellular suspension, which is then submitted to a first benzonase (50 U/ml) treatment to initiate DNA degradation and to reduce viscosity. The resulting extract was clarified by centrifugation (17,000 × g, 30 min at 20°C), and residual DNA was further digested by a second incubation with freshly added benzonase (50 U/ml). The final suspension was filtered through a cartridge unit (0.45-μm-pore-size prefilter and 0.2-μm-pore-size final filter; Sartobran P; Sartorius Ltd.). OMVs were separated from contaminants by diafiltration on a 300-kDa membrane (Pellicon 2-C screen; Millipore) and permeation on Sephacryl S-400 HR. The purified vesicles were sterilized by filtration through a 0.20-μm membrane (Sartobran 300; Sartorius) and stored at 4°C.
Mouse immunizations and rabbit pyrogenicity assay.
Outbred OF1 mice (also known as CF1; female, 6 to 8 weeks of age; from Charles River, Lyon, France) received three injections with OMVs via the intramuscular route on days 0, 21, and 28. Each injection contained OMV antigen normalized to 5 μg of protein and was either formulated with the GlaxoSmithKline proprietary AS04 adjuvant system (AlPO4 plus 3-O-desacyl-4′ monophosphoryl lipid A) or adsorbed onto aluminum hydroxide alone, in a final volume of 50 μl. Control mice received either adjuvant or vehicle. Blood samples were collected 14 days after the third injection. Rabbit pyrogenicity assays were performed according to the standard assay described in the European Pharmacopeia (6a). The animal experiments complied with the relevant national guidelines of Belgium and institutional policies of GlaxoSmithKline Biologicals.
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was used to characterize the anti-LOS response in mice immunized with OMVs. Microplates were precoated for 2 h at room temperature with 1 μg/ml poly-l-lysine diluted in phosphate-buffered saline (PBS), then washed with PBS-0.05% Tween 20, and coated overnight at room temperature with 2 μg/ml purified L3,7 LOS diluted in PBS. The microwells were blocked with PBS-1% bovine serum albumin. After the microwells were washed, serial dilutions of sera were added for 30 min at room temperature. The microwells were washed again, and peroxidase-labeled goat anti-mouse IgG (Jackson) was added for 30 min at room temperature. The plates were then washed and developed using o-phenylenediamine and H2O2 in citrate buffer. After 30 min, the reaction was stopped with HCl and the absorbance was measured at 490 to 620 nm.
An LNnT ELISA was used to detect the presence of anti-LNnT antibodies. Microplates were coated overnight with 5 μg/ml of LNnT-acetylphenylenediamine-human serum albumin conjugate (IsoSep) diluted in PBS at 4°C. The plates were washed and blocked, and serial dilutions of sera were added to the plates. Following this washing, biotin-labeled rabbit anti-mouse Ig (Prosan) and streptavidin-horseradish peroxidase complex (Amersham) were successively added. The plates were then washed and developed as described for the LOS ELISA. Serum pools were tested on a single-sample basis.
Serum bactericidal assay (SBA).
Wild-type N. meningitidis strains were grown overnight on either tryptic soy broth agar plates or brain heart infusion agar plates in a 5% CO2 atmosphere. Bacteria from one plate were scraped with a sterile swab and streaked onto fresh agar plates. After 4 h of culture at 37°C in 5% CO2, the plates were swabbed and bacteria were resuspended in PBS containing 0.5 mM MgCl2, 0.9 mM CaCl2, and 0.1% glucose (PBS-glucose) in order to reach an optical density at 600 nm of 0.4 (bacterial suspension). The sera were heat inactivated (40 min at 56°C) and subsequently diluted in PBS-glucose. In wells of sterile flat-bottom microtiter plates (Nunc, Denmark), 25 μl of diluted test serum was mixed with 12.5 μl of baby rabbit complement (selected for their absence of bactericidal activity against the test strains; Cedarlane Laboratories) and 12.5 μl of bacterial suspension. Serial dilutions of test sera were treated similarly. Controls included bacteria plus complement, bacteria plus heat-inactivated complement, and serum plus bacteria plus heat-inactivated complement. Antiserum from mice immunized three times with whole bacterial cells (5 μg protein-AS04 per injection) was used as a positive control in the bactericidal assays but also for validation of each microtiter plate. The microtiter plates were sealed and incubated while shaking (520 rpm) for 75 min at 37°C without CO2. After incubation, 50 μl MH-0.9% agar was added to each well followed by a second layer of 50 μl PBS-0.9% agar 30 min later. After overnight incubation at 33°C in 5% CO2, the colonies were counted. The average CFU number of the controls corresponding to bacteria plus complement was set to 100%. Bactericidal titers were reported as the reciprocal value of the serum dilution yielding 50% killing of bacteria. Individual sera or serum pools were tested as single samples.
Antibody depletion experiments were performed as described previously (44). Briefly, microplates were coated with serial dilutions of the test antigens and then washed and blocked. The plates were incubated with diluted sera (in order to target ∼50% of killing) for 4 h. Finally, the depleted sera were transferred into SBA microplates, in which bacteria and complement were added to complete the bactericidal test.
Intracellular cytokine staining.
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated and cryopreserved using standard procedures. Thawed and washed PBMCs were cultured in U-bottom microplates at a density of 1 × 106 cells per well. Serial dilutions of OMV preparations (or 0.5 ng of lipopolysaccharide from Escherichia coli; Sigma) were added. After 30 min of incubation at 37°C and 5% CO2, brefeldin A was added to the plates. PBMCs were recovered after 6 h of incubation, and CD14+ cells were marked using anti-CD14-fluorescein isothiocyanate (BD Biosciences). The cytokine flow cytometry protocol from the manufacturer was followed using anti-interleukin 6 (IL-6)-phycoerythrin, anti-tumor necrosis factor alpha (TNF-α)-allophycocyanin, and Cytofix Cytoperm and Permash solutions from BD Biosciences. A FACSCalibur instrument (BD Biosciences) was used, and CD14+ cells were gated using the CellQuest software (BD Biosciences).
Statistical analysis.
Differences in bactericidal antibody titers were determined by the Kruskal-Wallis nonparametric test with a one-tailed Dunn test. A P value of <0.05 was indicative of statistical significance.
RESULTS
L3,7 LOS-enriched but not ΔgalE LOS-enriched OMVs induce bactericidal antibody responses against invasive MenB strains.
OMVs were purified from the wild-type L3,7 LOS-expressing H44/76 strain and its galE mutant (Fig. 1) according to two different methods using either the conventional concentration of deoxycholate (DOC; 0.5%) or a lower concentration (0.1%) in order to modify the LOS content in OMVs. The purified OMVs contained approximately 5% of L3,7 or ΔgalE LOS via 0.5% DOC extraction and 15% L3,7 or ΔgalE LOS via 0.1% DOC extraction. These different preparations were formulated in the AS04 adjuvant system and administered to OF1 mice (10 mice per group). Serum samples were obtained 2 weeks after the third injection. Pools of sera (10 sera per pool, one pool per group) were tested in an ELISA for their anti-LOS IgG responses. The bactericidal activities of individual sera against three invasive strains that expressed PorA, either homologous or heterologous to that of the vaccine strain, were analyzed. A fourth strain, derived from H44/76 but not expressing PorA, was also analyzed in an SBA.
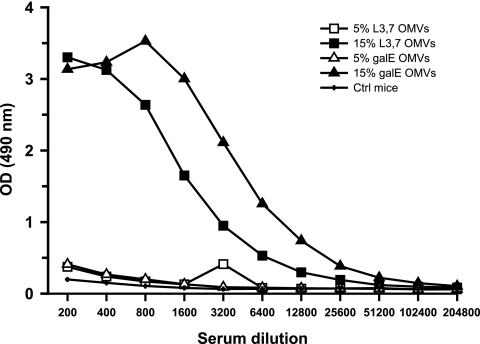
Only mice immunized with OMVs containing 15% LOS (L3,7 or ΔgalE) produced detectable amounts of anti-LOS IgG antibodies (Fig. 2). This indicates that more than 5% LOS in OMVs was required to induce the production of anti-LOS antibodies in mice and that both L3,7 and ΔgalE LOSs were immunogenic.
FIG. 2.
Anti-LOS IgG response as measured by ELISA. Mice (10 per group) were immunized with either 15% LOS OMVs (0.1% DOC extraction), 5% LOS OMVs (0.5% DOC extraction), or AS04 adjuvant alone (Ctrl). OMVs were produced from the ΔgalE strain (galE) or the L3,7 strain. Pooled sera from after the third immunization were serially diluted and tested in ELISA plates coated with purified L3,7 LOS. OD, optical density.
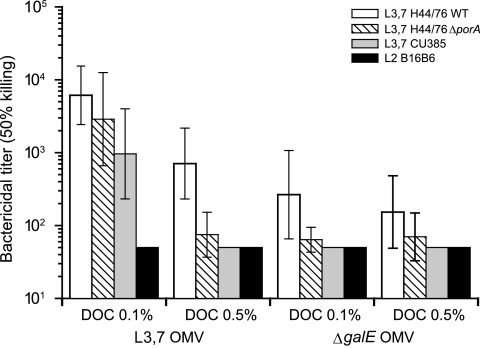
Both L3,7 OMVs and ΔgalE OMVs induced bactericidal antibodies able to mediate killing of the homologous H44/76 strain regardless of the LOS content of the vaccine (Fig. 3). However, the 5% LOS OMV preparations (L3,7 and ΔgalE) induced much less bactericidal activity against the PorA-deficient H44/76 strain than the 15% L3,7 OMV preparation. This observation, together with the lack of anti-LOS antibody response, suggests that the bactericidal antibodies elicited by these 5% LOS OMV preparations were not directed against LOS but were targeted against other OMV antigens, most likely PorA. With the 15% LOS OMV preparations, the bactericidal titer detected in the pool of sera from mice immunized with L3,7 OMVs was higher than that of mice immunized with ΔgalE OMVs. The L3,7 OMVs, but not the ΔgalE OMVs, induced a bactericidal response against the PorA-heterologous L3,7 Cu385 strain and elicited a high bactericidal titer against the ΔporA H44/76 strain, which emphasizes the protective role played by anti-L3,7 LOS antibodies.
FIG. 3.
Bactericidal titers (geometrical mean titers and 95% confidence intervals) of mouse sera against four MenB strains. Sera were from mice (10 per group) immunized with either 5% (DOC, 0.5%) or 15% (DOC, 0.1%) LOS OMVs produced from either the ΔgalE or the L3,7 LOS-expressing H44/76 (PorA+) strain. Strains tested in an SBA were the L3,7 LOS-expressing H44/76 wild type (WT; PorA, P1.7,16), its PorA-deficient mutant, and two PorA-heterologous strains (L3,7 CU385 [P1.19,15] and L2 B16B6 [P1.2]). Bactericidal titers of ≤50 were considered negative.
Potential cross-bactericidal activity between the two LOS immunotypes of interest, namely, L3,7 and L2, was investigated using the L2 strain B16B6. This strain expresses a heterologous PorA, which allowed us to avoid avoid interference from anti-PorA bactericidal responses. No bactericidal activity was found against the L2 strain B16B6 in sera of mice immunized with L3,7 OMVs, indicating the absence of L3,7 LOS and L2 LOS cross-bactericidal activity.
In summary, although both L3,7 LOS and ΔgalE LOS were shown to be immunogenic, the results suggest that only the L3,7 LOS was able to elicit the production of bactericidal antibodies against the most prevalent invasive MenB strains that express L3,7 LOS.
Impact of the lgtB mutation (TrL3 LOS) on the induction of anti-LOS antibodies.
The LNnT (Gal-GlcNAc-Gal-Glc) tetrasaccharide is shared by the L3 and L7 LOSs and some cryptic antigens localized on the surface of red blood cells. Since the shorter ΔgalE LOS, which contains only the glucose of LNnT, fails to induce a bactericidal response against L3,7 strains, we have evaluated the impact of lgtB mutation on the immunogenicity of LOS. The lgtB mutation results in a truncated L3 LOS, designated TrL3 LOS, lacking the terminal galactose of LNnT (Fig. 1). To avoid the presence of residual capsular polysaccharide in OMV preparations and the induction of PorA-specific bactericidal antibodies, siaD and porA deletion mutations were performed in both L3,7 and TrL3 H44/76 strains.
OMVs containing approximately 15% TrL3 LOS or L3,7 LOS were produced from these modified strains (ΔsiaD ΔporA) and were formulated either in AS04 or on aluminum hydroxide and administered to OF1 mice (n = 30 per group). Individual postimmunization sera were tested in an SBA against the wild-type strain H44/76, and serum pools (30 sera per pool, one pool per group) were tested against a panel of L3,7 and L2 heterologous MenB strains isolated from disease cases. Whatever the formulation, TrL3 OMVs elicited the production of bactericidal antibodies against the parental H44/76 strain and other PorA-heterologous L3,7 strains but not against the L2 strain (Table 2). TrL3 and L3,7 OMVs elicited similar amounts of bactericidal antibodies against the H44/76 strain when formulated in AS04 (P = 0.51) but not in the aluminum hydroxide formulation (P = 0.02). In general, OMVs adsorbed onto aluminum hydroxide induced fewer bactericidal antibodies than OMVs formulated in AS04 (P < 0.01).
TABLE 2.
Impact of lgtB mutation (TrL3 LOS) on the induction of complement-mediated killing by bactericidal antibodies in mice
| Vaccine formulation (15% LOS OMVs) | Adjuvant | Titer for indicated bacterial strainc
|
|||
|---|---|---|---|---|---|
| H44/76a (P1.7,16; L3,7) | Cu385b (P1.19,15; L3,7) | M97-250687b (P1;19,15; L3,7) | B16B6b (P1;2; L2) | ||
| L3,7 | AS04 | 4,125 (2,293-7,421) | 2,192 | 11,188 | <100 |
| Al(OH)3 | 2,029 (1,204-3,319) | 1,090 | 1,656 | <100 | |
| TrL3 | AS04 | 3,204 (1,870-5,489) | 4,314 | 4,826 | <100 |
| Al(OH)3 | 828 (477-1,435) | 904 | 871 | <100 | |
Geometric mean titers for 50% killing (calculated on 30 individual sera) and confidence intervals at 95%.
SBA titers of pooled sera (n = 30).
PorA and LOS immunotypes are in parentheses following the strain names.
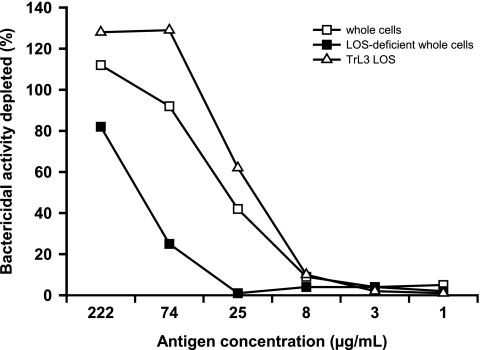
In order to demonstrate the role of anti-TrL3 LOS antibodies in the bactericidal response induced by immunization with TrL3 LOS-enriched OMVs, antibody depletion experiments were performed. A pool of sera was depleted either (i) with purified TrL3 LOS, (ii) with a whole-cell preparation obtained from a capsule-deficient H44/76 mutant expressing a L3,7 LOS in order to deplete antibodies directed against LOS in its native conformation and antibodies directed against OMPs, or (iii) with a whole-cell preparation obtained from a capsule- and LOS-deficient H44/76 mutant in order to deplete the antibodies directed against OMPs only. The antibody-depleted sera were used in an SBA against the L3,7 M97-250687 strain. Purified TrL3 LOS and the capsule-deficient H44/76 whole-cell preparation were both able to inhibit the bactericidal activity of pooled sera in a dose-dependent manner, while the capsule- and LOS-deficient whole-cell preparation was clearly less effective (Fig. 4). This suggests that TrL3 LOS in OMVs was able to elicit the production of bactericidal antibodies reacting with LOS expressed by invasive L3,7 strains, whereas ΔgalE LOS in OMVs was not effective.
FIG. 4.
Antibody depletion experiment. A pool of sera from mice (n = 30) immunized with 15% TrL3 OMVs was incubated in microwells coated with whole-cell preparations from either the capsule-deficient L3,7 LOS-expressing H44/76 strain or the capsule- and LOS-deficient H44/76 mutant or purified TrL3 LOS. After 4 h of incubation, sera were tested in an SBA using the L3,7 LOS-expressing strain M97-250687 (P1.19,15). The percentage of inhibition of the bactericidal activity is shown for each antigen (representative data from one of three experiments).
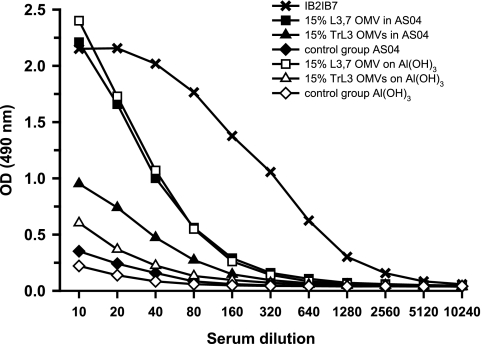
Since the lgtB mutation results in the synthesis of an LOS containing a truncated LNnT structure (with the absence of the terminal galactose), the ability of TrL3 OMVs to induce anti-LNnT antibodies was investigated by ELISA. The 1B2-1B7 monoclonal antibody specific to the LNnT epitope was used as a reference (Fig. 5). With both formulations tested (AS04 and aluminum hydroxide), the amount of anti-LNnT antibodies observed in the pools of sera from mice immunized with L3,7 OMVs (1,433 and 1,426 ELISA units/ml, respectively) was greater than the amount observed for sera of mice treated with TrL3 OMVs (381 and 176 ELISA units/ml, respectively).
FIG. 5.
Anti-LNnT Ig response as measured by ELISA. Mice (30 per group) were immunized with 15% LOS OMVs either formulated with AS04 adjuvant or adsorbed onto aluminum hydroxide. LOS-enriched OMVs were produced from the TrL3 strain or L3,7 strain. Control mice received only the adjuvant. Pooled sera after the third immunization were serially diluted and tested in ELISA plates coated with LNnT conjugated to human serum albumin. The monoclonal antibody 1B2-1B7 was used as reference to calculate the anti-LNnT ELISA antibody titers (see the text). OD, optical density.
In conclusion, OMVs containing approximately 15% TrL3 LOS were able to elicit the production of anti-LOS bactericidal antibodies but not anti-LNnT antibodies.
Impact of LOS detoxification by msbB mutation on the reactogenicity and immunogenicity of OMVs.
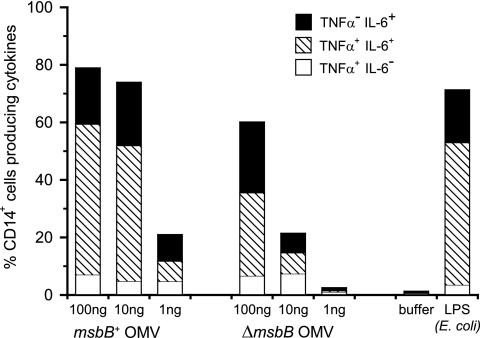
As suggested by the pyrogenicity data for rabbits, OMVs containing 15% LOS were too reactogenic to be used as a vaccine (Table 3). In order to reduce the toxicity, attempts to generate OMVs with a reduced amount of LOS but still sufficient to induce an anti-LOS bactericidal response were made. These attempts were unsuccessful. A more effective strategy that was followed in the present study was to genetically detoxify lipid A by deleting the msbB gene. Mass spectrometry analysis of purified LOS from the msbB mutant strain confirmed the penta-acylation of lipid A (data not shown), as previously described (Fig. 1) (41). The pyrogenicity data for rabbits (Table 3) and the frequency of CD14+ human blood cells producing proinflammatory cytokines (TNF-α and/or IL-6) (Fig. 6) indicated that detoxified LOS-enriched OMVs were at least 10 times less reactogenic than OMVs containing similar amounts of nondetoxified LOS. The strains used to compare these different OMVs had a common background with or without the additional msbB mutation.
TABLE 3.
Impact of msbB mutation and DOC extraction (0.5% or 0.1%) on the pyrogenicity of OMVs in rabbits
| OMV type | Pyrogenicity assay result with indicated DOC and LOS/protein concna
|
|||
|---|---|---|---|---|
| 0.5% DOC
|
0.1% DOC
|
|||
| 0.025/0.5 | 0.075/0.5 | 0.150/1.0 | 0.300/2.0 | |
| msbB+ | Passed (0.7) | Failed (3.3) | NT | NT |
| ΔmsbB | NT | Passed (0.4) | Passed (0.8) | Passed (0.6) |
Results of the pyrogenicity assay according to European Pharmacopoeia. Sum body temperature increases measured in three rabbits are given in parentheses after the results. Concentrations of LOS and protein injected into rabbits are expressed in μg/kg body weight. NT, not tested.
FIG. 6.
Percentage of human CD14+ cells producing intracellular cytokines (TNF-α and/or IL-6) after stimulation of PBMCs with different concentrations of OMVs from msbB+ and ΔmsbB strains. Data are representative of three experiments made with PBMCs from three different donors.
Mice (30 per group) were immunized with different ΔporA OMV preparations containing approximately 15% TrL3 LOS, detoxified (ΔmsbB LOS) or left alone (msbB+ LOS), and formulated on aluminum hydroxide. Serum samples obtained after the third dose were tested in an SBA individually against three L3,7 MenB strains, and pooled sera (30 sera per pool, one pool per group) were tested in an SBA against one L2 MenB strain (Table 4). Bactericidal titers measured against L3,7 strains in the sera from mice immunized with msbB+ and ΔmsbB OMVs were similar, indicating that the msbB mutation had no impact on the immunogenicity of OMVs in mice (P > 0.3). As observed previously (Fig. 3 and Table 2), the response was immunotype specific, as the two pools of sera from mice immunized with L3,7-derived LOS (msbB+ and ΔmsbB) did not show a cross-bactericidal response against the tested L2 strain (Table 4).
TABLE 4.
Impact of detoxification of TrL3 LOS (msbB mutation) on the induction of complement-mediated killing by bactericidal antibodies in mice
| Vaccine formulation (15% LOS OMVs)a | Titer for indicated bacterial straind
|
|||
|---|---|---|---|---|
| H44/76b (P1.7,16; L3,7) | M97-250687b (P1.19,15; L3,7) | NZ124b (P1.7,4; L3,7) | 2986c (P1.5,2; L2) | |
| TrL3 msbB+ | 1,095 (566-2,118) | 1,399 (669-2,927) | 123 (72-212) | <100 |
| TrL3 ΔmsbB | 1,577 (853-2,913) | 1,123 (571-2,209) | 183 (98-342) | <100 |
OMVs were adsorbed onto aluminum hydroxide.
Geometric mean titers for 50% killing (calculated on 30 individual sera) and 95% confidence intervals.
SBA titers of pooled sera.
PorA and LOS immunotypes are in parentheses following the strain names.
Broad cross-bactericidal response induced by bivalent OMV vaccines.
With a limited panel of meningococcal strains, we have observed that immunization with either L3,7 or TrL3 OMVs elicited a cross-bactericidal response against the L3,7 strains but not against the L2 strains. To broaden the cross-bactericidal response of a vaccine based on ΔmsbB LOS-enriched OMVs, we have evaluated a bivalent OMV vaccine composed of ΔmsbB TrL3-enriched OMVs and ΔmsbB TrL2-enriched OMVs. The TrL2 OMVs were produced from a modified L2 760676 strain (ΔsiaD ΔporA ΔlgtB ΔmsbB) via 0.1% DOC extraction.
Mice (30 per group) were immunized with either monovalent (TrL3 or TrL2) or bivalent (TrL3 and TrL2) OMV vaccine. Pooled sera taken after the third injection (30 sera per pool, one pool per group) were tested in an SBA against a panel of 19 meningococcal strains from the five pathogenic serogroups (A, B, C, W-135, and Y) and producing one of the LOS immunotypes studied (L3,7, L2, L3v, L4, L10, and L11) (Table 5) . The SBA threshold titer for a positive result using rabbit complement was defined as ≥1/128. TrL3 OMVs induced the production of bactericidal antibodies mainly against strains expressing L3,7 LOS (as observed before with the limited panel of strains), while TrL2 OMVs induced a protective response against strains producing L2 or L3v LOS. Compared with monovalent vaccines, the combination of TrL3 OMVs and TrL2 OMVs induced complementary bactericidal activities against the panel of strains tested while not affecting the bactericidal titers. In order to validate the process of purification/formulation, different OMV preparations were tested. To this end, sera from vaccinated mice (monovalent or bivalent formulations) were tested against a limited number of strains in an SBA (data not shown). The limited number of L4, L10, and L11 strains tested in the SBA did not allow us to draw a conclusion on cross-bactericidal responses against these LOS types.
TABLE 5.
Serum bactericidal titers of pooled mice sera against 19 N. meningitidis strainsa
| Treatment | Serum bactericidal titer for strain (serogroup, LOS type):
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 760676 (B, L2) | B16B6 (B, L2) | 2986 (B, L2) | 3356 (B, L2) | 3151 (W, L2) | H44/76 (B, L3,7) | M687 (B, L3,7) | NZ124 (B, L3,7) | S3446 (B, L3,7) | S4383 (W, L3,7) | C11 (C, L3v) | 6275 (B, L3v) | 2991 (B, L3v) | 608B (B, L3v) | 3193 (W, L3v) | S1975 (Y, L3v) | C19 (C, L4) | 3125 (A, L10) | F8238 (A, 11) | |
| TrL3 OMVs | <50 | <50 | <50 | 109 | 502 | 8,591 | 4,542 | 919 | 159 | 320 | 50 | <50 | 348 | 50 | <50 | 443 | <50 | 61 | 323 |
| TrL2 OMVs | 1,195 | 790 | 1,566 | 118 | 966 | 67 | 816 | <50 | <50 | 1,304 | 608 | 5,090 | 25,600 | 25,600 | 11,921 | 11,487 | <50 | <50 | 4,376 |
| TrL3 and TrL2 OMVs | 1,040 | 415 | 3,929 | <50 | 4,178 | 8,517 | 3,974 | 504 | 112 | 2,164 | 1,105 | 5,985 | 25,600 | 9,371 | 18,153 | 25,600 | <50 | <50 | 4,348 |
| Control | <50 | <50 | <50 | <50 | <50 | 54 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 |
Mice (30 animals per group) were immunized with monovalent vaccines (TrL3 or TrL2 OMVs), a bivalent vaccine (TrL3 OMVs and TrL2 OMVs), or the formulation buffer alone. Bactericidal titers from pooled sera (30 sera per pool) are expressed as the reciprocal value of the serum dilution yielding 50% killing of bacteria. Titers above the threshold for a positive result (titer, ≥128) are shown in bold.
DISCUSSION
New approaches to afford large cross-protection against MenB must be envisaged. In this context, the development of a LOS-based vaccine might be a valuable alternative. LOS of the immunotype L3,7 is observed in at least 70% of invasive MenB cases (29, 33). In addition, it was already documented that L3,7 LOS induces the production of bactericidal antibodies in humans (5, 44). For these reasons, L3,7 LOS appears to be a suitable vaccine antigen with the potential to elicit interstrain cross-protective responses. Immunization with conventional OMV vaccines, such as VA-MENGOC-BC (Finlay Institute, Cuba), MenBVac (Norwegian Institute of Public Health, Norway), and MeNZB (Novartis, Basel, Switzerland), is known to induce the production of antibodies directed against LOS even though the LOS antigen is present at low levels in these vaccines (approximately 5% of protein content) (9, 21). In order to improve the cross-protection elicited by LOS-containing OMV-based vaccines, we developed a strategy to increase the LOS content while decreasing LOS-associated toxicity.
By using a mouse model developed to mimic the vaccine response elicited in infants by conventional OMV vaccines (42), the immunogenicities of OMVs containing standard concentrations of LOS (approximately 5%) and LOS (approximately 15%)-enriched OMVs were compared. We observed that the cross-bactericidal antibody response was clearly enhanced in mice immunized with 15% L3,7 LOS OMVs compared to the response in mice immunized with 5% L3,7 LOS OMVs. Based on the observation that a monoclonal antibody (B5) directed against the inner core of L3,7 LOS (i.e., corresponding to ΔgalE LOS) has been described as being bactericidal, opsonic, and protective in infant rats (28), we have compared the bactericidal responses induced in mice immunized with L3,7 LOS OMVs and ΔgalE LOS OMVs. Even though an anti-LOS antibody response was measured in the sera from both groups of mice (Fig. 2), only L3,7 LOS OMVs induced a bactericidal response (Fig. 3). The difference in bactericidal activity between anti-L3,7 LOS and anti-ΔgalE LOS antibodies relates to the absence of a crucial epitope in ΔgalE LOS, since the protective activity of monoclonal antibody B5 is observed only against strains that express ΔgalE LOS. Such strains are not representative of invasive strains, which are mainly of the L3,7 immunotype. Therefore, only L3,7 OMVs, and not ΔgalE OMVs, induced a cross-bactericidal response against invasive L3,7 strains.
Intramuscular immunization of mice with 15% LOS OMVs induced a bactericidal response directed in part against LOS, as demonstrated by an antibody depletion experiment (Fig. 4). Surprisingly, intraperitoneal immunization of mice with the same OMV preparation adsorbed onto aluminum salts did not induce such a bactericidal response (data not shown). Although the reason for this observation is not known, we may speculate that the affinity of the induced anti-LOS antibodies varies depending on the immunization route and that the avidity of anti-LOS antibodies generated via the intraperitoneal route is low, as has been suggested by Granoff's team (19). The impact of the immunization route on the quality of the LOS antibody response could explain the discrepancy between our results and those recently published concerning native OMVs obtained from an L3 strain (13, 19).
Some limitations may arise from the use of L3,7 LOS. For example, LNnT, present on the L3,7 structure, is also the precursor of human blood group antigens (22). Some IgM monoclonal antibodies obtained from mice immunized with neisserial antigens have been shown to react against L3,7 LOS-bearing strains and to agglutinate human red blood cells at 4°C (22). These characteristics are typical of cold agglutinins even though associated diseases are not observed after meningococcal diseases (14). We further investigated the immunogenicity of a truncated structure of L3,7 LOS lacking the terminal galactose of the LNnT. This was obtained by deleting the lgtB gene coding for a glycosyltransferase (41). By antibody depletion experiments, we have demonstrated that this structure, designated TrL3, induced the production of anti-LOS bactericidal antibodies able to recognize the wild-type L3,7 LOS at the surface of the bacteria and to mediate killing, whereas the shorter ΔgalE LOS was not effective. The levels of antibodies directed against LNnT measured in pooled sera from mice immunized with TrL3 OMVs were low compared to those from sera of L3,7 OMV-immunized mice. However, the amount of anti-LNnT antibodies in sera from mice immunized with L3,7 OMVs was very low and most probably without biological significance, since these pooled sera were unable to agglutinate human red blood cells even at 4°C (data not shown).
Another limitation of using LOS-enriched OMVs is their reactogenicity due to the lipid A moiety of the LOS molecule. To reduce this reactogenicity, conventional OMVs were obtained using 0.5% DOC during their purification, which removes most of the LOS molecules from the vaccine preparations (8). These preparations induce low levels of anti-LOS antibodies. In order to generate LOS-enriched OMVs, we reduced the percentage of DOC to 0.1% in the purification process. Although the resulting OMVs induced a broader cross-bactericidal response than conventional OMVs, they were reactogenic, as observed both in the rabbit pyrogenicity assay (Table 3) and through the production of proinflammatory cytokines by human monocytic cells (Fig. 6) or when assessed by local reactogenicity in mice and rabbits (data not shown). To maintain a high percentage of LOS in OMVs while reducing their reactogenicity, we genetically modified the OMV-producing strains. Several mutations of genes involved in LOS biosynthesis have been described previously to reduce toxicity in in vitro or in vivo assays. However, most of these genes were not selected because such mutants either negatively impacted the immunogenicity of OMVs, such as that containing the lpxL2 mutation, which results in a tetra-acyl lipid A (7, 37), or affected the synthesis of the saccharidic moiety of LOS (kdtA or kpsF deletion/inactivation) (35, 36, 46, 47), which is crucial for the induction of a broad bactericidal antibody response against invasive meningococcal strains, as demonstrated in the present study. We have chosen to detoxify lipid A via the inactivation of the msbB gene, also designated lpxL1 (37). This gene codes for an acyltransferase involved in the late acylation step of lipid A, and its inactivation results in a penta-acyl lipid A, as reported by others (7, 37), without affecting the synthesis of the glycan moiety of LOS. Previous reports showed that, when added to outer membrane complexes produced from an LOS-deficient strain, purified ΔmsbB LOS showed a reduced toxicity while maintaining adjuvant activity in mice (41). Our preclinical results are in line with these studies, since ΔmsbB LOS-enriched OMVs retained their immunogenicity (i.e., induced a level of cross-bactericidal antibodies similar to that of msbB+ LOS-enriched OMVs), while the reactogenicity and the pyrogenicity of ΔmsbB OMVs were greatly reduced compared to those of msbB+ OMVs.
The bactericidal antibody responses induced by our L3,7 OMVs or TrL3 OMVs were shown to mediate the complement killing of the majority of the L3,7 strains tested but not most of the tested L2 strains. On the other hand, we demonstrated that immunization of mice with either L2 OMVs (data not shown) or TrL2 OMVs induced a protective bactericidal response against most of the L2 strains tested but not against the majority of the L3,7 strains. The fact that some cross-protection between L3,7 and L2 immunotypes was observed suggests that, in addition to anti-LOS antibodies, antibodies directed against OMPs were also involved in the bactericidal response induced by TrL3 and TrL2 OMVs. Immunotypes L3 and L2 represent the great majority of invasive serogroup B, C, W-135, and Y strains isolated from disease cases, with 70 to 80% expressing immunotype L3 and 20 to 30% expressing L2 (29, 33). A L2/L3 bivalent formulation would have the potential to protect against the majority of invasive B, C, W-135, and Y strains.
A new LOS immunotype was recently described by two different groups. It was named L3v, and some strains previously immunotyped as L3 can now be discriminated as L3v strains by use of new monoclonal antibodies (12, 31). The L3,7 LOS inner core is characterized by the presence of a PEA group at position 3 on the HepII β chain, while L2 LOS displays a PEA on position 6 of HepII and a glucose on position 3. L3v strains display characteristics of both L3 and L2 types, since their LOS inner cores are characterized by a PEA in position 3 and a second PEA in position 6 (Fig. 1). The LOS outer core α chains are similar in L2, L3,7, and L3v immunotypes and are composed of an LNnT tetrasaccharide, sialylated or unsialylated (11, 12, 26, 31). In our study, sera from mice immunized with L3,7 or TrL3 OMVs failed to induce complement-mediated killing of L3v strains, while the sera from mice immunized with L2 OMVs (data not shown) or TrL2 OMVs (Table 5) did, which suggests that the PEA at position 6 of HepII has a major impact on the antigenicity of LOS. L2-derived OMV vaccines did not induce a bactericidal antibody response against the tested L4 strain that differs from L2 and L3v only by the absence of a residue on position 3 of HepII (20). This suggests that the presence/absence of a PEA at position 3 of HepII could affect the antigenicity of the LOS molecule. As the structures of L10 and L11 LOS are not fully elucidated, it is not possible to explain the absence or presence of killing activity of anti-bivalent OMV mouse sera against these L10 and L11 LOS-expressing strains. Together, these results strongly suggest that the inner-core composition has a major impact on LOS antigenicity, either by altering the α-chain conformation or by being part of the bactericidal epitope.
In conclusion, we have developed a bivalent OMV vaccine derived from an L3,7 strain and an L2 strain. The preclinical evaluation of this candidate vaccine in mice has demonstrated its low-reactogenicity profile and its potential to induce a broad cross-bactericidal response against most of the MenB clinical isolates and also against strains from serogroups C, W-135, and Y.
Acknowledgments
We thank Colin Tinsley for the gift of the spectinomycin resistance cassette, Martine Bos for the kind gift of the capsule- and LOS-deficient H44/76 strain, and Ulrike Krause for editorial assistance.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 16 March 2009.
REFERENCES
- 1.Bos, M. P., and J. Tommassen. 2005. Viability of a capsule- and lipopolysaccharide-deficient mutant of Neisseria meningitidis. Infect. Immun. 736194-6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandtzaeg, P., and M. van Deuren. 2002. Current concepts in the role of the host response in Neisseria meningitidis septic shock. Curr. Opin. Infect. Dis. 15247-252. [DOI] [PubMed] [Google Scholar]
- 3.Braun, J. M., J. Beuth, C. C. Blackwell, S. Giersen, P. G. Higgins, G. Tzanakaki, H. Unverhau, and D. M. Weir. 2004. Neisseria meningitidis, Neisseria lactamica and Moraxella catarrhalis share cross-reactive carbohydrate antigens. Vaccine 22898-908. [DOI] [PubMed] [Google Scholar]
- 4.Drabick, J. J., B. L. Brandt, E. E. Moran, N. B. Saunders, D. R. Shoemaker, and W. D. Zollinger. 1999. Safety and immunogenicity testing of an intranasal group B meningococcal native outer membrane vesicle vaccine in healthy volunteers. Vaccine 18160-172. [DOI] [PubMed] [Google Scholar]
- 5.Estabrook, M. M., G. A. Jarvis, and J. McLeod Griffiss. 2007. Affinity-purified human immunoglobulin G that binds a lacto-N-neotetraose-dependent lipooligosaccharide structure is bactericidal for serogroup B Neisseria meningitidis. Infect. Immun. 751025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estabrook, M. M., J. McLeod Griffiss, and G. A. Jarvis. 1997. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect. Immun. 654436-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.European Directorate for the Quality of Medicines and Health Care (EDQM). 2009. European pharmacopeia, 6th ed., section 2-06.08. Council of Europe, Strasbourg, France. http://online.edqm.eu/entry.htm.
- 7.Fisseha, M., P. Chen, B. Brandt, T. Kijek, E. Moran, and W. Zollinger. 2005. Characterization of native outer membrane vesicles from lpxL mutant strains of Neisseria meningitidis for use in parenteral vaccination. Infect. Immun. 734070-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frasch, C. E., L. van Alphen, J. Holst, J. T. Poolman, and E. Rosenqvist. 2001. Outer membrane protein vesicle vaccines for meningococcal disease, p. 81-107. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 9.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Frøholm, A. K. Lindbak, B. Møgster, E. Namork, U. Rye, G. Stabbetorp, R. Winsnes, B. Aase, and O. Closs. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 1467-80. [PubMed] [Google Scholar]
- 10.Frosch, M., E. Schultz, E. Glenn-Calvo, and T. F. Meyer. 1990. Generation of capsule-deficient Neisseria meningitidis strains by homologous recombination. Mol. Microbiol. 41215-1218. [DOI] [PubMed] [Google Scholar]
- 11.Gamian, A., M. Beurret, F. Michon, J.-R. Brisson, and H. J. Jennings. 1992. Structure of the L2 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J. Biol. Chem. 267922-925. [PubMed] [Google Scholar]
- 12.Gidney, M. A. J., J. S. Plested, S. Lacelle, P. A. Coull, J. C. Wright, K. Makepeace, J.-R. Brisson, A. D. Cox, E. R. Moxon, and J. C. Richards. 2004. Development, characterization, and functional activity of a panel of specific monoclonal antibodies to inner core lipopolysaccharide epitopes in Neisseria meningitidis. Infect. Immun. 72559-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou, V. C., O. Koeberling, J. A. Welsch, and D. M. Granoff. 2005. Protective antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed genome-derived neisserial antigen 1870. J. Infect. Dis. 192580-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howitz, M., T. G. Krause, J. B. Simonsen, S. Hoffmann, M. Frisch, N. M. Nielsen, J. Robbins, R. Schneerson, K. Mølbak, and M. A. Miller. 2007. Lack of association between group B meningococcal disease and autoimmune disease. Clin. Infect. Dis. 451327-1334. [DOI] [PubMed] [Google Scholar]
- 15.Jennings, M. P., D. W. Hood, I. R. A. Peak, M. Virji, and E. R. Moxon. 1995. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol. Microbiol. 18729-740. [DOI] [PubMed] [Google Scholar]
- 16.Jennings, M. P., P. van der Ley, K. E. Wilks, D. J. Maskell, J. T. Poolman, and E. R. Moxon. 1993. Cloning and molecular analysis of the galE gene of Neisseria meningitidis and its role in lipopolysaccharide biosynthesis. Mol. Microbiol. 10361-369. [PubMed] [Google Scholar]
- 17.Jones, D. H., and S. C. Winistorfer. 1992. Recombinant circle PCR and recombination PCR for site-specific mutagenesis without PCR product purification. BioTechniques 12528-535. [PubMed] [Google Scholar]
- 18.Jones, D. M., R. Borrow, A. J. Fox, S. Gray, K. A. Cartwright, and J. T. Poolman. 1992. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb. Pathog. 13219-224. [DOI] [PubMed] [Google Scholar]
- 19.Koeberling, O., A. Seubert, and D. M. Granoff. 2008. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J. Infect. Dis. 198262-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kogan, G., D. Uhrín, J.-R. Brisson, and H. J. Jennings. 1997. Structural basis of the Neisseria meningitidis immunotypes including the L4 and L7 immunotypes. Carbohydr. Res. 298191-199. [DOI] [PubMed] [Google Scholar]
- 21.Kristiansen, P. A., I. S. Aaberge, K. Møyner, L. M. Næss, K. Bryn, K. Nord, A. G. Skryten, E. Namork, E. Rosenqvist, and K. Harbak. 2002. Tailor-made outer membrane vesicle (OMV) vaccine against the group B meningococcal epidemic in New Zealand—manufacturing and characterisation, p. 268. 13th Int. Pathog. Neisseria Conf., Oslo, Norway, 1 to 6 September 2002.
- 22.Mandrell, R. E., J. McLeod Griffiss, and B. A. Macher. 1988. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J. Exp. Med. 168107-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mieszala, M., G. Kogan, and H. J. Jennings. 2003. Conjugation of meningococcal lipooligosaccharides through their lipid A terminus conserves their inner epitopes and results in conjugate vaccines having improved immunological properties. Carbohydr. Res. 338167-175. [DOI] [PubMed] [Google Scholar]
- 24.Moran, E. E., B. L. Brandt, and W. D. Zollinger. 1994. Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity. Infect. Immun. 625290-5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oster, P., D. Lennon, J. O'Hallahan, K. Mulholland, S. Reid, and D. Martin. 2005. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 232191-2196. [DOI] [PubMed] [Google Scholar]
- 26.Pavliak, V., J.-R. Brisson, F. Michon, D. Uhrín, and H. J. Jennings. 1993. Structure of the sialylated L3 lipopolysaccharide of Neisseria meningitidis. J. Biol. Chem. 26814146-14152. [PubMed] [Google Scholar]
- 27.Peak, I. R. A., Y. Srikhanta, M. Dieckelmann, E. R. Moxon, and M. P. Jennings. 2000. Identification and characterisation of a novel conserved outer membrane protein from Neisseria meningitidis. FEMS Immunol. Med. Microbiol. 28329-334. [DOI] [PubMed] [Google Scholar]
- 28.Plested, J. S., S. L. Harris, J. C. Wright, P. A. Coull, K. Makepeace, M.-A. J. Gidney, J.-R. Brisson, J. C. Richards, D. M. Granoff, and E. R. Moxon. 2003. Highly conserved Neisseria meningitidis inner-core lipopolysaccharide epitope confers protection against experimental meningococcal bacteremia. J. Infect. Dis. 1871223-1234. [DOI] [PubMed] [Google Scholar]
- 29.Poolman, J. T., C. T. P. Hopman, and H. C. Zanen. 1982. Problems in the definition of meningococcal serotypes. FEMS Microbiol. Lett. 13339-348. [Google Scholar]
- 30.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29303-313. [DOI] [PubMed] [Google Scholar]
- 31.Rahman, M. M., M. A. Monteiro, T. Minnini, and V. Pavliak. 2004. Antigenic variation in the inner core region of lipooligosaccharides (LOSs) from Neisseria meningitidis strains representing the L3 immunotype, p. 173. 14th Int. Pathog. Neisseria Conf., Milwaukee, WI, 5 to 10 September 2004.
- 32.Ram, S., A. D. Cox, J. C. Wright, U. Vogel, S. Getzlaff, R. Boden, J. Li, J. S. Plested, S. Meri, S. Gulati, D. C. Stein, J. C. Richards, E. R. Moxon, and P. A. Rice. 2003. Neisserial lipooligosaccharide is a target for complement component C4b. Inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 27850853-50862. [DOI] [PubMed] [Google Scholar]
- 33.Scholten, R. J., B. Kuipers, H. A. Valkenburg, J. Dankert, W. D. Zollinger, and J. T. Poolman. 1994. Lipo-oligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J. Med. Microbiol. 41236-243. [DOI] [PubMed] [Google Scholar]
- 34.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Høiby, J. Holst, H. Nøkleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 2811520-1527. [DOI] [PubMed] [Google Scholar]
- 35.Tzeng, Y.-L., A. Datta, V. K. Kolli, R. W. Carlson, and D. S. Stephens. 2002. Endotoxin of Neisseria meningitidis composed only of intact lipid A: inactivation of the meningococcal 3-deoxy-d-manno-octulosonic acid transferase. J. Bacteriol. 1842379-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzeng, Y.-L., A. Datta, C. Strole, V. S. K. Kolli, M. R. Birck, W. P. Taylor, R. W. Carlson, R. W. Woodard, and D. S. Stephens. 2002. KpsF is the arabinose-5-phosphate isomerase required for 3-deoxy-d-manno-octulosonic acid biosynthesis and for both lipooligosaccharide assembly and capsular polysaccharide expression in Neisseria meningitidis. J. Biol. Chem. 27724103-24113. [DOI] [PubMed] [Google Scholar]
- 37.van der Ley, P., L. Steeghs, H. J. Hamstra, J. ten Hove, B. Zomer, and L. van Alphen. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 695981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Deuren, M., P. Brandtzaeg, and J. W. M. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verheul, A. F. M., H. Snippe, and J. T. Poolman. 1993. Meningococcal lipopolysaccharides: virulence factor and potential vaccine component. Microbiol. Rev. 5734-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virji, M., K. Makepeace, I. R. A. Peak, D. J. P. Ferguson, M. P. Jennings, and E. R. Moxon. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18741-754. [DOI] [PubMed] [Google Scholar]
- 41.Wakarchuk, W., A. Martin, M. P. Jennings, E. R. Moxon, and J. C. Richards. 1996. Functional relationships of the genetic locus encoding the glycosyltransferase enzymes involved in expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. J. Biol. Chem. 27119166-19173. [DOI] [PubMed] [Google Scholar]
- 42.Weynants, V. E., C. M. Feron, K. K. Goraj, M. P. Bos, P. A. Denoël, V. G. Verlant, J. Tommassen, I. R. A. Peak, R. C. Judd, M. P. Jennings, and J. T. Poolman. 2007. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect. Immun. 755434-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong, S., D. Lennon, C. Jackson, J. Stewart, S. Reid, S. Crengle, S. Tilman, I. Aaberge, J. O'Hallahan, P. Oster, K. Mulholland, and D. Martin. 2007. New Zealand epidemic strain meningococcal B outer membrane vesicle vaccine in children aged 16-24 months. Pediatr. Infect. Dis. J. 26345-350. [DOI] [PubMed] [Google Scholar]
- 44.Zollinger, W., E. Moran, D. Schmiel, and B. Brandt. 2006. Specificity of cross-reactive bactericidal antibodies in normal and convalescent human sera (P8.3.19), abstr. no. P8.3.19, p. 124. 15th Int. Pathog. Neisseria Conf., Cairns, Queensland, Australia, 10 to 15 September 2006.
- 45.Zollinger, W. D., E. E. Moran, S. J. N. Devi, and C. E. Frasch. 1997. Bactericidal antibody responses of juvenile rhesus monkeys immunized with group B Neisseria meningitidis capsular polysaccharide-protein conjugate vaccines. Infect. Immun. 651053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zughaier, S., S. Agrawal, D. S. Stephens, and B. Pulendran. 2006. Hexa-acylation and KDO2-glycosylation determine the specific immunostimulatory activity of Neisseria meningitidis lipid A for human monocyte derived dendritic cells. Vaccine 241291-1297. [DOI] [PubMed] [Google Scholar]
- 47.Zughaier, S., L. Steeghs, P. van der Ley, and D. S. Stephens. 2007. TLR4-dependent adjuvant activity of Neisseria meningitidis lipid A. Vaccine 254401-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]