Abstract
Indole has been proposed to act as an extracellular signal molecule influencing biofilm formation in a range of bacteria. For this study, the role of indole in Vibrio cholerae biofilm formation was examined. It was shown that indole activates genes involved in vibrio polysaccharide (VPS) production, which is essential for V. cholerae biofilm formation. In addition to activating these genes, it was determined using microarrays that indole influences the expression of many other genes, including those involved in motility, protozoan grazing resistance, iron utilization, and ion transport. A transposon mutagenesis screen revealed additional components of the indole-VPS regulatory circuitry. The indole signaling cascade includes the DksA protein along with known regulators of VPS production, VpsR and CdgA. A working model is presented in which global control of gene expression by indole is coordinated through σ54 and associated transcriptional regulators.
Bacterial cells synthesize myriad small organic molecules to signal and adapt to environmental, physiological, and population structure changes. These molecules include extracellular signals such as acyl homoserine lactones, butyrolactones, quinolones, a furanosyl borate diester, oligopeptides, 3-hydroxypalmitic acid methyl ester, and a hydroxyketone and intracellular signals including cyclic nucleotides and ppGpp (25; reviewed in reference 11). The phenotypic response to signaling compounds often involves traits that are beneficial under adverse conditions, such as biofilm formation, virulence, motility, bioluminescence, sporulation, competence, modification of carbon and energy utilization, and macromolecule biosynthesis.
Indole is a relatively recent addition to the list of signaling molecules used by bacteria and is produced as a by-product of the breakdown of tryptophan by the enzyme tryptophanase (TnaA) (18). Since the expression of the tnaA gene is controlled by catabolite repression, it is transcribed only during carbon limitation (57). As a result of this regulation, large quantities of indole are produced during the stationary phase of growth. Indole has long been known to act as a chemorepellent of Escherichia coli (52), but only more recently has indole also been shown to control the expression of a wide assortment of genes and phenotypes unrelated to chemotaxis in many different bacteria. For example, in E. coli indole controls the expression of genes involved in amino acid metabolism (54), plasmid maintenance (13), and quorum sensing (32), among other functions (14). Additionally, indole may function as an interspecies signal contributing to biofilm formation in an assortment of different bacteria known to carry a copy of the tryptophanase gene (36). In E. coli specifically, the indole-driven transcriptional response, which controls subsequent biofilm formation, is known to be controlled by the quorum-sensing transcriptional regulator SdiA (32).
It was previously shown that control of biofilm formation by indole also extends to the etiological agent of the pandemic disease cholera, Vibrio cholerae. Transposon insertions in the tryptophanase gene contained within the genomes of two environmental strains of V. cholerae were shown to result in diminished biofilm formation by each mutant, and supplementation of indole in the growth medium was able to complement the biofilm defect of these strains (38). Due to the reliance of these strains on the production of Vibrio polysaccharide (VPS) for biofilm formation, it was theorized that indole was influencing the regulation of VPS production.
The genes encoding the enzymes that catalyze VPS synthesis are contained within two operons within the V. cholerae genome (vps-I and vps-II predicted operons), and their regulation involves multiple transcriptional activators and repressors. The main activator of VPS production appears to be the σ54-dependent transcriptional activator VpsR, which is essential for vps gene expression (58) and is a distant homolog of SdiA from E. coli. A secondary activator of the vps genes is VpsT, which is not essential for vps transcription but acts synergistically with VpsR to activate expression (6). Antagonizing these activities is the master transcriptional regulator of quorum sensing in V. cholerae, HapR, which is translated when autoinducer molecules accumulate in the extracellular environment. Since HapR is a repressor of vps expression, it is thought that quorum sensing acts to downregulate VPS production and biofilm formation once the cell density increases above a given threshold (23). Superimposed on these regulatory mechanisms are proteins with GGDEF and/or EAL domains, which modulate intracellular levels of the second messenger cyclic diguanylic acid (c-di-GMP) (34, 51). c-di-GMP influences the regulators described above and ultimately many genes involved with motility, chemotaxis, virulence, and biofilm formation (7). Thus, the regulation of processes such as biofilm formation is multifaceted, depending on a variety of extracellular and intracellular signal molecules.
In this study it is shown that extracellular indole is also used as a signal in V. cholerae and that it influences the expression of many different types of genes, including those involved in transport, virulence, biofilm formation, and motility. Evidence is also provided that indole signaling proceeds through the RNA polymerase regulatory protein DksA, the VPS regulators VpsR and VpsT, and the c-di-GMP second messenger system.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
All strains and plasmids used in this study are listed in Table 1. Wild-type strains SIO and TP of V. cholerae were isolated from southern California coastal waters. All E. coli and V. cholerae strains were grown in LB broth (37) supplemented with appropriate antibiotics at 37°C, except when stated otherwise. Antibiotics used in this study were kanamycin ([Km] 50 μg/ml for E. coli and 200 μg/ml for V. cholerae), chloramphenicol (20 μg/ml for E. coli and 5 μg/ml for V. cholerae), gentamicin ([Gm] 50 μg/ml), and ampicillin (Ap) and rifampin at a concentration of 100 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or description | Source or reference |
|---|---|---|
| E. coli strain | ||
| S17-1λπ | recA pro hsdR RP4-2-Tc::Mu-m::Tn7 | 46 |
| V. cholerae strains | ||
| 92A1552R | Wild-type 92A1552; rugose variant | 60 |
| AR2101 | 92A1552R ΔtnaA | This study |
| 92A1552S | Wild-type 92A1552; smooth variant | 60 |
| AS2101 | 92A1552S ΔtnaA | This study |
| N16961 | Wild-type N16961 | 24 |
| N2101 | N16961 ΔtnaA | This study |
| SIO | Wild-type SIO | 44 |
| S1101 | SIO tnaA::Tn5 Kmr | 38 |
| S2101 | SIO ΔtnaA | 38 |
| S2148 | SIO ΔlacZ | This study |
| S2150 | SIO ΔlacZ ΔtnaA | This study |
| S4100 | SIO rRNA::gfp Gmr | This study |
| S4101 | SIO tnaA::Tn5 rRNA::gfp Kmr Gmr | This study |
| S9149 | SIO ΔlacZ ΔtnaA ΔvpsL::lacZ | This study |
| S9170 | SIO ΔlacZ ΔtnaA ΔvpsR ΔvpsL::lacZ | This study |
| S9171 | SIO ΔlacZ ΔvpsL::lacZ | This study |
| S9185 | SIO ΔlacZ ΔtnaA ΔrpoE ΔvpsL::lacZ | This study |
| S9190 | SIO ΔlacZ ΔtnaA ΔcpxA ΔvpsL::lacZ | This study |
| S9209 | SIO ΔlacZ ΔtnaA ΔcpxA ΔrpoE ΔvpsL::lacZ | This study |
| S9211 | SIO ΔlacZ ΔtnaA Δfis ΔvpsL::lacZ | This study |
| S9213 | SIO ΔlacZ ΔtnaA ΔluxO ΔvpsL::lacZ | This study |
| S9216 | SIO ΔlacZ ΔtnaA VC1376::Tn5 ΔvpsL::lacZ | This study |
| S9218 | SIO ΔlacZ ΔtnaA VC1673::Tn5 ΔvpsL::lacZ | This study |
| S9219 | SIO ΔlacZ ΔtnaA cdgA::Tn5 ΔvpsL::lacZ | This study |
| S9224 | SIO ΔlacZ ΔtnaA dksA::Tn5 ΔvpsL::lacZ | This study |
| S9225 | SIO ΔlacZ ΔtnaA VCA0075::Tn5 ΔvpsL::lacZ | This study |
| S9226 | SIO ΔlacZ ΔtnaA VC0338::Tn5 ΔvpsL::lacZ | This study |
| S9227 | SIO ΔlacZ ΔtnaA hmpA::Tn5 ΔvpsL::lacZ | This study |
| S9228 | SIO ΔlacZ ΔtnaA VC0143::Tn5 ΔvpsL::lacZ | This study |
| S9229 | SIO ΔlacZ ΔtnaA VC1731::Tn5 ΔvpsL::lacZ | This study |
| S9230 | SIO ΔlacZ ΔtnaA ΔdksA ΔvpsL::lacZ | This study |
| TP | Wild-type TP | 44 |
| T1101 | TP tnaA::Tn5 Kmr | 38 |
| T1137 | TP vgrG-1::Tn5 Kmr | 38 |
| T1144 | TP VCA0109::Tn5 Kmr | 38 |
| Plasmids | ||
| pCC12 | pRS415 vpsL promoter, Apr | 12 |
| pFLcm | pFL122L containing cat gene from pBSL181 | 38 |
| pFLcdgA | pFLcm, cdgA operon, Cmr | This study |
| pGPKm | pGP704sac28 containing the Kmr gene | 38 |
| pGPcpxA | ΔcpxA in pGPKm | This study |
| pGPfis | Δfis in pGPKm | This study |
| pGPlacZ | ΔlacZ in pGPKm | This study |
| pGPluxO | ΔluxO in pGPKm | This study |
| pGPrpoE | ΔrpoE in pGPKm | This study |
| pGPtnaA | ΔtnaA in pGPKm | This study |
| pGPvpsL::lacZ | ΔvpsL::lacZ in pGPKm | This study |
| pGPvpsR | ΔvpsR in pGPKm | This study |
| pRL27 | Tn5-Rl27 oriR6K, Kmr | 30 |
| pMCM11 | pGP704::mTn7-GFP, Gmr Apr | 7 |
| pUX-BF13 | oriR6K helper plasmid, mob-oriT, provides the Tn7 transposition function in trans; Apr | 4 |
V. cholerae mating.
E. coli strain S17-1 λpir (46) was used as a donor for all conjugation experiments with strains of V. cholerae. All strains were grown overnight to stationary phase at 37°C. V. cholerae cells were subcultured at a 1:100 dilution and grown in LB medium at 22°C until the mid-exponential phase of growth was reached (optical density at 600 nm [OD600] of ∼0.7). E. coli and V. cholerae (if necessary) were washed of antibiotics by centrifugation (2 min at 13,000 × g) and resuspended in equal amounts of fresh LB medium. One milliliter of washed E. coli was then added to 4 ml of V. cholerae and briefly vortexed. This mixture was then vacuum filtered onto a sterile membrane (0.45-μm pore size; 47-mm diameter), which was placed on top of an LB agar plate and left overnight at 37°C. Membranes were then transferred to tubes containing 10 ml of LB medium, and cells were removed by vortexing. Dilutions were plated onto thiosulfate citrate bile salts sucrose (89 g/liter; Difco) or LB agar, both supplemented with appropriate antibiotics, and grown at 37°C overnight.
DNA manipulations.
All PCRs were carried out with Expand High-Fidelity PCR kits (Roche) or Taq polymerase (Invitrogen). PCR purification was carried out using a MolBio PCR purification kit according to the manufacturer's specifications, and DNA sequencing was performed by SeqXcel, Inc. (San Diego, CA). Qiaprep Spin Miniprep kits (Qiagen) were used for plasmid purifications, and restriction enzymes were obtained from New England Biolabs, Inc. (Ipswich, MA).
The method of gene splicing by overlap extension PCR as developed by Horton (26) was used to engineer in-frame deletions of specific genes within the V. cholerae genomes. PCR amplicons were designed and subcloned into plasmid pGPKm as previously described (38). After sequence verification, each plasmid was electroporated into E. coli S17-1 λpir and conjugated into the desired V. cholerae strain (see above). Subsequent screening for plasmid integration events and in-frame deletion verification was then performed as explained in Mueller et al. (38).
Transposon mutagenesis library generation, screening, and mutant identification.
Conjugations transferring plasmid pRL27 (30) into V. cholerae strain S9149 were carried out as described above. Transposon mutant libraries were made by arraying recovered exconjugants from the LB agar plates containing 100 μg/ml rifampin and 200 μg/ml Km onto petri dishes with LB medium supplemented with 200 μg/ml Km in 49-sample (7 by 7) grid format. After overnight incubation of these plates at 37°C, each cell patch was replica plated onto one LB agar plate supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 100 μg/ml) and one containing X-Gal (100 μg/ml) and indole (500 μM). Each plate was grown at 37°C, and the following day individual patches were qualitatively assessed for production of blue colonies with and without indole. Putative mutants altered in lacZ expression were repatched onto LB-X-Gal (100 μg/ml) plates with or without indole (500 μM). Mutants displaying phenotypes different from the parental S9149 strain were preserved at −80°C in LB medium containing 15% glycerol (vol/vol).
The sequences of flanking DNA surrounding transposon insertions within these mutant strains were then retrieved using an arbitrary PCR technique first described by O'Toole et al. (41). PCRs resulting in amplicons of approximately 500 bp were cleaned and sequenced using Sanger's dideoxy chain termination method (45). Recovered sequence data were analyzed by performing BLAST analysis (1) against the V. cholerae strain N16961 genomic sequence (24).
Crystal violet quantification of biofilm formation.
Strains were initially streaked onto LB agar and allowed to grow overnight at 37°C. Biological replicates originating from three unique colonies for each strain were inoculated into 5-ml liquid cultures of LB medium and grown for ∼16 h. Five microliters of each culture was then inoculated into 5 ml of fresh LB medium and grown overnight at 37°C with moderate shaking. Biofilms formed by each strain were then quantified by a method described by O'Toole et al. (41). For each culture, 50 μl of crystal violet (0.1%, wt/vol) was then added to allow for staining of the adherent cells. The medium was then washed from each tube and replaced with 5 ml of 95% ethanol (vol/vol), and the OD570 values of individual replicates were recorded. Pairwise comparisons of the data of each sample were then analyzed for significance using a Student's t test (α = 0.05).
Auto-aggregation assay.
Strains were grown as above to obtain three biological replicates. After overnight growth in 5 ml of LB medium, each sample was allowed to settle for at least 1 h. Once the flocculent particles within each culture had settled to the bottom of each tube, 200 μl was removed from the top portion of each culture, and the OD600 of this sample was read using a Spectramax M2 microplate reader (Molecular Devices).
Miller assays for β-galactosidase activity.
A protocol similar to that as described by Miller (37) was used to assay for alterations in lacZ expression. In brief, three biological replicates of each strain were grown overnight in LB broth and subcultured at 1:1,000 in fresh LB medium (with or without 500 μM indole from 1 M stock in methanol) and grown for an additional 20 h, at which point the OD600 was measured. One-milliliter aliquots were then removed from each of these cultures, and cells were centrifuged (2 min at 13,000 × g) and resuspended in 500 μl of CPRG lysis buffer (250 mM Tris, pH 7.4, 2.5 mM EDTA, 0.25% Igepal [vol/vol]). For samples where the indole concentration was estimated, the supernatant of each was retained for later analysis. Cells were then lysed by freezing at −20°C for 30 min and subsequently thawing at room temperature. One hundred microliters of lysate was then added to 900 μl of Z buffer (0.06 M Na2HPO4·7H2O, 0.04 M NaH2PO4·H2O, 0.01 M KCl, 0.85 M β-mercaptoethanol), and β-galactosidase activity was assayed as outlined previously (37). Control experiments performed with cells grown in the presence of LB medium supplemented with methanol (0.05%, vol/vol), which is the indole solvent, demonstrated no appreciable change in vpsL::lacZ expression (data not shown).
For the coculture experiment, three biological replicates of each strain were grown overnight in LB medium from single colonies and subcultured together in fresh LB medium at a 1:5,000 dilution. Cultures were grown for 48 h, and ratios of each strain were monitored throughout using plating experiments. For all cell combinations, the Miller units calculated were normalized to the percentage of S9149 cells in the total OD, as represented by the percentage of S9149 colony counts obtained for each two-strain culture. In this manner, the calculated β-galactosidase activity was not reflective of all of the cells harvested but only of those producing LacZ.
For the conditioned medium experiment, biological replicates of S9149 (without indole) and S9171 (with indole) were grown overnight in 11 ml of LB medium at 37°C. One-milliliter aliquots were removed from each tube for later use in inoculating new cultures. The remaining 10 ml of each culture was centrifuged to pellet the cells (10 min at 3,640 × g), and the resulting supernatants were filter sterilized through 0.22-μm-pore-size membranes. To 3.8 ml of each of these conditioned supernatants, 200 μl of 20× YT medium (100 g/liter yeast extract and 200 g/liter tryptone) was added. At this point, each biological replicate of S9149 was inoculated at a 1:1,000 dilution into fresh LB medium, conditioned medium lacking indole, and conditioned medium with indole. After 24 h of growth, Miller assays were performed as described above.
Indole concentration measurements.
The concentration of indole in the supernatants of cell cultures was measured by mixing 250 μl of supernatant with 250 μl of trichloroacetic acid (20% wt/vol). After incubation on ice for 15 min, each sample was centrifuged to remove precipitated proteins (10 min at 13,000 × g). This supernatant was then added to 500 μl of Kovac's reagent (Sigma-Aldrich Co.) and vortexed, and the OD571 was measured for 200 μl of the top layer. A standard curve of known indole concentrations was recorded and used to estimate the amount of indole in each sample.
RNA isolation and transcription analysis using whole-genome transcription profiling.
V. cholerae strains were initially grown on LB agar plates at 37°C overnight. Individual colonies from each plate were inoculated into 5 ml of LB medium and grown overnight at 37°C. Two colonies for each strain were picked and subcultured as biological replicates at a 1:1,000 dilution in fresh LB medium (with or without 350 μM indole) and grown for 20 h at 37°C with moderate shaking. Two separate 1-ml samples from each culture were harvested as technical replicates, and RNA was isolated as described previously (59).
The microarrays used in this study representing the open reading frames present in the V. cholerae genome were composed of 70-mer oligonucleotides and were printed at the University of California, Santa Cruz. Whole-genome expression analysis was performed using a common reference RNA, which was a 1:1 mixture of total RNA isolated from wild-type cells grown in LB medium and wild-type cells grown in LB medium supplemented with indole. cDNA synthesis, microarray hybridization, and scanning were performed as described previously (7). Signal ratios were normalized with LOWESS print-tip normalization using the Bioconductor packages (21) in the R environment. The significance analysis of microarrays method (53) was used to determine differentially regulated genes using ≥1.5-fold differences in gene expression and ≤1% false discovery rate as cutoff values.
For meta-analysis of microarray experiments, a χ2 test was used to evaluate statistically significant differences between over- and underexpressed genes in previously published expression data sets and the results obtained in these current experiments. Data sets were downloaded from the supplemental tables of published reports (Table 4), and genes were grouped together if they demonstrated a significant upregulation or significant downregulation under the treatment conditions. The resulting gene sets from each report were then individually compared to the sets of differentially regulated genes found in the study to determine the overlap between the two data sets. From this, a two-by-two χ2 test was performed, and significance was assessed as having a P value of ≤0.01.
TABLE 4.
χ2 analysis of the tryptophanase/indole expression profiles compared to published data sets
| Expression profile | SIO/S1101 comparisona | Reference |
|---|---|---|
| c-di-GMP | 102.31 | 7 |
| ΔvpsR | 73.58 | 6 |
| ΔvpsT | 50.95 | 6 |
| ΔcdgA | 48.38 | 6 |
| Δhfq | 13.43 | 16 |
| ΔmbaA | 21.82 | 35 |
| ΔrpoN | 44.12 | 59 |
| ΔrseA | 17.73 | 16 |
| ΔhapR | 8.04 | 6 |
| ΔrpoE | 3.19 | 16 |
| ΔtoxR | 1.01 | 8 |
Critical values of ≥11.35 represent a P value of ≤ 0.01 with three degrees of freedom and are shown in boldface.
GFP tagging of V. cholerae strains and CLSM.
Triparental conjugations for inserting the green fluorescent protein (GFP) gene into the V. cholerae chromosome were performed as described previously (7). Biofilm formation by GFP-expressing strains within coverglass chambers (Nalge Nunc International) was assessed using confocal laser scanning microscopy (CLSM). Biological replicates for each strain were inoculated from stationary phase cultures into chambers containing fresh LB medium (with or without 500 μM indole) at a final concentration of 106 cells/ml. Static growth was allowed to proceed for 6 h at 37°C, at which point medium was removed from the chambers, and attached cells were washed twice with 100 mM phosphate-buffered saline (pH 7.0). After resuspension in 100 mM phosphate-buffered saline, biofilms were visualized with a Nikon C1si microscope, and analysis of Z-stacks and three-dimensional rendering was performed with NIS Elements software (Nikon Instruments, Inc.).
RESULTS
Indole controls biofilm-associated phenotypes in V. cholerae.
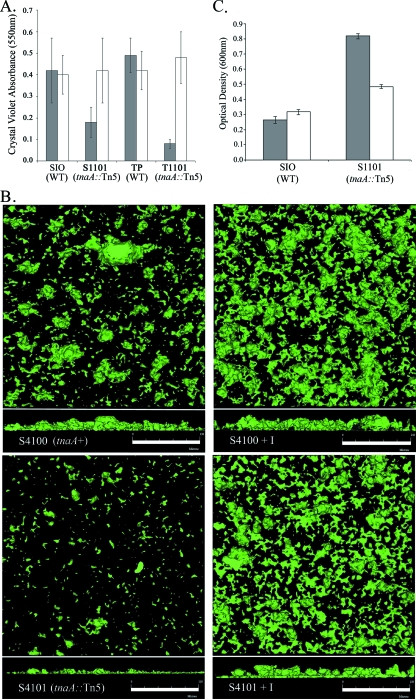
A previous transposon mutagenesis screen for biofilm mutants in two environmental strains of V. cholerae, SIO and TP, identified the tryptophanase gene as being essential for proper biofilm formation (38). When the biofilms of these tnaA::Tn5 mutants were examined with crystal violet staining, it was noted that the SIO mutant (S1101) formed ∼2.3-fold less biofilm than its parental wild-type and the TP mutant (T1101) formed ∼6-fold less biofilm than the wild-type (Fig. 1A). Since indole, a by-product of the tryptophanase reaction, had previously been identified as a factor controlling biofilm formation in other bacteria (36), the effect of exogenous indole addition on the biofilms of these tnaA mutants was tested. In this experiment, indole added exogenously at a concentration of 350 μM was able to fully complement the biofilm formation of these mutants to wild-type levels (Fig. 1A).
FIG. 1.
Phenotypes of V. cholerae tryptophanase mutants. (A) Biofilm accumulation of wild-type (WT) SIO and TP strains and their corresponding tnaA::Tn5 mutants S1101 and T1101 grown in LB medium (gray bars) and LB medium supplemented with indole (350 μM; white bars). (B) Confocal micrographs of the biofilms of S4100 and S4101 grown in LB without indole or with 500 μM indole (+I) for 6 h under static conditions. The top panels are the x-y axis, and bottom panels are the z axis. (C) Measure of the auto-aggregation phenotype of SIO and S1101 grown in LB medium with 500 μM indole (white bars) or without (gray bars). Error bars represent 1 standard deviation from the mean of three biological replicates for each strain.
The influence of indole on various properties of strain SIO was studied in more detail due to its genetic tractability and the clear phenotypic differences between the smooth colonial phenotype of VPS-defective mutants and the rugose colony structure of the wild-type strain. The biofilms formed by GFP-expressing SIO and S1101 strains (strains S4100 and S4101, respectively) were examined further using CLSM to investigate the morphological effects of indole on their biofilms. As seen in Fig. 1B, the biofilms of the parental S4100 strain grown on glass coverslips for 6 h in LB broth under static conditions were distinctly different from the biofilms of S4101, the tryptophanase mutant. Without the addition of indole to the biofilm chamber, the S4100 strain began to form regular microcolonies covering the glass substratum with an average height of 25.8 ± 5.0 μm. In contrast, the microcolonies of S4101 biofilms were much smaller and exhibited sparse surface coverage. Additionally, these biofilms were thinner and reached an average height of only 15.5 ± 2.6 μm. When indole was added back to each strain, an up-shift in biofilm formation was discovered. While strain S4100 produced a slight increase in surface coverage when exogenous indole was added, a clear difference was seen for S4101 under these conditions. The biofilms of S4101 with exogenous indole nearly doubled in thickness to an average height of 28.0 ± 4.4 μm, and the surface coverage increased considerably and was not significantly different from that of the parental strain with indole.
Another notable phenotypic difference among the SIO-derived strains was that the tryptophanase mutant did not exhibit the auto-aggregative phenotype of its parental strain. Under stationary growth conditions, wild-type cultures form multicellular clumps that settled to the bottom of the tube within liquid medium. When liquid growth of the tryptophanase mutant was examined, a lack of aggregation within the medium was evident. Figure 1C, which shows the OD600 of the cultures of the SIO and S1101 strains with and without indole upon settling, demonstrates the aggregation effect. This figure also shows that when indole is added back to the tnaA mutant strain, the auto-aggregation phenotype is restored to nearly wild-type levels.
Indole regulates VPS gene expression in V. cholerae.
In addition to affecting biofilm formation and auto-aggregation, indole induced the smooth colonies of S1101 to revert to the rugose phenotype of the SIO parental wild type when grown on LB agar plates (38). It has previously been shown that all three of these phenotypes are linked to overproduction of exopolysaccharides (2, 55, 61). Therefore, it was hypothesized that indole is exerting its effects, at least in part, by regulating vps gene expression. Therefore, the β-galactosidase activity of modified SIO and S1101 parental strains carrying lacZ reporter fusions to the vpsL genes and deletions of the native lacZ genes was monitored (S9171 and S9149, respectively). In these strains, β-galactosidase activity serves as a proxy for vps gene expression in the presence and absence of endogenously produced indole.
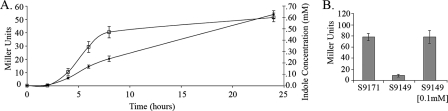
The initial experiment examined the simultaneous production of endogenous indole and β-galactosidase activity throughout the growth phases of S9171 grown in batch culture (Fig. 2A). It was observed that indole production by this tnaA+ strain mirrored vpsL::lacZ expression. During early-exponential-phase growth, both indole and β-galactosidase levels are low. However, consistent with tnaA catabolite repression control (57), late-exponential- and stationary-phase cultures produced increased amounts of indole and, concomitantly, increased amounts of β-galactosidase. While this experiment showed that extracellular levels of indole and vpsL induction correlate with one another, it did not address whether indole is responsible for vpsL induction.
FIG. 2.
Indole production correlates to increased vpsL::lacZ transcription. (A) Measured endogenous indole (open boxes) and β-galactosidase (filled circles) production by strain S9171 (ΔlacZ vpsL::lacZ) over a 24-h period. (B) Comparison of the β-galactosidase production in response to endogenous indole production in strain S9171 and exogenous addition of indole to strain S9149 (ΔlacZ ΔtnaA vpsL::lacZ). Error bars represent 1 standard deviation from the mean of three biological replicates for each strain.
To address this possibility, strain S9149 (ΔtnaA) was used to monitor vpsL::lacZ expression under conditions of exogenous indole addition. Similar to the biofilm results reported above, S9149 vpsL::lacZ expression was approximately 10-fold less than its indole-producing parental strain, S9171 (Fig. 2B). Full complementation of vpsL::lacZ induction occurred with concentrations of exogenous indole as low as 100 μM, which is well below the observed concentration of endogenous indole produced by S9171. These data illustrate that indole supplied exogenously can regulate VPS expression when it is supplied at levels below physiologically relevant concentrations.
Indole acts as an extracellular signal regulating VPS production.
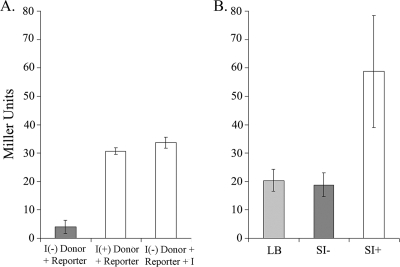
To further address the hypothesis that endogenously produced indole acts as a signal controlling VPS in V. cholerae, experiments were performed to determine whether indole produced from an originating cell can be taken up by a different cell and elicit a response (i.e., vpsL::lacZ upregulation). First, coculture experiments were performed with LacZ-deficient indole-negative (S2150; ΔlacZ ΔtnaA) or indole-positive (S2148; ΔlacZ tnaA+) donor strains and an indole-negative reporter strain (S9149; ΔtnaA vpsL::lacZ). As shown in Fig. 3A, the indole reporter strain produced very little β-galactosidase activity when grown in coculture with the indole-negative donor. However, when grown with the indole-positive donor, the β-galactosidase activity increased approximately eightfold. This increase is strikingly similar to the activity achieved when indole is added exogenously to the coculture conditions with the indole negative donor and reporter strains, demonstrating that exogenously and endogenously produced indole have the same VPS regulatory effect.
FIG. 3.
Endogenously produced indole acts as a signal to stimulate the expression of vpsL in recipient cells. (A) In the coculture experiment, an indole-negative donor strain, S2150 [I(−)], does not upregulate vpsL::lacZ expression in the reporter strain S9149 (gray bar), while the indole-positive donor strain S2148 [I(+)] can complement vpsL::lacZ expression to similar levels as when 0.5 mM indole (I) is added exogenously (white bars). (B) The same upregulation by indole is seen when the S9149 reporter strain is grown in conditioned medium grown from indole-positive supernatant from S2148 (SI+) and indole-negative supernatant from S2150 (SI−) conditions. Growth of S9149 in LB medium alone also did not stimulate vpsL::lacZ upregulation. For the coculture experiment Miller units are normalized to the percentage of S9149 cells from the total OD (see Materials and Methods). Error bars represent 1 standard deviation from the mean of three biological replicates for each strain.
An experiment was also performed in which strain S9149 was grown by itself in conditioned or unconditioned medium (Fig. 3B). S9149 grown in fresh LB medium produced very little vpsL::lacZ reporter activity, which was similar to the result when S9149 was grown in a supernatant from an indole-negative strain (Fig. 3B) (derived from strain S2150). However, when S9149 was grown in conditioned medium derived from an indole-producing strain (Fig. 3B) (derived from strain S2148), the level of β-galactosidase production rose significantly (more than threefold). These results reinforce the conclusion that the VPS regulatory response is produced specifically by indole and not another component of the supernatant.
It was also investigated whether indole influences biofilm formation and VPS production in clinical strains of V. cholerae in addition to the non-O1/O139 environmental strains tested. It was found that addition of indole to cells of V. cholerae O1 El Tor strain N16961 carrying an in-frame deletion of the tnaA gene (smooth variant) (24) caused significant increases in biofilm formation and vpsL transcription compared to cells grown without indole (data not shown). Thus, indole regulates biofilm formation and vps transcription in both clinical and environmental strains of V. cholerae.
Transcriptional regulation by TnaA activity and indole production extends beyond VPS genes.
To explore whether indole regulation of gene expression extends beyond genes involved in VPS biosynthesis, whole-genome expression profiles from the wild-type SIO strain and strain S1101 (tnaA::Tn5) grown to stationary phase in LB medium in either the presence or absence of exogenously added indole were obtained and compared. Significantly regulated genes were defined using the significance analysis of microarrays (53) as having ≤1% false-positive discovery rates and ≥1.5-fold transcript abundance differences between each sample. Four sets of pairwise comparisons were made from the resulting transcriptome profiles in order to assess the influence of indole and/or tryptophanase activity on gene expression. Table S1 of the supplemental material provides the complete list of differentially regulated genes within each data set. The first comparison made was between the transcriptomes of SIO and S1101 (pairwise comparisons are indicated using the form SIO/S1101) to understand the effects of a tryptophanase mutation on gene expression. The second comparison examined the effects of indole alone by matching the expression data of the tryptophanase mutant, S1101, grown with or without exogenous indole [S1101(+I)/S1101]. The next comparison evaluated the expression changes of the indole-producing SIO strain grown in the presence of additional exogenous indole to SIO grown solely in LB medium, [SIO(+I)/SIO]. Here, the consequences of artificially high indole concentrations on gene expression were evaluated. Finally, the expression data from the SIO strain and S1101 grown with indole were compared to determine whether indole alone could complement the expression changes resulting from a tnaA mutation [SIO/S1101(+I)]. Table 2 summarizes the results of these comparisons noting the number of differentially regulated genes in each pair.
TABLE 2.
Whole genome expression profiles of SIO and S1101 strains
| Pairwise comparisona | Total no. of genes
|
||
|---|---|---|---|
| Regulated | Upregulated | Downregulated | |
| SIO/S1101 | 507 | 255 | 252 |
| S1101(+I)/S1101 | 64 | 45 | 19 |
| SIO(+I)/SIO | 218 | 83 | 135 |
| SIO/S1101(+I) | 242 | 128 | 114 |
+I, cells grown in the presence of exogenous indole at 350 μM.
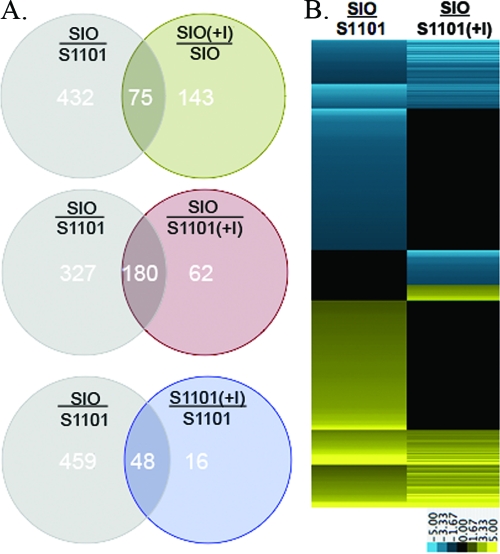
The data set comparing the wild-type SIO strain to S1101 contained the most genes demonstrating significant induced/reduced expression, suggesting that both indole production and tryptophanase activity can alter gene expression. Figure 4A displays Venn diagrams illustrating the overlap between each additional expression profile comparison and the SIO/S1101 data set. When the SIO(+I)/SIO expression profile was compared to SIO/S1101, only 34% of genes differentially expressed were shared between data sets, possibly due to the artificially high indole conditions present in the former. In contrast, many of the genes from the indole complementation expression profiles [SIO/S1101(+I) and S1101(+I)/S1101] are shared with the SIO/S1101 data set (74% and 75%, respectively). The complementation effect of indole can be seen in the heat map of Fig. 4B, where 327 of the 507 genes significantly reduced/induced in expression in the SIO/S1101 data set show no significant difference in expression in the SIO/S1101(+I) data set.
FIG. 4.
Overlap of genes found to be significantly altered in expression between the SIO/S1101 expression profile and remaining pairwise comparisons. (A) Venn diagrams show the shared and unique genes found between the differentially expressed genes of SIO/S1101 grown in LB medium compared with SIO(+I)/SIO, SIO/S1101(+I), and S1101(+I)/S1101. (B) Heat map depicting differences in expression between all genes within the union of the SIO/S1101 and SIO/S1101(+I) expression profiles (569 genes) (see middle diagram of panel A). Compact views of genes are presented using the log2-based color scale shown at the bottom of the panels Yellow, induced; blue, repressed; black, no significant change.
Differentially expressed genes were grouped according to their annotated functional roles in order to determine which categories may be influenced by indole production. Not surprisingly, genes involved in cell envelope maintenance and production were strongly upregulated by indole, which is clearly demonstrated by the heat map of the VPS genes shown in Fig. 5A. In total, 16 of the 19 genes contained within the two vps operons were upregulated by indole when all gene profile comparisons were considered. Additionally, some of these demonstrate the strongest differential expression between the SIO and S1101 expression profiles, with six demonstrating at least ∼10-fold activation in the SIO profile.
FIG. 5.
Heat maps of selected functionally relevant genes that are significantly altered in transcriptional levels from each expression profile examined. (A) Selected genes of the V. cholerae genome believed to be responsible for VPS biosynthesis. (B) Selected genes functionally categorized as having a role in cellular processes, including many genes thought to be involved in flagellar biosynthesis, motility, and chemotaxis. (C) Genes involved in iron transport and VAS. Compact views of genes are presented using the log2-based color scale shown at the top right. Yellow, induced; blue, repressed; black, no significant change.
Another notable functional category that was differentially regulated by indole is the group containing genes responsible for general cellular processes (Fig. 5B). Included within this functional class are many genes that have a role in chemotaxis and flagellar biosynthesis. These include many of the annotated chemotaxis (Che) proteins and methyl-accepting chemotaxis proteins of the V. cholerae genome (24). The vast majority of these genes demonstrate significant downregulation in the presence of exo- or endogenous indole, coinciding with microarray studies performed with E. coli showing a similar downregulation chemotaxis gene transcription in the presence of indole (27).
The transport and binding protein functional category also demonstrated overall downregulation in response to indole. This group includes genes involved in amino acid transport, iron uptake, and carbohydrate transport. Figure 5C shows genes from the largest subgroup of genes within this category that function as iron transport systems and in siderophore production and transport. Most of these genes were repressed only under artificially high indole conditions where gene expression of wild-type SIO grown in LB supplemented with exogenous indole was compared to SIO grown solely in LB medium.
Figure 5C also shows a set of genes that demonstrate a relative increase in expression and are annotated to fall within a group of genes with previously unknown functions. However, recent work has shown that the hcp (VC1415 and VCA0017), vgrG (VCA0018 and VCA0123), and vasK (VCA0120) genes of this group are involved in virulence-associated secretion (VAS), which has a role in infection and virulence toward eukaryotic cells (for reviews, see references 9 and 19). For example, it has been shown that the vas genes and their homologues within other species function in mediating (i) effective infection and nitrogen fixation by Rhizobium leguminosarum within nodules of the pea plant (10), (ii) disease in fish by the piscine pathogen Edwardsiella tarda (47), (iii) death of the phagocytic eukaryote Dictyostelium discoideum by V. cholerae (43), and (iv) pellicle and biofilm formation in Vibrio parahaemolyticus and Actinobacillus actinomycetemcomitans, respectively (17, 27).
In addition to the above five genes, the array results indicate that all but one of the surrounding genes of the VAS operon (VCA0107 to VCA0123) (see Table S1 in the supplemental material) are indole induced (Fig. 5C). Seven of these genes demonstrated moderate to strong significant activation by indole (greater than ∼2.5-fold). Although the vas genes did not show a significant increase in transcript abundance in the pairwise comparison between S1101 grown on LB supplemented with indole versus growth on LB medium alone, semiquantitative reverse transcription-PCR measuring the abundance of VCA0108 transcript demonstrated a 1.7-fold increase in S1101 cells grown in the presence of indole relative to growth without indole. Therefore, it appears that in the S1101 background, the VAS operon can be upregulated by indole although its detection may be below the limits of the microarray experiments performed.
Tryptophanase and indole have a role in grazing resistance.
Since indole induces genes involved in VAS secretion and toxin production, it was hypothesized that the tryptophanase gene may be important for grazing resistance. For these experiments using the D. discoideum grazing model (43), strain SIO could not be used due to its high sensitivity to grazing by this amoeba. Therefore, V. cholerae strain TP was used since it was discovered to be resistant to D. discoideum grazing. Standard plaque assays were performed on nutrient agar supplemented with indole at levels that were not toxigenic to D. discoideum (data not shown).
Table 3 shows that when strain T1101 (tnaA::Tn5) was examined for its resistance, a dramatic decrease in its ability to survive under grazing pressure was observed relative to the wild-type TP strain. However, when indole was supplied exogenously, partial grazing resistance was restored in T1101. In addition, it was observed that a strain (T1144) carrying a mutation in the vas operon was also susceptible to grazing whether it was grown with or without indole. These results suggest that indole may stimulate VAS production leading to increased grazing resistance.
TABLE 3.
Qualitative assessment of the plaque assay demonstrating the resistance and/or susceptibility of multiple strains of bacteria to grazing by the phagocytic eukaryotic predator D. discoideum in the presence and absence of indole
| Strain | Grazing resistance phenotypea
|
|
|---|---|---|
| No indole | With indole | |
| V. cholerae | ||
| TP | ++ | ++ |
| T1101 (tnaA::Tn5) | − | + |
| T1144 (VCA0109::Tn5) | − | − |
| N16961 | − | + |
| K. aerogenes | − | + |
Indole was added at a concentration of 500 μM. Scoring is as follows: ++, no significant plaque formation covering the majority of the plate; + small circular zones of clearing covering less than half of the plate; −, no plaques observed.
It was also found that exogenous indole could slightly increase grazing resistance in V. cholerae strain N16961 and Klebsiella aerogenes, both of which carry full copies of the vas operon within their genomes yet are grazing susceptible when grown without indole. It should be noted, though, that a VAS connection to the phenotypes of V. cholerae N16961 and K. aerogenes has not been conclusively established.
χ2 analysis suggests patterns of regulation involved in the indole response.
Various activators and repressors that are known to influence exopolysaccharide production were differentially regulated in the transcriptome comparisons. Included within these are genes involved in (i) direct transcriptional activation of vps (vpsR and vpsT), (ii) quorum sensing (cqsA, luxQ, fis, and hapR), (iii) c-di-GMP biosynthesis and degradation (mbaA and cdgA), and (iv) membrane stress sensing and response (cpxPAR, rpoE, and rseA). To determine whether the gene sets regulated by indole and tryptophanase are significantly similar to the regulons of some of these other known transcriptional regulators of V. cholerae and to predict possible key players of the indole response, a χ2 analysis was performed.
The results of this analysis are shown in Table 4. Several patterns emerge from these analyses that suggest roles for specific transcriptional regulators in the indole response. The data sets with the most significant correlation to the SIO/S1101 expression profile are from experiments performed under conditions of altered VPS production. These include transcriptome analyses performed with mutant strains of the vpsT and vpsR transcriptional activators and with GGDEF protein mutants (mbaA and cdgA) or cells grown with artificially high levels of c-di-GMP. Even when the 18 vps genes were excluded from these analyses, there was still significant overlap between the data sets, indicating that the similarities extend beyond VPS production (data not shown).
Other regulons that appear to be similar to the indole-tnaA-regulated gene sets are regulons controlled by the nucleic acid binding protein Hfq and the alternative sigma factor σ54 (RpoN). Hfq has been shown previously to be involved in quorum sensing in V. cholerae (33), and σ54 regulates motility and virulence and other global regulators including HapR and σ38 (RpoS) (43, 59), many of which are also regulated in the presence of indole.
Other regulators are not significantly correlated with the indole-tnaA regulon, however. These include HapR, the master regulator of quorum sensing; ToxR, a master regulator of pathogenesis in virulent strains of V. cholerae; and RpoE, a regulator of the membrane stress response. Whole-genome hybridization microarray experiments indicate that strain SIO does not contain many of the genes (e.g., VPI-1 and CTX elements) found within the traditionally defined ToxR regulon of V. cholerae (8; also M. Miller, personal communication), which may account for some of these results.
Since lack of a significant correlation between the microarray experiments could be due to various reasons (e.g., experimental design or strain variations), the roles of the quorum-sensing and membrane stress response systems in contributing to indole-driven gene expression were tested. In-frame deletions of key genes for each system were constructed (for quorum sensing mutants, fis and luxO; for membrane stress, rpoE and cpxA), and the ability of these mutant strains to respond to indole was examined using the vpsL::lacZ reporter gene system. When strains were grown under conditions with or without exogenous indole, none of them was impaired in the upregulation of the vpsL::lacZ fusion in the presence of indole (data not shown).
To also ensure that indole, due to its hydrophobic nature, is not triggering a membrane stress response as seen in other bacteria when indole is provided at artificially high concentrations (40), we investigated the ability of indole to depolarize the membrane of V. cholerae cells. Here, it was found that physiologically relevant concentrations of indole (0.5 mM) did not have a measurable effect on membrane polarity (data not shown). Taken together, the above results support the view that neither the membrane stress nor quorum-sensing system plays a role in the indole signaling cascade.
Indole regulation of VPS proceeds through DksA, VpsR, and GGDEF domain-containing proteins.
In order to further develop the details of the indole-signaling circuit, a transposon mutagenesis screen was performed to look for mutants that display an indole-nonresponsive phenotype. Of the approximately 11,000 transposon mutants screened, four unique genes with transposon insertions were recovered which demonstrated a consistent low level of vpsL::lacZ expression that did not change significantly upon the addition of exogenous indole (Table 5). Interestingly, three of these four strains carried mutations in sequences coding for GGDEF domain-containing proteins (VCA0074 and VC1376) or in a hypothetical protein immediately upstream of one of these genes (VCA0075). Furthermore, each of these genes was recovered multiple times from independent transposition events (VCA0075, five independent mutations; VCA0074 and VC1376, two independent mutations each), demonstrating the importance of these genes in controlling the indole-induced VPS response and suggesting a near-saturation of the genome by the transposon mutagenesis screen.
TABLE 5.
Transposon mutants altered in vpsL::lacZ production
| Gene category and/or TIGR no. | Strain | Altered gene name or conserved domain(s) | Predicted function | β-Galactosidase activity ina:
|
|
|---|---|---|---|---|---|
| LB medium | LB medium with indole | ||||
| Parental | S9149 | ΔtnaA ΔlacZ ΔvpsL::lacZ | 16 ± 3.1 | 31 ± 4.9 | |
| Indole-nonresponsive mutants | |||||
| VC0596 | S9224 | dksA mutant; dnaK suppressor protein | Regulatory functions | 10.3 ± 2.3 | 14 ± 3.3 |
| VC1376 | S9216 | GGDEF family protein | Cell Signaling | 16 ± 2.8 | 21 ± 1.8 |
| VCA0074 | S9218 | cdgA mutant; GGDEF family protein | Cell Signaling | 4.7 ± 0.7 | 5.2 ± 2.2 |
| VPS mutants | |||||
| VC0338 | S9226 | Sodium symporter | Ion transport | 0 | 0 |
| VC1673 | S9219 | AcrB-D-F transporter | Ion transport | 0 | 0 |
| VCA0183 | S9227 | hmpA mutant; flavohemoglobin | Electron transport | 0 | 0 |
| VC0665 | S9170 | vpsR mutant; σ54-dependent transcriptional regulator | Regulatory functions | 0 | 0 |
| VC0143 | S9228 | Hypothetical protein | Unknown | 0 | 0 |
| VC1731 | S9229 | Conserved hypothetical protein | Unknown | 0 | 0 |
Values are in Miller units.
VCA0074, which has been named cdgA, has diguanylate cyclase activity and increases intracellular c-di-GMP levels, which leads to the production of VPS and the formation of biofilms in V. cholerae (34). On the V. cholerae genome, the cdgA gene resides within a two-gene operon and is located immediately downstream of VCA0075. A transposon mutation in VCA0075 has previously been shown to also affect the biofilm formation of V. cholerae (38) although it is not clear whether this is a direct consequence of VCA0075 mutation by the transposon or an indirect result of polar effects on downstream cdgA expression.
Previous transcriptome experiments were reviewed to understand what genes potentially control the expression of the cdgA operon (Table 4, references). These data show that the cdgA operon is only differentially regulated in response to mutations in known regulators of VPS expression (ΔhapR, ΔvpsR, ΔvpsT, ΔrpoN, and ΔvpvC) or in cells grown under conditions of artificially high intracellular c-di-GMP concentrations. The cdgA operon is always upregulated by activators of VPS production and downregulated by repressors. This operon did not exhibit any differential regulation in microarray experiments comparing rpoE, hfq, toxR, and rhyB mutants that also do not alter the overall regulation of the VPS operons. Considering that there are 41 genes encoding GGDEF-domain-containing proteins in the V. cholerae genome, it is striking that cdgA is consistently transcribed under conditions of VPS gene activation.
Only one mutant was recovered that does not respond to indole and contains an insertion in a gene or operon whose product(s) is not known to affect the production of GGDEF proteins. This mutant contains an insertion in the dnaK suppressor protein gene, dksA, which is the third of eight genes in an operon and codes for a protein thought to interact directly with the RNA polymerase holoenzyme (E). Through this physical interaction, DksA promotes the dissociation of E from the housekeeping sigma factor σ70, allowing the RNA polymerase molecule to interact with alternative sigma factors such as σ54 (RpoN) (reviewed in reference 22). Interestingly, rpoN was not differentially regulated in any of the whole-genome profile comparisons made, suggesting that indole's effect is posttranscriptional, such as at the level of DksA activity. As with previous failed attempts to complement transposon mutations in strain SIO (38), we were not able to restore indole responsiveness when the wild-type dksA gene was supplied in trans. However, construction of an in-frame deletion of the dksA gene resulted in an indole-nonresponsive phenotype (data not shown), suggesting that polar effects within the operon where dksA is found are not responsible for this observed phenotype.
A second class of mutants was found to produce no measurable β-galactosidase activity. Included in this class are two hypothetical proteins, one of which is predicted to be membrane localized, and two transporter genes. None of these genes has a known role in VPS production. A fifth mutant contained a transposon insertion in the hmpA gene, which encodes a soluble flavohemoglobin known to counteract nitrosative stress in Salmonella enterica (3). As VPS production has previously been shown to counteract oxidative stress (61), HmpA may play a role as a sensor that can regulate VPS production in response to oxidative stress. The last mutant in this group contained a disruption in the vpsR gene, which is a key regulator of VPS production (58) and is known to combine with other regulators including VpsT and GGDEF domain proteins to direct vps transcription (6). Therefore, it was not surprising that a vpsR mutant demonstrated no measurable β-galactosidase activity. These results indicate that the indole induction of VPS requires VpsR, c-di-GMP, DksA, and additional factors.
DISCUSSION
Indole signaling controls physiologically important functions in V. cholerae.
Recently, it was proposed that the molecule indole, which is a natural product of the breakdown of tryptophan by the enzyme tryptophanase, can act as a stationary phase signal molecule that induces biofilm formation (36, 54). Previous work showed that a mutation in the tryptophanase gene of V. cholerae strains influences biofilm formation and the ability to produce rugose colonies, and it was speculated that this was a result of reduced VPS production (38). In this current study, it has been shown that indole secreted by one cell can act as an extracellular signal that is sensed by others within a population and that the perception of this indole signal leads to the coordinated upregulation of vps gene expression and the associated phenotypes of biofilm formation and rugose colonial morphology.
These results correspond with those of Martino et al. (36), who demonstrated that indole enhances the biofilm formation of many different microorganisms carrying the tryptophanase gene. However, they are in contrast to a report indicating that indole downregulates biofilm formation in E. coli (32). Similarly, we have found that biofilm formation by the rugose variant of V. cholerae strain 92A1552 is not affected by deletion of the tnaA gene (unpublished results), suggesting that indole's effects may be strain specific. While the underlying reason for this difference is unknown, comparison of transcriptome expression patterns of the tnaA mutant of V. cholerae strain that we used and the tnaA mutant of E. coli K-12 (32) provides a possible partial explanation. Both V. cholerae SIO and E. coli K-12 appear to downregulate genes involved in cell motility and chemotaxis (27 genes in V. cholerae and 7 in E. coli). While motility enhances biofilm formation in E. coli (56), it is not required for biofilm development on glass or plastic by V. cholerae strain SIO (38), which may account for the observed differences in biofilm formation in response to indole by these species.
Another set of genes that was differentially regulated by indole in the transcriptome experiments localizes to the VAS operon, which is important for resistance of grazing by protozoa (43). These genes are often carried by plant- or gut-associated bacteria (15), which are exposed to high levels of indolic compounds within these environments (28, 49). Thus, the evidence presented here linking VAS operon regulation to indole is compelling and deserves further investigation for other bacteria in different ecological settings (e.g., VAS-mediated root or pathogenic infection model systems).
From the microarray experiments it was noted that the expression of genes involved in flagellar biosynthesis, the vps I and II operons, and genes of the VAS operon was changed by indole. In each case, the regulation of these genes is known to depend on specific σ54-dependent transcriptional activators: FlrC for flagellar biosynthesis (42), VpsR for vps transcription (58, 59), and VasH as a regulator of the VAS operon (43). Since indole does not appear to influence the expression levels of the rpoN gene (which encodes the σ54 protein), it appears that this effect may be in part due to indole-induced alterations in σ54-dependent transcriptional regulator gene expression. Indole appears to downregulate flrC (∼1.7-fold in the SIO/S1101 comparison) and upregulate vasH (∼2.3-fold in the SIO/S1101 comparison), consistent with the patterns of motility and VAS gene expression found in response to indole. In contrast, vpsR does not show a significant expression change in response to indole, and indole-controlled VPS regulation may result from posttranscriptional effects on VpsR.
Indole signaling involves known regulators of VPS synthesis and the dnaK suppressor protein, DksA.
It is apparent that the indole regulon is composed of many of the same genes that are governed by known regulators of VPS synthesis, such as VpsR, VpsT, and multiple GGDEF-domain-containing proteins (e.g., CdgA and MbaA). However, the expression profile of genes controlled by HapR, another known VPS regulator, did not correlate with the microarray results in any of the comparisons performed. Although HapR is the major quorum-sensing transcriptional regulator in some strains of V. cholerae (62) and is known to affect the transcription of numerous regulators of VPS, such as vpsT, vpsR, and cdgA, these regulators can also function independently of HapR to control VPS production (6). In fact, many strains of V. cholerae often carry mutations in the hapR gene, rendering the gene nonfunctional and presumably inactivating the quorum-sensing mechanisms of these strains (24, 29). This information combined with our results showing that quorum-sensing mutations had no effect on the upregulation of VPS by indole led us to conclude that indole regulation is dependent on regulators such as VpsR, VpsT, and GGDEF domain proteins rather than on quorum-sensing regulators.
Previous studies also have shown that VpsR and CdgA are involved in a regulatory cascade controlling VPS production, with VpsR acting to upregulate cdgA expression and CdgA increasing the intracellular concentrations of the second messenger c-di-GMP through its guanylate cyclase activity (6). Rising c-di-GMP concentrations then appear to signal increased transcription of genes involved in VPS production and repression of genes responsible for motility (7). Combining these results with ours implicates indole as an inducer of this c-di-GMP regulatory cascade.
A third gene that is thought to be a component of this signal cascade is vpsT, another known transcriptional activator of vps (12). vpsT shows consistent upregulation by indole in the transcriptome comparisons (∼2.8-fold in the SIO/S1101 comparison); therefore, it could also have a role in indole induction of VPS. To test its role, multiple attempts to make mutations within the vpsT gene were performed, but the mutant was never recovered. Although ΔvpsT derivatives of clinical strains of V. cholerae have previously been made, VpsT may have an essential role in the genetic background of the strains used in this study. A role for VpsT in the indole response cannot be ruled out.
Another significant finding is that the gene dksA appears to be involved in the indole response. Recently, it was shown that the protein DksA along with the intracellular alarmone ppGpp can bind directly to RNA polymerase (RNAP) and indirectly modulate its associations with different sigma factors (reviewed in reference 22). This binding increases the available intracellular pool of RNAP able to interact with σ54. As more RNAP holoenzyme E-containing σ54 (Eσ54) is produced, increased interactions with σ54-dependent transcriptional activators takes place, and transcription from σ54 promoters proceeds at higher levels. It has been proposed that DksA can act in concert with ppGpp to passively promote transcription from σ54 promoters (5). No previous findings have demonstrated that indole can influence ppGpp production or DksA activity. However, it has been shown that various indole derivatives, such as indole-based antimicrobial compounds and indole-3-acetic acid, can influence RelA-based production of ppGpp (48, 50). The role of dksA in the indole response and the fact that σ54-dependent transcriptional regulators control indole-controlled genes provide circumstantial evidence for a role for σ54 in the indole response.
A proposed model of the indole signaling cascade in V. cholerae.
Based on the data presented in this report, a model to guide future investigation of indole regulation is proposed in Fig. 6. In this scenario catabolite-repressed tryptophanase activity increases as carbon and energy supplies begin to dwindle, leading to a concomitant increase in indole production and excretion by individual cells. Once in the extracellular environment, it is unclear how the cells receive the indole signal. It has been suggested the Mtr transporter can actively transport indole (57) or, due to its hydrophobic nature, that indole might directly diffuse through the membrane (20). Once the signal is received, though, we propose that this indole signal may promote DksA-mediated interactions between RNAP and σ54, possibly in a ppGpp-dependent manner. Another possibility is that indole can interact directly with DksA to promote differences in RNAP availability although both hypotheses deserve future evaluation. As the association between RNAP and σ54 is enhanced, many genes under the direct control of σ54 are then activated including the VAS and VPS operons. Downregulation of the rpoS (which encodes the σ38 protein) gene is also observed, which could result from the ability of σ54 to negatively regulate the transcription of rpoS (59). Further, since the σ38 regulon is known to include genes involved in siderophore production and iron acquisition in other strains of V. cholerae (39, 59) and in Vibrio vulnificus (31), it is possible that the observed downregulation of the iron acquisition machinery is due to decreased rpoS expression (Fig. 6).
FIG. 6.
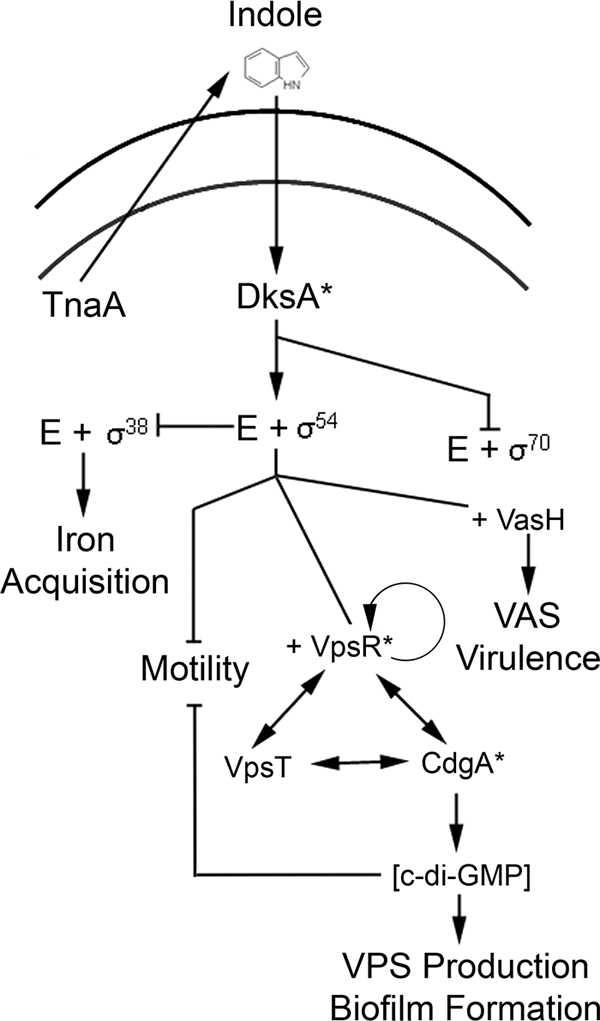
Model of indole-regulated gene expression. Indole affects DksA activity and downstream associations between RNA polymerase holoenzyme and σ54. This transcriptional complex can (i) repress rpoS transcription and its activation of iron acquisition mechanisms, (ii) couple with VasH to enhance VAS operon transcription, and (iii) combine with VpsR to autoregulate VpsR expression in addition to upregulation of CdgA and VpsT expression. Upregulation of CdgA leads to increases in c-di-GMP levels and subsequent repression of motility and chemotaxis genes and activation of vps genes. An asterisk marks the corresponding protein products of genes that are non-indole responsive and were recovered in the transposon mutagenesis. No arrowhead proceeds from RpoN to VpsR due to the lack of experimental evidence establishing this link.
The indole regulation of genes involved in motility and VPS production is known to result also from downstream effects on the c-di-GMP signal system of V. cholerae (7, 51). The results of the transposon mutagenesis screen support this model since the majority of indole nonresponsive mutations are in genes coding for GGDEF-domain-containing proteins. It is proposed that once VpsR is activated, the protein can interact with Eσ54 to enhance the expression of cdgA and vpsT. Both genes appear to have VpsR promoter binding sites (59) and are upregulated in the expression profiles. The increase in CdgA leads to an increase in c-di-GMP levels within the cell, which in turn stimulates vps expression and represses motility gene expression, leading to the overall enhancement of biofilm formation in V. cholerae strain SIO. Thus, it appears that ultimate regulation of many of the indole-responsive genes is dependent on the c-di-GMP intracellular signaling system.
Given the multiple roles of biofilms and VAS in stress protection, predator-prey interactions, and virulence toward eukaryotic hosts, the production of the indole signaling molecule by the tryptophanase enzyme during carbon and energy limitation must have an important role in environmental survival.
Supplementary Material
Acknowledgments
We thank Ilham Naili for help in the preparation of the tryptophanase mutant strain, and we thank Federico Lauro, Fiona Tomas, and Xavier Mayali for aiding with statistical analysis.
This work was supported by NIH grant AI46600-02 and funding from grant CEQI0047 provided by the UC Marine Council Coastal Environmental Quality Initiative.
Footnotes
Published ahead of print on 27 March 2009.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 2.Aslim, B., D. Onal, and Y. Beyatli. 2007. Factors influencing autoaggregation and aggregation of Lactobacillus delbrueckii subsp. bulgaricus isolated from handmade yogurt. J. Food Prot. 70223-227. [DOI] [PubMed] [Google Scholar]
- 3.Bang, I.-S., L. Liu, A. Vazquez-Torres, M.-L. Crouch, J. S. Stamler, and F. C. Fang. 2006. Maintenance of nitric oxide and redox homeostasis by the Salmonella flavohemoglobin Hmp. J. Biol. Chem. 28128039-28047. [DOI] [PubMed] [Google Scholar]
- 4.Bao, Y., D. P. Lies, H. Fu, and G. P. Roberts. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109167-168. [DOI] [PubMed] [Google Scholar]
- 5.Bernardo, L. M. D., L. U. M. Johansson, D. Solera, E. Skarfstad, and V. Shingler. 2006. The guanosine tetraphosphate (ppGpp) alarmone, DksA and promoter affinity for RNA polymerase in regulation of sigma54-dependent transcription. Mol. Microbiol. 60749-764. [DOI] [PubMed] [Google Scholar]
- 6.Beyhan, S., K. Bilecen, S. R. Salama, C. Casper-Lindley, and F. H. Yildiz. 2007. Regulation of rugosity and biofilm formation in Vibrio cholerae: comparison of VpsT and VpsR regulons and epistasis analysis of vpsT, vpsR, and hapR. J. Bacteriol. 189388-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beyhan, S., A. D. Tischler, A. Camilli, and F. H. Yildiz. 2006. Transcriptome and phenotypic responses of Vibrio cholerae to increased cyclic di-GMP level. J. Bacteriol. 1883600-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bina, J., J. Zhu, M. Dziejman, S. Faruque, S. Calderwood, and J. Mekalanos. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc. Natl. Acad. Sci. USA 1002801-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bingle, L. E. H., C. M. Bailey, and M. J. Pallen. 2008. Type VI secretion: a beginner's guide. Curr. Opin. Microbiol. 113-8. [DOI] [PubMed] [Google Scholar]
- 10.Bladergroen, M. R., K. Badelt, and H. P. Spaink. 2003. Infection-blocking genes of a symbiotic Rhizobium leguminosarum strain that are involved in temperature-dependent protein secretion. Mol. Plant Microbe Interact. 1653-64. [DOI] [PubMed] [Google Scholar]
- 11.Camilli, A., and B. L. Bassler. 2006. Bacterial small-molecule signaling pathways. Science 3111113-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casper-Lindley, C., and F. H. Yildiz. 2004. VpsT is a transcriptional regulator required for the rugose colonial morphology of Vibrio cholerae O1 El Tor. J. Bacteriol. 1861574-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chant, E. L., and D. K. Summers. 2007. Indole signalling contributes to the stable maintenance of Escherichia coli multicopy plasmids. Mol. Microbiol. 6335-43. [DOI] [PubMed] [Google Scholar]
- 14.Collet, A., S. Vilain, P. Cosette, G. Junter, T. Jouenne, R. Phillips, and P. Di Martino. 2007. Protein expression in Escherichia coli S17-1 biofilms: impact of indole. Antonie van Leeuwenhoek 9171-85. [DOI] [PubMed] [Google Scholar]
- 15.Das, S., and K. Chaudhuri. 2003. Identification of a unique IAHP (IcmF associated homologous proteins) cluster in Vibrio cholerae and other proteobacteria through in silico analysis. In Silico Biol. 3287-300. [PubMed] [Google Scholar]
- 16.Ding, Y., B. M. Davis, and M. K. Waldor. 2004. Hfq is essential for Vibrio cholerae virulence and downregulates σE expression. Mol. Microbiol. 53345-354. [DOI] [PubMed] [Google Scholar]
- 17.Enos-Berlage, J. L., Z. T. Guvener, C. E. Keenan, and L. L. McCarter. 2005. Genetic determinants of biofilm development of opaque and translucent Vibrio parahaemolyticus. Mol. Microbiol. 551160-1182. [DOI] [PubMed] [Google Scholar]
- 18.Evans, W. C., W. Richard, C. Handley, and F. C. Happold. 1941. The tryptophanase-indole reaction: some observations on the production of tryptophanase by Esch. coli; in particular the effect of the presence of glucose and amino acids on the formation of tryptophanase. Biochem. J. 35207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filloux, A., A. Hachani, and S. Bleves. 2008. The bacterial type VI secretion machine: yet another player for protein transport across membranes. Microbiology 1541570-1583. [DOI] [PubMed] [Google Scholar]
- 20.Gaede, H. C., W. M. Yau, and K. Gawrisch. 2005. Electrostatic contributions to indole-lipid interactions. J. Phys. Chem. B 10913014-13023. [DOI] [PubMed] [Google Scholar]
- 21.Gentleman, R., V. Carey, D. Bates, B. Bolstad, M. Dettling, S. Dudoit, B. Ellis, L. Gautier, Y. Ge, J. Gentry, K. Hornik, T. Hothorn, W. Huber, S. Iacus, R. Irizarry, F. Leisch, C. Li, M. Maechler, A. Rossini, G. Sawitzki, C. Smith, G. Smyth, L. Tierney, J. Yang, and J. Zhang. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gourse, R. L., W. Ross, and S. T. Rutherford. 2006. General pathway for turning on promoters transcribed by RNA polymerases containing alternative sigma factors. J. Bacteriol. 1884589-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammer, B. K., and B. L. Bassler. 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50101-104. [DOI] [PubMed] [Google Scholar]
- 24.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. Mcdonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Salzberg, H. O. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins, D. A., M. E. Pomianek, C. M. Kraml, R. K. Taylor, M. F. Semmelhack, and B. L. Bassler. 2007. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature 450883-886. [DOI] [PubMed] [Google Scholar]
- 26.Horton, R. 1997. In vitro recombination and mutagenesis of DNA. SOEing together tailor-made genes. Methods Mol. Biol. 67141-149. [DOI] [PubMed] [Google Scholar]
- 27.Kachlany, S. C., P. J. Planet, M. K. Bhattacharjee, E. Kollia, R. DeSalle, D. H. Fine, and D. H. Figurski. 2000. Nonspecific adherence by Actinobacillus actinomycetemcomitans requires genes widespread in bacteria and archaea. J. Bacteriol. 1826169-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchi, M., and K. Syono. 1996. The excessive production of indole-3-acetic acid and its significance in studies of the biosynthesis of this regulator of plant growth and development. Plant Cell Physiol. 371043-1048. [DOI] [PubMed] [Google Scholar]
- 29.Kovacikova, G., and K. Skorupski. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 461135-1147. [DOI] [PubMed] [Google Scholar]
- 30.Larsen, R. A., M. M. Wilson, A. M. Guss, and W. W. Metcalf. 2002. Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch. Microbiol. 178193-201. [DOI] [PubMed] [Google Scholar]
- 31.Lee, H.-J., K.-J. Park, A. Y. Lee, S. G. Park, B. C. Park, K.-H. Lee, and S.-J. Park. 2003. Regulation of fur expression by RpoS and Fur in Vibrio vulnificus. J. Bacteriol. 1855891-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, J., A. Jayaraman, and T. Wood. 2007. Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol. 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenz, D. H., K. C. Mok, B. N. Lilley, R. V. Kulkarni, N. S. Wingreen, and B. L. Bassler. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 11869-82. [DOI] [PubMed] [Google Scholar]
- 34.Lim, B., S. Beyhan, J. Meir, and F. H. Yildiz. 2006. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol. Microbiol. 60331-348. [DOI] [PubMed] [Google Scholar]
- 35.Lim, B., S. Beyhan, and F. H. Yildiz. 2007. Regulation of vibrio polysaccharide synthesis and virulence factor production by CdgC, a GGDEF-EAL domain protein, in Vibrio cholerae. J. Bacteriol. 189717-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martino, P. D., R. Fursy, L. Bret, B. Sundararaju, and R. S. Phillips. 2003. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Can. J. Microbiol. 49443-449. [DOI] [PubMed] [Google Scholar]
- 37.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Mueller, R. S., D. McDougald, D. Cusumano, N. Sodhi, S. Kjelleberg, F. Azam, and D. H. Bartlett. 2007. Vibrio cholerae strains possess multiple strategies for abiotic and biotic surface colonization. J. Bacteriol. 1895348-5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen, A. T., N. A. Dolganov, G. Otto, M. C. Miller, C. Y. Wu, and G. K. Schoolnik. 2006. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathog. 2e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norman, K. E., and H. Nymeyer. 2006. Indole localization in lipid membranes revealed by molecular simulation. Biophys. J. 912046-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 31091-109. [DOI] [PubMed] [Google Scholar]
- 42.Prouty, M. G., N. E. Correa, and K. E. Klose. 2001. The novel sigma-54 and sigma-28 dependent flagellar gene transcription hierarchy of Vibrio cholerae. Mol. Microbiol. 391595-1609. [DOI] [PubMed] [Google Scholar]
- 43.Pukatzki, S., A. T. Ma, D. Sturtevant, B. Krastins, D. Sarracino, W. C. Nelson, J. F. Heidelberg, and J. J. Mekalanos. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA 1031528-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purdy, A., F. Rohwer, R. Edwards, F. Azam, and D. H. Bartlett. 2005. A glimpse into the expanded genome content of the Vibrio cholerae species through the identification of genes present in environmental strains. J. Bacteriol. 1873305-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 745463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 47.Srinivasa Rao, P. S., Y. Yamada, Y. P. Tan, and K. Y. Leung. 2004. Use of proteomics to identify novel virulence determinants that are required for Edwardsiella tarda pathogenesis. Mol. Microbiol. 53573-586. [DOI] [PubMed] [Google Scholar]
- 48.Sundar, L., and F. N. Chang. 1993. Antimicrobial activity and biosynthesis of indole antibiotics produced by Xenorhabdus nematophilus. J. Gen. Microbiol. 1393139-3148. [DOI] [PubMed] [Google Scholar]
- 49.Swanson, K. S., C. M. Grieshop, E. A. Flickinger, L. L. Bauer, B. W. Wolf, J. Chow, K. A. Garleb, J. A. Williams, and G. C. Fahey, Jr. 2002. Fructooligosaccharides and Lactobacillus acidophilus modify bowel function and protein catabolites excreted by healthy humans. J. Nutr. 1323042-3050. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi, K., K. Kasai, and K. Ochi. 2004. Identification of the bacterial alarmone guanosine 5′-diphosphate 3′-diphosphate (ppGpp) in plants. Proc. Natl. Acad. Sci. USA 1014320-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tischler, A. D., and A. Camilli. 2004. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 53857-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tso, W.-W., and J. Adler. 1974. Negative chemotaxis in Escherichia coli. J. Bacteriol. 118560-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 985116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, D., X. Ding, and P. N. Rather. 2001. Indole can act as an extracellular signal in Escherichia coli. J. Bacteriol. 1834210-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood, T. K., A. González Barrios, M. Herzberg, and J. Lee. 2006. Motility influences biofilm architecture in Escherichia coli. Appl. Microbiol. Biotechnol. 72361-367. [DOI] [PubMed] [Google Scholar]
- 57.Yanofsky, C., V. Horn, and P. Gollnick. 1991. Physiological studies of tryptophan transport and tryptophanase operon induction in Escherichia coli. J. Bacteriol. 1736009-6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yildiz, F. H., N. A. Dolganov, and G. K. Schoolnik. 2001. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPSETr-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 1831716-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yildiz, F. H., X. S. Liu, A. Heydorn, and G. K. Schoolnik. 2004. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol. Microbiol. 53497-515. [DOI] [PubMed] [Google Scholar]
- 60.Yildiz, F. H., and G. K. Schoolnik. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. USA 964028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 993129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.