Abstract
Us3 is a serine/threonine protein kinase encoded by herpes simplex virus 1 (HSV-1). We recently identified serine at Us3 position 147 (Ser-147) as a physiological phosphorylation site of Us3 (A. Kato, M. Tanaka, M. Yamamoto, R. Asai, T. Sata, Y. Nishiyama, and Y. Kawaguchi, J. Virol. 82:6172-6189, 2008). In the present study, we investigated the effects of phosphorylation of Us3 Ser-147 on regulation of Us3 catalytic activity in infected cells and on HSV-1 pathogenesis. Our results were as follows. (i) Only a small fraction of Us3 purified from infected cells was phosphorylated at Ser-147. (ii) Us3 phosphorylated at Ser-147 purified from infected cells had significantly higher kinase activity than Us3 not phosphorylated at Ser-147. (iii) Phosphorylation of Us3 Ser-147 in infected cells was dependent on Us3 kinase activity. (iv) Replacement of Us3 Ser-147 by alanine significantly reduced viral replication in the mouse cornea and the development of herpes stromal keratitis and periocular skin disease in mice. These results indicated that Us3 catalytic activity is tightly regulated by autophosphorylation of Ser-147 in infected cells and that regulation of Us3 activity by autophosphorylation appeared to play a critical role in viral replication in vivo and in HSV-1 pathogenesis.
The herpes simplex virus 1 (HSV-1) Us3 gene encodes a serine/threonine protein kinase with an amino acid sequence that is conserved in the subfamily Alphaherpesvirinae (8, 24, 36). The consensus target sequence of an HSV-1 Us3 homologue encoded by pseudorabies virus (PRV) is RnX(S/T)YY, where n is ≥2; X can be Arg, Ala, Val, Pro, or Ser; and Y can be any amino acid except an acidic residue (20, 21, 35). The phosphorylation target site specificity of HSV-1 and other alphaherpesvirus Us3 kinases has been reported to be similar to that of the PRV homologue and to that of protein kinase A (PKA), a cellular cyclic AMP-dependent protein kinase (1, 4, 6, 13). Based on studies showing that recombinant Us3 null mutant viruses have impaired growth properties in cell cultures and virulence in mouse models, Us3 has been suggested to be a positive regulator of viral replication and pathogenicity (25, 40). Additional data have indicated other putative Us3 functions, including (i) to block apoptosis (22, 28-30); (ii) to promote nuclear egress of progeny nucleocapsids through the nuclear membrane (40, 42); (iii) to redistribute and phosphorylate nuclear membrane-associated viral nuclear egress factors UL31 and UL34 (14, 38, 39) and cellular proteins, including lamin A/C and emerin (19, 26, 27); (iv) to control infected cell morphology (13, 29); and (v) to downregulate cell surface expression of viral envelope glycoprotein B (12). In addition, Us3 has been reported to mediate phosphorylation of several viral and cellular proteins, such as histone deacetylase 1 (HDAC1) and HDAC2 (14, 34, 37). However, the linkage between these putative Us3 functions and their roles in viral replication and pathogenesis remains to be elucidated.
The intracellular activity of protein kinases is tightly regulated: they are turned on or off by phosphorylation, binding of regulatory subunits, interaction with small molecules, or their subcellular localization (41). Therefore, information on the mechanism by which the activity of a protein kinase is regulated is necessary for understanding its overall features, as well as its functional sequelae. However, data on the regulation of Us3 catalytic activity has been limited.
We previously reported that UL13, another HSV-1-encoded protein kinase, phosphorylates Us3 in infected cells (15). However, the biological significance of UL13-mediated phosphorylation of Us3 for the regulatory activity of Us3 has not been determined. In addition, we recently identified serine at Us3 residue 147 (Us3 Ser-147) to be a physiological phosphorylation site of Us3 in infected cells and demonstrated that alanine replacement of Us3 Ser-147 (Us3-S147A) significantly impaired protein kinase activity in in vitro kinase assays of purified recombinant Us3 expressed by a baculovirus expression system (13). Furthermore, the Us3-S147A mutation affected the ability of Us3 to induce wild-type cytopathic effects and to localize correctly in HSV-1-infected cells. These observations suggested that phosphorylation of Us3 Ser-147 is involved in upregulation of Us3 catalytic activity and that this regulation is critical for control of infected cell morphology and Us3 localization (13). However, we also obtained data that were not consistent with this hypothesis: the total protein kinase activity of Us3 carrying the S147A mutation, purified by immunoprecipitation from Vero cells infected with recombinant virus carrying the Us3-S147A mutation, was identical to that of wild-type Us3 from wild-type HSV-1(F)-infected Vero cells (13). In agreement with this observation, other Us3 functions, such as viral growth properties in cells, regulation of UL34 localization, and modification of UL31 and HDAC2, were not affected by the Us3-S147A mutation in infected Vero cells (13). We hypothesized from these observations that only a small fraction of Us3 molecules are phosphorylated at Ser-147 at any one time in infected cells and/or that most Us3 molecules are only phosphorylated transiently at specific times during viral infection. However, it is possible that phosphorylation of Us3 Ser-147 only regulates Us3 kinase activity in vitro during artificial overexpression of recombinant Us3 in the baculovirus system. Thus, the physiological relevance of phosphorylation of Us3 at Ser-147 in infected cells remains to be elucidated.
To resolve the function of phosphorylation of Us3 Ser-147 in infected cells, a probe(s) that specifically detects phosphorylated Us3 at Ser-147 (Us3-S147P) in infected cells is necessary. In the present study, a monoclonal antibody that specifically recognized Us3-S147P was developed and used to show that phosphorylation of Us3 Ser-147 in infected cells, which was mediated by the kinase activity of Us3 itself, was tightly regulated and that the catalytic activity of Us3 was controlled by autophosphorylation in infected cells. We also present data showing that regulation of Us3 catalytic activity by autophosphorylation played a critical role in viral replication in vivo and in HSV-1 pathogenesis.
MATERIALS AND METHODS
Cells and viruses.
Vero cells were described previously (44), as was HSV-1 wild-type strain HSV-1(F) (44). A Us3 deletion mutant virus, R7041 (36), was kindly provided by B. Roizman. The SP2/O murine myeloma cell line was kindly provided by K. Miyake. Recombinant virus YK511, encoding an enzymatically inactive Us3 mutant in which lysine at position 220 was replaced with methionine (Us3-K220M); recombinant virus YK513, in which the K220M mutation in YK511 was repaired (Us3-KM-repair); recombinant virus YK515, encoding a Us3-S147A mutant; and recombinant virus YK517, in which the S147A mutation in YK515 was repaired (Us3-SA-repair) were described previously (13).
Viral infection.
Vero cells were infected with indicated viruses at a multiplicity of infection (MOI) of 5 and subjected to various assays in this study.
Antibodies.
Peptides corresponding to Us3 residues 141 to 154, with or without phosphorylation of Ser-147, were purchased from GL Biochem for generation of mouse monoclonal antibodies that specifically recognized Us3-S147P. Peptides were covalently bound to keyhole limpet hemocyanin at the N terminus. BALB/c mice were immunized by repeated injections of 50 μg phosphopeptide with either Freund's complete or incomplete adjuvant. Spleen cells from the immunized mice were fused with SP2/O murine myeloma cells, and supernatants of the hybridoma cells were screened using an enzyme-linked immunosorbent assay with either phosphorylated or unphosphorylated peptide. Rabbit polyclonal antibody to Us3 was described previously (13). Mouse monoclonal antibodies to α-tubulin (DM1A) and ICP8 (10A3) were purchased from Sigma-Aldrich and Chemicon, respectively.
Immunoblotting and immunofluorescence.
Immunoblotting was performed as described previously (17), except that Can Get Signal immunoreaction enhancer solution A (Toyobo) was used when membranes were probed with monoclonal antibody to Us3-S147P. The amount of protein in the bands was quantitated, as described in the figure legends, by using the Dolphin Doc image capture system with Dolphin-1D software (Wealtec). Indirect immunofluorescence was performed as described previously (12), except that Can Get Signal immunostain solution A (Toyobo) was used when samples were reacted with a mouse monoclonal antibody to Us3-S147P.
Phosphatase treatment.
Lysates of HSV-1(F)-infected Vero cells were treated with λ phosphatase (λ-PPase) as described previously (13).
Immune complex kinase assays.
Immune complex kinase assays were performed as described previously (13), except that Vero cells were infected with either HSV-1(F), YK515, or YK517 at an MOI of 5 and monoclonal antibody to Us3-S147P and anti-Us3 polyclonal antibody were used for immunoprecipitation. To reduce the possibility that the antibodies might coprecipitate a protein kinase in addition to Us3, the precipitated material containing Us3 protein kinase was washed with a high-salt buffer containing 1 M NaCl prior to the assays, as described previously (13). The amount of radioactivity in phosphorylated substrate bands was quantitated in autoradiographs, as described in the figure legends, using the Dolphin Doc system with Dolphin-1D software.
Animal studies.
Five-week-old female ICR mice were purchased from Charles River and handled in accordance with the requirements of the Animal Care and Use Committee of the Institute of Medical Science, University of Tokyo. Mice were infected with HSV-1 strains under deep anesthesia. Corneas of mice were lightly scarified with a 27-gauge needle, the tear film was blotted, and a 3-μl drop containing 1 × 106 or 1 × 105 PFU of the indicated virus was applied to the eye as described previously (9, 33). To determine viral titers in tear films, tear film samples were collected from both eyes using a single cotton-tipped applicator. The cotton tip was transferred to 1 ml medium 199 supplemented with 1% fetal calf serum and frozen at −80°C. Frozen samples were later thawed and thoroughly mixed, and infectious virus was quantitated by standard plaque assays on Vero cells. Mice were monitored daily until 16 days postinfection, and the clinical severity of herpes stromal keratitis (HSK) or periocular skin disease was scored as described previously (9, 33). The HSK scoring system was as follows: 0, normal cornea; 1, mild corneal haze; 2, moderate corneal opacity or scarring; 3, severe corneal opacity, iris not visible; 4, opaque cornea; and 5, necrotizing stromal keratitis. The periocular skin disease scoring system was as follows: 0, no lesions; 1, minimal eyelid swelling; 2, moderate eyelid swelling accompanied by crusty ocular discharge; 3, severe eyelid swelling and moderate hair loss in periocular skin; and 4, severe swelling with eyes crusted shut, severe periocular hair loss, and skin lesions.
Induction of apoptosis and measurement of caspase 3/7 activity.
Induction of apoptosis in HSV-1-infected cells was performed as described previously, except that Vero cells were used instead of SK-N-SH cells (15). Caspase 3/7 activity was assayed using a Caspase-Glo 3/7 assay kit (Promega) as described previously (15).
Electron microscopic analysis.
Vero cells infected with HSV-1(F), R7041, or YK515 at an MOI of 5 for 20 h were fixed with 1% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4) for 1 h on ice. The cells were then harvested and fixed for 1 h with the same fixative. Small pieces of fixed pellets were washed with the same buffer containing 3% sucrose and postfixed with 1% osmium tetroxide in the same buffer for 1 h on ice. The samples were then dehydrated with an ethanol gradient series followed by propylene oxide, embedded in Epon 812 resin mixture, and polymerized at 70°C for 2 days. Thin sections were stained with uranyl acetate and lead citrate and examined with a Hitachi H7500 electron microscope.
RESULTS
Production and characterization of monoclonal antibodies to Us3-S147P.
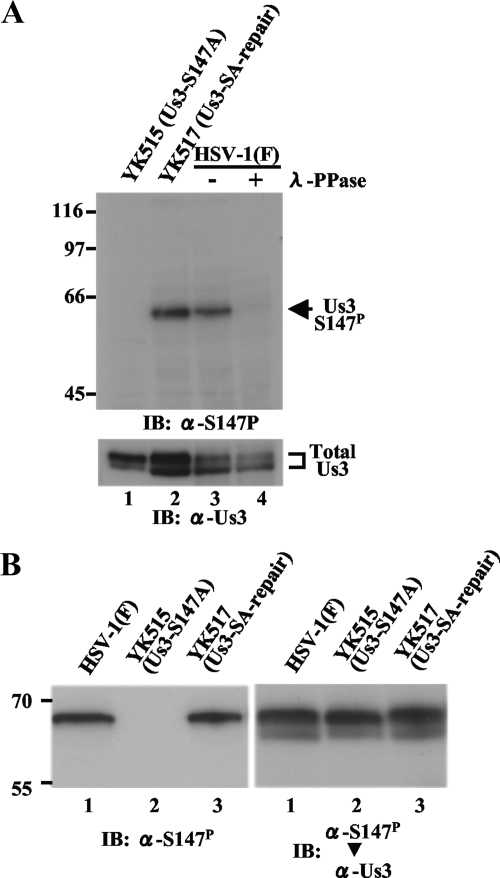
For studies of phosphorylation of Us3 Ser-147 in infected cells, monoclonal antibodies were raised against a synthetic phosphopeptide corresponding to Us3 residues 141 to 153 in which Ser-147 was phosphorylated. Phosphorylated Ser-147 was designated S147P in this study. Three hybridoma clones (1.65.4, 3.21.1, and 1.3.1) that secreted antibodies that reacted with the phosphopeptide but not with unphosphorylated peptide in an enzyme-linked immunosorbent assay were isolated. Clone 1.65.4 was characterized further. As shown in Fig. 1A, in immunoblots of infected Vero cell lysates, the monoclonal antibody (anti-Us3-S147P antibody) reacted with wild-type HSV-1(F) Us3 but not with YK515 Us3, which carries a S147A mutation. Immunoblots of lysates of Vero cells infected with YK517, in which the Us3-S147A mutation in YK515 was repaired, showed restoration of the reactivity of the antibody with Us3. These results indicated that the antibody recognized a Us3 epitope containing Ser-147. Furthermore, reactivity of the monoclonal antibody to wild-type Us3 was dependent on phosphorylation of Us3 Ser-147, based on the observation that phosphatase treatment of lysates of Vero cells infected with HSV-1(F) abolished the anti-Us3-S147P reactivity. Taken together, these results indicated that the anti-Us3-S147P antibody was able to specifically detect Us3-S147P in infected cells.
FIG. 1.
(A) Immunoblots of electrophoretically separated lysates from Vero cells infected with YK515 (Us3-S147A) (lane 1), YK517 (Us3-SA-repair) (lane 2), or wild-type HSV-1(F) (lanes 3 and 4). Infected cells were harvested at 18 h postinfection, solubilized, mock treated (lanes 1, 2, and 3) or treated with λ-PPase (lane 4), and immunoblotted with anti-Us3-S147P monoclonal antibody (upper panel) or anti-Us3 polyclonal antibody (lower panel). +, present; −, absent. (B) Immunoblots of electrophoretically separated lysates from Vero cells infected with wild-type HSV-1(F), YK515 (Us3-S147A), or YK517 (Us3-SA-repair). Infected cells were harvested at 18 h postinfection and immunoblotted with anti-Us3-S147P monoclonal antibody (left panel). The same membrane was reprobed with anti-Us3 polyclonal antibody (right panel). Molecular sizes are shown on the left of the panels. α, anti; IB, immunoblotting. Total Us3, Us3 protein detected by anti-Us3 polyclonal antibody.
As described previously (27), Us3 protein from HSV-1(F)-infected cell lysates was detected in a denaturing gel as a doublet with anti-Us3 polyclonal antibody (Fig. 1A, lower panel). In contrast, the anti-Us3-S147P antibody reacted with only a single isoform of Us3 (Fig. 1A, upper panel). To determine which Us3 isoforms the anti-Us3-S147P antibody recognized, lysates of Vero cells infected with HSV-1(F), YK515 (Us3-S147A), or YK517 (Us3-SA-repair) were subjected to immunoblotting with the anti-Us3-S147P antibody (Fig. 1B, left panel) and then the immunoblot was reprobed with anti-Us3 polyclonal antibody (Fig. 1B, right panel). As shown in Fig. 1B, the anti-Us3 Us3-S147P antibody recognized the more-slowly migrating isoform of Us3. We note that the more-slowly migrating band seen in the immunoblot from HSV-1(F)-infected cells was detected even after phosphatase treatment (Fig. 1A, lower panel), suggesting that not all Us3 in the band represented the phosphorylated species at Ser-147 and that other and unknown modifications and/or isoforms were included in the band. Furthermore, we observed that the electrophoretic pattern of Us3 isoforms from YK515 (Us3-S147A)-infected cell lysates was different from that of Us3 isoforms from HSV-1(F)- or YK517 (Us3-SA-repair)-infected cell lysates (Fig. 1A, lower panel). In YK515 (Us3-S147A)-infected cells, the lower band was shifted up slightly and the upper band became the dominant species compared to those in HSV-1(F)- or YK517 (Us3-SA-repair)-infected cells. Similar results were also obtained in the experiments whose results are shown in Fig. 2E and Fig. 3A. These observations suggested that posttranslational modification of the Us3-S147A mutant was different from that of wild-type Us3 and further support our previous conclusion (13) that Ser-147 is a physiological phosphorylation site in Us3.
FIG. 2.
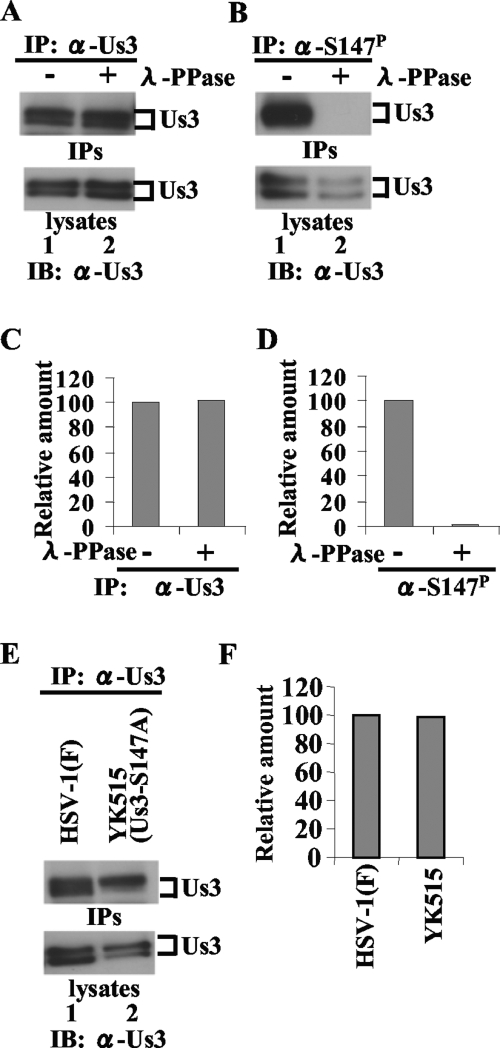
(A and B) Immunoblots of electrophoretically separated Us3 immunoprecipitates (upper panel) and lysates (lower panel) from Vero cells infected with HSV-1(F). Infected cells were harvested at 18 h postinfection, solubilized, and either mock treated (lanes 1) or treated with λ-PPase (lanes 2). Part of the treated cell lysates was analyzed by immunoblotting with anti-Us3 antibody (lower panels). The remainder of each lysate was immunoprecipitated with anti-Us3 (A) or anti-Us3-S147P antibody (B). The immunoprecipitates were separated in a denaturing gel and analyzed by immunoblotting with anti-Us3 antibody (upper panel). (C and D) Amount of Us3 protein immunoprecipitated with anti-Us3 (B, upper panel) and anti-Us3-S147P antibody (C, upper panel) relative to the amount of Us3 protein in the corresponding HSV-1(F)-infected cell lysates (A and B, lower panels). The data were normalized to the value for HSV-1(F)-infected cells without phosphatase treatment in panels A and B, lanes 1. (E) Immunoblots of electrophoretically separated Us3 immunoprecipitates (upper panel) and lysates (lower panel) from Vero cells infected with HSV-1(F) (lane 1) or YK515 (Us3-S147A) (lane 2). Infected Vero cells were harvested at 18 h postinfection and solubilized. Part of the cell lysates was immunoblotted with anti-Us3 polyclonal antibody (lower panel). The remainder of each lysate was immunoprecipitated with anti-Us3. The immunoprecipitates were separated in a denaturing gel and analyzed by immunoblotting with anti-Us3 antibody (upper panel). (F) Amount of Us3 protein immunoprecipitated with anti-Us3 antibody (E, upper panel) relative to the amount of Us3 protein in the corresponding HSV-1(F)- or YK515 (Us3-S147A)-infected cell lysates (E, lower panel). These data were normalized relative to the value for HSV-1(F)-infected cells in panel E, lane 1. IP, immunoprecipitation; IPs, immunoprecipitates; IB, immunoblotting; α, anti; +, present; −, absent.
FIG. 3.
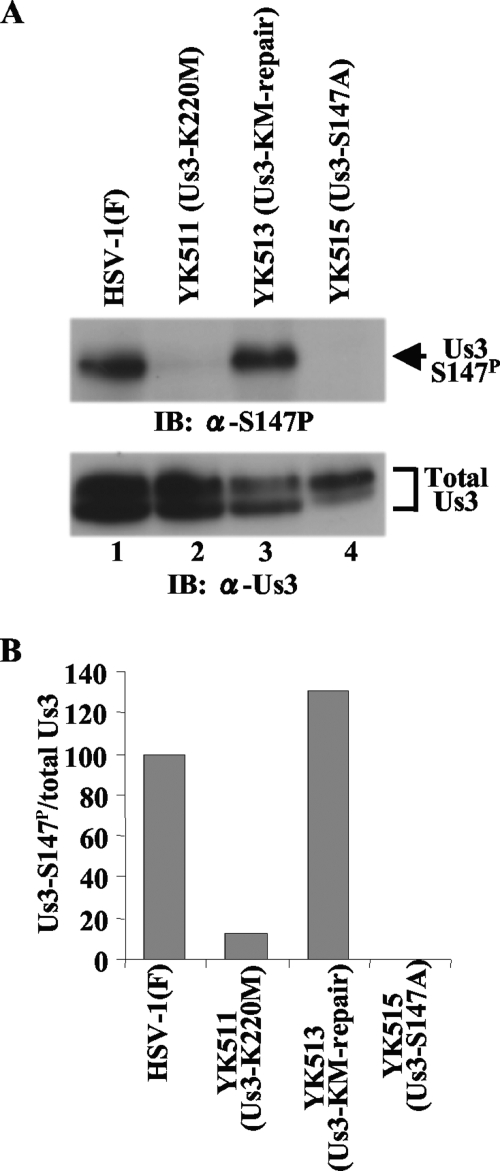
(A) Immunoblots of electrophoretically separated lysates from Vero cells infected with wild-type HSV-1(F) (lane 1), YK511 (Us3-K220M) (lane 2), YK513 (Us3-KM-repair) (lane 3), or YK515 (Us3-S147A) (lane 4). Infected Vero cells were harvested at 18 h postinfection and analyzed by immunoblotting with anti-Us3-S147P antibody (upper panel) or anti-Us3 antibody (lower panel). (B) Amount of Us3-S147P protein detected with anti-Us3-S147P antibody (A, upper panel) relative to the amount of Us3 protein detected with anti-Us3 antibody (A, lower panel) in HSV-1(F)-infected cells. The data were normalized to the value for HSV-1(F)-infected cells in panel A, lane 1. α, anti; Total Us3, Us3 protein detected by anti-Us3 polyclonal antibody.
In addition, the anti-Us3-S147P antibody efficiently precipitated Us3 proteins from lysates of Vero cells infected with HSV-1(F) but not from lysates of Vero cells infected with HSV-1(F) treated with phosphatase (Fig. 2B and D) or lysates of Vero cells infected with YK515 (Us3-S147A) (data not shown), which confirmed that the anti-Us3-S147P antibody efficiently and specifically precipitated Us3-S147P from infected cell lysates. Furthermore, the efficiency of Us3 immunoprecipitation with anti-Us3 polyclonal antibody from infected Vero cell lysates was not affected by phosphatase treatment of the lysates (Fig. 2A and C) or by an S147A mutation in Us3 (Fig. 2E and F), indicating that the anti-Us3 polyclonal antibody precipitated both phosphorylated and unphosphorylated Us3 proteins from infected cell lysates with similar efficiencies.
Us3 autophosphorylation of Us3 Ser-147.
We previously reported that Us3 Ser-147 was phosphorylated by Us3 and PKA in vitro (13). However, it is known that some protein kinases are somewhat promiscuous in vitro if allowed sufficient reaction time and provided with enough substrate. Therefore, to identify the protein kinase(s) that mediates phosphorylation of Us3 Ser-147 in infected cells, Vero cells were infected with wild-type HSV-1(F); YK511, encoding the enzymatically inactive Us3 mutant Us3-K220M; YK513, in which the Us3 K220M mutation in YK511 had been repaired; and YK515 (Us3-S147A). At 18 h postinfection, infected cell lysates were analyzed by immunoblotting with anti-Us3-S147P monoclonal antibody. As shown in Fig. 3, phosphorylation of Us3 Ser-147 decreased significantly in YK511 (Us3-K220M)-infected cells compared to the level in wild-type-virus-infected cells, with no detectable phosphorylation of Us3 from YK515 (Us3-S147A)-infected cells. The wild-type phenotype was restored in cells infected with the repaired virus YK513 (Us3-KM-repair). These results indicated that Us3 protein kinase activity mediated phosphorylation of Us3 Ser-147 in infected cells. However, we noted that Us3 Ser-147 was slightly phosphorylated in the absence of Us3 protein kinase activity in YK511-infected cells (Fig. 3B), indicating that a protein kinase(s) other than Us3, probably a cellular protein kinase(s), also mediated Us3 Ser-147 phosphorylation in infected cells.
Accumulation of Us3 phosphorylated at Ser-147 in infected cells.
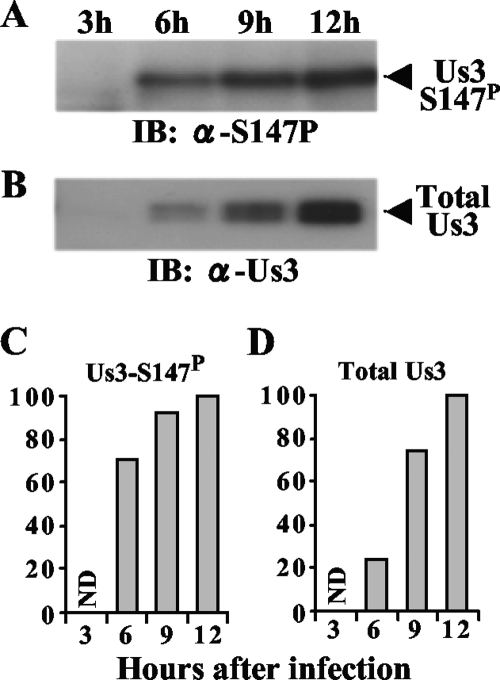
To monitor accumulation of Us3-S147P in infected cells, Vero cells were infected with wild-type HSV-1(F); harvested at 3, 6, 9, and 12 h postinfection; and analyzed by immunoblotting with anti-Us3-S147P and anti-Us3 antibody. As shown in Fig. 4, both Us3-S147P and total Us3 proteins were not detectable at 3 h postinfection and the levels of both proteins increased from 6 to 12 h postinfection. The amount of both Us3-S147P and total Us3 peaked at 12 h postinfection and remained relatively constant thereafter (data not shown). However, there was a significant difference in the rate of accumulation of Us3-S147P and total Us3 proteins, with Us3-S147P increasing more rapidly than total Us3. At 6 h postinfection, the amount of Us3-S147P was 71% of that at its peak at 12 h postinfection, while the amount of total Us3 was only 23% of that at its peak at 12 h postinfection. These observations indicated that more of the Us3 Ser-147 present early in infection (i.e., by 6 h postinfection) than of the Us3 Ser-147 present at later infection times was phosphorylated.
FIG. 4.
(A and B) Immunoblots of electrophoretically separated lysates from Vero cells infected with wild-type HSV-1(F). Infected Vero cells were harvested at the indicated times postinfection and analyzed by immunoblotting with anti-Us3-S147P antibody (A) or anti-Us3 antibody (B). (C and D) Amount of Us3-S147P protein shown in panel A (C) and total Us3 protein shown in panel B (D) as a function of time postinfection, relative to the amounts at 12 h postinfection. The data were normalized to the values at 12 h postinfection. α, anti; ND, not detected; Total Us3, Us3 protein detected by anti-Us3 polyclonal antibody.
Effect of the Us3-S147A mutation on Us3 accumulation in infected cells.
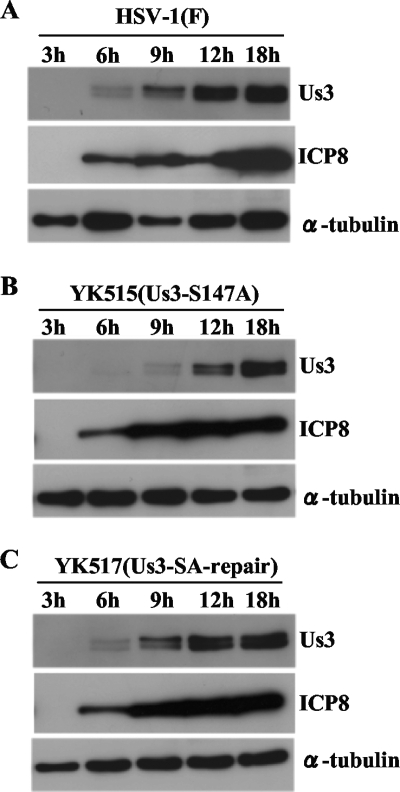
We previously demonstrated that phosphorylation of Us3 Ser-147 is not required for proper accumulation of Us3 proteins in HSV-1-infected Vero cells at 18 h postinfection (13). However, the results described above indicated that most phosphorylation of Us3 Ser-147 occurred earlier, by 6 h postinfection, and led us to investigate the accumulation of Us3 proteins at times earlier than 18 h postinfection. As shown in Fig. 5A and B, the accumulation of the mutant Us3-S147A protein in YK515 (Us3-S147A)-infected Vero cells was delayed by several hours compared to the accumulation of wild-type Us3 in HSV-1(F)-infected cells. The wild-type phenotype was restored in cells infected with the repaired virus YK517 (Us3-SA-repair) (Fig. 5C). In contrast, delay in the accumulation of another viral protein, ICP8, in YK515 (Us3-S147A)-infected Vero cells was not observed. Consistent with our earlier report (13), the level of mutant Us3-S147A protein in YK515-infected cells at 18 h postinfection was similar to that of wild-type Us3 protein in HSV-1(F)-infected cells. These results indicated that the Us3 Ser-147 phosphorylation site was required for proper accumulation of Us3 in infected cells at an early phase of HSV-1 infection.
FIG. 5.
Immunoblots of electrophoretically separated lysates from Vero cells infected with wild-type HSV-1(F) (A), YK515 (Us3-S147A) (B), and YK517 (Us3-SA-repair) (C). Infected Vero cells were harvested at the indicated times postinfection and analyzed by immunoblotting with anti-Us3, anti-ICP8, and anti-α-tubulin antibodies.
Intracellular localization of Us3-S147P in infected cells.
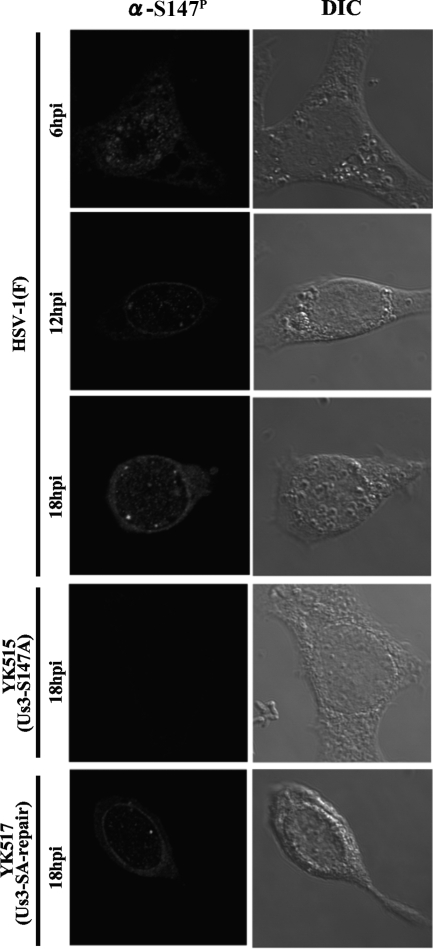
To investigate the localization of Us3-S147P in infected cells, Vero cells were infected with HSV-1(F), YK515 (Us3-S147A), or YK517 (Us3-SA-repair) and analyzed by immunofluorescence with confocal microscopy. In wild-type HSV-1(F)-infected Vero cells, Us3-S147P accumulated in both the nucleus and cytoplasm throughout the infection (Fig. 6). We note that nuclear rim staining of Us3-S147P proteins was especially evident at 12 or 18 h after infection. No specific staining of Us3-S147P proteins was detected in YK515 (Us3-S147A)-infected cells throughout the infection, and wild-type staining of Us3-S147P was restored in YK517 (Us3-SA-repair)-infected cells (Fig. 6 and data not shown). As reported previously (13, 40), polyclonal antibody to Us3 had a consistent background fluorescence in cells infected with HSV-1 Us3 null mutants, making it difficult to draw conclusions about the exact localization of Us3. Therefore, we could only compare the results for immunofluorescence with the anti-Us3-S147P antibody to previous reports (11, 13, 42) where total Us3 localization was analyzed. The comparison suggested that there was no apparent difference in staining patterns between Us3-S147P and total Us3 proteins.
FIG. 6.
Digital confocal images showing the localization of Us3-S147P. Vero cells were infected with wild-type HSV-1(F), YK515 (Us3-S147A), or YK517 (Us3-SA-repair). Infected Vero cells were fixed at the indicated times postinfection, permeabilized, stained with anti-Us3-S147P antibody, and examined by confocal microscopy. hpi, hours postinfection; α, anti; DIC, digital interference contrast.
Effect of phosphorylation of Us3 Ser-147 on Us3 catalytic activity in infected cells.
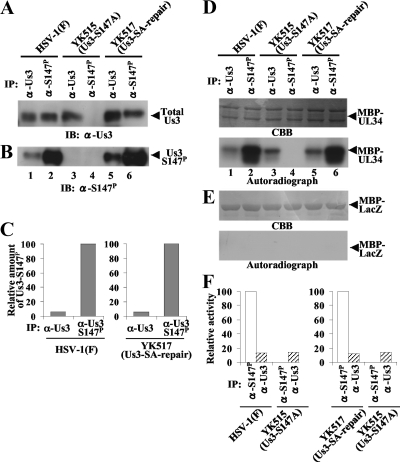
To determine whether phosphorylation of Us3 Ser-147 regulates Us3 catalytic activity in infected cells, Vero cells were infected with wild-type HSV-1(F), YK515 (Us3-S147A), or YK517 (Us3-SA-repair) at an MOI of 5, harvested at 18 h postinfection, solubilized, and immunoprecipitated with anti-Us3-S147P monoclonal or anti-Us3 polyclonal antibody. As described above (Fig. 2), in infected cell lysates, anti-Us3-S147P immunoprecipitated Us3-S147P and anti-Us3 immunoprecipitated phosphorylated Us3, unphosphorylated Us3, and Us3-S147A equivalently. To obtain approximately equal amounts of immunoprecipitated Us3 proteins for each of the antibodies in these experiments, twofold dilutions of cell lysates were immunoprecipitated with anti-Us3 antibody and compared to the immunoprecipitates with anti-Us3-S147P antibody. The immunoprecipitates were then divided into four aliquots. Two of the immunoprecipitate aliquots were separated in a denaturing gel and analyzed by immunoblotting with anti-Us3 (Fig. 7A) or anti-Us3-S147P (Fig. 7B) antibody. When approximately equal amounts of total Us3 proteins from HSV-1(F)- and YK517 (Us3-SA-repair)-infected cells were immunoprecipitated with anti-Us3-S147P or anti-Us3 antibody (Fig. 7A), the anti-Us3-S147P immunoprecipitates, with only Us3-S147P, contained much more Us3-S147P than the anti-Us3 immunoprecipitates, with both phosphorylated and unphosphorylated Us3 at Ser-147 (Fig. 7B). Quantitation of these results showed that only about 6% of total Us3 protein was phosphorylated at Ser-147 in cells infected with either wild-type HSV-1(F) or YK517 (Us3-SA-repair) (Fig. 7C).
FIG. 7.
(A and B) Immunoblots of electrophoretically separated Us3 immunoprecipitates from Vero cells infected with wild-type HSV-1(F) (lanes 1 and 2), YK515 (Us3-S147A) (lanes 3 and 4), and YK517 (Us3-SA-repair) (lanes 5 and 6). Infected Vero cells were harvested at 18 h postinfection, solubilized, and immunoprecipitated with anti-Us3 (lanes 1, 3, and 5) or anti-Us3-S147P antibody (lanes 2, 4, and 6). The immunoprecipitates were then divided into four aliquots. Two immunoprecipitate aliquots were separated in a denaturing gel and analyzed by immunoblotting with anti-Us3 (A) and anti-Us3-S147P (B) antibodies. (C) Amount of Us3-S147P protein shown in panel B relative to the amount of total Us3 protein shown in panel A. In the left graph, the data were normalized to the relative amount for immunoprecipitates with anti-Us3-S147P antibody for cells infected with HSV-1(F) shown in panels A and B, lanes 2. In the right graph, the data were normalized to the relative amount for immunoprecipitates with anti-Us3-S147P antibody for cells infected with YK517 (Us3-SA-repair) shown in panels A and B, lanes 6. (D and E) For in vitro kinase assays of the other two immunoprecipitate aliquots, the immunoprecipitates described for panels A and B were incubated in kinase buffer containing [γ-32P]ATP and MBP-UL34 (D) or MBP-LacZ (E) and separated in denaturing gels. The gels were stained with Coomassie brilliant blue (CBB) (upper panels) and analyzed by autoradiography (lower panels). (F) Amount of radioactivity in 32P-labeled MBP-UL34 produced in a kinase reaction whose results are shown in panel D, lower panel, relative to the amount of total Us3 protein shown in panel A. In the left graph, the data were normalized to the relative value for the kinase activity of anti-Us3-S147P immunoprecipitates from cells infected with HSV-1(F) shown in panels D, lower panel, and A, lanes 2. In the right graph, the data were normalized to the relative value for the kinase activity of anti-Us3-S147P immunoprecipitates from cells infected with YK517 (Us3-SA-repair) shown in panels D, lower panel, and A, lanes 6. α, anti; IP, immunoprecipitation; IB, immunoblotting; Total Us3, Us3 protein detected by anti-Us3 polyclonal antibody.
The other two immunoprecipitate aliquots described above were used for in vitro kinase assays with maltose binding protein (MBP)-UL34 and MBP-LacZ. In agreement with our previous report (13), MBP-UL34 was phosphorylated in kinase assays using anti-Us3 immunoprecipitates from HSV-1(F)-infected cells at a level similar to that in kinase assays using anti-Us3 immunoprecipitates from YK515 (Us3-S147A)-infected cells (Fig. 7D, lanes 1 and 3). In contrast, MBP-UL34 was efficiently phosphorylated in kinase assays using anti-Us3-S147P immunoprecipitates from cells infected with HSV-1(F) and YK517 (Us3-SA-repair) (Fig. 7D, lanes 2 and 6), but little phosphorylation was detected in kinase assays using anti-Us3 immunoprecipitates from YK515 (Us3-S147A)-infected cells (Fig. 7D, lane 3). The relative kinase activity of anti-Us3-S147P immunoprecipitates from cells infected with HSV-1(F) or YK517 (Us3-SA-repair) (Us3-S147P) was about sevenfold higher than that of anti-Us3 immunoprecipitates from YK515 (Us3-S147A)-infected cells (Us3-S147A) (Fig. 7F). Similarly, MBP-UL34 was phosphorylated in kinase assays using anti-Us3-S147P immunoprecipitates from cells infected with HSV-1(F) and YK517 (Us3-SA-repair) (Us3-S147P) much more efficiently than in kinase assays using anti-Us3 immunoprecipitates from cells infected with HSV-1(F) and YK517 (Us3-SA-repair), in which most Us3 proteins were unphosphorylated at Ser-147 (Fig. 7D, lanes 1, 2, 5, and 6). The relative kinase activity of anti-Us3 immunoprecipitates from cells infected with HSV-1(F) or YK517 (Us3-SA-repair) was about eight- or ninefold lower than that of anti-Us3-S147P immunoprecipitates from cells infected with HSV-1(F) or YK517 (Us3-SA-repair) (Us3-S147P), while the activity of the anti-Us3 immunoprecipitates was similar to that of anti-Us3 immunoprecipitates from YK515 (Us3-S147A)-infected cells (Us3-S147A) (Fig. 7F). None of the immunoprecipitates phosphorylated MBP-LacZ (Fig. 7E). These results indicated that Us3-S147P from infected cells had a much higher protein kinase activity than Us3 not phosphorylated at Ser-147.
Effect of the Us3-S147A mutation on viral replication and pathogenesis in mice.
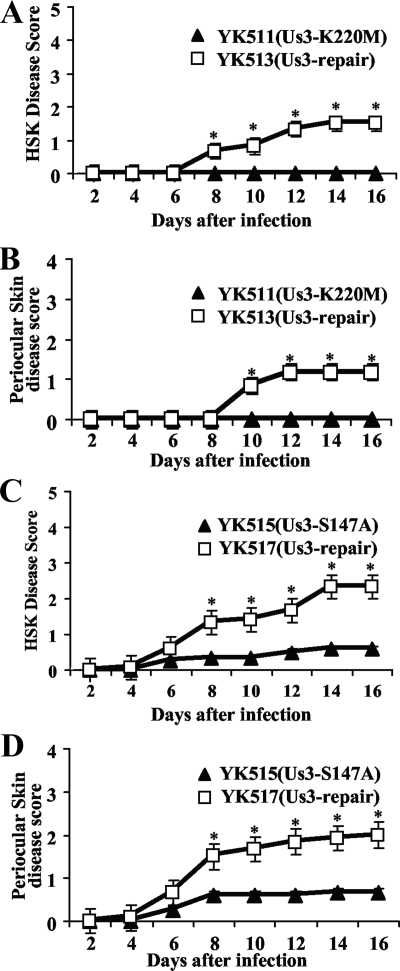
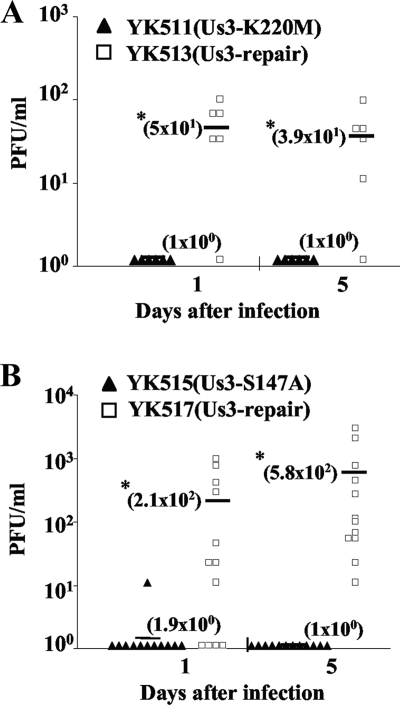
Us3 has been reported to play a critical role(s) in HSV-1 pathogenesis in experimental animals (25). To determine the effect(s) of the Us3-S147A mutation on viral replication and pathogenesis in vivo, we employed the murine model of HSK. Mice were inoculated ocularly with 1 × 106 PFU/eye of YK511 (Us3-K220M), YK513 (Us3-KM-repair), YK515 (Us3-S147A), or YK517 (Us3-SA-repair) and observed daily for 16 days postinfection for HSK development and periocular skin disease. In addition, to examine viral replication at the infection site, tear film samples were collected at the times indicated in Fig. 8 and viral titers were determined. As shown in Fig. 8, mice infected with YK511 (Us3-K220M) and YK515 (Us3-S147A) exhibited a reduced severity of HSK and periocular skin disease compared to mice infected with the YK513 (Us3-KM-repair) and YK517 (Us3-SA-repair) repaired viruses. Similar results were also obtained when mice were infected ocularly with 1 × 105 PFU of each of the viruses (data not shown). In addition, YK511 (Us3-K220M) and YK515 (Us3-S147A) replicated significantly less efficiently in the tear films of these infected mice, with titers approximately 10- to 100-fold lower than those of the YK513 and YK517 repaired viruses, respectively (Fig. 9). These results indicated that both the Us3-Ser147 phosphorylation site and Us3 protein kinase activity were required for efficient viral replication and development of HSK and periocular skin disease in mice. We note that titers in the tear films and disease scores in mice infected with YK513 (Us3-KM-repair) were lower than those in mice infected with YK517 (Us3-SA-repair). The difference might be due to introduction of a secondary mutation(s) other than the K220M mutation in Us3 into the viral genome of YK511 (Us3-K220M).
FIG. 8.
Effect of the Us3-S147A and Us3-K220M mutations on HSK and periocular skin disease in mice. (A and B) Six 5-week-old female ICR mice were infected with 1 × 106 PFU YK511 (Us3-K220M) or YK513 (Us3-KM-repair) by corneal scarification and scored for HSK and periocular skin disease every other day for 16 days. The data shown are the averages and standard errors of the observations. A statistically significant difference between HSK and periocular skin disease scores in mice infected with YK511 (Us3-K220M) and those infected with YK513 (Us3-KM-repair) was noted (*, P < 0.01). (C and D) Two independent experiments were done, each using six 5-week-old female ICR mice infected with 1 × 106 PFU YK515 (Us3-S147A) or YK517 (Us3-SA-repair) by corneal scarification and scored for HSK and periocular skin disease every other day for 16 days. The results from two independent experiments (each with six mice) were combined. The data shown are the averages and standard errors of the observations. A statistically significant difference between HSK and periocular skin disease scores in mice infected with YK515 (Us3-S147A) and those infected with YK517 (Us3-SA-repair) was noted (*, P < 0.01).
FIG. 9.
Effects of the Us3-S147A and Us3-K220M mutations on virus growth in the tear film of mice following corneal infection. In the experiment whose results are shown in Fig. 6, viral titers in the tear film of infected mice at 1 and 5 days postinfection were determined by standard plaque assays. Each data point represents the titer in the tear film of one mouse. The horizontal bars and figures in parentheses show the average for each group. A statistically significant difference in viral titers between mice infected with YK511 (Us3-K220M) and YK513 (Us3-KM-repair) (A) and between mice infected with YK515 (Us3-S147A) and YK517 (Us3-SA-repair) (B) was noted (*, P < 0.05).
Effects of the Us3-S147A mutation on Us3 functions in infected cells.
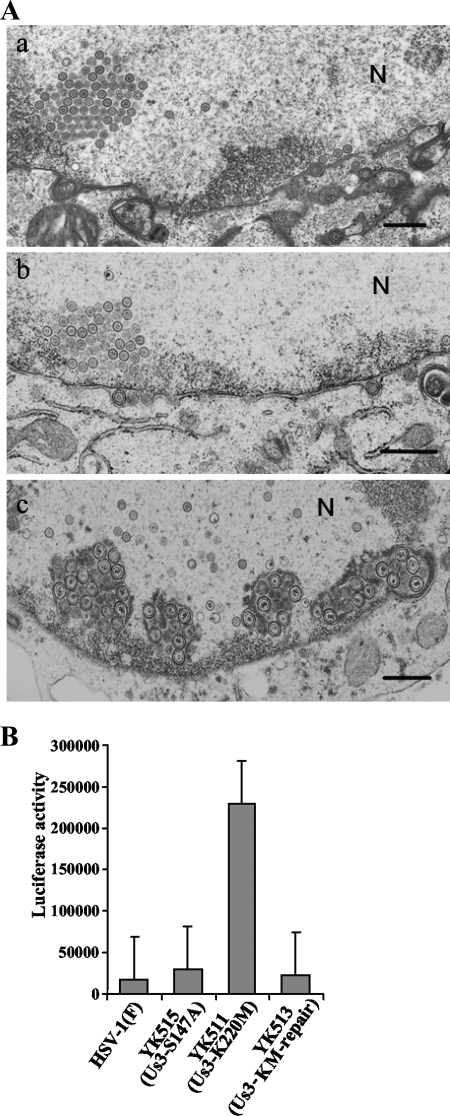
Two series of experiments were performed to investigate whether phosphorylation of Us3 Ser-147 affected regulation of Us3 functions other than those previously reported (13), including nuclear egress of nucleocapsids and prevention of apoptosis. In the first series of experiments, Vero cells were infected with HSV-1(F), R7041 (ΔUs3), or YK515 (Us3-S147A) at an MOI of 5 for 20 h, fixed, and processed for electron microscopy. It has been reported that, in the absence of Us3 protein or Us3 catalytic activity, enveloped virions accumulated within membranous structures which appeared to be invaginations of the inner nuclear membrane into the nucleus, probably leading to a delay in transit from the nucleus to the cytoplasm (40, 42). In agreement with that report, membranous invaginations retaining several enveloped virions were frequently observed in the nucleus of Vero cells infected with the Us3 null mutant virus R7041 (Fig. 10A-c). However, membranous invaginations were observed much more rarely in Vero cells infected with wild-type HSV-1(F) or YK515 (Us3-S147A) (Fig. 10A-a and A-b), indicating that the Us3-S147A mutation had no effect on nuclear membrane morphology in infected cells. Thus, it is likely that phosphorylation of Us3 Ser-147 was not necessary for efficient nuclear egress of nucleocapsids in infected cells.
FIG. 10.
(A) Electron microscopy of Vero cells infected with wild-type HSV-1(F) (a), YK515 (Us3-S147A) (b), or R7041 (ΔUs3) (c) for 20 h. Scale bars, 500 nm; N, nucleus. (B) Caspase 3/7 activity of infected Vero cells after induction of apoptosis by osmotic shock. Vero cells were infected with wild-type HSV-1(F), YK511 (Us3-K220M), YK513 (Us3-KM-repair), or YK515 (Us3-S147A). At 12 h postinfection, the cells were exposed to sorbitol for 1 h, incubated for an additional 5 h, harvested, and assayed for caspase 3/7 activity using a Z-DEVD-aminoluciferin substrate (15). The values are the averages and standard deviations of the results of three independent experiments.
In the second series of experiments, Vero cells were infected with HSV-1(F), YK511 (Us3-K220M), YK513 (Us3-KM-repair), or YK515 (Us3-S147A) and, at 12 h postinfection, apoptosis was induced by osmotic shock for 1 h. The cells were incubated for an additional 5 h and then harvested and assayed for caspase 3/7 activity. As shown in Fig. 8B, caspase 3/7 activity in YK511 (Us3-K220M)-infected cells was significantly higher than that in HSV-1(F)-infected cells, probably due to the lack of Us3 antiapoptotic activity. Similar results were previously obtained with the Us3 null mutant virus R7041 (15). Wild-type caspase 3/7 activity was restored in cells infected with YK513 (Us3-KM-repair) (Fig. 10B). In YK515 (Us3-S147A)-infected cells, caspase 3/7 activity was similar to that in HSV-1(F) infected cells. The differences in caspase 3/7 activity among HSV-1(F)-, YK511-, YK513-, and YK515-infected cells were not detected without osmotic shock (data not shown). These results indicated that the Us3-S147A mutation did not affect caspase 3/7 activity in infected Vero cells that had received an osmotic shock. Since upregulation of caspase 3/7 activity is a typical indicator of apoptosis, these observations suggested that phosphorylation of Us3 Ser-147 was not required for the antiapoptotic activity of Us3 in infected cells.
DISCUSSION
Herpesviruses commonly encode at least one protein kinase (31). Protein phosphorylation by protein kinases is one of the most common and important strategies used by cells and viruses to regulate protein activity (5, 16, 23). Thus, viral protein kinases have been reported to play multiple roles in viral replication by regulation of viral gene expression (37), encapsidation (45), nuclear egress of nucleocapsids (10, 18, 40, 42), replication of viral genomes (45), apoptosis (22, 32), intracellular trafficking of viral and cellular membrane proteins (7, 12), and axonal transport of capsids (3). While the downstream effects of herpesvirus protein kinases have been extensively studied, there is a lack of information on how herpesvirus protein kinase activity is regulated in infected cells. The key finding reported here is that the catalytic activity of HSV-1 protein kinase Us3 was regulated by its autophosphorylation of Us3 Ser-147 in infected cells. To our knowledge, these are the first direct data on the mechanism of regulation of herpesvirus protein kinase activity in infected cells.
For these studies, we developed a monoclonal antibody that specifically recognized Us3-S147P in infected cells, to study phosphorylation of Us3 Ser-147 and its function in infected cells. This monoclonal antibody enabled us to purify Us3-S147P by immunoprecipitation from infected cells, while anti-Us3 polyclonal antibody, which we generated previously (13), allowed purification of phosphorylated Us3, unphosphorylated Us3, and a Us3-S147A mutant by immunoprecipitation from infected cell lysates. The specificity of these antibodies enabled us to compare the catalytic activity of Us3-S147P from infected cells with that of Us3 not phosphorylated at Ser-147. In the present study, we showed that Us3-S147P purified from infected cells had much higher kinase activity than Us3 not phosphorylated at Ser-147. The protein kinase activity of Us3-S147P from infected cells was about sevenfold higher than that of Us3 not phosphorylated at Ser-147. These observations indicated that phosphorylation of Us3 Ser-147 upregulated the catalytic activity of Us3 in HSV-1-infected cells. We need to note that the possibility that the phosphorylation of Us3 at Ser-147 alters substrate specificity is less likely, but we cannot eliminate the possibility completely.
The anti-Us3-S147P antibody also enabled us to examine the relative amounts of total Us3 and Us3-S147P in wild-type HSV-1(F)-infected cells. These experiments showed that only about 6% of total Us3 protein purified by immunoprecipitation from wild-type HSV-1(F)-infected cells with anti-Us3 polyclonal antibody was phosphorylated at Ser-147. These results indicated that only a small fraction of Us3 is phosphorylated at Ser-147 in infected cells. Furthermore, the data showed that, although Us3 accumulated continuously throughout infection in HSV-1(F)-infected cells, most Us3 Ser-147 phosphorylation (71% of the total Us3-S147P formed) had occurred by 6 h postinfection, a time when only 23% of total Us3 had accumulated. Consistent with these observations, Us3-S147A accumulation in YK515 (Us3-S147A)-infected cells during early infection (until 12 h postinfection) was delayed by several hours postinfection compared to Us3 accumulation in HSV-1(F)-infected cells. Taken together, these results support the hypothesis that phosphorylation of Us3 at Ser-147 is tightly regulated in infected cells. At present, the mechanism for regulation of phosphorylation of Us3 Ser-147 in HSV-1-infected cells remains unknown. We previously showed that, when Us3 was artificially overexpressed in a baculovirus expression system, the S147A mutation in Us3 had a dramatic inhibitory effect on Us3 protein kinase activity in insect cells (13). These observations suggested that the majority of Us3 protein expressed in insect cells was efficiently phosphorylated at Ser-147, unlike the Us3 expression in HSV-1-infected Vero cells observed in the present study. There is a possibility that a Us3 cofactor(s), perhaps an HSV-1 factor(s) and/or a cellular factor(s) specifically expressed in mammalian cells, might tightly regulate Us3 autophosphorylation of Ser-147 in HSV-1-infected cells, as reported for a subset of cellular protein kinases, such as calcium/calmodulin-dependent kinase II (2, 46).
In general, identification of a protein kinase responsible for phosphorylation of a substrate requires demonstration that the protein kinase specifically and directly phosphorylates the substrate in vitro and that phosphorylation of the substrate is altered in cells in which the protein kinase activity is downregulated. We previously reported that Us3 specifically and directly phosphorylated Us3 Ser-147 in vitro (13). In the present study, we have shown that phosphorylation of Us3 Ser-147 was significantly decreased in cells infected with YK511, encoding a catalytically inactive mutant of Us3 (Us3-K220M). We conclude from all these results that Us3 is the protein kinase responsible for phosphorylation of Us3 Ser-147 in infected cells and, therefore, that it is highly likely that Us3 autophosphorylates its Ser-147 in HSV-1-infected cells. However, we cannot completely eliminate the possibility that a protein kinase(s) other than Us3 is regulated by Us3 and is involved in phosphorylation of Us3 Ser-147. In agreement with this possibility, we previously reported that PKA, whose activity has been suggested to be regulated by Us3 (1), phosphorylated Us3 Ser-147 in vitro (13).
We previously reported that the S147A mutation in Us3 had no effect on viral growth in cell cultures (13). Although the mutation affected some Us3 functions, including regulation of infected cell morphology and Us3 accumulation and localization in infected cells, the biological significance of Us3 Ser-147 phosphorylation in viral replication and pathogenesis remained unclear. In the present study, we have shown that the S147A mutation in Us3 significantly reduced viral replication in the cornea and pathogenic sequelae of HSK and periocular skin disease in mice. We conclude that the regulation of Us3 kinase activity by autophosphorylation of its Ser-147 plays a critical role in viral replication in vivo and HSV-1 pathogenesis, based on the results described above and the observation that Ser-147 is a physiological phosphorylation site in Us3 whose phosphorylation is tightly regulated and controls Us3 catalytic activity. At present, the mechanism by which regulation of Us3 kinase activity contributes to HSV-1 pathogenesis remains unclear. The reduced replication capability of YK515 (Us3-S147A), compared to that of YK517 (Us3-SA-repair), may account for its lower pathogenicity in HSK. Since phosphorylation of Us3 Ser-147 stimulated its kinase activity about sevenfold, strict regulation of Us3 Ser-147 phosphorylation may be necessary to avoid too many Us3 target proteins being phosphorylated by Us3, which might be disadvantageous for proper viral replication in vivo. It has been reported that periocular HSV-1 infection involves zosterlike spread from the cornea to periocular skin via sensory ganglia (33, 43). The observation that the periocular disease score in mice infected with YK515 (Us3-S147A) was lower than that in mice infected with YK517 (Us3-SA-repair) suggested that regulation of Us3 catalytic activity by autophosphorylation of its Ser-147 was required for proper axonal transport of the virus. In agreement with this suggestion, it has been reported that a PRV Us3 homologue plays a role in axonal transport (3).
In conclusion, the data presented here have shown that Us3 catalytic activity was tightly regulated by autophosphorylation in HSV-1-infected cells and that this regulation played a critical role in viral replication in vivo and pathogenesis. Thus far, studies of herpesvirus protein kinases have concentrated on downstream effects of the protein kinases. The present study indicated that the regulation of Us3 enzyme activity is as important as the downstream effects of the enzyme activity. Studies of Us3 regulatory mechanisms should include the identification of cofactor(s), other protein kinase(s), and protein phosphatase(s) that play roles in regulating the Us3 enzyme activity. Finally, we note that, based on the observation that the Us3-S147A mutation affected only limited functions of Us3, the protein kinase activity of Us3 with unphosphorylated Ser-147 seemed to regulate most Us3 functions, including viral replication in cell cultures, blocking of apoptosis, promotion of nuclear egress of progeny nucleocapsids though the nuclear membrane, and redistribution and phosphorylation of viral and cellular factors associated with nuclear membranes. It is possible that there is a regulatory mechanism(s) that controls the basal Us3 protein kinase activity for most Us3 functions. One possibility is that phosphorylation of Us3 by UL13 plays a partial role in the regulation of the basal Us3 protein kinase activity, since a phenotype of UL13 null mutant virus resembles that of Us3 null mutant virus with respect to the localization of UL31 and UL34 (15), whose localization has been shown to be regulated by the Us3 catalytic activity (41). Elucidation of Us3 regulatory mechanism(s), as well as further definition of previously unreported functional consequences of Us3, is needed to understand the overall features of this viral enzyme.
Acknowledgments
We thank Bernard Roizman for kindly providing R7041 virus and Kensuke Miyake for SP/O cells. We are grateful to Shihoko Koyama for excellent technical assistance.
This study was supported in part by Grants for Scientific Research and Grants for Scientific Research in Priority Areas from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan and a grant from the Takeda Science Foundation.
Footnotes
Published ahead of print on 18 March 2009.
REFERENCES
- 1.Benetti, L., and B. Roizman. 2004. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Natl. Acad. Sci. USA 1019411-9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colbran, R. J. 2004. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem. J. 3781-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coller, K. E., and G. A. Smith. 2008. Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus. Traffic 91458-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daikoku, T., Y. Yamashita, T. Tsurumi, K. Maeno, and Y. Nishiyama. 1993. Purification and biochemical characterization of the protein kinase encoded by the US3 gene of herpes simplex virus type 2. Virology 197685-694. [DOI] [PubMed] [Google Scholar]
- 5.Edelman, A. M., D. K. Blumenthal, and E. G. Krebs. 1987. Protein serine/threonine kinases. Annu. Rev. Biochem. 56567-613. [DOI] [PubMed] [Google Scholar]
- 6.Eisfeld, A. J., S. E. Turse, S. A. Jackson, E. C. Lerner, and P. R. Kinchington. 2006. Phosphorylation of the varicella-zoster virus (VZV) major transcriptional regulatory protein IE62 by the VZV open reading frame 66 protein kinase. J. Virol. 801710-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisfeld, A. J., M. B. Yee, A. Erazo, A. Abendroth, and P. R. Kinchington. 2007. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J. Virol. 819034-9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frame, M. C., F. C. Purves, D. J. McGeoch, H. S. Marsden, and D. P. Leader. 1987. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J. Gen. Virol. 68(Pt. 10)2699-2704. [DOI] [PubMed] [Google Scholar]
- 9.Gangappa, S., J. S. Babu, J. Thomas, M. Daheshia, and B. T. Rouse. 1998. Virus-induced immunoinflammatory lesions in the absence of viral antigen recognition. J. Immunol. 1614289-4300. [PubMed] [Google Scholar]
- 10.Gershburg, E., S. Raffa, M. R. Torrisi, and J. S. Pagano. 2007. Epstein-Barr virus-encoded protein kinase (BGLF4) is involved in production of infectious virus. J. Virol. 815407-5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goshima, F., T. Daikoku, H. Yamada, S. Oshima, T. Tsurumi, and Y. Nishiyama. 1998. Subcellular localization of the US3 protein kinase of herpes simplex virus type 2. Arch. Virol. 143613-622. [DOI] [PubMed] [Google Scholar]
- 12.Kato, A., J. Arii, I. Shiratori, H. Akashi, H. Arase, and Y. Kawaguchi. 2009. Herpes simplex virus 1 protein kinase Us3 phosphorylates viral envelope glycoprotein B and regulates its expression on the cell surface. J. Virol. 83250-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato, A., M. Tanaka, M. Yamamoto, R. Asai, T. Sata, Y. Nishiyama, and Y. Kawaguchi. 2008. Identification of a physiological phosphorylation site of the herpes simplex virus 1-encoded protein kinase Us3 which regulates its optimal catalytic activity in vitro and influences its function in infected cells. J. Virol. 826172-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato, A., M. Yamamoto, T. Ohno, H. Kodaira, Y. Nishiyama, and Y. Kawaguchi. 2005. Identification of proteins phosphorylated directly by the Us3 protein kinase encoded by herpes simplex virus 1. J. Virol. 799325-9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato, A., M. Yamamoto, T. Ohno, M. Tanaka, T. Sata, Y. Nishiyama, and Y. Kawaguchi. 2006. Herpes simplex virus 1-encoded protein kinase UL13 phosphorylates viral Us3 protein kinase and regulates nuclear localization of viral envelopment factors UL34 and UL31. J. Virol. 801476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi, Y., and K. Kato. 2003. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev. Med. Virol. 13331-340. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 717328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krosky, P. M., M. C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leach, N., S. L. Bjerke, D. K. Christensen, J. M. Bouchard, F. Mou, R. Park, J. Baines, T. Haraguchi, and R. J. Roller. 2007. Emerin is hyperphosphorylated and redistributed in herpes simplex virus type 1-infected cells in a manner dependent on both UL34 and US3. J. Virol. 8110792-10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leader, D. P. 1993. Viral protein kinases and protein phosphatases. Pharmacol. Ther. 59343-389. [DOI] [PubMed] [Google Scholar]
- 21.Leader, D. P., A. D. Deana, F. Marchiori, F. C. Purves, and L. A. Pinna. 1991. Further definition of the substrate specificity of the alpha-herpesvirus protein kinase and comparison with protein kinases A and C. Biochim. Biophys. Acta 1091426-431. [DOI] [PubMed] [Google Scholar]
- 22.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 947891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning, G., D. B. Whyte, R. Martinez, T. Hunter, and S. Sudarsanam. 2002. The protein kinase complement of the human genome. Science 2981912-1934. [DOI] [PubMed] [Google Scholar]
- 24.McGeoch, D. J., and A. J. Davison. 1986. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 141765-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meignier, B., R. Longnecker, P. Mavromara-Nazos, A. E. Sears, and B. Roizman. 1988. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162251-254. [DOI] [PubMed] [Google Scholar]
- 26.Morris, J. B., H. Hofemeister, and P. O'Hare. 2007. Herpes simplex virus infection induces phosphorylation and delocalization of emerin, a key inner nuclear membrane protein. J. Virol. 814429-4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mou, F., T. Forest, and J. D. Baines. 2007. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 816459-6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munger, J., A. V. Chee, and B. Roizman. 2001. The US3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 755491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munger, J., and B. Roizman. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. USA 9810410-10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogg, P. D., P. J. McDonell, B. J. Ryckman, C. M. Knudson, and R. J. Roller. 2004. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 319212-224. [DOI] [PubMed] [Google Scholar]
- 31.Pellett, P. E., and B. Roizman. 2007. The family Herpesviridae: a brief introduction, p. 2479-2499. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott-Williams & Wilkins, Philadelphia, PA.
- 32.Perkins, D., E. F. Pereira, M. Gober, P. J. Yarowsky, and L. Aurelian. 2002. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 761435-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polcicova, K., P. S. Biswas, K. Banerjee, T. W. Wisner, B. T. Rouse, and D. C. Johnson. 2005. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc. Natl. Acad. Sci. USA 10211462-11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poon, A. P., Y. Liang, and B. Roizman. 2003. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J. Virol. 7712671-12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purves, F. C., A. D. Deana, F. Marchiori, D. P. Leader, and L. A. Pinna. 1986. The substrate specificity of the protein kinase induced in cells infected with herpesviruses: studies with synthetic substrates [corrected] indicate structural requirements distinct from other protein kinases. Biochim. Biophys. Acta 889208-215. [DOI] [PubMed] [Google Scholar]
- 36.Purves, F. C., R. M. Longnecker, D. P. Leader, and B. Roizman. 1987. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J. Virol. 612896-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 906701-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purves, F. C., D. Spector, and B. Roizman. 1991. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J. Virol. 655757-5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds, A. E., B. J. Ryckman, J. D. Baines, Y. Zhou, L. Liang, and R. J. Roller. 2001. UL31 and UL34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 758803-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 768939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roux, P. P., and J. Blenis. 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68320-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryckman, B. J., and R. J. Roller. 2004. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J. Virol. 78399-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Summers, B. C., T. P. Margolis, and D. A. Leib. 2001. Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread. J. Virol. 755069-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanaka, M., H. Kagawa, Y. Yamanashi, T. Sata, and Y. Kawaguchi. 2003. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J. Virol. 771382-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf, D. G., C. T. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. USA 981895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zayzafoon, M. 2006. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell. Biochem. 9756-70. [DOI] [PubMed] [Google Scholar]