Abstract
Human T-cell leukemia virus (HTLV) regulatory protein, Rex, functions to increase the expression of the viral structural and enzymatic gene products. The phosphorylation of two serine residues (S151 and S153) at the C terminus is important for the function of HTLV-2 Rex (Rex-2). The Rex-2 phosphomimetic double mutant (S151D, S153D) is locked in a functionally active conformation. Since rex and tax genes overlap, Rex S151D and S153D mutants were found to alter the Tax oncoprotein coding sequence and transactivation activities. Therefore, additional Rex-2 mutants including P152D, A157D, S151Term, and S158Term were generated and characterized (“Term” indicates termination codon). All Rex-2 mutants and wild-type (wt) Rex-2 localized predominantly to the nucleus/nucleolus, but in contrast to the detection of phosphorylated and unphosphorylated forms of wt Rex-2 (p26 and p24), mutant proteins were detected as a single phosphoprotein species. We found that Rex P152D, A157D, and S158Term mutants are more functionally active than wt Rex-2 and that the Rex-2 C terminus and its specific phosphorylation state are required for stability and optimal expression. In the context of the provirus, the more active Rex mutants (A157D or S158Term) promoted increased viral protein production, increased viral infectious spread, and enhanced HTLV-2-mediated cellular proliferation. Moreover, these Rex mutant viruses replicated and persisted in inoculated rabbits despite higher antiviral antibody responses. Thus, we identified in Rex-2 a novel C-terminal inhibitory domain that regulates functional activity and is positively regulated through phosphorylation. The ability of this domain to modulate viral replication likely plays a key role in the infectious spread of the virus and in virus-induced cellular proliferation.
Human T-cell leukemia virus type 1 (HTLV-1) and type 2 (HTLV-2) are related complex oncogenic retroviruses that transform primary human T cells in culture and are associated with leukemia and neurological disorders in humans (54). In addition to the typical retrovirus structural and enzymatic genes gag, pol, and env, HTLV encodes two transregulatory gene products, Tax and Rex, in partially overlapping open reading frames, as well as accessory gene products (29). The viral oncoprotein Tax increases the rate of transcription from the viral long terminal repeat (LTR) (12, 15, 23). In addition, Tax can modulate the expression or activities of numerous cellular proteins involved in cell proliferation and differentiation, cell cycle regulation, and DNA repair processes (11, 30, 36, 37, 42). The pleiotropic effects of Tax on cellular processes are required for its transforming and oncogenic capabilities and play a critical role in HTLV-induced cellular transformation (16, 39-41).
Rex is a key regulator of viral replication. At the molecular level, the basic role of Rex is to regulate cytoplasmic levels of viral genomic unspliced mRNA (gag/pol) and singly spliced (env) mRNA, thus controlling the expression of the structural and enzymatic gene products that are essential for production of viral progeny (21, 28). Rex functions by binding viral mRNAs via a cis-acting RNA Rex response element (RxRE) and facilitating the export of these mRNA species from the nucleus to the cytoplasm (5, 7, 10). Previous studies revealed that HTLV-1 Rex (Rex-1), HTLV-2 Rex (Rex-2), and their RxREs are structurally similar and functionally interchangeable (27, 52). Mutational analyses of Rex-1 and Rex-2 have defined several domains critical for their functional properties. These include the arginine-rich N-terminal sequences that serve both as an RNA binding domain and as a nuclear localization signal, the central leucine-rich activation domain encompassing the nuclear export signal, and the multimerization domain composed of two regions flanking the nuclear export signal (9, 10, 20, 22, 32, 34, 38, 44, 47, 48).
Both Rex-1 and Rex-2 are phosphoproteins, and phosphorylation has been shown to be critical for their function (1, 2, 19). In HTLV-2-infected cells, as well as in cell lines transfected with Rex-2 expression plasmids, two major species of Rex-2 (p24 and p26) have been detected. Both Rex-2 species have the same amino acid backbone and differ by a conformational change that is induced by serine phosphorylation (18, 29, 31). This is unique to Rex-2 as Rex-1 presents as a single 27-kDa protein. Rex-2 p24 is found primarily in the cytoplasm, whereas the p26 phosphorylated form localizes predominantly to the nucleus and nucleolus (14, 53). In addition, phosphorylation of Rex-2 correlates with its binding to RxRE-containing RNA and inhibition of mRNA splicing (4, 19). A mutational analysis of Rex-2 that targeted all serines and threonines revealed a novel C-terminal functional domain containing two critical phosphorylated residues at serine 151 and 153 (31, 32). Rex-2 mutants containing alanine substitutions at either of these two serines (S151A, S153A) displayed reduced phosphorylation, impaired RNA binding capacity, diffused cytoplasmic localization, and decreased functional activity. In contrast, replacement of both serine residues with phosphomimetic aspartic acids (S151D, S153D) resulted in detection of only the p26 species in cells, enhanced RNA binding capacity of Rex-2, and an intense speckled nucleolar localization (31, 32). Interestingly, this Rex-2 mutant was locked in a phosphorylated active conformation since it could not be altered by phosphatase treatment in vitro. These results suggest an important role of the Rex-2 C terminus in its functional regulation.
We have proposed that the regulation of Rex-2 through phosphorylation provides a critical control in HTLV-2 replication cycle at the cellular level, which would allow the virus to better adjust to environmental stimuli (31, 55). Mutant Rex (S151D, S153D) is locked in an active form, potentially removing at least one of its key regulatory controls, thus providing a unique reagent with which to evaluate the role of Rex-2 in regulating viral replication and cellular transformation in vitro and viral persistence in vivo. One caveat is that the mutations (S151D, S153D) in Rex-2 affected the amino acid sequence and disrupted the transactivation activities of the viral oncoprotein Tax. Therefore, to facilitate our studies, several new Rex-2 mutants were generated that do not significantly affect Tax function. These mutants include two with phosphomimetic or charged mutations, P152D and A157D, and two with deletion mutations, S151Term and S158Term. We found that the introduction of aspartic acid into the C terminus or deletion of the C-terminal sequences downstream of serine 158 resulted in a highly functional Rex protein, a phenotype consistent with the disruption or removal of a carboxy-terminal inhibitory domain. Our data indicate that the C terminus is indispensable for Rex-2 protein stability, whereas the phosphorylation status of the C terminus dictates the function of the Rex-2 protein but does not affect protein stability. In the context of full-length infectious virus, the more functionally active Rex-2 mutants, the A157D and S158Term mutants, showed increased viral gene expression in infected primary T cells, enhanced viral infectivity, and promoted HTLV-2-mediated cellular proliferation of primary T lymphocytes. Lastly, HTLV-2 mutant viruses containing the Rex-2 mutations at either A157D or S158Term successfully replicated and persisted in inoculated rabbits and resulted in stronger antibody responses to viral antigens compared to wild-type (wt) HTLV-2. Thus, we identified a novel C-terminal inhibitory domain in Rex-2 that regulates functional activity, and this domain itself is positively regulated through phosphorylation or charge-induced conformation alterations. The ability of this domain to modulate viral replication likely plays a key role in HTLV infectious spread and virus-mediated cellular proliferation and cell survival.
MATERIALS AND METHODS
Cells.
293T and HeLa cells were maintained in Dulbecco's modified Eagle's medium, and 729 human B-cell and Jurkat T-cell lines were maintained in Iscove's medium and RPMI 1640 medium. Medium was supplemented with 10% fetal bovine serum, 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml). Human peripheral blood mononuclear cells (PBMCs) were isolated and cultured as described previously (50).
Plasmids.
The Rex-2 expression vector BC20.2, containing the HTLV-2 tax/rex cDNA expressed from the cytomegalovirus (CMV) immediate-early gene promoter, and the Rex-1 expression vector SE356, containing the HTLV-1 tax/rex cDNA expressed from the CMV immediate-early gene promoter, have been described previously (18, 52). The rex mutations were generated in either BC20.2 (Rex-2) or SE356 (Rex-1) using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Various Rex-2 mutants were transferred to the HTLV-2 proviral clone pH6neo (13). Mutations were confirmed by DNA sequencing. The human immunodeficiency virus type 1 (HIV-1) Tat expression vector, pcTat, and the Rex-2 reporter plasmid (pCgagRxRE-II) were described previously (31). The LTR-2-luciferase Tax reporter plasmid, κB-Luc Tax reporter plasmid, CMV-luciferase (firefly) plasmid, and thymidine kinase-Renilla luciferase plasmid were described previously (52). Mutant and wt Rex-2-green fluorescent protein (GFP) constructs were generated by inserting Rex-2 sequences into the enhanced GFP (EGFP)-N3 vector (Promega, Madison, WI) upstream of the GFP open reading frame. FLAG-tagged Rex-2 constructs were generated by insertion of the FLAG-tag sequence into vector BC20.2 upstream of the Rex-2 open reading frame using primers SphI-flag (sense) (5′-GCATGCTCGATTACAAGGATGATGATGATAAGGGCGGCATGC-3′) and SphI-flag (antisense) (5′-GCATGCCGCCCTTATCATCATCATCCTTGTAATCGAGCATGC-3′).
Tax and Rex functional reporter assays.
The ability of Tax to activate CREB/ATF (viral LTR) or NF-κB was determined by using a dual luciferase assay as described previously (50). The Rex functional assay was performed as described previously with a slight modification (31). Briefly, Rex cDNA expression plasmids were cotransfected into 293T cells with 0.05 μg of CMV-Luc, 0.25 μg of pcTat, and 0.5 μg of Rex reporter plasmid pCgag-RxRE. Cell lysates were prepared at 48 h posttransfection, and luciferase activity was determined to control for transfection efficiency. The HIV-1 p24 Gag level in the cell lysates was determined by using an enzyme-linked immunosorbent assay (ELISA; Beckman-Coulter, Fullerton, CA). All transfection experiments were performed in triplicate in three independent experiments.
p19 Gag ELISA and isolation of HTLV-2 stable producer cell lines.
Virion production of HTLV proviral clones from transiently transfected 293T cells was measured by a commercially available p19 matrix antigen ELISA (ZeptoMetrix, Buffalo, NY). To generate stable transfectants, proviral plasmid clones containing the Neor gene were introduced into 729 B cells by electroporation as described previously (17). Stable transfectants containing the desired proviral clones were isolated and characterized as previously described (49).
DNA preparation and PCR analysis.
Genomic DNA was isolated from permanently transfected cell clones or from immortalized PBMCs using the Puregene DNA purification system (Gentra, Minneapolis, MN). Genomic DNA (1 μg) was subjected to 30-cycle PCR analysis. The forward primer 670 (28) and the reverse primer PG201 (5′-GCTGGTATAGGTATAGGCAT-3′) were used to amplify a specific 437-bp fragment from the HTLV-2 tax/rex region. The PCR-amplified product was separated on agarose gels and visualized by ethidium bromide staining. Mutations were confirmed by DNA sequencing. For infected rabbit PBMCs, 1 μg DNA was subjected to 40-cycle PCR using primers 670 and 671 (28) to amplify a 159-bp fragment specific for the HTLV-1/HTLV-2 tax/rex region. In addition, 40 cycles of real-time TaqMan PCR were conducted to quantitate the proviral copy number per cell as described previously (3). Rabbit PBMC DNA was subjected to PCR analysis in duplicate using the HTLV-specific primer pair AAM.001 (5′-CGGATACCCAGTCTACGTGTTT-3′) and AAM.002 (5′-CTGAGCCGATAACGCGTCCA-3′) and probe (5′-6-carboxyfluorescein-ATCACCTGGGACCCCATCGATGGA-6-carboxytetramethylrhodamine-3′), and final values were averaged. The 25-μl reaction mixtures contained 500 ng of DNA, 100 ng (25 ng/ml) of each primer, and a probe concentration of 100 pmol/μl. The copy number was determined based on a standard curve generated from duplicate samples of dilutions of a plasmid containing the tax gene sequences. The copy number per cell value for a sample was generated based on the estimation that 1 μg PBMC DNA is equivalent to 134,600 cells.
Western blot analysis, antibodies, and pulse-chase immunoprecipitation assay.
Cells were lysed with modified RIPA buffer (0.05 M Tris-Cl [pH 8.0], 0.15 M NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 2 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, 1 mM Na3VO4, and 1 mM NaF) on ice for 30 min. After being centrifuged, the cell lysates were subjected to 12% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (Schleicher and Schuell Biosciences, Keene, NH). Western blot analyses were performed as recommended by the manufacturer. Proteins were visualized using the enhanced chemiluminescence Western blot analysis system (Santa Cruz Biotechnology, Santa Cruz, CA).
Rex-1, Rex-2, and Tax-2 were detected using protein-specific rabbit polyclonal antisera. Anti-EGFP antibody, anti-FLAG M2 monoclonal antibody, anti-actin monoclonal antibody, goat anti-rabbit immunoglobulin G (IgG) antibody, and goat anti-mouse IgG antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
The half-life of Rex was determined by pulse-chase experiments. Briefly, 106 293T cells were transfected with 10 μg of wt rex or various rex mutant expression plasmids or a negative control using Lipofectamine Plus reagent (Invitrogen, Carlsbad, CA). Cells were metabolically labeled with [35S]methionine-cysteine (Trans-35S label; 100 mCi/ml; Amersham) in methionine-cysteine-free RPMI 1640 medium supplemented with 20% dialyzed fetal calf serum for 6 h. Cells were chased with cold medium for the indicated time points and then harvested and lysed in RIPA buffer on ice for 30 min. Lysates were clarified by centrifugation at 17,000 × g for 30 min at 4°C. Subsequently, equal amounts of cell lysates from the different time points were immunoprecipitated using rabbit anti-Rex antiserum for 16 h at 4°C. The immune complexes were collected using protein A-Sepharose CL-4B (Sigma) and subjected to 12% SDS-PAGE; 35S-labeled proteins were visualized and quantified by Typhoon analysis (Molecular Dynamics).
Short-term coculture microtiter proliferation and long-term immortalization assays.
Short-term microtiter proliferation assays were performed as described previously with some modifications (35). Briefly, freshly isolated human PBMCs were prestimulated with 2 μg/ml phytohemagglutinin and 10 U/ml interleukin-2 (IL-2; Roche Diagnostic Corporation, Indianapolis, IN) for 3 days. One hundred 729 HTLV producer cells were irradiated (10,000 rad) and cocultured with 104 prestimulated PBMCs in the presence of IL-2 in round-bottom 96-well plates. Wells were enumerated for growth and split at a ratio of 1:4 at weekly intervals. At week 7, cell proliferation was confirmed by MTS assays using CellTiter 96 AQueous one solution reagent as recommended by the manufacturer (Promega, Madison, WI). The long-term immortalization assays were performed as described previously (50).
Detection of HTLV-2-infected T cells by flow cytometry analysis.
Irradiated 729 HTLV producer cells (2 × 105) were cocultured with 106 PBMCs in the presence of IL-2. Four days after plating, cells were washed with PAB (PBS, 0.1% NaN3, 1% bovine serum albumin) and stained with phycoerythrin-Cy5-conjugated mouse anti-human CD3 antibody. The samples were washed, fixed, and permeabilized with Fix&Perm reagents (Serotec, Raleigh, NC). For detection of intracellular viral protein, cells were first incubated with HTLV-2 Gag p19 detector antibody (ZeptoMetrix) and then washed, incubated with fluorescein isothiocyanate-conjugated secondary antibody goat anti-mouse IgG2b, and analyzed for CD3/p19 double-positive cells using a BD Biosciences (San Jose, CA) fluorescence-activated cell scanner (FACScan).
Rabbit inoculation procedures.
Twelve-week-old, specific-pathogen-free New Zealand White rabbits (Hazelton, Kalamazoo, MI) were inoculated via the lateral ear vein with approximately 1 × 107 gamma-irradiated (7,500 rad) 729wtHTLV-2, 729HTLV-2/RexA157D, or 729HTLV-2/Rex S158Term mutant cells or 729 uninfected control cells (six rabbits per group). Cell inocula were equilibrated based on their p19 Gag production per cell (a surrogate for virion production) prior to inoculation. At 0, 1, 2, 4, 6, and 8 weeks after inoculation, 10 ml of blood was drawn from the central ear artery of each animal. Serum reactivity to specific viral antigenic determinants was detected using a commercial ELISA (1:100 dilution; BioMerieux, Inc., Durham, NC) and an HTLV Western blot assay (1:200 dilution; ZeptoMetrix) adapted for rabbit plasma by use of avidin-conjugated goat anti-rabbit IgG (1:2,000 dilution; Sigma, St. Louis, MO) (43). Serum showing reactivity to Gag (p24 CA or p19 MA) and Env (gp21 TM or gp46 SU) antigens was classified as HTLV-2 seropositive. Estimated proviral loads were determined and quantified using genomic DNA from PBMC samples by PCR and real-time TaqMan PCR analyses as described above.
RESULTS
Generation of Rex-2 mutants.
Rex-2 is detected in virus-infected cells as two distinct protein species or conformations as detected by Western blot analysis (p26 and p24). Both Rex-2 species have the same amino acid backbone and differ by serine phosphorylation (p26 is nuclear and the active species). In particular, serines 151 and 153 were identified as two phosphorylation sites that play critical roles in the functional regulation of Rex-2 (19, 31). Replacement of these two serines with alanines not only disrupted Rex-2 function but shifted the Rex-2 steady-state concentration to the p24 Rex species; the p26/p24 steady-state ratio for wt Rex is approximately 1, whereas for the Rex S151, S153A mutant the ratio was approximately 0.5. Moreover, phosphomimetic aspartic acids (S151D, S153D) locked Rex-2 in a phosphorylated and active p26 conformation (31, 32). The primary objectives of this study were to understand the functional role of the carboxy-terminal Rex-2 phosphorylation domain and to address whether the ability to regulate Rex function was important for HTLV-2-mediated cellular proliferation and immortalization in vitro and survival in a rabbit model of infection. Numerous studies have demonstrated the essential role of the viral oncoprotein Tax in HTLV-mediated cellular proliferation and transformation. Since the Tax and Rex proteins are encoded by separate but partially overlapping reading frames, mutations in rex could alter the amino acid sequence of Tax and disrupt critical transcriptional activities required for viral replication and cellular transformation. Indeed, our previously described highly active Rex mutations (S151D and S153D) resulted in alteration of the Tax coding sequence and significantly impaired Tax CREB/ATF and NF-κB transactivation activities (40) (Table 1).
TABLE 1.
Rex-2 mutants and their Tax transactivation activities
| Rex-2 mutantsa | Tax-2 mutation(s) | Steady-state ratio of Tax transactivation activityb
|
|
|---|---|---|---|
| CREB/ATF | NF-κB | ||
| S151, S153A | L131P, F133R | 0.25 | <0.05 |
| S151D | L131G, A132S | 0.50 | <0.05 |
| S153D | F133G | 0.44 | <0.05 |
| P152D | A132G | 0.45 | 0.86 |
| A157D | wt | 1.0 | 1.0 |
| S151Term | wt | 1.0 | 1.0 |
| S158Term | wt | 1.0 | 1.0 |
The Rex-2 mutants generated were named based on the mutated amino acid and its position within the 170-amino-acid HTLV-2 Rex. For example, S151D indicates that the serine residue at amino acid 151 was substituted with aspartic acid.
Tax transactivation activities toward CREB/ATF- and NF-κB-responsive luciferase reporter genes were examined using the dual luciferase assay described in Materials and Methods. The data are based on three independent experiments and normalized to wt Rex-2 activity (set as 1.0).
It has been well documented that phosphorylation events can result in a charge-induced conformational change of a protein, which may alter its stability or function (8, 33, 45, 51). Therefore, we hypothesized that introducing a phosphomimetic amino acid into the Rex-2 C terminus, but not necessarily at serine 151 or serine 153, might result in the same conformational alteration as that reported for the Rex S151D, S153D mutant (31). Based on this hypothesis, two Rex-2 mutations were generated in the 170-amino-acid Rex-2 polypeptide, P152D and A157D. The Rex A157D mutant did not have any alteration in the Tax amino acid sequence, whereas the Rex P152D mutant resulted in a single amino acid change in Tax (A132G). Using LTR-Luc and κB-Luc reporter assays, we showed that the Tax A132G mutation resulted in a 50% reduction in CREB/ATF transactivation activity but induction of nearly wt levels of NF-κB activity (Table 1). The Rex A157D mutant did not show altered Tax expression or function. One hypothesis consistent with our previous Rex-2 phosphorylation and functional data was that the C terminus of Rex-2, when nonphosphorylated, inhibits protein function but can be positively regulated or activated by phosphorylation (31, 32). To test this hypothesis, we generated two Rex C-terminal deletion mutants, the Rex S151Term and S158Term mutants, in which the codon for serine 151 or serine 158, respectively, was mutated to a termination codon. These mutations maintained the Tax amino acid sequence and its transactivation function (Table 1).
Protein expression and functional activities of Rex-2 mutants.
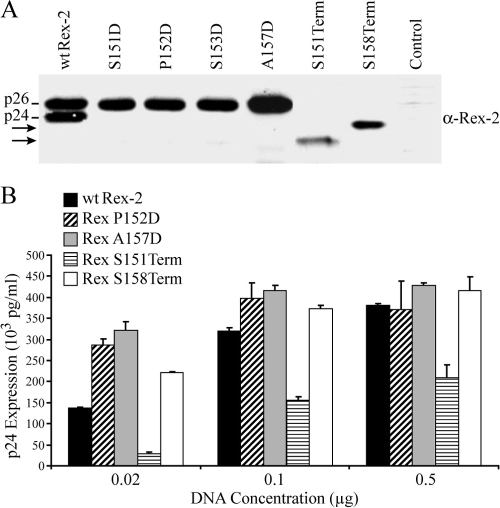
We initially evaluated protein expression of Rex-2 mutants in transfected 293T cells by Western blot analysis using rabbit polyclonal anti-Rex antisera. As expected, in the cells transfected with the wt Rex-2 expression construct, both p24 and phosphorylated p26 protein species were detected (Fig. 1A). Consistent with our previous report, expression of the Rex S151D or S153D mutation resulted in the detection of only the phosphorylated p26 (31) (Fig. 1A). Rex mutants that contained a phosphomimetic or charged residue within this region, P152D and A157D, also exhibited a single phosphorylated p26 species. The two C-terminal Rex deletion mutants, the Rex S151Term and S158Term mutants, displayed a single protein form consistent with their predicted gel mobility (Fig. 1A). However, it was notable that the amount of steady-state protein for the two termination mutants was lower than that for the wt Rex-2 or aspartic acid point mutants (Fig. 1A).
FIG. 1.
Expression and functional activities of Rex-2 mutants. (A) Western blot analysis of Rex-2 protein expressed from 293T cells transiently transfected with Rex-2 cDNA plasmids. Proteins were detected using rabbit Rex-2-specific antisera. wt p24 and p26 are indicated, and arrows identify truncated Rex proteins. α, anti. (B) Functional activities of Rex-2 cDNA mutants were determined using the modified HIV p24 Gag reporter assay. 293T cells were transfected with 0.25 μg pcTat, 0.5 μg pcGagRxRE-II, 0.05 μg CMV-Luc, and increasing concentrations of wt Rex or mutant Rex plasmids as indicated (0.02 to 0.5 μg). Forty-eight hours after transfection, cells were harvested and assayed for p24 Gag. The values represent actual p24 Gag production from a representative experiment performed in triplicate. Error bars indicate standard deviations.
We next tested the Rex-2 mutants for their abilities to function in our quantitative reporter bioassay in which HIV-1 p24 Gag production was measured and used as a read-out of Rex functional activity (31). The Rex P152D, A157D, and S158Term mutants were significantly more active than wt Rex-2 at the lowest Rex-expression plasmid concentration tested. This difference was gradually minimized and disappeared at higher concentrations, which was likely due to a plateau effect (outside the linear range of the assay). These results were consistent with our hypothesis that the Rex-2 C terminus inhibits its own function and that this inhibition can be removed either through deletion of the C-terminal sequence or via introduction of a phosphomimetic amino acid (negative charge) within the C terminus. In contrast, the activity of the Rex S151Term mutant was approximately 25 to 40% that of the wt over the range of Rex expression plasmid concentrations tested (0.02 to 0.5 μg), suggesting that the C-terminal sequence from amino acid 151 to amino acid 157, which includes serines 151 and 153, was required for optimal Rex function.
It is also worthy to note that Rex functional activity of the Rex S158Term mutant is higher than that of wt Rex-2, despite its lower steady-state level of Rex protein, which suggests that the C terminus inhibits Rex-2 activity. This inhibitory effect of the Rex C terminus can act either in cis through induction of conformational change or in trans through competition of cellular factors inhibitory to Rex-2 function. To distinguish between these two possibilities, we performed a simple experiment in which a GFP-Rex C-terminal fusion construct (GFP-Rex151-170 or GFP-Rex154-170) was cotransfected into 293T cells with either wt Rex-2 or the Rex S158Term mutant. We did not observe any significant inhibition of Rex-2 function (data not shown), consistent with our hypothesis that the Rex-2 C terminus negatively regulates Rex-2 through a conformational change or spatial effect.
Subcellular localization of Rex-2 carboxy-terminal mutants.
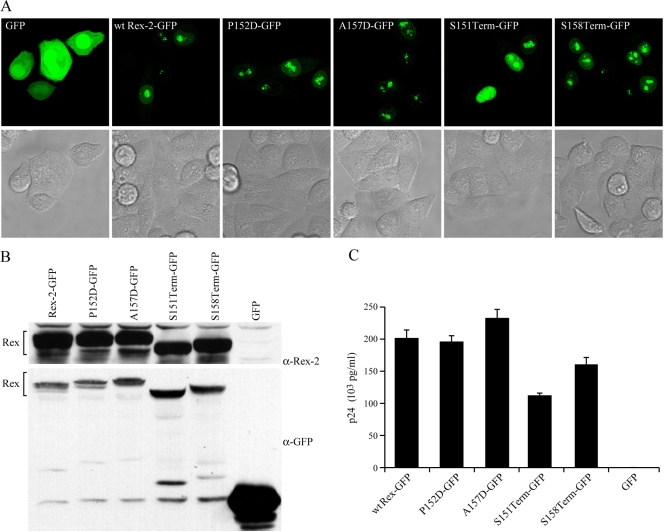
Previous studies indicated that the phosphorylated form of Rex-2, p26, is found primarily in the nucleus and nucleolus, whereas the p24 inactive form displayed diffuse cytoplasmic and nuclear localization, indicating that the proper subcellular localization of Rex-2 correlated with its function (4, 14, 19). We determined the subcellular localization of the newly generated Rex-2 mutants in transiently transfected HeLa-Tat cells using Rex-EGFP fusion proteins. EGFP alone displayed bright, diffuse staining throughout the cytoplasm as well as in the nucleus. In contrast, all of the Rex-EGFP fusion proteins tested, including S151Term-EGFP, exhibited predominant nuclear localization with some weak cytoplasmic staining. This result indicated that the capacity of Rex-2 to localize to the nucleus was not significantly affected by the C-terminal mutations (Fig. 2A). We confirmed the relatively equal stable protein expression of these Rex-EGFP fusion proteins by Western blot analysis using either anti-Rex or anti-EGFP specific antisera. Furthermore, their functional activities were comparable to those of their untagged protein forms (compare Fig. 1B and 2C). It is important to note that the addition of the GFP tag at the carboxy terminus of S151Term and S158Term stabilized these deletion mutants in comparison to their untagged counterparts. We attribute this to the location and possibly the size of the tag since FLAG-tagged S158Term at the amino terminus resulted in a protein stability similar to that of the untagged protein (Fig. 3C).
FIG. 2.
Subcellular localization of Rex-2 mutants. HeLa-Tat cells were transfected with 1 μg of various Rex-2-EGFP plasmids or the EGFP-N3 negative control as indicated using Lipofectamine Plus (Invitrogen, Carlsbad, CA). (A) For EGFP detection, cells were plated and visualized using a Zeiss LSM 510 microscope (GFP and the light field are shown). (B) Expression of Rex-2-EGFP fusion proteins was detected by Western blot analysis using anti-Rex-2 antisera or anti-EGFP antibody. α, anti. (C) The functional activities of Rex-2-EGFP fusion proteins were determined by using an HIV p24 Gag reporter assay. The values represent actual p24 Gag production from a representative experiment performed in triplicate. Error bars indicate standard deviations.
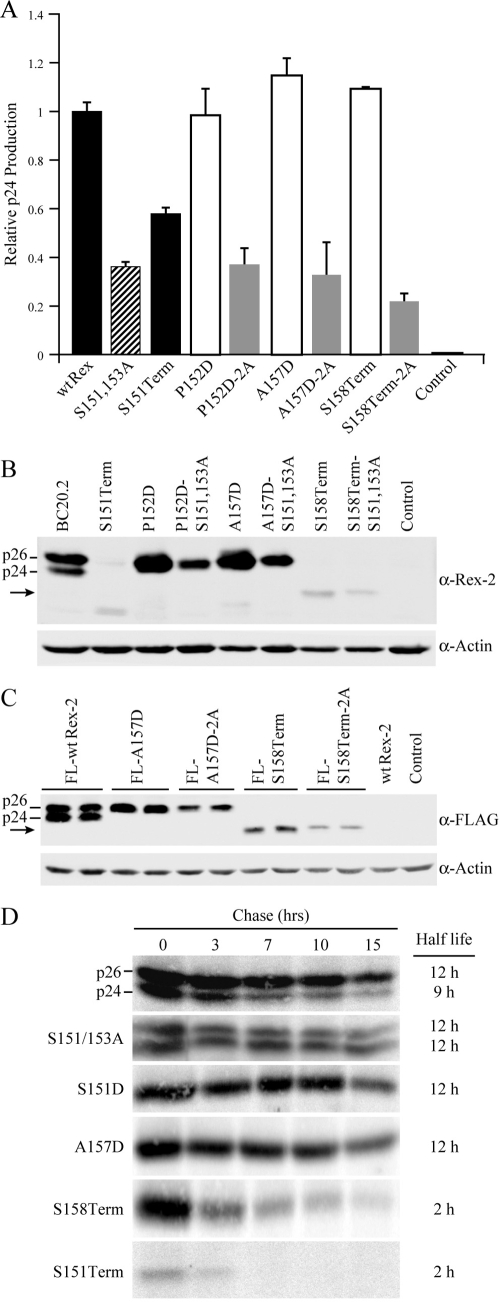
FIG. 3.
The C terminus is crucial for Rex protein expression and stability. (A) Disruption of phosphorylation at serines 151 and 153 (by deletion or alanine substitution mutation) consistently impairs Rex functional activity. 293T cells were transfected with 0.25 μg pcTat, 0.5 μg pcGagRxRE-II, 0.05 μg CMV-Luc, and 0.5 μg wt Rex or mutant Rex plasmids as indicated. Forty-eight hours after transfection, cells were harvested and assayed for p24 Gag. The values, which are normalized and shown relative to wt Rex-2, represent relative p24 Gag production from a representative experiment performed in triplicate. Error bars indicate standard deviations. (B) Rex-2 proteins expressed from transiently transfected 293T cells were detected by Western blot analysis using anti-Rex-2 specific antisera. wt p24 and p26 are indicated, and the arrow identifies the truncated Rex. Detection of cellular β-actin was used as a loading control. (C) The altered expression levels of Rex-2 mutants are not attributable to the detection sensitivity of our anti-Rex-2 antisera. Flag-tagged Rex protein expression (the FLAG-wt Rex, FLAG-RexA157D, FLAG-RexA157D-2A, FLAG-RexS158Term, and FLAG-RexS158Term-2A mutants) was detected from transiently transfected 293T cells using anti-FLAG monoclonal antibody M2. wt p24 and p26 are indicated, and the arrow identifies the truncated Rex. (D) The half-lives of wt Rex, S151/S153A, S151D, A157D, S158Term, and S151Term mutants were determined by pulse-chase experiments as described in Materials and Methods. Quantification of protein at different time points using the Typhoon imaging system was utilized to determine the protein half-life. α, anti.
Phosphorylation at Ser151 and/or Ser153 enhances Rex-2 functional activity.
Our results demonstrated that only S151Term and not the other three C-terminal mutants, including the Rex P152D, A157D, and S158Term mutants, exhibit significantly impaired activity as detected by reporter assay. One important difference between these mutants was that serines 151 and 153 were deleted and not available to be phosphorylated in the Rex S151Term mutant. In order to determine whether it was the loss of phosphorylation at serines 151 and 153 that contributed to the impaired functional activity of the S151Term mutant, we introduced serine 151 and 153 alanine encoding mutations in combination with P152D, A157D, and S158Term; mutants were termed P152D-2A, S157D-2A, and S158Term-2A, respectively. The functional activities of these Rex mutants were examined by quantitative reporter assay (Fig. 3A). Consistent with our previous report (31), the functional activity of the Rex S151 and 153A mutants was approximately 30% that of wt Rex-2 (Fig. 3A). Similarly, the functional activities of the Rex P152D-2A, A157D-2A, and S158Term-2A mutants were about 70% lower than those of their parental mutants, suggesting that phosphorylation at serine 151 and/or 153 was required for a fully functional Rex-2 (Fig. 3A). We next compared the expression profile and steady-state levels of Rex from the parental mutants with the serine 151 and 153 alanine mutants. Introduction of the additional S151/153A mutations into Rex P152D, A157D, and S158Term mutants did not alter the mobility of the Rex proteins on SDS-PAGE (Fig. 3B). As noted above, we detected increased Rex protein expression of phosphomimetic point mutants but decreased protein expression of the C-terminal deletion mutants. Interestingly, additional mutation of the phospho-acceptor serine residues 151 and 153 by alanine substitution appeared to significantly decrease the steady-state amounts of all Rex mutants. To eliminate the possibility that the decreased steady-state levels of protein seen by using Western blot analysis was not a reflection of altered epitopes and recognition by the anti-Rex-2 antisera, we generated FLAG-tagged Rex constructs and examined Rex protein expression using anti-FLAG M2. Consistently, Rex steady-state protein levels detected in the A157D-2A and S158Term-2A mutants were significantly lower than in the A157D and S158Term mutants, respectively (Fig. 3C). To determine the half-life of the wt and Rex-2 mutant proteins, we performed pulse-chase experiments. As shown in Fig. 3D, the half-life of the functionally active wt p26 was approximately 12 h and more stable than the inactive p24 species (half-life is approximately 9 h). In mutant S151, S153A, the half-life of both p26 and p24 is approximately 12 h. Thus, mutation of these two serine residues to alanine alone does not result in an unstable p26 or p24 protein species. Indeed, the slight increase in the S151, S153A p24 species is consistent with the overall steady-state increase of p24 relative to p26 and the idea that phosphorylation at one or both of these sites on p24 contributes to the conformational shift to p26. For mutants S151Term and S158Term, deletion of the C-terminal sequence not only decreased Rex steady-state level, but also substantially decreased Rex half-life to approximately 2 h. Together, these results indicated that in addition to its role in regulation of Rex-2 functional activity, the C terminus and phosphorylation status of Rex-2 played an important role in protein stability and optimal expression of steady-state protein levels.
Establishment and characterization of stable virus producer cell lines.
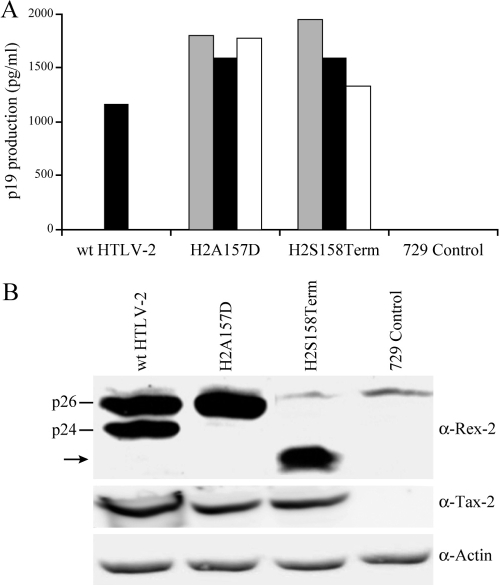
Next, we generated mutant HTLV-2 proviral clones including HTLV-2/RexA157D and HTLV-2/RexS158Term (termed H2A157D and H2S158Term, respectively) to assess how viral replication would be affected by the unique phenotypes of these Rex mutants. Both H2A157D and H2S158Term are competent for viral protein production as determined by p19 Gag ELISA in transiently transfected 293T cells. Consistent with the more functionally active phenotype displayed by these Rex mutants, both show increased levels of p19 production relative to that of the wt (data not shown). In order to determine the capacity of H2A157D and H2S158Term proviral clones to replicate and induce cellular immortalization/transformation in primary human T cells, permanent 729 B-cell transfectants expressing the two mutant viruses were isolated and characterized. For each of the stable transfectants, the expected mutations were confirmed by diagnostic genomic DNA PCR analysis and DNA sequencing (data not shown). To monitor the production of viral proteins in these stable transfectants, the concentration of p19 Gag in the culture supernatant of several cell clones was quantified by ELISA. As shown in Fig. 4A, the amount of p19 Gag expression from each stable cell clone tested was variable. This is likely attributable to the chromosomal location of integrated proviral sequences and the overall proviral copy number in each cellular clone. All stable cell clones expressing the Rex mutant viruses produced slightly more p19 Gag compared to our well-characterized HTLV-2 producer cell line, 729pH6neo. This result is also consistent with proviral clone transfection data and the more functionally active phenotype displayed by these Rex mutants. We confirmed the expression of Rex and Tax by Western blot analysis in the cell lines selected for subsequent coculture assays (Fig. 4B). We consistently observed slightly lower Tax expression in the two mutant producer cell lines relative to that in the wt, which again correlates with a more functional Rex and redistribution of viral mRNA (greater gag/pol and env mRNA at the expense of tax/rex).
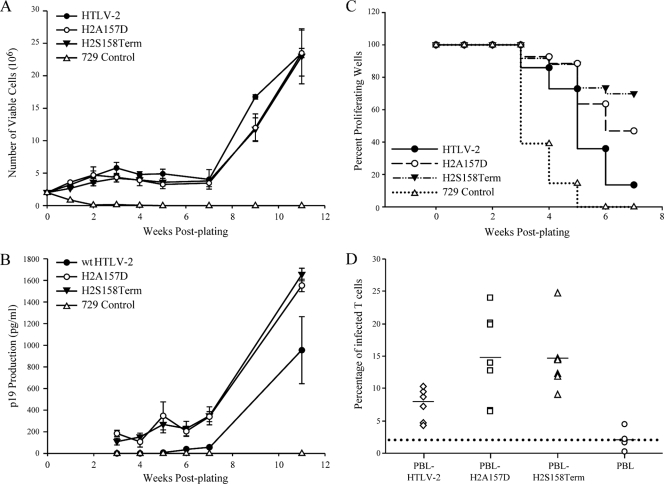
FIG. 4.
Establishment of permanent producer cell lines of HTLV-2 mutants. (A) Viral protein expression in permanent transfectants. Three independently isolated 729 stable producer cell clones for the Rex H2A157D and H2S158Term mutants were isolated as described in Materials and Methods. Cells (5 × 106) were plated in each well of six-well plates, and p19 Gag production was measured in 48-h culture supernatants by ELISA. The 729wtHTLV-2 cell line was used as the positive control. (B) Viral protein expression, including Rex and Tax, and β-actin as a loading control were detected by Western blot analysis for selective stable producer cell lines (black bars in panel A) to be used in coculture analysis. wt p24 and p26 are indicated, and the arrow identifies the truncated Rex. α, anti.
Functionally more active Rex promoted HTLV-2 infectivity and virus-induced cellular proliferation.
We performed short-term microtiter proliferation assays and long-term immortalization assays to assess the capacity of the Rex-2 mutant viruses to infect and immortalize human PBMCs (17, 35, 50). These coculture assays use freshly isolated PBMCs and cell-associated virus transmission designed to mimic the in vivo infection. The in vitro long-term coculture immortalization assays were carried out initially in 24-well plates in order to monitor the immortalization process and the characteristic expansion of infected PBMCs. After 7 weeks of being cocultured, infected PBMCs were transferred to 25-cm2 flasks to expand. The growth curve shown in Fig. 5A indicated that both Rex H2A157D and H2S158Term mutants induce progressive growth patterns consistent with wt HTLV-2 immortalization. Viral protein production from the infected PBMCs was quantified by Gag p19 ELISA. As shown in Fig. 5B, PBMCs infected with HTLV-2 Rex mutants continuously produced more p19 Gag than those infected with wt HTLV-2, indicating more robust viral replication and virion production due to the more active Rex. As previously reported, the immortalized PBMCs were shown to harbor and express HTLV-2 as determined by immunofluorescence for p19 (50), and the genetic mutations were confirmed by PCR amplification of rex-specific sequences from genomic DNA, followed by DNA sequencing (data not shown). These results indicated that although cells infected with Rex H2A157D and H2S158Term mutant viruses produce more virions, this phenotype did not translate into a significant increase in immortalization of PBMCs. It is important to note that HTLV long-term immortalization of T lymphocytes is relatively inefficient, involves a selection for growth of a rare number of infected cell clones, and is not quantitative.
FIG. 5.
HTLV-2 T-lymphocyte immortalization and proliferation assays. PBMCs (2 × 106) were cultured with irradiated donor cells (1 × 106) in each well of 24-well plates. (A) Representative growth curves for HTLV-2-infected cells are shown. Cell viability was determined weekly by trypan blue exclusion (0 to 11 weeks postcocultivation). The mean and standard deviation for each time point were determined from three independent wells. (B) HTLV-2 gene expression was confirmed by detection of p19 Gag protein in the culture supernatant using ELISA. (C) Representative Kaplan-Meier plots for T-lymphocyte proliferation in a short-term microtiter assay. Prestimulated PBMCs (104) were cocultured with 100 irradiated 729 stable producer cells per well in 96-well plates. The Kaplan-Meier plot shows the percentage of proliferating wells as a function of time (weeks). (D) Functionally more active Rex enhances viral infectivity in coculture assays. Irradiated 729 stable producer cells (2 × 105) were cocultured with 106 PBMCs in the presence of IL-2. The percentages of newly infected T cells (CD3+, p19+) were enumerated 2 days postplating by using immunofluorescence analysis. The mean and standard deviation for each sample were determined from three independent experiments using PBMCs from three different healthy donors. The mean values are indicated by the horizontal lines. The percentages of Rex H2A157D mutant- and H2S158Term mutant-infected T cells are both significantly higher than that of wt HTLV-2-infected T cells (P < 0.001) as determined by using analysis of variance (ANOVA) followed by Tukey's posttest.
In an effort to obtain a more quantitative measure of the abilities of these viruses to infect and immortalize PBMCs, 104 PBMCs were cultured with 100 virus-producing cells in 96-well plates. At weekly intervals, individual wells were assayed for proliferation as measured microscopically by increased cell number or by MTS assay. In addition, the cells in individual wells were split weekly at a ratio of 1:4. Therefore, slowly growing or nondividing cells are eliminated very quickly, and the percentage of surviving wells is an accurate measure of the immortalization efficiency of viruses. The Kaplan-Meier plot of HTLV-2-induced T-cell proliferation demonstrated that the percentage of wells containing proliferating lymphocytes in coculture with HTLV-2 mutants with the more active Rex was substantially higher than those in coculture with wt HTLV-2 (Fig. 5C). Furthermore, flow cytometry analysis revealed that the percentages of HTLV-2 Rex mutant-infected T cells were significantly higher than those of wt HTLV-2-infected T cells at 2 days postplating (Fig. 5D). These data indicate that maintenance of Rex-2 in the active state can enhance infectivity and increase proliferation of HTLV-2-infected cells.
HTLV-2 mutants with more active Rex persisted in the inoculated rabbit model.
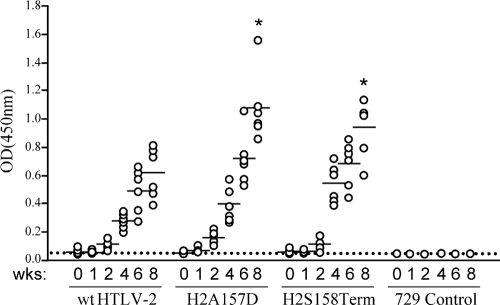
In order to evaluate the role of Rex functional regulation in HTLV-2 infection and replication in vivo, we inoculated rabbits with 729wtHTLV-2, 729H2A157D, 729H2S158Term, or 729 control cells. Rabbits were inoculated with lethally irradiated cell lines, and rabbit blood was sampled at 0, 1, 2, 4, 6, and 8 weeks postinoculation. Serum antibody titers to viral antigens increased over the course of the study in the majority of the rabbits (Fig. 6). Statistical analysis confirmed a significantly higher antibody response to HTLV-2 antigens in the two groups of Rex HTLV-2 mutant-infected rabbits than in the wt HTLV-2-infected group at 8 weeks postinoculation. In addition, proviral loads were examined by amplification of specific HTLV-2 genomic fragments from rabbit PBMCs. We detected proviral signals in all inoculated rabbits, which was consistent with the seroconversion data. However, quantitative real-time TaqMan PCR analysis over time revealed that proviral loads in rabbits infected with either Rex-2 mutant virus were not significantly different from the proviral loads of rabbits inoculated with wt HTLV-2 (Table 2). Interestingly, the enhanced antibody responses in Rex mutant virus-infected rabbits did not attenuate viral infection (represented as proviral load) over the time course of this study, indicating that HTLV-2 Rex mutant viruses could successfully infect and persist in inoculated rabbits. We did not observe any in vivo reversion to the wt HTLV-2 sequence in Rex mutant virus-infected rabbits (data not shown).
FIG. 6.
Assessment of HTLV-2 infection in inoculated rabbits. Antibody responses against HTLV-2 from each rabbit were measured by an anti-HTLV-2 ELISA, using both HTLV-2 Gag and envelope proteins as antigens. Each dot represents the absorbance value of a single inoculated rabbit at 0, 1, 2, 4, 6, and 8 weeks postinoculation within each group. The inocula as indicated below include 729wtHTLV-2 (n = 6), 729H2A157D (n = 6), 729H2S158Term (n = 6), or 729 (n = 2). The horizontal line represents the average of the rabbit group at each weekly time point. Statistical analysis (ANOVA followed by Tukey's posttest) of titers at 4 and 8 weeks after inoculation revealed significantly higher antibody responses to HTLV-2 antigens in the 729H2A157D-inoculated (P < 0.01) and 729H2S158Term-inoculated (P < 0.05) rabbits (denoted by an asterisk) than in the wt control group. Week 6 displayed borderline significance (P = 0.058). OD(450nm), optical density at 450 nm.
TABLE 2.
Detection and quantification of HTLV-1 DNA in rabbit PBMCs
| Inoculum and rabbit no. | Presence of amplified PCR fragment (copy no., 102) at indicated wk postinoculationa
|
|||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 8 | 11 | |
| 729/HTLV-2 | ||||||
| R1 | − | + | + (0.29) | + (1.74) | + (1.36) | + (1.20) |
| R2 | − | + | + (0.23) | + (0.48) | + (0.35) | + (0.83) |
| R3 | − | + | + (0.24) | + (0.54) | + (0.9) | + (0.53) |
| R4 | − | + | + (1.05) | + (0.32) | + (1.5) | + (3.28) |
| R5 | − | + | + (0.55) | + (0.36) | + (0.67) | + (1.07) |
| R6 | − | + | + (0.83) | + (0.4) | + (0.09) | + (0.42) |
| 729/HTLV-2 A157D | ||||||
| R7 | − | + | + (0.16) | + (1.27) | + (0.41) | + (1.12) |
| R8 | − | + | + (0.15) | + (0.69) | + (0.35) | + (1.22) |
| R9 | − | + | + (0.08) | + (0.44) | + (0.33) | + (0.20) |
| R10 | − | + | + (0.26) | + (2.76) | + (0.27) | + (1.25) |
| R11 | − | + | + (0.22) | + (1.37) | + (0.28) | + (0.76) |
| R12 | − | + | + (0.93) | + (1.57) | + (1.55) | + (2.20) |
| 729/HTLV-2 S158Term | ||||||
| R13 | − | + | + (0.14) | + (0.30) | + (0.26) | + (0.94) |
| R14 | − | + | + (0.47) | + (0.47) | + (1.76) | + (4.31) |
| R15 | − | + | + (0.47) | + (4.74) | + (0.91) | + (1.23) |
| R16 | − | + | + (0.05) | + (1.52) | + (0.13) | + (0.46) |
| R17 | − | + | + (0.24) | + (0.30) | + (3.48) | + (4.16) |
| R18 | − | + | + (0.05) | + (0.42) | + (0.27) | + (0.81) |
| 729 | ||||||
| R19 | − | − | − (0.05) | − (0.05) | − (0.01) | − (0.00) |
| R20 | − | − | − (0.03) | − (0.03) | − (0.04) | − (0.03) |
Genomic DNA isolated from rabbit PBMCs was subjected to PCR and to real-time TaqMan PCR analyses (0, 2, 4, 8, and 11 weeks) using HTLV-2-specific primers (670/671). −, no amplified PCR fragment; +, amplified PCR fragment. As determined by ANOVA, copy numbers in rabbits inoculated with the mutant proviruses were not significantly different from those of the wt. P values for weeks 2, 4, 8, and 11 are 0.216, 0.517, 0.498, and 0.443, respectively.
DISCUSSION
The goal of this study was to better understand the functional role of the carboxy-terminal Rex-2 phosphorylation domain and to address whether the ability to regulate Rex function is important for HTLV-2-mediated cellular proliferation and immortalization in vitro and virus survival in a rabbit model of infection. We have shown that introducing a phosphomimetic amino acid (negative charge) into the Rex-2 C terminus or deletion of certain C-terminal sequences could maintain Rex-2 in a highly functional state. Interestingly, we also found that the C terminus played an important role in Rex-2 protein stability and/or protein expression. Thus, our data provided evidence that the Rex-2 C terminus contains a functional inhibitory domain that also is regulated by the phosphorylation status. Cells harboring HTLV-2 mutants with more active Rex showed enhanced infection in vitro and increased virion production. Furthermore, these mutants promoted HTLV-2-induced proliferation of human primary T cells and displayed increased replication in inoculated rabbits as measured by significantly stronger antibody responses compared to those in wt HTLV-2-infected animals. However, proviral load levels over time and the abilities of the mutant viruses to persist were similar to those in wt HTLV-2-inoculated rabbits. Thus, although HTLV requires Rex to efficiently replicate and persist in inoculated rabbits, the ability to modulate Rex function does not significantly alter the in vivo course of infection, including the proviral load set point and the establishment of persistence.
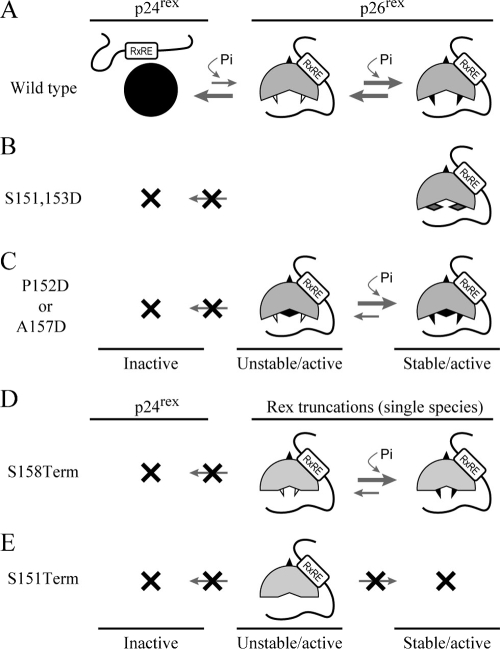
We have shown that deletion of the C terminus destabilizes the Rex-2 protein by significantly decreasing its half-life. In addition, we observed that substitution of negatively charged amino acids (aspartic acid) within the Rex-2 carboxy terminus increased steady-state protein levels and functional activity, whereas specific mutation or substitution of serines 151 and 153 with alanine resulted in decreased protein steady-state levels and impaired Rex-2 function. Presented in Fig. 7 is a model consistent with our Rex-2 biochemical and functional data and the published literature. The initial translation product of Rex-2, p24, is located primarily in the cytoplasm. We propose that phosphorylation of this inactive p24 at an unidentified serine(s) removes the inhibitory effect of the C terminus and results in a conformational change, giving rise to an active p26 intermediate that can translocate to the nucleus and interact with target RNA. This intermediate is likely unstable and a potential substrate for dephosphorylation (return to the p24 conformation) or subsequent phosphorylation at serine 151 and/or 153, resulting in a more stable p26 conformation (Fig. 7A). Substitution of serine 151 and serine 153 with phosphomimetic aspartic acid residues (S151D, S153D) disrupts the C-terminal inhibitory domain and results only in the detection of a stable (phosphatase-resistant) and highly active p26 (Fig. 7B) (31). Substitution of negatively charged aspartic acid for other residues in the C terminus of Rex-2 (P152D or A157D) can override the initial phosphorylation at an unidentified serine(s), remove the inhibitory effect of the C terminus, and quickly drive the equilibrium forward. As a result of this open conformation, serines 151 and/or 153 are likely more easily accessible and quickly phosphorylated, resulting in the detection of only the stable and active p26 (Fig. 7C). In S158Term, the inhibitory C-terminal domain is removed by the deletion of sequences downstream of serine 158, and as a result of this open conformation, serines 151 and/or 153 are quickly phosphorylated, generating a single stable active protein (Fig. 7D). Similarly, although the Rex S151Term mutant is active in a single detectable protein conformation, its functional activity is significantly attenuated, likely due to the loss of the phospho-acceptor serines located at positions 151 and 153 (Fig. 7E). This is consistent with the phenotype of combination mutants containing additional serine 151 and 153 alanine substitutions (P152D-2A, A157D-2A, and S158Term-2A), which display reduced stability and functional activities. Studies currently ongoing in our laboratory to identify other key phosphorylation sites and their respective cellular kinases may further facilitate our understanding of the functional regulation of Rex-2.
FIG. 7.
Model for Rex-2 phosphorylation and functional regulation. (A) The primary Rex-2 translation product p24 is inactive. An initial phosphorylation on an unidentified serine(s) induces a conformational alteration and results in an unstable but functionally active p26 intermediate. This intermediate can be further stabilized by subsequent phosphorylation on serine 151 and/or 153, generating a fully functional, stable p26 form. “Pi” represents phosphorylation, open triangles denote nonphosphorylated serines, and filled triangles denote phosphorylated serines. (B) The Rex S151 and 153D mutant disrupts the equilibrium between inactive p24rex and active p26rex because the aspartic acids (solid diamonds) are not subjected to dephosphorylation. (C) In Rex P152D and A157D mutants, introduction of a phosphomimetic aspartic acid (solid diamond) into the carboxy terminus functionally overrides the initial phosphorylation on an unidentified serine(s), removes the inhibitory effect of the carboxy terminus, and results in an unstable p26rex active form. Rex-2 is locked in the p26rex form because the aspartic amino acid is not subjected to dephosphorylation. (D) Deletion of the sequence downstream of Ser158 permanently removes the inhibitory carboxy terminus and interrupts the equilibrium between the p24rex inactive form and the p26rex active form. The p26rex intermediate can be stabilized by phosphorylation on serine 151 and/or 153, whereas Rex expressed from the S151Term mutant (E) is conformationally unstable because serines 151 and 153 are deleted.
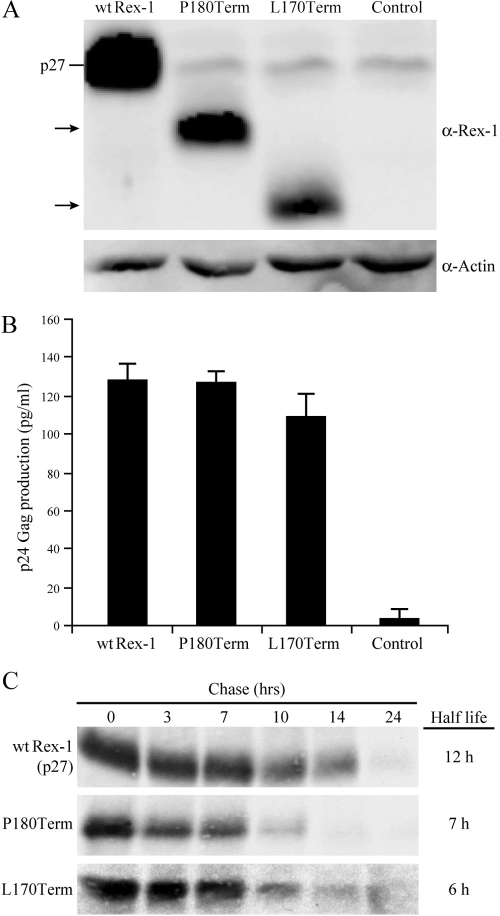
The question arises as to whether this C-terminal regulatory domain is unique to Rex-2 or if it is also contained in the highly related Rex-1. Rex-1 is a 189-amino-acid protein, and although Rex-1 and Rex-2 have the capacity to functionally substitute for each other (27, 52), the carboxy termini of the two related proteins at the amino acid level are quite divergent (31, 32). In cells, Rex-1 is detected as a single phosphoprotein species of 27 kDa, and one study using the H7 kinase inhibitor suggested that phosphorylation is important for its function (1, 2). Our initial studies revealed that two Rex-1 deletion mutants (P180Term and L170Term) have reduced steady-state protein levels compared to those of wt Rex-1, yet their functions remain similar (Fig. 8A and B). Moreover, deletion of part of the C terminus reduces the half-life of Rex-1 from 12 h to approximately 7 h (Fig. 8C). Thus, this phenotype is nearly identical to that of Rex-2 S158Term, suggesting that Rex-1 also contains a carboxy-terminal inhibitory/stability domain. Additional studies will be required to understand what, if any, role phosphorylation plays in the regulation of this domain.
FIG. 8.
Expression and functional activity of Rex-1 mutants. (A) Western blot of Rex-1 protein expressed from 293T cells transiently transfected with wt Rex-1 and deletion mutants, the P180Term L170Term mutant, and cDNA plasmids. Proteins were detected using rabbit Rex-1-specific antisera. wt p27 Rex is indicated, and the arrows identify the truncated Rex proteins. α, anti. (B) Functional activity of Rex-1 was determined by using the modified HIV p24 Gag reporter assay. 293T cells were transfected with 0.25 μg pcTat, 0.5 μg pcGagRxRE-I, 0.05 μg CMV-Luc, and 0.1 mg wt and mutant Rex-1 DNA. Forty-eight hours after transfection, cells were harvested and assayed for p24 Gag production. The values represent actual p24 Gag production from a representative experiment performed in triplicate. Error bars indicate standard deviations. (C) The half-lives of wt Rex-1 and the P180Term mutant were determined by pulse-chase experiments as described in Materials and Methods. Proteins were quantified at different time points using the Typhoon imaging system.
In the context of the provirus, the functionally more active Rex-2 mutants, A157D and S158Term, led to increased production of p19 Gag as measured in the culture supernatant, ultimately increasing HTLV-2 infectious spread and HTLV-2-mediated cellular proliferation as determined in our short-term quantitative assays. Both HTLV-2 Rex mutants can successfully infect and persist in inoculated rabbits. Consistent with increased expression of structural proteins in vitro, rabbits inoculated with Rex-2 A157D and S158Term mutants exhibited significantly higher antibody responses to viral antigens than wt HTLV-2-infected rabbits. The common expectation is that a higher antibody titer will more efficiently limit viral replication, leading to lower proviral loads and ultimately the elimination of viral infection. However, we did not observe any significant difference in proviral loads between the wt and Rex-2 mutants. Our Western blot analysis indicated that the increased antibody response was directed primarily against the viral Env and Gag. Although antibody response is a strong indicator of viral replication, there is no evidence that these antibodies are neutralizing or significantly control the infection. Previous studies provided strong evidence that an efficient cytotoxic T-lymphocyte response is the major host immune surveillance against HTLV-1-infected cells and possibly pathogenesis (6). Tax protein has been identified as the dominant target of HTLV-1-specific cytotoxic T lymphocytes (24, 26), which is expressed from double-spliced viral mRNA and minimally affected by alterations in Rex functional activity (28). More importantly, various studies have suggested that host genetic polymorphisms play a critical role in determining the efficacy of an individual immune response to HTLV-1 or HTLV-2 (25, 46). Consistently, we detected highly variant serological responses in inoculated rabbits, irrespective of the inoculum.
Overall, our studies characterized the C terminus of Rex-2 and emphasized its important role in regulation of Rex-2 protein function and expression and in HTLV-2 replication and infectious spread, along with viral induction of cellular proliferation. This finding also provides some important information regarding HTLV-2 replication in the rabbit animal model and its cross-talk with the host immune system.
Acknowledgments
We thank Joshua Arnold and Romi Doueiri for their assistance with the rabbit experiment, Tim Vojt for preparation of the figures, and Kate Hayes-Ozello for editorial comments.
This work was supported by a grant from the National Institutes of Health (CA100730) to P.L.G.
Footnotes
Published ahead of print on 11 March 2009.
REFERENCES
- 1.Adachi, Y., T. D. Copeland, C. Takahashi, T. Nosaka, A. Ahmed, S. Oroszlan, and M. Hatanaka. 1992. Phosphorylation of the Rex protein of human T-cell leukemia virus type I. J. Biol. Chem. 26721977-21981. [PubMed] [Google Scholar]
- 2.Adachi, Y., T. Nosaka, and M. Hatanaka. 1990. Protein kinase inhibitor H-7 blocks accumulation of unspliced mRNA of human T-cell leukemia virus type I (HTLV-II). Biochem. Biophys. Res. Commun. 169469-475. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, J., B. Yamamoto, M. Li, A. J. Phipps, I. Younis, M. D. Lairmore, and P. L. Green. 2006. Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood 1073976-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker, A. L. X., C. T. Ruland, D. W. Stephens, A. C. Black, and J. D. Rosenblatt. 1996. Human T-cell leukemia virus type 2 Rex inhibits pre-mRNA splicing in vitro at an early stage of spliceosome formation. J. Virol. 705511-5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballaun, C., G. R. Farrington, M. Dobrovnik, J. Rusche, J. Hauber, and E. Bohnlein. 1991. Functional analysis of human T-cell leukemia virus type I Rex-response element: direct RNA binding of Rex protein correlates with in vivo binding activity. J. Virol. 654408-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bangham, C. R., and M. Osame. 2005. Cellular immune response to HTLV-1. Oncogene 246035-6046. [DOI] [PubMed] [Google Scholar]
- 7.Black, A. C., C. T. Ruland, M. T. Yip, J. Luo, B. Tran, A. Kalsi, E. Quan, I. S. Y. Chen, and J. D. Rosenblatt. 1991. Human T-cell leukemia virus type II Rex binding and activity requires an intact splice donor site and a specific RNA secondary structure. J. Virol. 656645-6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bockus, B. J., and B. Schaffhausen. 1987. Phosphorylation of polyoma large T antigen: effects of viral mutations and cell growth state. J. Virol. 611147-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogerd, H., and W. C. Greene. 1993. Dominant negative mutants of human T-cell leukemia virus type I Rex and human immunodeficiency virus type 1 Rev fail to multimerize in vivo. J. Virol. 672496-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogerd, H. P., G. L. Huckaby, Y. F. Ahmed, S. M. Hanly, and W. C. Greene. 1991. The type 1 human T-cell leukemia virus (HTLV-I) Rex trans-activator binds directly to the HTLV-I Rex and the type 1 human immunodeficiency virus Rev RNA response elements. Proc. Natl. Acad. Sci. USA 885704-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boxus, M., J. C. Twizere, S. Legros, J. F. Dewulf, R. Kettmann, and L. Willems. 2008. The HTLV-1 Tax interactome. Retrovirology 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cann, A. J., J. D. Rosenblatt, W. Wachsman, N. P. Shah, and I. S. Y. Chen. 1985. Identification of the gene responsible for human T-cell leukemia virus transcriptional regulation. Nature 318571-574. [DOI] [PubMed] [Google Scholar]
- 13.Chen, I. Y., J. McLaughlin, J. C. Gasson, S. C. Clark, and D. W. Golde. 1983. Molecular characterization of genome of a novel human T-cell leukaemia virus. Nature 305502-505. [DOI] [PubMed] [Google Scholar]
- 14.Ciminale, V., L. Zotti, D. M. D'Agostino, and L. Chieco-Bianchi. 1997. Inhibition of human T-cell leukemia virus type 2 Rex function by truncated forms of Rex encoded in alternately spliced mRNAs. J. Virol. 712810-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felber, B. K., H. Paskalis, C. Kleinman-Ewing, F. Wong-Staal, and G. N. Pavlakis. 1985. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science 229675-679. [DOI] [PubMed] [Google Scholar]
- 16.Gatza, M. L., J. C. Watt, and S. Marriott. 2003. Cellular transformation by the HTLV-I Tax protein, a jack-of-all-trades. Oncogene 225141-5149. [DOI] [PubMed] [Google Scholar]
- 17.Green, P. L., T. M. Ross, I. S. Y. Chen, and S. Pettiford. 1995. Human T-cell leukemia virus type II nucleotide sequences between env and the last exon of tax/rex are not required for viral replication or cellular transformation. J. Virol. 69387-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green, P. L., Y. Xie, and I. S. Y. Chen. 1991. The Rex proteins of human T-cell leukemia virus type II differ by serine phosphorylation. J. Virol. 65546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green, P. L., M. T. Yip, Y. Xie, and I. S. Y. Chen. 1992. Phosphorylation regulates RNA binding by the human T-cell leukemia virus Rex protein. J. Virol. 664325-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammes, S. R., and W. C. Green. 1993. Multiple arginine residues within the basic domain of HTLV-1 Rex are required for specific RNA binding and function. Virology 19341-49. [DOI] [PubMed] [Google Scholar]
- 21.Hidaka, M., J. Inoue, M. Yoshida, and M. Seiki. 1988. Post transcriptional regulator (rex) of HTLV-I initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 7519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hope, T. J., B. L. Bond, B. McDonald, N. P. Klein, and T. G. Parslow. 1991. Effector domains of human immunodeficiency virus type 1 Rev and human T-cell leukemia virus type I Rex are functionally interchangeable and share an essential peptide motif. J. Virol. 656001-6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue, J. I., M. Yoshida, and M. Seiki. 1987. Transcriptional (p40x) and post-transcriptional (p27xIII) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc. Natl. Acad. Sci. USA 843653-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson, S., H. Shida, D. E. McFarlin, A. S. Fauci, and S. Koenig. 1990. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature 348245-248. [DOI] [PubMed] [Google Scholar]
- 25.Jeffery, K. J., K. Usuku, S. E. Hall, W. Matsumoto, G. P. Taylor, J. Procter, M. Bunce, G. S. Ogg, K. I. Welsh, J. N. Weber, A. L. Lloyd, M. A. Nowak, M. Nagai, D. Kodama, S. Izumo, M. Osame, and C. R. Bangham. 1999. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc. Natl. Acad. Sci. USA 963848-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kannagi, M., H. Shida, H. Igarashi, K. Kuruma, H. Murai, Y. Aono, I. Maruyama, M. Osame, T. Hattori, H. Inoko, et al. 1992. Target epitope in the Tax protein of human T-cell leukemia virus type I recognized by class I major histocompatibility complex-restricted cytotoxic T cells. J. Virol. 662928-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, J. H., P. A. Kaufman, S. M. Hanly, L. T. Rimsky, and W. C. Greene. 1991. Rex transregulation of human T-cell leukemia virus type II gene expression. J. Virol. 65405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kusuhara, K., M. Anderson, S. M. Pettiford, and P. L. Green. 1999. Human T-cell leukemia virus type 2 Rex protein increases stability and promotes nuclear to cytoplasmic transport of gag/pol and env RNAs. J. Virol. 738112-8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lairmore, M., and G. Franchini. 2007. Human T-cell leukemia virus types 1 and 2, p. 2071-2106. In B. Fields, D. Knipe, P. Howley, R. Chanock, T. Monath, J. Melnick, B. Roizman, and S. Straus (ed.), Fields virology, vol. 5. Lippincott Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 30.Mulloy, J. C., T. Kislyakova, A. Cereseto, L. Casareto, A. LoMonico, J. Fullen, M. V. Lorenzi, A. Cara, C. Nicot, C. Giam, and G. Franchini. 1998. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J. Virol. 728852-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narayan, M., K. Kusuhara, and P. L. Green. 2001. Phosphorylation of two serine residues regulates human T-cell leukemia virus type 2 Rex function. J. Virol. 758440-8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narayan, M., I. Younis, D. M. D'Agostino, and P. L. Green. 2003. Functional domain structure of human T-cell leukemia virus type 2 Rex. J. Virol. 7712829-12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen, T., P. J. Sherratt, H. C. Huang, C. S. Yang, and C. B. Pickett. 2003. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element: degradation of Nrf2 by the 26 S proteasome. J. Biol. Chem. 2784536-4541. [DOI] [PubMed] [Google Scholar]
- 34.Palmeri, D., and M. H. Malim. 1996. The human T-cell leukemia virus type 1 posttranscriptional trans-activator Rex contains a nuclear export signal. J. Virol. 706442-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persaud, D., J. L. Munoz, S. L. Tarsis, E. S. Parks, and W. P. Parks. 1995. Time course and cytokine dependence of human T-cell lymphotropic virus type 1 T-lymphocyte transformation as revealed by a microtiter infectivity assay. J. Virol. 696297-6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramadan, E., M. Ward, X. Guo, S. S. Durkin, A. Sawyer, M. Vilela, C. Osgood, A. Pothen, and O. J. Semmes. 2008. Physical and in silico approaches identify DNA-PK in a Tax DNA-damage response interactome. Retrovirology 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ressler, S., G. F. Morris, and S. J. Marriott. 1997. Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J. Virol. 711181-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rimsky, L., M. Duc Dodon, E. P. Dixon, and W. C. Greene. 1989. Trans-dominant inactivation of HTLV-I and HIV-1 gene expression by mutation of the HTLV-I Rex transactivator. Nature 341453-456. [DOI] [PubMed] [Google Scholar]
- 39.Robek, M. D., and L. Ratner. 1999. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 734856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross, T. M., M. Narayan, Z. Y. Fang, A. C. Minella, and P. L. Green. 2000. Human T-cell leukemia virus type 2 Tax mutants that selectively abrogate NFκB or CREB/ATF activation fail to transform primary human T cells. J. Virol. 742655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross, T. M., S. M. Pettiford, and P. L. Green. 1996. The tax gene of human T-cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J. Virol. 705194-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitt, I., O. Rosin, P. Rohwer, M. Gossen, and R. Grassmann. 1998. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J. Virol. 72633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverman, L. R., A. J. Phipps, A. Montgomery, L. Ratner, and M. D. Lairmore. 2004. Human T-cell lymphotropic virus type 1 open reading frame II-encoded p30II is required for in vivo replication: evidence of in vivo reversion. J. Virol. 783837-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siomi, H., H. Shida, S. H. Nam, T. Nosaka, M. Maki, and M. Hatanaka. 1988. Sequence requirements for nucleolar localization of human T cell leukemia virus type I pX protein, which regulates viral RNA processing. Cell 55197-209. [DOI] [PubMed] [Google Scholar]
- 45.Smith, C. L., C. Debouck, M. Rosenberg, and J. S. Culp. 1989. Phosphorylation of serine residue 89 of human adenovirus E1A proteins is responsible for their characteristic electrophoretic mobility shifts, and its mutation affects biological function. J. Virol. 631569-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vine, A. M., A. D. Witkover, A. L. Lloyd, K. J. Jeffery, A. Siddiqui, S. E. Marshall, M. Bunce, N. Eiraku, S. Izumo, K. Usuku, M. Osame, and C. R. Bangham. 2002. Polygenic control of human T lymphotropic virus type I (HTLV-I) provirus load and the risk of HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Infect. Dis. 186932-939. [DOI] [PubMed] [Google Scholar]
- 47.Weichselbraun, I., J. Berger, M. Dobrovnik, H. Bogerd, R. Grassmann, W. C. Greene, J. Hauber, and E. Böhnlein. 1992. Dominant-negative mutants are clustered in a domain of the human T-cell leukemia virus type I Rex protein: implications for trans dominance. J. Virol. 664540-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weichselbraun, I., G. K. Farrington, J. R. Rusche, E. Bohnlein, and J. Hauber. 1992. Definition of human immunodeficiency virus type 1 Rev and human T-cell leukemia virus type 1 Rex protein activation domain by functional exchange. J. Virol. 662583-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie, L., and P. L. Green. 2005. Envelope is a major viral determinant of the distinct in vitro cellular transformation tropism of human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2. J. Virol. 7914536-14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie, L., B. Yamamoto, A. Haoudi, O. J. Semmes, and P. L. Green. 2006. PDZ binding motif of HTLV-1 Tax promotes virus-mediated T-cell proliferation in vitro and persistence in vivo. Blood 1071980-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, X. J. 2005. Multisite protein modification and intramolecular signaling. Oncogene 241653-1662. [DOI] [PubMed] [Google Scholar]
- 52.Ye, J., L. Xie, and P. L. Green. 2003. Tax and overlapping Rex sequences do not confer the distinct transformation tropisms of human T-cell leukemia virus types 1 and 2. J. Virol. 777728-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yip, M. T., W. S. Dynan, P. L. Green, A. C. Black, S. J. Arrigo, A. Torbati, S. Heaphy, C. Ruland, J. D. Rosenblatt, and I. S. Y. Chen. 1991. Human T-cell leukemia virus (HTLV) type II Rex protein binds specifically to RNA sequences of the HTLV long terminal repeat but poorly to the human immunodeficiency virus type 1 Rev-responsive element. J. Virol. 652261-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida, M. 2005. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene 245931-5937. [DOI] [PubMed] [Google Scholar]
- 55.Younis, I., and P. L. Green. 2005. The human T-cell leukemia virus Rex protein. Front. Biosci. 10431-445. [DOI] [PMC free article] [PubMed] [Google Scholar]