Abstract
Purpose
This review is intended to bring to the informed reader the current state of knowledge about meibomian lipids and the art for analyzing them.
Methods
At the forefront of any endeavor, there are controversies, and these, along with future directions in the field, are brought to the reader's attention.
Results
Function and anatomy of meibomian glands are briefly covered, giving insight into possible mechanisms for secretory controls. Anatomically, some anomalies in meibomian gland distribution of different species, such as whales versus dolphins, are presented, and, for the first time, the structure of the meibomian glands in a selection of marsupials is presented. In attempting to make the literature more accessible, lipid structure and nomenclature are described, and these structures are related to their possible effects on the physicochemical properties of meibomian lipids. The advantages and disadvantages of various collection and storage techniques are described, as well as how gas chromatography and combined HPLC and mass spectrometry coupled with fragmentation are currently enabling us to determine the nature of the lipids in very small samples.
Conclusions
This review extends to discussing the lipids in tears (as opposed to meibomian gland lipids) and briefly highlights new thoughts about the interactions between proteins of the tear film and meibomian lipids. A model that includes proteins in the outer layer of the tear film is also presented. This model is currently being critically analyzed by the ocular community. It concludes briefly by highlighting possible further areas of research in this area.
Keywords: meibomian lipids, HPLC, mass spectroscopy, fragmentation, marsupial
INTRODUCTION
Meibomian lipids are of great interest because of their association with a stable tear film. They are traditionally thought to form a ~100 nm thick blanket (a minimum of 20 molecules thick based on end-on-end alignment of acyl chains of lipids, but likely to be many more if disordered) over the aqueous part of the tear film, and this film can be observed in situ using interference microscopy.1-4 The interference pattern and colors have been graded and associated with tear film stability. In particular, an amorphous thick lipid film has been associated with a stable tear film, whereas a thin patterned film has been associated with dry eye. Logic suggests that variance in these lipid films is associated with variations in the individual lipid components. Consequently, there have been many studies analyzing the composition of meibomian lipids. Until very recently, the technology and techniques available for analyzing lipids have been limited, and only a few dedicated research groups have made inroads into the chemical composition and physicochemical analysis of meibomian lipids. A major advantage of the newer techniques and technology has been the ability to cope with small sample volumes. Formerly, there has been a primary reliance on animal samples and pooled samples from humans. In parallel have been developments in commercially available solvents and lipid standards, which have eliminated the need for in-house distillation and purification of solvents and synthesis of standards. While contaminants in solvents can generally be subtracted HPLC-mass spectrometric analysis (HPLC-MS) because they form a stable background signal, in physical measurements, such as surface tension, they cannot. Analysis of proteins has also had similar advancements, resulting in more detailed knowledge about tear proteins in the tear film, often including their three-dimensional structure. Consequently, more intricate models of the tear film, which incorporate interactions of lipids and proteins, are now being considered.
Although these improvements have enabled better analysis of lipid composition, they have also meant that minimizing contamination at all stages of the sampling and analysis process is paramount to obtaining valid results. The state of the art in the field is to determine the detailed composition of meibomian lipids and their molar ratios, and to try to relate variants with the performance of the lipid layer. Being a thin film, it theoretically requires only small differences in a small number of molecules to cause large changes in performance. Currently, neither the importance of slight variants in major components nor possible roles of minor components is known, and hence the necessity for careful and detailed evaluation. Consequently, the literature is full of details that are difficult for the casual reader to appreciate. Therefore, a main purpose of this review is to facilitate interpretation of the literature and how to deal with results which have apparent differences between groups. It is highly recommended that in conjunction with this review, the papers by McDonald1 (a readable view and explanation of terminology associated with the physical aspects of surface activity of the lipid layer of the tear film), Bron et al.4 (an overview of the relationship between the lipid layer and the tear film) should be read, and a couple of older but relevant reviews by Tiffany.5,6
Meibomian Glands and Their Control
Meibomian lipids are released onto the ocular surface from small openings in the eyelid margins. In humans, there are 30-40 openings in the upper lid and 20-30 in the lower lid corresponding to meibomian glands located in the tarsal plates.7-9 These are holocrine glands (whole contents of cells are secreted), and in rats there is a continuous division of stem cells at the base of the acini. It takes about 9 days for a cell to mature and be secreted.10 It has been shown that lipid release occurs during blinking due to the gentle pressure that occurs on the lids.11-13 However, morphological studies show that human meibomian glands are innervated by nerves positive for vasoactive intestinal polypeptide,14 and these could have a role in control of secretion. In contrast, detection of expression of the purinergic receptor (P2Y2) gene in meibomian gland cells from rabbit and rhesus macaque15 is more likely to indicate that ATP stimulation causes changes to lipid composition. This is because P2Y2 receptors are typically coupled to G proteins that effect second messenger pathways associated with enzyme activity modification, rather than simply stimulate secretion.15
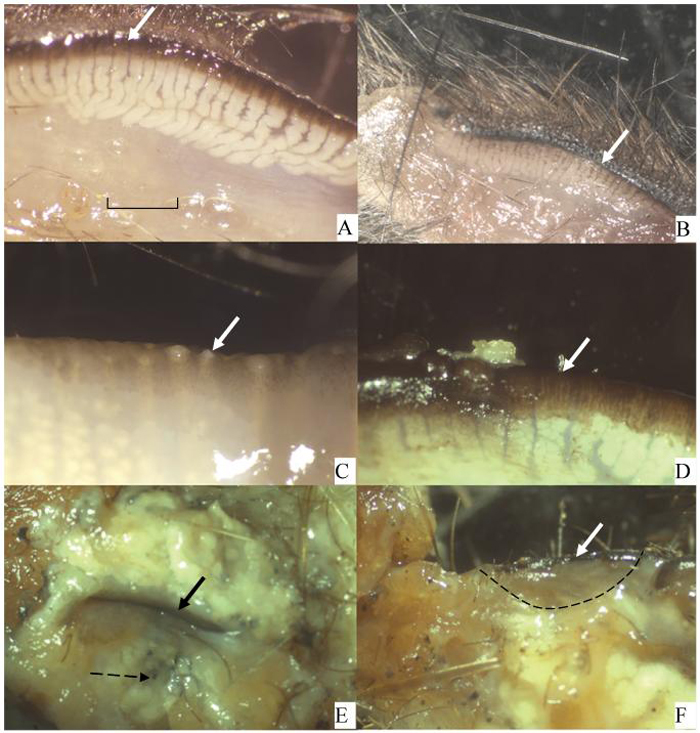
Given that one of the prime functions assigned to the outer lipid layer of the tear film is to prevent evaporation, it would be unsurprising to read that whales have no meibomian glands;16 however, dolphins do.17 Another group of animals, the voles and muskrats, provide further curiosity. Most of these species are like us, with glands spread across both the upper and lower lids, but others, such as Microtus richardsoni (water vole), have practically no glands in the lower lids, and some Microtus oregoni (creeping vole) and Ondatra zibethica (American muskrat) have glands only at the inner and outer canthi.18 Such unusual distribution of the glands does not readily support the idea that meibomian lipids prevent skin lipids from contaminating the ocular surface and preventing spillage of tears over the lid margin, whereas an evenly distributed row of meibomian gland orifices across the whole eyelid does. In preparing for this review, there did not seem to be information available on marsupials, so we have now examined several species. A limited selection of marsupials* show that they all have meibomian glands distributed across the upper and lower lids (Fig. 1). The one monotreme studied, Tachyglossus aculeatus (echidna), had no meibomian glands, but instead a large fat pad at the base of the eyelids formed from sebaceous glands (Fig. 1F). Many species, e.g., rabbit, but not primates, have a harderian gland that also contributes oily secretions to the ocular surface.19,20 This variance of anatomical structure between species is sufficient to prevent convincing conclusions about functions of meibomian lipids, and hence it has been necessary to return to detailed analysis of their composition to attempt to obtain functional information.
FIGURE 1.
View of inner tarsal plate from various marsupials and an echidna. Bold arrow indicates eyelid margin. The meibomian glands are visible below this. (A) Kultarr (Antechinmys laniger). An endangered desert dwelling species. (B) Antechinus (Antechinus stuartii). Coastal forest dweller. (C) Koala (Phascolarctos cinereus). Tree dweller living in coastal forests. Just left of the arrow are large meibomian gland openings. (D) Red necked wallaby (Macropus rufogriseus). A coastal plain dweller. Just left of the arrow is a meibomian gland opening with a small amount of meibomian oil extruded. (E) Echidna (Tachyglossus aculeatus). Both eyelids are present surrounding the ocular slit. This region has a massive fat pad consisting of sebaceous glands. The broken arrow points to the end of a hair shaft that is surrounded by the sebaceous gland. (F) Echidna. The edge of one eyelid has been dissected (from lid margin to dotted line) revealing no meibomian glands. Scale in (A) to be used for all figures according to this legend: A = 0.5 mm; B = 2.5 mm; C = 2.0 mm; D = 2.0 mm; E = 2.5 mm; F = 2.5 mm.
Lipid Structure
Meibomian lipids in humans and domestic and laboratory animals have been found to consistently comprise non-polar lipids (wax esters and steroid esters) and a smaller amount of polar lipids. It is important to understand that these are actually families of lipids rather than individual lipids, and variance within a family of lipids is likely to have profound effects on the packing of the lipids at the surface. As a result, an area at the forefront meibomian lipid research is not simply to identify the families of lipids but also the exact chemical structures of different family members and their ratios. It is believed that particular ratios might be associated with disease states, or a more stable lipid layer. To facilitate an understanding of these endeavors, an overview of lipid structure and nomenclature is given. For more details, some very well maintained Web sites are commended (http://www.lipidlibrary.co.uk/index.html; http://www.cyberlipid.org/index.htm), (http://www.lipidmaps.org), and a publication by Fahy et al.,21 outlining systematic naming of lipids.
Fatty Acids and Fatty Alcohols
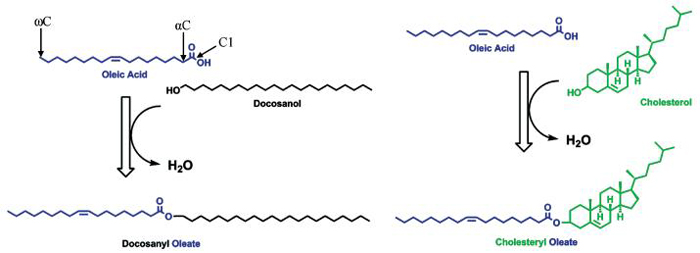
Acetic acid (CH3COOH) is a carboxylic acid soluble in water. In water, it dissociates into its conjugate base, acetate (CH3COO-), and a proton (H+). It is the carboxyl group that is water soluble (or hydrophilic), and as the carbon chain extends (CH3(CH2)nCOOH), the whole molecule becomes more lipid soluble (or hydrophobic). Hence, those with carbon chains of 12 or more (n ≥ 10) are called fatty acids. These have one end that is soluble in water and the other in lipids (amphiphilic), and the presence of such molecules in tears are of interest as they are able to create an interface between aqueous and lipid environments, thus helping to stabilize the inherently thermodynamically unstable mixtures of lipids and water. These types of molecules are also called surface active compounds (or surfactants). A similar situation occurs for alcohols. Methanol (CH3OH), ethanol (CH3CH2OH), and propanol (CH3CH2CH2OH) are infinitely soluble in water due to the very hydrophilic hydroxyl group and their relatively short carbon chains, while extending the carbon chain (CH3(CH2)nCH2OH) progressively makes alcohols more lipid soluble. These are then known as fatty alcohols. Biochemically, the basic building block for fatty acids and alcohols is acetic acid, and hence they typically have an even number of carbon atoms. It should be noted that significant amounts of odd-carbon and branched-chain fatty acids have been reported from meibomian lipid extracts.22,23 Some fatty acids and alcohols have one or more double bonds and are called mono- or polyunsaturated. Carbons are not free to rotate around the double bond and hence has a cis- (new standard nomenclature is Z) or a trans- (new standard nomenclature is E) conformation. Biologically, this bond is predominantly in a cis-conformation which places a fixed bend in the molecule. The presence of this bend makes it difficult for these molecules to pack closely with other fatty acids or fatty alcohols, especially saturated ones, and hence they have a lower melting temperature. This is important for meibomian lipids because, to form a compact strong film, they would be expected to pack densely with each other (saturated), but to be able to spread readily across the ocular surface they would be expected to be very fluid (unsaturated). The position of the double bonds can be defined in two different ways: one counts the bonds from the first carbon (most common chemically; e.g., 6Z in the name means the sixth bond from C1 is a cis double bond) or bonds are counted backwards from the last carbon. The last carbon, irrespective of the chain length, is known as the omega carbon (most common for nutritional biochemistry; e.g., an omega-3 (read omega minus three) fatty acid has a cis double bond 3 bonds back from the last carbon). Oleic acid, common in meibomian lipids, is an omega-9 fatty acid (Fig. 2). Double bonds, especially multiple ones, are a very important consideration for storing the lipids before analysis. They are subject to oxidation and, if this were to occur, it would often result in a cascade of oxygen-free radicals being produced, which could lead to uncontrolled formation of multiple oxidation products that include hydroperoxides, hydroxides, epoxides, ketones, and various carbon- and oxygen-centered free radicals.24,25 Spontaneous Z → E isomerization of double bonds, cleavage of lipids, removal of double bonds, and other structural changes that would give misleading details about the lipid structure can also occur. If there is enough sample, these processes can be monitored by reanalyzing the sample over a period of time.
FIGURE 2.
Wax and sterol esters. Oleic acid C18:1:9Z is an 18-carbon fatty acid with a cis double bond 9 bonds away from C1. The same double bond is 9 bonds from the omega or last carbon (ωC), so it is an omega-9 fatty acid. It condenses with a long-chain alcohol, which results in an ester bond to form a wax ester. Similarly, a condensation with a sterol (in this case, cholesterol) will form a sterol ester. These are families of molecules because the fatty acid, alcohol, or sterol may vary.
Waxes and Sterol Esters
Waxes are formed by a condensation between a fatty acid and a fatty alcohol (Fig. 2). The bond formed by a condensation between an acid and alcohol is called an ester bond. Although the ester bond is slightly hydrophilic, it is overwhelmed by the hydrophobic carbon chains on either side of the bond, and hence waxes are very hydrophobic. Sterol esters are similar, except a sterol replaces the fatty alcohol (Fig. 2). In meibomian lipids, the major sterol appears to be cholesterol and/or epi-cholesterol, although dihydrolanosterol has been reported as the major sterol in rabbits.22 Hence, most of the sterol esters are referred to as cholesterol esters, which are again very hydrophobic due to the dominance of the sterol ring and the hydrocarbon chain. The meibomian gland enzymes, which catalyze these condensations, appear to be nonspecific, and hence predominating wax or sterol ester (a combination of a particular fatty acid with a particular alcohol or sterol) reflect the pool of fatty acids and fatty alcohols synthesized by the meibomian gland of the particular species.22,23 Cholesterol levels in meibomian lipids reportedly bear no relationship to plasma cholesterol levels.26 This means that lipids are not being taken up from the blood by the meibomian gland cells, but instead are being synthesized locally. This has been shown in the rabbit using radioactively labeled acetate and some amino acids27 and is consistent with findings showing that androgens control the levels of the enzymes in the pathway for steroid synthesis in meibomian gland cells.28
Glycerolipids (Monoacyl-, Diacyl- and Triacylglycerols) and Phospholipids
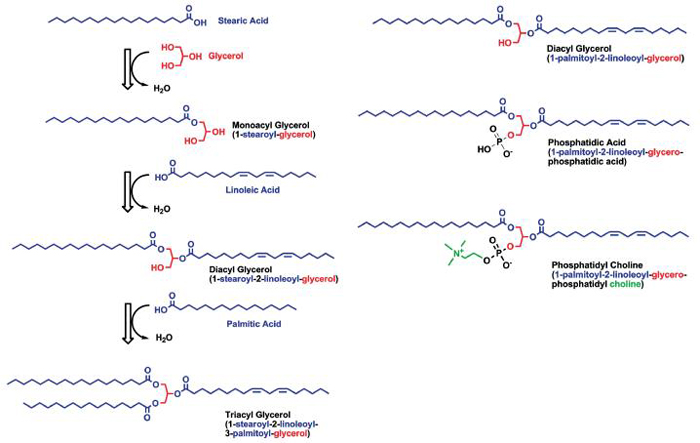
The alcohol can also be glycerol, which is a three-carbon backbone with a hydroxyl group on each carbon. Ester bonds can be formed at each of these carbons with different (or the same) fatty acids. With one fatty acid attached, it is a monoacylglycerol, and with two a diacylglycerol (Fig. 3). The free hydroxyl group/s make this part of the molecule remain water soluble, so these molecules are amphiphilic and expected to lie at water lipid interfaces. When glycerol has three fatty acids attached, it is a triacylglycerol, and having no free hydroxyl group makes it very hydrophobic.
FIGURE 3.
Glycerolipids are formed by a condensation of various fatty acids to glycerol. For monoacyl- and diacylglycerols, the free hydroxyl groups make this part of the molecule water soluble, while triacylglycerols, with no free hydroxyl, are very hydrophobic. Phospholipids have a phosphate condensed to C3 of glycerol, and this head group is very hydrophilic. An alcohol can further condense onto the phosphate group to form phospholipids. If the acid group were removed at C2 (common) or C1 (uncommon), it would be called a lysophospholipid.
With phosphoric acid esterified to the third carbon instead of a fatty acid, a phospholipid is formed. This general form is called phosphatidic acid. Since phosphoric acid is a trivalent acid, it can form an ester (phosphoester bond) with hydroxyl groups of various alcohols. Some examples of such molecules, which can be bound to the phosphate of phosphatidic acid, are choline to form phosphatidylcholine, serine to form phosphatidylserine, or another glycerol to form phosphatidylglycerol. These all carry some negative charge due to the easily ionizable phosphate group and may carry additional charge depending on the nature of the attached alcohol. The lipids that have both the positive and the negative charges are called zwitterions with a net zero charge. The best-known examples of such phospholipids are phosphatidylcholine and sphingomyelin whose choline residues (with their permanently positively charged quaternary amino group) render the net charge of the phospholipids zero. Others lead to a net negative charge and are called anionic phospholipids, e.g., phosphatidylglycerol. If such phospholipids are lacking an acyl group esterified to the second carbon, they are called lysophospholipids. These are of particular interest because some bacteria have lipases capable of hydrolyzing the ester bonds in glycerides and phospholipids, which would increase the pool of fatty acids and lysophospholipids. There is some evidence that this occurs in blepharitis and meibomianitis, and it could be that this alteration of the normal lipid ratios due to bacterial lipases may be the cause of dry eye in these cases.29-31
Collection Techniques, Contamination, and Storage of Meibomian Lipids
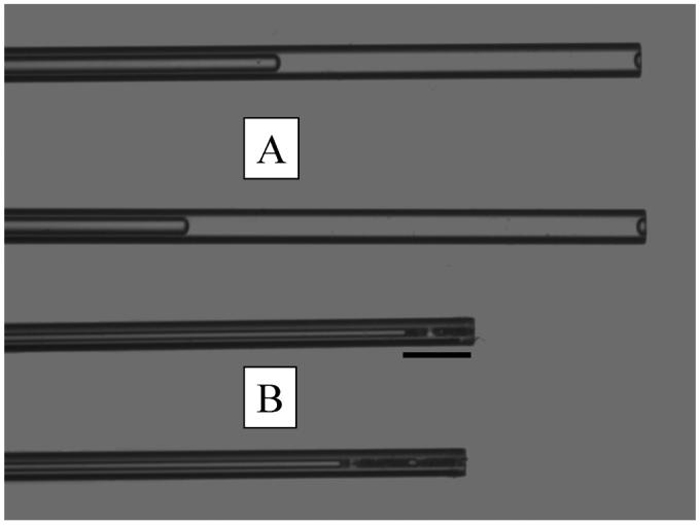
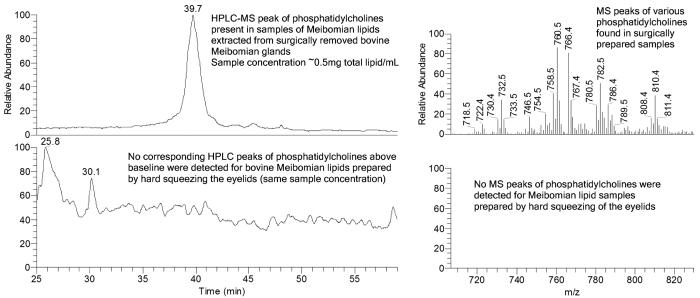
From the above, it is clear that, to obtain accurate data, great care has to be taken to protect the lipids from contamination or breakdown before final analysis. The collection method is the first step that is likely to affect the results, and there has been great variance. The main questions regarding collection are: Is the collection technique more or less likely to be contaminated with skin or hair lipids? Are aqueous tears also being collected? Are cell membranes likely to be part of the sample? Are the samples large? and Are pooled samples being used? Using a microcapillary to collect fluid at the meibomian gland orifices32 has the advantage of being relatively non-invasive and causing little trauma to the eyelid. It has been reported that when microcapillary tubes have been used for meibomian lipid sampling, they rapidly solidify and block the capillary33 because of their relatively high melting point (32°C to 34°C).11,33 However, if Meibomian lipids are mechanically pushed out of the gland using gentle pressure on the outside of the lid, they can be drawn into the microcapillary as a viscous liquid through capillary action (Fig. 4), but it does usually solidify after being drawn into the microcapillary. Therefore, the amounts of lipid material collected this way are typically small (a few micrograms).32 A small amount of tears is sometimes observed, and, therefore, analysis of such samples may include lipids from the aqueous part of the tear film. Schirmer's strips can also be used to collect tear lipids.34 This collection method has similar advantages and disadvantages to capillary tube collection. An additional advantage is that they are routinely used in ophthalmic practices, while a disadvantage is that there is a considerable risk of contamination of the sample with cellular lipids due to conjunctival surface damage. The lipids collected by this technique represent the entire lipid pool in the tear film. Hard expression of Meibomian lipids by squeezing the eyelids has long been used to collect relatively pure samples of meibomian lipids.33,35-38 Such expression results in a worm-like paste being extruded from the meibomian gland orifices (Fig. 1D), which can be collected by running a metal spatula or similar dry device along the eyelid margin. This technique, noted by Nicholaides as “hard squeeze” production,23 has the advantages that it can be used on both live and post-mortem material and results in relatively large amounts of meibomian lipids being collected (~1 mg).37,38 Importantly, no contamination of samples with ceramides-typical markers of skin cells39-has been observed in such samples.37,38 The ability to collect large samples with this method enables the same sample to be chemically analyzed in a number of different ways and tested for its physicochemical properties. It also allows access to detecting minor lipid components. This collection method can be traumatic, and often causes pain. Local anesthetic is relatively ineffective in reducing the pain, but some people suffer no pain by this process. Other disadvantages of this process are that there is risk of skin lipid contamination from the dry collection process, and these samples theoretically would be expected to have an excess of membrane phospholipids due to less mature meibomian gland cells being excreted because of the large force applied to the glands. In practice, membrane phospholipids do not seem to be a contaminant.37,38 There have been a few reports where whole meibomian glands have been removed, homogenized, and extracted for lipid analysis.39,40 This process inevitably leads to uncontrollable contamination by lipids from surrounding tissues. We have done some comparisons where whole bovine meibomian glands have been carefully dissected from the lids, homogenized, and extracted. The purpose of this study was to determine if cellular debris were contaminating samples collected by the forced extrusion method. This did not seem to be the case (Fig. 5), nor did it appear to be the case from previously published work from rabbit.22
FIGURE 4.
Comparative appearance and volumes of tear samples (A) and meibomian lipid samples (B) collected with a micropipette at room temperature. Scale = 1.46 mm.
FIGURE 5.
Comparisons of HPLC and MS spectra obtained from homogenized bovine glands versus extruded lipids.
For all methods, likely and poorly controllable sources of contamination are cell debris, skin cells, and various cosmetic products. These, however, can be easily distinguished by monitoring specific lipids that are pertinent to each of the types of contamination. Cell debris, or whole tears, for example, are the most likely source of phospholipids, and large quantities of ceramides and squalene would be a typical marker of skin lipid contamination.38,39 Hydrocarbons, e.g., squalene, that had been found in some studies41,42 are likely to be contaminants from skin, make-up, and sometimes the laboratory environment. Bacteria could theoretically be a problem because some species have lipases that could break down meibomian lipids. In practice, this never occurs because the lipids are stored in organic solvents immediately after collection, and this not only kills bacteria but also denatures and dilutes proteins.
Incorrect storage can also lead to contamination or breakdown of lipid samples. The samples should be stored anaerobically to prevent oxidation. This can be achieved by storing in an organic solvent or in dry state under argon or nitrogen. Other precautions are avoiding sample exposure to moisture and light; maintaining the sample temperature -20°C or below; and using plastic-free containers (only glass, Teflon®, and stainless steel are recommended).43
In summary, (micro)capillary and Schirmer's test strip techniques are best suited for gathering and analyzing the entire lipid pool that is present in aqueous tears, while hard expressing the meibomian glands produces purer samples of lipids that are biosynthesized by the glands, but cannot be used to evaluate changes to the lipids that might occur after they have been secreted onto the ocular surface, nor does it evaluate tear film lipids originating from sources other than the meibomian gland. The surgical procedure is of limited use for the purpose of lipid analyses unless the researchers need large quantities of the secretions and are willing to accept the risks discussed above.
Analytical Procedures and Their Limitations
Considering the small size of meibomian lipid samples, comprehensive lipidomic analysis of individual human subjects requires methods that have both high sensitivity and selectivity. Many early studies characterized meibomian lipids by thin-layer chromatography (TLC),23,42 nuclear magnetic resonance spectroscopy (NMR),40 gas chromatography (GC),42 and GC mass spectrometry (GC-MS).44-46 Though useful, many of the above techniques have their shortcomings and are not well suited for analyzing small samples. TLC-based experiments22,47,48 lack resolution power and specificity, generally limiting identification to the main classes (all triacylglycerols present, all wax esters present, all sterol esters present) (Table 1). The broad range (last column) from the different studies is a result of variance in analytical methodology employed and the inherent variability in almost all biological samples. Other problems associated with TLC are prolonged exposure of the sample to air, which can lead to sample degradation particularly if acids or bases are used in the developing phase, and destructive charring of the plate for spot identification, which prevents further analysis of the lipids.
TABLE 1.
Comparison of the major lipid composition of normal human meibomian lipids
| Lipid | Cory35 (1973) (%) | Tiffany42 (1978) (%) | Nicolaides23 (1981) (%) | McCulley49 (1997) (%) | Mathers50 (1998) (%) | Range (%) |
|---|---|---|---|---|---|---|
| HC | - | 26-38 | - | 1 | - | 1-38 |
| WE | 64a | 13-23 | 35 | 68 | 51 | 13-68 |
| SE | Seea | 8-34 | 30 | 16 | 39 | 8-39 |
| Diesters | 18 | - | 8 | - | 2 | 2-18 |
| TG | 2 | 11-43 | 4 | 6 | 3 | 2-43 |
| Alcs | 5 | 0-2 | 2 | - | - | 0-5 |
| FFA | 10 | 0-24 | 2 | 1.0 | 3 | 0-24 |
| Ch | - | - | - | - | 1 | 0-1 |
| Polar | - | 0-5 | 16 | 4 | - | 0-16 |
HC hydrocarbon, WE wax ester, SE sterol ester, TG triglycerol, Alcs alcohols, FFA free fatty acids, Ch cholesterol, Polar polar lipids.
Composite of WE and SE.
For further analysis of the major lipid classes, the spots are scraped from the plate after non-destructive staining. Iodine vapor can be used for spot identification, but saturated lipids give little or no signal so could be missed altogether. Once scraped, the lipids are extracted and commonly analyzed with GC, GC-MS, or NMR. Before the GC and GC-MS analyses, the samples must be derivatized with other reagents to increase volatility or the samples are labeled with a chromophore or fluorophore for detection in a spectrophotometer. This process typically involves partial hydrolysis and transesterification and/or silylation of complex higher molecular weight lipids, e.g., wax esters and cholesteryl esters.42,51 Sample recovery during the derivatization procedures can be an issue, because lipid species in low quantities may not be recovered in sufficient amount to be detected. Although GC and GC-MS are sensitive, the analysis may take hours and is conducted at high (destructive) temperatures, which could compromise the analytes by inadvertent isomerization and/or decomposition of labile molecules. Reportedly, higher molecular weight analytes (above C30 equivalent chain length) have not been successfully determined by this method.42 Another serious problem arises from the hydrolysis because it is very difficult, if not impossible, to find which combination of products existed in the parent compounds. Hence, the hydrolysis products such as fatty acids and alcohols in case of cholesteryl esters and wax esters46,51,52 are reported, and not the parent compounds. By contrast, NMR is non-destructive but lacks the necessary sensitivity, necessitating very long NMR signal accumulation times40,53 to resolve lipids, which are in low amounts.
It is now possible to overcome most of these problems by combining technologies. HPLC can be used to separate main lipid classes. The peaks can be observed using either ultraviolet light or evaporative light scattering, or atmospheric pressure ionization mass spectrometry (API MS). In recent studies on meibomian lipids, two major types of API MS have been used: electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) MS.38,52,54,55 Choosing a particular MS method (ESI or APCI) strongly depends on the type of lipid samples that are to be analyzed. General consensus among mass-spectrometrists is that ESI is best suited for analyzing polar and/or easily ionizable compounds like carbohydrates, nucleotides, amino acids, peptides, and proteins, while APCI works best for relatively non-polar compounds like triacylglycerols, wax esters, aromatic compounds, etc. Due to their ionic nature, phospholipids are frequently analyzed using the ESI technique, especially in direct infusion experiments.55,56 However, APCI has been equally successfully used to detect various (phospho)lipids in HPLC-style experiments with human meibomian lipids37,38 and is much more suitable than ESI for analyzing non-polar lipids. A sensitivity of below the nanogram level can be achieved with API MS methods, and it is relatively fast if ion trap technology is used. Ion trap instruments capable of multi-stage fragmentation of ions (a.k.a. MSn-capable mass spectrometers) enable the parent ions to be sequentially fragmented within the same run, enabling structural elucidation of complex molecules. Importantly, the current higher-resolution MSn instruments are able to resolve complex mixtures of analytes without prior chromatographic separation. This is an invaluable tool when analyzing mixtures of compounds that belong to different groups, e.g., polar and non-polar lipids that are notoriously difficult to separate in one run using modern chromatographic equipment.
Though qualitative analysis of lipid mixtures is relatively straightforward, quantifying the individual components of the same lipid mixtures is a formidable task. Ideally, a number of pure chemical standards are chosen to be as close as possible chemically to the analytes (their 2H- and 13C-labeled derivatives are the best, but expensive, standards). These are used to establish a calibration (dose-signal) curve for the particular lipid. Importantly, the dose-signal curves are not always linear and may dramatically differ even for two closely related compounds. This necessitates using as many standards as possible. In most reports on meibomian lipids, there is no evidence that such calibration has been done, and so the quantitative data available needs to be treated with caution.
Current Areas of Interest and Controversies
Whole Tear Film Lipids
One area of interest is whether the lipid composition in whole tears differs from that of meibomian lipids, and whether there is transport of lipids from one compartment (the aqueous mucous layer) to the other (lipid layer) or vice versa. Associated with this are the questions: How are the lipids in the aqueous phase of the tear film emulsified? and If they are substantially different from the meibomian lipid composition, where do they come from?
Semi-quantitative studies of human tear lipids have reported the major classes observed in meibomian gland lipids (Table 2). Wollensak et al.,57 using high-performance thin-layer chromatography (HPTLC), found a mean total tear lipid amount of 1.8 ± 0.8 g/L, which was substantially different from a preliminary study by Stuchell et al.,58 who reported a concentration of 0.13 g of lipids/L. However, they used a combination of silicic acid column fractionation and quantitative thin-layer chromatography. Notable was the relatively high levels of free cholesterol in whole tears and the high levels of glycerolipids by Stuchell at 55%. In a recent study,59 electrospray tandem mass spectrometry was used to identify and compare the non-polar lipids of rabbit tears, showing that the major species of mono-acyl, diacyl, and triacylglycerols increased in the dry eye condition (Table 3). In subsequent work,55,60 they used matrix-assisted laser desorption ionization (MALDI) and a unique extraction procedure to further characterize the phosphorylated lipids of the tears from rabbits and some in human. The presence of polar lipids in human tears has been confirmed by a different technique: Fourier transform infrared (FTIR) spectroscopy.61 In this study, tears were collected using Schirmer strips and the lipids extracted from these. They were pooled data from several people, but clearly indicated a possibility of phospholipids being present in human tears.
TABLE 2.
Comparison of normal human meibomian gland secretion and whole tear lipid composition
| Lipid | Meibum (%) from Table 1 | Tear (%) Stuchell et al.58 | Tear (%) Wollensak et al.57 |
|---|---|---|---|
| Wax esters | 13-68 | - | 45a |
| Cholesterol esters | 8-39 | 9.7 | Seea |
| Free cholesterol | 0-1 | 7.1 | 15 |
| Free fatty acids | 0-24 | 18.3 | <15 |
| Triacylglycerols | 2-43 | 6.9 | <15 |
| Diesters | 2-18 | - | - |
| Hydrocarbons | 1.38 | - | - |
| Polar lipids | 0-16 | 0.9 | 15 |
| Alcohols | 0-5 | - | - |
| Glycolipids | - | 55.0 | - |
Wax and cholesterol esters combined.
TABLE 3.
Comparison of quantitative results of normal and dry eye rabbit tear lipid extractsa
| Compound identification | Li+ Adduct (m/z) | Normal eye (mg/μL) | Dry eye (mg/μL) | Percent increase (%) |
|---|---|---|---|---|
| Free glycerol | 99 | 9.6 × 10-4 ± 2.3 × 10-4 | 1.5 × 10-3 ± 2.7 × 10-4 | 56 |
| Glyceryl-isoprene acetal | 153 | 6.8 × 10-4 ± 2.7 × 10-4 | 1.7 × 10-3 ± 8.5 × 10-4 | 150 |
| Glyceryl-isoprene acetal | 287 | 7.5 × 10-4 ± 2.9 × 10-4 | 1.8 × 10-3 ± 9.2 × 10-4 | 140 |
| Monopalmitin | 337 | 7.4 × 10-4 ± 1.6 × 10-4 | 1.1 × 10-3 ± 2.0 × 10-4 | 49 |
| Monostearin | 365 | 1.6 × 10-3 ± 3.4 × 10-4 | 2.5 × 10-3 ± 2.5 × 10-4 | 56 |
| Glyceryl-isoprene acetal | 421 | 1.2 × 10-3 ± 3.7 × 10-4 | 2.9 × 10-3 ± 1.6 × 10-3 | 142 |
| 1,3-dipalmitin (major) 1-stearin-3-myristin (minor) 1-myristin-2-stearin (minor) | 575 | 1.4 × 10-4 ± 3.1 × 10-5 | 2.1 × 10-4 ± 2.3 × 10-5 | 50 |
| 1-stearin-3-palmitin (major) | 603 | 5.2 × 10-4 ± 9.5 × 10-5 | 7.3 × 10-4 ± 2.7 × 10-5 | 40 |
| 1,3-distearin (major) | 631 | 5.1 × 10-4 ± 9.0 × 10-5 | 7.2 × 10-4 ± 1.3 × 10-5 | 41 |
| Tristearin (major) 1-arachidin-2-stearin-3-palmitin (minor) | 897 | 1.3 × 10-4 ± 5.5 × 10-5 | 1.8 × 10-4 ± 9.9 × 10-5 | 38 |
| TOTAL | 7.3 × 10-3 ± 7.1 × 10-4 | 1.4 × 10-2 ± 2.1 × 10-3 | 92 |
Analyzed by ESI mass spectroscopy as described earlier.59
Meibomian Gland Lipids
For meibomian gland secretions, an issue of contention centers on the nature of polar lipids, particularly phospholipids, and what role they might play in stabilizing the lipid film. Some publications have reported very low to no phospholipids,37,38,47,61 while others report their presence.52,62-64 This issue is particularly important for modeling the lipid layer, because phospholipids are strong surfactants and can act as the interface between the aqueous layer and the non-polar lipid surface layer. A model of this kind was proposed by McCulley and Shine,49 and this model has provided a significant foundation for further understanding and modeling of the lipid layer. If phospholipids were not a component of meibomian lipids, then would the phospholipids reported in whole tears adsorb to the lipid layer and act as surfactants, or would other molecules of the aqueous (proteins) act as surfactants?
In an attempt to clarify this issue, we carried out a study where one of us collected samples of meibomian lipids from different species and whole tears from humans, and another analyzed the material blind. Rabbit meibomian lipids had substantial amounts of phospholipids, whereas steers, cats, and humans had levels that were extremely low and very difficult to resolve from the noise (to be published elsewhere). Some reports from the literature40,53,65 are shown in Table 4.
TABLE 4.
Comparison of human and rabbit meibomian gland secretion phospholipid composition
| Phospholipid | Human65 (%) | Rabbit40 tarsal plate only (mole% ± SD) | Rabbit32 meibum oil only (mole% ± SD) | Rabbit53 tarsal plate + meibum (mole% ± SD) |
|---|---|---|---|---|
| PG | - | - | - | 0.18 ± 0.08 |
| LEPLAS | - | 0.27 ± 0.21 | - | - |
| LPE | - | 0.30 ± 0.22 | - | 0.22 ± 0.11 |
| LPS | - | 0.23 ± 0.14 | - | - |
| PA | - | - | - | 0.39 ± 0.17 |
| DPG | - | 3.38 ± 0.36 | 2.42 ± 0.36 | 3.17 ± 0.26 |
| DHSM | - | 3.09 ± 0.37 | 5.34 ± 0.36 | 1.88 ± 0.16 |
| EPLAS | - | 13.33 ± 0.46 | 8.55 ± 0.36 | 9.37 ± 0.26 |
| PE | 13 | 13.09 ± 0.55 | 18.02 ± 6.9 | 18.29 ± 032 |
| PS | - | 9.23 ± 0.42 | 6.92 ± 0.36 | 6.99 ± 0.33 |
| SM | 5 | 9.52 ± 1.12 | 8.40 ± 0.36 | 7.70 ± 0.23 |
| DIMEPE | - | 0.27 ± 0.21 | - | - |
| LPC | - | 1.35 ± 0.26 | 1.69 ± 0.36 | 1.01 ± 0.04 |
| PI | - | 5.16 ± 0.36 | 4.79 ± 0.36 | 5.89 ± 0.17 |
| SPC | - | 0.31 ± 0.08 | - | 0.54 ± 0.06 |
| AAPC | - | 2.71 ± 0.47 | 3.53 ± 0.36 | 1.94 ± 0.78 |
| PC | 27 | 37.78 ± 0.48 | 40.34 ± 0.36 | 42.43 ± 0.75 |
| CE | 6 | - | - | - |
| CB | 24 | - | - | - |
| Unknowns | 27 | - | - | - |
PG = phosphatidylglycerol, LEPLAS = lysoethanolamine plasmalogen, LPE = lysophosphatidylethanolamine, LPS = lysophosphatidylserine, PA = phosphatidic acid, DPG diphosphatidylglycerol, DHSM = dihydrosphingomyelin, EPLAS = ethanolamine plasmalogen, PE = phosphatidylethanolamine, PS = phosphatidylserine, SM = sphingomyelin, DIMEPE = dimethylphosphatidylethanolamine, LPC = lysophosphatidylcholine, PI = phosphatidylinositol, SPC = sphingosylphosphorylcholine, AAPC = alkylacylphosphatidylcholine, PC = phosphatidylcholine, CE = ceramides, CB = cerebrosides.
In contrast to meibomian lipids, the blind study confirmed the presence of phospholipids in whole human tears similar to that previously reported.55,60 It is presumed that other mammals also have phospholipids in their tears, but this is yet to be shown. The results from this blind study help to clarify the variability in the literature. Relatively large quantities of phospholipids have consistently been found in rabbit meibomian lipid samples,40,53 but not from human material.37,38,61 Butovich et al.37,38 have recently revisited the major wax esters in normal human meibomian lipids using HPLC ion trap mass spectrometry. Oleic acid was found to be the main fatty acid associated with wax esters, and fatty alcohol moieties ranged from C18:0 to C30:0. The sterol esters were cholesterol esters, and free cholesterol was also found. Importantly, they did not observe any phosphorylated lipid signals above background noise in human meibomian lipid samples. Borchman et al.61 supported these observations by finding no phosphate signal in extruded human meibomian gland lipids using Fourier transform infrared (FTIR) spectroscopy. When phospholipids have been detected, it is likely that some tear sample has been collected as well. In other cases, some of the meibomian gland cells might have been sampled and so contaminated the sample with membrane phospholipids.
Some specific lipids have also provoked interest. High cholesterol esters were always found in patients with blepharitis, but only in some normals, with other normals having low levels of cholesterol esters,45 and in our own studies, some supposedly normal subjects have been found with high cholesterol ester levels; otherwise, this is normally associated with dry eye conditions. Similarly, phosphatidylcholine and sphingomyelin were found to be more prevalent in keratoconjunctivitis sicca.62,63 Also of interest are fatty acid amides. Nichols and coworkers recently identified fatty acid amides and confirmed the presence of free fatty acids in meibomian lipids, with initial evidence that oleamide is one of the predominant lipids present in meibomian lipids,32 but this was not supported by other work37,67 and is an area worthy of further investigation. If the fatty acid amides are indeed present, then there is a possibility that these might be acting as surfactants at the lipid aqueous interface.
Meibum and Tear Protein-Lipid Interactions
In parallel with developments in meibomian lipid chemistry, there have been major advances in understanding the proteins in tears. As a result, evidence is accumulating that strongly suggests that proteins are a major part of the lipid layer of the tear film, which is more reminiscent of the fluid mosaic model of cell membranes. It is likely that the outer layer of the tear film is rich with domains of proteins and lipids. Some of the evidence has led to the proposal of a radical model of the outer layer of the tear film.36 This model was developed on the basis of physicochemical experiments using a Langmuir trough67 and a pendant drop,68 where it was found that the major proteins of the tear film (lactoferrin, lysozyme, lipocalin, and IgA) were surface active and capable of penetrating bovine meibomian lipid films or phospholipid films. Although these experiments demonstrate that tear proteins can penetrate a meibomian lipid film, they were limited in that they were carried out at 20°C (below the melting point of meibomian lipids), the meibomian lipids used were bovine, and the penetration was slow. Temperature is important because the surface of the eye and the eyelids are between 33°C and 37°C, and the higher the temperature the less viscous the meibomian lipids.22,69 This has been supported by experimental measures of meibomian oil release onto the eyelids after heating or cooling the eyelids: levels of meibomian lipids on the eyelid margin correlate directly with temperature.69 Although the increase could be due to increased blood flow, it is much more likely to be due to decreasing the viscosity of the meibomian lipids, making for easier secretion. The softening and melting range of whole meibomian lipids is in the region of (15°C-34°C), with the melting range 30°C-34°C. This depends on the ratios of individual lipid components because these have quite varied melting points, e.g., from fatty acids at around 9°C to cholesterol at 148°C, with steryl and wax esters having 25°C-44°C.22 Although this leads to cautious interpretation of these experiments, if they were done at a higher temperature, one would only expect increased penetration due to increased kinetic energy in the system. It is worthwhile noting that the proteins used in these experiments were about 100 times less concentrated than that found in the tears, and penetration time included diffusion through the 80-mL subphase. Since physiologically, the proteins would already be at the interface at higher concentrations and higher temperature, it is hard to imagine how they are kept from penetrating the lipid layer.
The concept of proteins being a part of the lipid layer has been strengthened recently by the discovery of surfactant proteins B and C.70 These are very similar to lung surfactant proteins B and C, and their role in the lungs has been thought to be to stabilize the surface of lung surfactant. By contrast, lung surfactant proteins A and D have also been found in tears, but these have antimicrobial properties.71
Lipocalin is a major protein in the tear film and binds with lipids in a 1:1 ratio,72,73 and hence its relationship with the lipid layer has attracted a great deal of attention. Among many of the functions proposed for tear lipocalin, one has been that it helps stabilize the tears by scavenging lipids from the corneal surface and depositing them into the lipid layer.74 This has been shown to decrease surface tension and therefore thought to help stabilize the tear film.75 The removal of lipids from the corneal surface decreases the build-up of hydrophobic regions on the surface, thus maintaining its wetability. Not all agree that this is the role of tear lipocalin. Saaren-Seppälä et al.76 indicated that it maintained the tear film interface through its surface activity and interaction with lipid membranes, but were unable to show that tear lipocalin was able to transfer neutral or polar lipids between lipoproteins and lipid vesicles. If this were true, then it would be unlikely that it would be depositing lipids into the meibomian lipid layer. Instead, it might be simply binding free lipids in the aqueous, and it is the abundance of lipocalin/lipid complex compared with free lipocalin which has been shown to change the viscosity of the tear film.77
A less abundant protein, phospholipid transfer protein (PLTP), has also been reported to be in human tears but not cholesteryl ester transfer protein (CETP).78 The authors' speculation that CETP would not be needed, as cholesteryl esters would be separated from the aqueous by a phospholipid layer, could only be true if there were indeed a phospholipid layer, and this is currently contentious for humans. On the other hand, PLTP, which can bind in a ratio 13 moles of cholesterol and 43 moles of PC per mole of PLTP,79 may be transferring polar phosphorylated lipids to the superficial lipid layer, suggesting a role for its presence in the tear film.
In addition to the proteins mentioned above, the role of proteins in the meibomian glands that are possibly co-secreted with the lipids has not been tackled. More than 90 proteins have been identified in the secretions of human meibomian gland.80 The proteins identified included cellular proteins such as membrane, mitochondrial, nuclear, and cytoplasmic, as would be expected from the holocrine nature and type of secretion of the meibomian gland. Also identified in the study were a number of lipid binding proteins such as potential phospholipid transporting ATPase IK and lipocalin 1, which binds to a number of lipophilic ligands. Once secreted, these proteins would be in very low concentrations in the tear film but still may have a significant role at the surface or binding to lipids in the aqueous.
Revised View of the Tear Film Structure Including Protein/Lipid Interaction
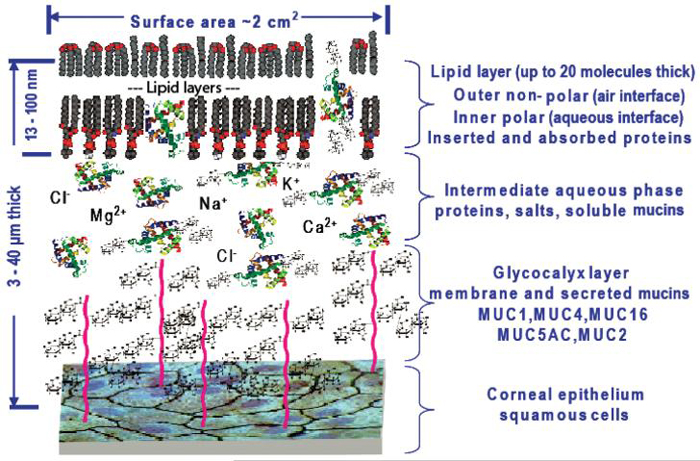
The three-layered structure of the tear film first provided by Wolff7,8 is still the generally accepted model for the layered compositional make-up of the tear film, while the specific thicknesses of the total tear film and its layers (see Tables 1 and 6 in Bron et al.4) and the extent and type of mucins are still under consideration.82 A biphasic outer lipid layer, where the polar lipids comprise the inner layer at the aqueous lipid interface and the non-polar lipids at the outer air-lipid interface layer, was first reported by Holly82 and later refined by McCulley and Shine,49 who attempted to more specifically describe the lipid arrangement based on the different lipid components. This model did not take into account proteins inserting into the lipid layer nor the measurements of an outer lipid layer that may be up to 20 molecules thick. An updated representation of the tear film, including a thicker outer lipid layer and an updated representation of the tear film, including proteins interacting with the lipid layer, is given in Figure 6.
FIGURE 6.
Revised tear film illustrating the closer interaction of the lipid-binding proteins of the tear and the outer lipid layer.
Future Directions
Meibomian Lipids and Tear Film-Associated Biomarker Studies
The new wave of “omics” (proteomics, metabolomics, glycomics, lipidomics) in biology and the technology associated with it have made the identification of disease state markers in meibomian lipid and tear film more readily accessible. An important advancement is the bioinformatics data bases associated with mass spectrometry and genetic sequencing. Some headway has already been made in tear biomarkers (Table 5). Some earlier studies have found decreases in polar lipids associated with chronic blepharitis (CB)62,65 in conjunction with evaporative keratoconjunctivitis sicca (KCS) as compared to individuals with CB and non-KCS-like symptoms. However, these studies were done before the newer techniques which suggest extremely low levels of phospholipids in normal human meibomian lipids, and hence it would be worthwhile reevaluating the results using lipidomics. Indeed, small changes in amphiphilic lipids, such as the phospholipids which are known to be in the aqueous of the human tear film, may affect surfactant interactions with the outer lipid layer. The levels of non-polar components of meibomian lipids, wax, and sterol esters have been found to change in women with complete androgen deficiency.52,54 Differences were also observed in the non-polar and polar lipid profiles associated with aging, suggesting that alterations in the lipid profiles may be representative of changes that describe the age-related increase of observed cases of dry eye syndrome.
TABLE 5.
Potential ocular surface tear-associated biomarkers identified by “omics” methodologies
| Biomarker | “Omics” | Method | Sample source | Marker type | Regulation |
|---|---|---|---|---|---|
| Diadenosine polyphosphates86 | Metabonomics | Schirmer I QHPLC | Tear (human) | Dry eye | Up |
| Phosphatidylethanolamine sphingomyelin63 | Lipidomics | TLC/HPLC CI-GC/MS | Meibum (human) | Chronic blepharitis KCS | Down |
| Polar and neutral lipids52,64 | Lipidomics | HPLC/MS | Meibum (human) | Age-related dry eye, androgen deficiency | Δ |
| Pyrophosphate sphingomyelin55 Phosphatidylserine | Lipidomics | MALDI-TOF/MS | Tear (rabbit) | Dry eye | Up |
| Acylglycerols59 | Lipidomics | ESI-QhQ/MS | Tear (rabbit) | Dry eye | Up |
| Cholesterol and ceramides87 | Lipidomics | TLC | Meibum (rabbit) | MGD | Up |
Interesting non-lipid examples that may be biological markers of ocular disease are the diadenosine polyphosphates. These are dinucleotides comprising two adenosines joined by a variable number of phosphates (abbreviated as ApnA, n= 2-7), and in rabbit models have been shown to modulate intraocular pressure,83 improve the rate of wound healing,84 and stimulate tear secretion.85 In a latter study of humantear,86 Ap4A and Ap5A were found to be increased over 100-fold in reduced tear secretion and dry eye, and hence could potentially be used as objective biomarkers for dry eye or monitoring the progress of dry eye under treatment. The test also indicated that the sex of the patient must also be taken into account, as higher values of diadenosine polyphosphates were measured in symptomatic women with normal tear secretion compared to similar male subjects.86
Animal Models
Wide variance in the human population means that most preliminary research is carried out on animal models. In this area of research, direct extrapolation from the animal results to human should be done with extreme caution. The meibomian gland lipids of rabbit, a commonly used laboratory model, appears to be fundamentally different from humans in that they have an abundance of phospholipids, where humans do not. Many animals also have a harderian gland that secretes lipids and can contribute to the lipids found in whole tears. However, once realized, this could be advantageous. For example, in a surgically induced dry-eye rabbit model, where the lacrimal and harderian glands were removed from one eye,55,59 there was a significant increase in the concentrations of three types of sphingomyelin lipids and a phosphatidylserine lipid in the dry eye tears compared with the normal. Similarly, a decrease in the percentage of sphingomyelins in the tear film has been correlated with the presence of dry eye in chronic blepharitis patients, although the different SM species within the tear film were not identified.63 Another rabbit dry-eye model caused by daily topical application of 2% epinephrine for a period of 6 months to a year87 produces a clinically evident meibomian gland disease similar to chronic posterior blepharitis in humans. In this model, both free sterols and ceramides were observed to be increased in content compared with controls suggesting hyperkeratinization of the meibomian gland ductal epithelium is involved with the meibomian gland disease.
CONCLUSION
There is still much to be done for us to understand the role of meibomian lipids. Once consistency is obtained in determining the classes of lipids within meibomian lipids, the sub-analysis of fatty acid and fatty alcohol composition will also be needed. The current evidence from animal models is that they, though useful, are sufficiently different from human, particularly rabbit. Therefore, workers in this field might want to treat the results obtained with animal models with caution and not directly extrapolate their findings to human material: our consensual opinion is that, currently, there is no adequate substitute for human studies in the area of dry eye research. Such studies will be of most benefit if they are linked to the physicochemical properties of lipid films and to clinical studies such as the effects of contact lenses and inflammatory responses on meibomian lipid composition. In terms of physicochemical properties, eventually experiments will be needed that are able to emulate the fast surface area changes that occur during a blink.
ACKNOWLEDGMENTS
The marsupial and monotreme material was provided by Dr. Julie Old and Dr. Robert Close of the University of Western Sydney. Research work of I.B. was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. (New York, NY) and a core NIH grant EY-016664. TJM was supported by the Australian Government Linkage Project Scheme and Alcon #LP0776482. We offer our special thanks to Dr. Jason J. Nichols and Dr. Kelly K. Nichols at The Ohio State University College of Optometry for the pictures of tear and meibomian lipid microcapillary samples.
Footnotes
The eyelids were obtained from frozen specimens that were part of other research projects, which all had animal ethics compliance.
Contributor Information
Igor A. Butovich, Department of Ophthalmology and Graduate School of Biomedical Sciences, University of Texas Southwestern Medical Center, Dallas, Texas, USA
Thomas J. Millar, School of Natural Sciences, Parramatta Campus, University of Western Sydney, Penrith South DC, Australia
Bryan M. Ham, Biological Sciences Division and Environmental Molecular Sciences Laboratory, Pacific Northwest National Lab, Richland, Washington, USA
REFERENCES
- [1].McDonald JE. Surface phenomena of tear films. Trans Am Ophthalmol Soc. 1968;66:905–939. [PMC free article] [PubMed] [Google Scholar]
- [2].McDonald JE. Surface phenomena of tear films. Am J Ophthalmol. 1969;67:56–64. doi: 10.1016/0002-9394(69)90008-7. [DOI] [PubMed] [Google Scholar]
- [3].Guillon J-P. Tear film photography and contact lens wear. J Br Contact Lens Assoc. 1982;5:84–87. [Google Scholar]
- [4].Bron AJ, Tiffany JM, Gouveia SM, et al. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- [5].Tiffany JM. Advances in Lipid Research. Academic Press; New York/London: 1987. The lipid secretion of the meibomian glands; pp. 1–62. [DOI] [PubMed] [Google Scholar]
- [6].Tiffany JM. Physiological functions of the meibomian glands. Prog Retinal Res. 1995;14:47–74. [Google Scholar]
- [7].Wolff E. The muco-cutaneous junction of the lid margin and the distribution of the tear fluid. Trans Ophthalmol Soc UK. 1946;66:291–308. [Google Scholar]
- [8].Wolff E. The Anatomy of the Eye and Orbit. 4th ed. H.K. Lewis and Co; London: 1954. p. 49. [Google Scholar]
- [9].Robin JB, Jester JV, Nobe J, Nicolaides N, Smith RE. In vivo transillumination biomicroscopy and photography of meibomian gland dysfunction. Ophthalmology. 1985;92:1423–1426. doi: 10.1016/s0161-6420(85)33848-4. [DOI] [PubMed] [Google Scholar]
- [10].Olami Y, Zajicek G, Cogan M, et al. Turnover and migration of meibomian gland cells in rats' eyelids. Ophthalmic Res. 2001;33:170–175. doi: 10.1159/000055665. [DOI] [PubMed] [Google Scholar]
- [11].Bron AJ, Tiffany JM. Meibomian gland and tear film lipids: Structure, function and control. In: Sullivan, editor. Tear Film and Dry Eye Syndromes. Vol. 2. Plenum; New York: 1998. pp. 281–295. [DOI] [PubMed] [Google Scholar]
- [12].Chew CKS, Hykin PG, Jansweijer C, et al. The casual level of meibomian lipids in humans. Curr Eye Res. 1993;12:255–259. doi: 10.3109/02713689308999471. [DOI] [PubMed] [Google Scholar]
- [13].Korb DK, Baron DF, Herman JP, et al. Tear film lipid layer thickness as a function of blinking. Cornea. 1994;13:354–359. doi: 10.1097/00003226-199407000-00012. [DOI] [PubMed] [Google Scholar]
- [14].Seifert P, Spitznas M. Vasoactive intestinal polypeptide (VIP) inner-vation of the human eyelid glands. Exp Eye Res. 1999;68:685–692. doi: 10.1006/exer.1999.0652. [DOI] [PubMed] [Google Scholar]
- [15].Cowlen MS, Zhang VZ, Warnock L, et al. Localization of ocular P2Y2 receptor gene expression by in situ hybridization. Exp Eye Res. 2003;77:77–84. doi: 10.1016/s0014-4835(03)00068-x. [DOI] [PubMed] [Google Scholar]
- [16].Kellogg R. The history of whales—Their adaptation to life in the water (concluded) Quart Rev Biol. 1928;3:174–208. [Google Scholar]
- [17].Young NM, Dawson WW. The ocular secretions of the bottlenose dolphin Tursiops truncatus. Mar Mammal Sci. 1992;8:57–68. [Google Scholar]
- [18].Quay WB. Misc Publ Museum Zool. Vol. 82. University of Michigan; 1954. The meibomian glands of voles and lemmings (Microtinae) pp. 5–17. [Google Scholar]
- [19].Payne AP. The harderian gland: A tercentennial review. J Anat. 1994;185:1–49. [PMC free article] [PubMed] [Google Scholar]
- [20].Millar TJ, Pearson ML. The effects of dietary and pharmacological manipulation on lipid production in the meibomian and harderian glands of the rabbit. Adv Exp Med Biol. 2002;506:431–440. doi: 10.1007/978-1-4615-0717-8_61. [DOI] [PubMed] [Google Scholar]
- [21].Fahy E, Subramaniam S, Brown HA, et al. A comprehensive classification system for lipids. J Lipid Res. 2005;46:839–861. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- [22].Tiffany JM, Marsden RG. The meibomian lipids of the rabbit. II. Detailed composition of the principal esters. Exp Eye Res. 1982;34:601–608. doi: 10.1016/0014-4835(82)90034-3. [DOI] [PubMed] [Google Scholar]
- [23].Nicolaides N, Kaitaranta JK, Rawdah TN, et al. Meibomian gland studies: Comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536. [PubMed] [Google Scholar]
- [24].Butovich IA, Luk'yanova SM, Reddy CC. Oxidation of linoleyl alcohol by potato tuber lipoxygenase: Kinetics and positional, stereo, and geometrical (cis, trans) specificity of the reaction. Arch Biochem Biophys. 2000;378:65–77. doi: 10.1006/abbi.2000.1816. [DOI] [PubMed] [Google Scholar]
- [25].Butovich IA, Luk'yanova SM, Reddy CC. Enzyme-catalyzed and enzyme-triggered pathways in dioxygenation of 1-monolinoleoylrac-glycerol by potato tuber lipoxygenase. Biochim Biophys Acta. 2001;1546:379–398. doi: 10.1016/s0167-4838(01)00162-5. [DOI] [PubMed] [Google Scholar]
- [26].van Haeringen NJ, Glasius E. Cholesterol in human tear fluid. Exp Eye Res. 1975;20:271–274. doi: 10.1016/0014-4835(75)90140-2. [DOI] [PubMed] [Google Scholar]
- [27].Duerden JM, Tiffany JM. Lipid sintesis in vitro by rabbit meibomian gland tissue and its inhibition by tetracycline. Biochim Biophys Acta. 1990;1042:13–18. doi: 10.1016/0005-2760(90)90050-8. [DOI] [PubMed] [Google Scholar]
- [28].Shirra F, Richards SM, Sullivan DA. Androgen influence on cholesterogenic enzyme mRNA levels in the mouse meibomian gland. Curr Eye Res. 2007;32:393–398. doi: 10.1080/02713680701316674. [DOI] [PubMed] [Google Scholar]
- [29].Dougherty JM, McCulley JP. Bacterial lipases and chronic blepharitis. Invest Ophthalmol Vis Sci. 1986;27:486–491. [PubMed] [Google Scholar]
- [30].Dougherty JM, McCulley JP, Silvany RE, Meyer DR. The role of tetracycline in chronic blepharitis: Inhibition of lipase production in Staphylococci. Invest Ophthalmol Vis Sci. 1991;32:2970–2975. [PubMed] [Google Scholar]
- [31].Shine WE, McCulley JP, Pandya AG. Minocycline effect on meibomian gland lipids in meibomianitis patients. Exp Eye Res. 2003;76:417–420. doi: 10.1016/s0014-4835(03)00005-8. [DOI] [PubMed] [Google Scholar]
- [32].Nichols KK, Ham BM, Nichols JJ, et al. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest Ophthalmol Vis Sci. 2007;48:34–39. doi: 10.1167/iovs.06-0753. [DOI] [PubMed] [Google Scholar]
- [33].Linton RG, Curnow DH, Riley WJ. The meibomian glands: An investigation into the secretion and some aspects of the physiology. Br J Ophthalmol. 1961;45:718–723. doi: 10.1136/bjo.45.11.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Borchman D, Foulks GN, Yappert MC, et al. Spectroscopic evaluation of human tear lipids. Chem Phys Lipids. 2007;147:87–102. doi: 10.1016/j.chemphyslip.2007.04.001. [DOI] [PubMed] [Google Scholar]
- [35].Cory CC, Hinks W, Burton JL, Shuster S. Meibomian gland secretion in the red eyes of rosacea. Br J Dermatol. 1973;89:25–27. doi: 10.1111/j.1365-2133.1973.tb01912.x. [DOI] [PubMed] [Google Scholar]
- [36].Millar TJ, Tragoulias ST, Anderton PJ, et al. The surface activity of purified ocular mucin at the air-liquid interface and interactions with meibomian lipids. Cornea. 2006;25:91–100. doi: 10.1097/01.ico.0000164779.87795.3c. [DOI] [PubMed] [Google Scholar]
- [37].Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 2007;42:765–776. doi: 10.1007/s11745-007-3080-2. [DOI] [PubMed] [Google Scholar]
- [38].Butovich IA, Uchiyama E, McCulley JP. Lipids of human meibum: Mass spectrometric analysis and structural elucidation. J Lipid Res. 2007;48:2220–2235. doi: 10.1194/jlr.M700237-JLR200. [DOI] [PubMed] [Google Scholar]
- [39].McMahon A, Butovich IA, Mata NL, et al. Retinal pathology and skin barrier defect in mice carrying a Stargardt disease-3 mutation in elongase of very long chain fatty acids-4. Mol Vis. 2007;13:258–272. [PMC free article] [PubMed] [Google Scholar]
- [40].Greiner JV, Glonek T, Korb DR, Leahy CD. Meibomian gland phospholipids. Curr Eye Res. 1996;15:371–375. doi: 10.3109/02713689608995827. [DOI] [PubMed] [Google Scholar]
- [41].Nicolaides N. Skin lipids. II. Lipid class composition of samples from various species and anatomical sites. J Am Oil Chem Soc. 1965;42:691–702. doi: 10.1007/BF02540042. [DOI] [PubMed] [Google Scholar]
- [42].Tiffany JM. Individual variations in human meibomian lipids. Exp Eye Res. 1978;27:289–300. doi: 10.1016/0014-4835(78)90164-1. [DOI] [PubMed] [Google Scholar]
- [43]. Avanti Web site: http://www.avantilipids.com/storageandhandlingoflipids.html (accessed December 2007)
- [44].Harvey DJ. Identification by gas chromatography/mass spectrometry of long-chain fatty acids and alcohols from hamster meibomian glands using picolinyl and nicotinate derivatives. Biomed Chromatogr. 1989;3:251–254. doi: 10.1002/bmc.1130030605. [DOI] [PubMed] [Google Scholar]
- [45].Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:2272–2280. [PubMed] [Google Scholar]
- [46].Shine WE, McCulley JP. Role of wax ester fatty alcohols in chronic blepharitis. Invest Ophthalmol Vis Sci. 1993;34:3515–3521. [PubMed] [Google Scholar]
- [47].Ehlers N. The precorneal film. Biomicroscopical, histological, and chemical investigations. Acta Ophthalmol. 1965;Suppl. 81:1–134. [PubMed] [Google Scholar]
- [48].Andrews JS. Human tear film lipids I. Composition of the principal non-polar component. Exp Eye Res. 1970;10:223–227. doi: 10.1016/s0014-4835(70)80032-x. [DOI] [PubMed] [Google Scholar]
- [49].McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophth Soc. 1997;45:79–93. [PMC free article] [PubMed] [Google Scholar]
- [50].Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–360. doi: 10.1007/978-1-4615-5359-5_50. [DOI] [PubMed] [Google Scholar]
- [51].Shine WE, McCulley JP. Association of meibum oleic acid with meibomian seborrhea. Cornea. 2000;19:72–74. doi: 10.1097/00003226-200001000-00014. [DOI] [PubMed] [Google Scholar]
- [52].Sullivan BD, Evans JE, Cermak JM, et al. Complete androgen insensitivity syndrome. Effect on human meibomian gland secretions. Arch Ophthalmol. 2002;120:1689–1699. doi: 10.1001/archopht.120.12.1689. [DOI] [PubMed] [Google Scholar]
- [53].Greiner JV, Glonek T, Korb DR, Leahy CD. Phospholipids in meibomian gland secretion. Ophthalmic Res. 1996;28:44–49. doi: 10.1159/000267872. [DOI] [PubMed] [Google Scholar]
- [54].Sullivan BD, Evans JE, Krenzer KL, et al. Impact of anti-androgen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J Clin Endocrinol Metab. 2000;85:4866–4873. doi: 10.1210/jcem.85.12.7066. [DOI] [PubMed] [Google Scholar]
- [55].Ham BM, Cole RB, Jacob JT. Identification and comparison of the polar phospholipids in normal and dry eye rabbit tears by MALDI-TOF mass spectrometry. Invest Ophthalmol Vis Sci. 2006;47:3330–3338. doi: 10.1167/iovs.05-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Brügger B, Erben G, Sandhoff R, et al. Quantitative analysis of biological membrane lipids at the low picomole level by nano-electrospray ionization tandem mass spectrometry. Proc Nat Acad Sci USA. 1997;94:2339–2344. doi: 10.1073/pnas.94.6.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wollensak G, Mur E, Mayr A, et al. Effective methods for the investigation of human tear film proteins and lipids. Graefes Arch Clin Exp Ophthalmol. 1990;228:78–82. doi: 10.1007/BF02764296. [DOI] [PubMed] [Google Scholar]
- [58].Stuchell RN, Slomiany BL, Joswiak Z, et al. Lipid composition of human tears. Invest Ophthalmol Vis Sci. (ARVO Abstracts) 1984;25:320. [Google Scholar]
- [59].Ham BM, Jacob JT, Keese MM, Cole RB. Identification, quantification, and comparison of major non-polar lipids in normal and dry eye tear lipidomes by electrospray tandem mass spectrometry. J Mass Spectr. 2004;39:1321–1336. doi: 10.1002/jms.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ham BM, Jacob JT, Cole RB. MALDI-TOF MS of phosphorylated lipids in biological fluids using immobilized metal affinity chromatography and a solid ionic crystal matrix. Anal Chem. 2005;77:4439–4447. doi: 10.1021/ac058000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Borchman D, Foulks GN, Yappert MC, Ho DV. Temperature-induced conformational changes in human tear lipids hydrocarbon chains. Biopolymers. 2007;87:124–133. doi: 10.1002/bip.20798. [DOI] [PubMed] [Google Scholar]
- [62].Shine WE, McCulley JP. Meibomianitis: Polar lipid abnormalities. Cornea. 2004;23:781–783. doi: 10.1097/01.ico.0000133995.99520.1f. [DOI] [PubMed] [Google Scholar]
- [63].Shine WE, McCulley JP. Keratoconjunctivitis sicca associated with meibomian secretion polar lipid abnormality. Arch Ophthalmol. 1998;116:849–852. doi: 10.1001/archopht.116.7.849. [DOI] [PubMed] [Google Scholar]
- [64].Sullivan BD, Evans JE, Dana MR, Sullivan DA. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Arch Ophthalmol. 2006;124:1286–1292. doi: 10.1001/archopht.124.9.1286. [DOI] [PubMed] [Google Scholar]
- [65].Shine WE, McCulley JP. Polar lipids in human meibomian gland secretions. Curr Eye Res. 2003;26:89–94. doi: 10.1076/ceyr.26.2.89.14515. [DOI] [PubMed] [Google Scholar]
- [66].Butovich IA. On the presence of oleamide in human meibum: Quantification by LC/MS. Invest Ophthalmol Vis Sci Lett. 2007 http://www.iovs.org/cgi/eletters/48/1/34 [Google Scholar]
- [67].Tragoulias ST, Anderton PJ, Dennis GR, et al. Surface pressure measurements of human tears and individual tear film components indicate that proteins are major contributors to the surface pressure. Cornea. 2005;24:189–200. doi: 10.1097/01.ico.0000138837.52694.37. [DOI] [PubMed] [Google Scholar]
- [68].Miano F, Calcara M, Millar TJ, Enea V. Insertion of tear proteins into a meibomian lipids film. Coll Surf B. 2005;44:49–55. doi: 10.1016/j.colsurfb.2005.05.011. [DOI] [PubMed] [Google Scholar]
- [69].Nagymihályi A, Dikstein S, Tiffany JM. The influence of eyelid temperature on the delivery of meibomian oil. Exp Eye Res. 2004;78:367–370. doi: 10.1016/s0014-4835(03)00197-0. [DOI] [PubMed] [Google Scholar]
- [70].Bräuer L, Johl M, Paulsen FP, et al. Detection and localization of the hydrophobic surfactant proteins B and C in human tear fluid and the human lacrimal system. Curr Eye Res. 2007;32:931–938. doi: 10.1080/02713680701694369. [DOI] [PubMed] [Google Scholar]
- [71].Bräuer L, Kindler C, Jäger K, et al. Detection of surfactant proteins A and D in human tear fluid and the human lacrimal system. Invest Ophthalmol Vis Sci. 2007;48:3945–3953. doi: 10.1167/iovs.07-0201. [DOI] [PubMed] [Google Scholar]
- [72].Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Structural changes in human tear lipocalins associated with lipid binding. Biochim Biophys Acta. 1998;1386:145–156. doi: 10.1016/s0167-4838(98)00092-2. [DOI] [PubMed] [Google Scholar]
- [73].Glasgow BJ, Marshall G, Gasymov OK, et al. Tear lipocalins: Potential lipid scavengers for the corneal surface. Invest Ophthalmol Vis Sci. 1999;40:3100–3107. [PubMed] [Google Scholar]
- [74].Gasymov OK, Abduragimov AR, Prasher P, et al. Tear lipocalin: Evidence for a scavenging function to remove lipids from the human corneal surface. Invest Ophthalmol Vis Sci. 2005;46:3589–3596. doi: 10.1167/iovs.05-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Nagyova B, Tiffany JM. Components responsible for the surface tension of human tears. Curr Eye Res. 1999;19:4–11. doi: 10.1076/ceyr.19.1.4.5341. [DOI] [PubMed] [Google Scholar]
- [76].Saaren-Seppälä H, Jauhiainen M, Tervo TMT, et al. Interaction of purified tear lipocalin with lipid membranes. Invest Ophthalmol Vis Sci. 2005;46:3649–3656. doi: 10.1167/iovs.05-0176. [DOI] [PubMed] [Google Scholar]
- [77].Gouveia SM, Tiffany JM. Human tear viscosity: An interactive role for proteins and lipids. Biochim Biophys Acta. 2005;1753:155–163. doi: 10.1016/j.bbapap.2005.08.023. [DOI] [PubMed] [Google Scholar]
- [78].Jauhianen M, Setala NL, Ehnholm C, et al. Phospholipid transfer protein is present in human tear fluid. Biochemistry. 2005;44:8111–8116. doi: 10.1021/bi050151k. [DOI] [PubMed] [Google Scholar]
- [79].Nashida HI, Nashida T. Phospholipid transfer protein mediates transfer of not only phosphatidylcholine but also cholesterol from phosphatidylcholine-cholesterol vesicles to high density lipoproteins. J Biol Chem. 1997;272:6959–6964. doi: 10.1074/jbc.272.11.6959. [DOI] [PubMed] [Google Scholar]
- [80].Tsai PS, Evans JE, Green KM, et al. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol. 2006;90:372–377. doi: 10.1136/bjo.2005.080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007;84:939–950. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Holly FJ. Formation and rupture of the tear film. Exp Eye Res. 1973;15:515–525. doi: 10.1016/0014-4835(73)90064-x. [DOI] [PubMed] [Google Scholar]
- [83].Pintor J, Peral A, Pelaez T, et al. Presence of diadenosine polyphosphates in the aqueous humor: Their effect on intraocular pressure. J Pharmacol Exp Ther. 2002;304:342–348. doi: 10.1124/jpet.102.041368. [DOI] [PubMed] [Google Scholar]
- [84].Pintor J, Bautista A, Carracedo G, Peral A. UTP and diadenosine tetraphosphate accelerate wound healing in the rabbit cornea. Ophthal Physiol Opt. 2004;24:186–193. doi: 10.1111/j.1475-1313.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- [85].Pintor J, Peral A, Hoyle CHV. Effects of diadenosine polyphosphates on tear secretion in New Zealand white rabbits. J Pharmacol Exp Ther. 2002;300:1–7. doi: 10.1124/jpet.300.1.291. [DOI] [PubMed] [Google Scholar]
- [86].Peral A, Carracedo G, Acosta MC, et al. Increased levels of diadenosine polyphosphates in dry eye. Invest Ophthalmol Vis Sci. 2006;47:4053–4058. doi: 10.1167/iovs.05-0980. [DOI] [PubMed] [Google Scholar]
- [87].Nicolaides N, Santos EC, Smith RE, Jester JV. Meibomian gland dysfunction III. Meibomian gland lipids. Invest Ophthalmol Vis Sci. 1989;30:946–951. [PubMed] [Google Scholar]