Abstract
Purpose
Hepatocyte growth factor (HGF) and its receptor Met are known to play diverse roles in both organogenesis and cancer. Wilms tumour is a prototype for the link between abrogated development and neoplasia, with dysregulation of growth factor/receptor pathways playing key roles. Despite this, an understanding of the HGF/Met axis in the process is lacking.
Experimental Design
Observing copy number alterations at the loci for these genes in Wilms tumours and their precursor lesions nephrogenic rests, we examined protein expression by immunohistochemistry, and investigated the effects of HGF on an in vitro model of kidney development.
Results
HGF was preferentially expressed in the blastemal cells of nephrogenic rests but not Wilms tumours. Met expression was infrequent, and restricted to well differentiated epithelial cells and stroma in both lesions. In an independent cohort of favourable histology Wilms tumours on a tissue microarray, HGF was expressed in 15/193 (8%) cases, and correlated with a predominance of epithelial cells, whilst Met expression was observed in 25/179 (14%) cases, and was associated with stromal subtypes. In a mouse mesonephric cell line model, we observed Met expression in culture conditions reflecting both mesenchymal and epithelial differentiation, whilst HGF was upregulated in association with acquisition of a more epithelial-like phenotype. This could be mimicked by exogenous exposure of mesenchymal-like cells to recombinant HGF.
Conclusions
These data demonstrate that the relatively infrequent expression of HGF and Met in Wilms tumorigenesis reflects their roles in nephrogenesis, particularly the mesenchymal-to-epithelial transition, rather than a dependence on oncogenic signalling pathways.
Keywords: HGF/SCF, c-Met, mesenchymal, epithelial, stromal, nephrogenesis
INTRODUCTION
Kidney development is a complex process, consisting of two distinct embryological origins, the nephrogenic (mesenchymal) and the ductogenic (ureteric) (1). Upon induction by the ureteric bud, the metanephric mesenchyme undergoes a series of morphogenic events resulting in ingrowth of the ureteric bud into the metanephric blastema, mesenchymal to epithelial transition, and formation of tubules of the mature nephron (2). Wilms tumour (nephroblastoma), the most common paediatric kidney cancer, can be considered a failure of this transition. It arises from pluripotent renal precursors that are undergoing excessive proliferation resulting in undifferentiated stromal components, blastemal cells similar to the condensing mesenchyme and primitive epithelial structures resembling comma and S-shaped bodies and glomeruli (3). The presence of associated nephrogenic rests (4) that consist of foci of persistent embryonal remnant tissues that failed to mature to normal renal parenchyma, further point towards impaired differentiation in early renal development.
A number of genes involved in nephrogenesis, especially in the mesenchymal to epithelial transition, have also been implicated in Wilms tumorigenesis (2, 3). We have previously identified dysregulation of the IGF2/IGF1R signalling network to be associated with perilobar nephrogenic rests (5) and Wilms tumour relapse (6). Other growth factor pathways active in the normal and developing kidney are also likely to play a role, with HGF/Met excellent candidates in this context (7).
Hepatocyte growth factor (HGF, scatter factor) and its high affinity tyrosine kinase receptor Met are both widely expressed early in development, and deletion of either gene causes lethal disruptions to embryogenesis (8, 9). During nephrogenesis, Met is preferentially expressed by the epithelium of the ureteric bud and the developing collecting duct, whilst HGF is expressed in mesenchymal cells and is subsequently localised to the distal tubules and collecting ducts, consistent with a role as a paracrine regulator for renal tubulogenesis (10). From the time of induction to the stage of metanephric condensation HGF is stimulating branching morphogenesis promoting motility, proliferation, invasion, morphogenesis and survival via its receptor’s multifunctional docking site, capable of recruiting signal transducers resulting in the activation of Ras/MAPK and PI3K/Akt signal transduction pathways (11, 12). Neutralizing antibodies against HGF blocked branching morphogenesis by the ureteric bud in organ cultures of kidney rudiments and inhibited the early steps in branching morphogenesis by immortalized ureteric bud cells in three-dimensional organ culture as well as glomerulogenesis and nephrogenesis in vivo (13, 14).
Dysregulation of HGF and Met signalling is a crucial feature of many human malignancies (15). Upon HGF binding, c-Met autophosphorylation occurs on two tyrosine residues (Y1234 and Y1235) within the activation loop of the kinase domain which regulate enzyme activity. Phosphorylation on two tyrosine residues near the COOH terminus (Y1329 and Y1356) forms a multifunctional docking site that recruits intracellular adapters via Src homology 2 domains and other recognition motifs, leading to downstream signalling. In particular, the direct binding of Grb2 to the c-Met docking site through Y1356 links the receptor to the Ras/MAPK pathway regulating cell cycle progression.
Multiple mechanisms of pathway activation including coexpression of HGF and Met, receptor amplification and point mutations have been described, with over- or misexpression often correlating with poor prognosis and, particularly, metastasis (12). In Wilms tumour, involvement of the HGF/Met pathway has been reported to correlate with the increased proliferation rate (16).although this has not been explored further. Little information is presently available on biochemical and biological responses induced by HGF on undifferentiated cells, such as those in nephrogenic rests.
We have noted copy number gains at the HGF and MET loci in Wilms tumours and nephrogenic rests, and sought to clarify their role in Wilms tumour biology. Contrary to their role in adult epithelial cancers, we observed that HGF/Met did not confer a worse clinical outcome in Wilms tumour patients, and were instead associated with a cell type-specific expression that reflects their role in renal development, and the mesenchymal to epithelial transition.
MATERIALS and METHODS
Samples
Archival pathology specimens of PLNRs and Wilms tumours were collected with full Ethical Committee approval, and have been described previously (5). Paediatric renal tumour tissue microarrays were constructed containing replicate representative cores (n=885) from all available cellular components from 274 Wilms tumours, 13 clear cell sarcomas of the kidney (CCSK), 10 mesoblastic nephromas (MN, seven classic and three cellular), and 7 rhabdoid tumours of the kidney (RTK), and has also been described previously (6, 17-20). Tumours were treated either with immediate nephrectomy or pre-operative chemotherapy and delayed surgery, and for the purpose of this analysis, all non-anaplastic Wilms tumours are described as favourable histology.
Array CGH
Array CGH was carried out using a 5.8K, 0.9Mb-spaced and/or a 16K, 100kb-spaced BAC array platform as reported previously (5). All raw and processed data have been deposited in Array Express (E-TABM-4361).
Immunohistochemistry
Immunohistochemistry was done on 5μm FFPE sections using a rabbit polyclonal antibody either to human HGF (JP18131, ImmunoBiological Laboratories, Gunma, Japan, directed against the N-terminal part of the Human HGF alpha chain) or Met (JP18321 Immuno-Biological Laboratories, directed against VDTRPASFWETS) using the Envision horseradish-peroxidase system (Dako, Ely, UK) at a dilution of 1:75 for HGF and 1:100 for Met according to the manufacturer’s instructions (21). An additional blocking step of 1% normal goat serum (Dako) was included for both antibodies. Antigen retrieval for HGF was carried out with 0.1% trypsin (Sigma, Poole, UK) in 0.05M Tris-HCl (pH7.4) at 37°C; for Met the slides were boiled for 10mins in 10mMol/L citrate buffer (pH6) in the microwave. Positive controls were pleomorphic adenoma (Abcam, Cambridge, UK) for HGF and invasive ductal breast carcinoma (Abcam) for Met. Tumour cell positivity and cellular distribution were assessed independently by three pathologists (NJS, GV, JSR-F).
Statistical analysis
All statistical tests were performed in R 2.6.2 2. Correlations between categorical values were performed using the Chi-square and Fishers exact tests. Cumulative survival probabilities were calculated using the Kaplan-Meier method, with differences between survival rates analysed with the log-rank test. Multivariate analysis was carried out using the Cox proportional hazards model.
Cell culture
M15 cells derived from mouse mesonephros, a kind gift from Melissa Little (University of Queensland, Brisbane, Australia), were cultured routinely in DMEM (Sigma) supplemented with 10% FCS and 1% L-glutamine (Invitrogen, Paisley, UK). Cells were grown on sterile 13mm glass coverslips at the cell densities of 30% confluence (sub-confluent) and 80% confluence (confluent) in a humidified 5% CO2 atmosphere at 37°C. For the stimulation experiments, cells were serum starved for 24h and incubated with 100ng/ml of human recombinant HGF (Merck, Hull, UK) for up to 48 hours.
Immunofluorescence
Cells were grown on glass coverslips in 24-well plates, with ice cold methanol used to fix cells for up to 5mins prior to incubation with the following primary antibodies; vimentin S-20 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) 1/50 dilution, wide-spectrum cytokeratin (Abcam) 1/75 dilution, WT1 C-19 (Santa Cruz Biotechnology) 1/50 dilution, HGF (Immuno-Biological Laboratories) 1/35 dilution and Met (Santa Cruz Biotechnology) 1/35 dilution. For the secondary detection, the following antibodies were used in 1/1000 dilution; Alexa Fluor Donkey anti-goat 488, Alexa Fluor Donkey anti-rabbit 555 and Alexa Fluor Donkey anti-rabbit 568 (Inivtrogen). 1% BSA, 2% FCS in PBS was used for blocking as well as diluting all the primary and secondary antibodies. Cell nuclei was stained with TOPRO-3. Fluorescence was examined using a laser scanning confocal microscope (Leica, Milton Keynes, UK).
RESULTS
Chromosomal rearrangements lead to recurrent gain of 7q21 in nephrogenic rests and Wilms tumours
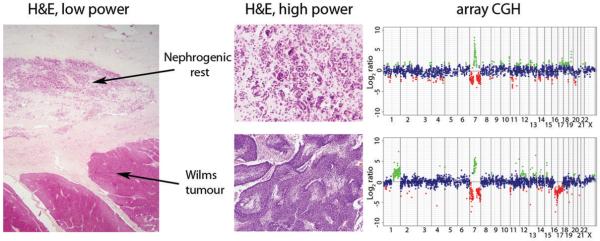
We have previously demonstrated the genomic changes associated with the development of Wilms tumours from their non-obligate precursor lesions nephrogenic rests by array CGH (5). In that study, we noted that in two cases, Wilms tumours and their associated rests harboured alterations on chromosome 7 which led to a gain of DNA copy number close to the centromere on the long arm. In one case, there was a low level focal gain in both the tumour and the rest of approx 5 Mb at 7q21, whilst in another we observed a complex rearrangement of chromosome 7 involving loss of the short arm, high level gain of 7q11-q21, and loss of 7q22-qter (Figure 1). Again, this genomic alteration was seen in both the nephrogenic rest and adjacent Wilms tumour, although there were additional changes in copy number seen only in the tumour, including concurrent gain of 1q and loss of 16q. In both of these cases, the minimal region spanned the HGF locus at 7q21.11.
Figure 1. Array CGH of a nephrogenic rest and Wilms tumour from the same patient harbouring high level gain of 7q21.
Haematoxylin and eosin stained sections (low power, original magnification ×12.5; high power, original magnification ×200) from a perilobar nephrogenic rest and associated blastemal type Wilms tumour alongside copy number profile by array CGH. Both lesions harbour a complex rearrangement on chromosome 7 leading to gain of 7q21 and concurrent loss of 7p and 7q22-qter. Additional alterations including gain of 1q and loss of 16q are present in the Wilms tumour. Genome plots show log2 ratios for each clone (x axis) plotted according to chromosomal location (y axis). The centromere is represented by a horizontal line. Points are coloured green and red to represent gains and losses, respectively.
HGF and MET are preferentially expressed in nephrogenic rests
With the focal copy number gain of the HGF locus observed in nephrogenic rests, and the known gains of the whole of chromosome 7q (involving both the HGF and MET loci) in Wilms tumours and rests, we sought to investigate the expression of the growth factor and its receptor in a series of tumours and precursor lesions by immunohistochemistry using fully optimised, reproducible and specific antibodies. In total, we assessed 46 nephrogenic rests and 36 Wilms tumours from 43 patients. HGF expression was observed in 25/46 (54%) of rests, including those with 7q21 gain (above) and was noted in all cellular components, including immature blastema, stroma and well differentiated tubules. Immunopositivity ranged from focal clusters comprising less than 10% of the whole lesion to 100% of rest cells (representative images are shown in Figure 2). By contrast, we observed no positive staining in any of the Wilms tumour cases in this series.
Figure 2. Expression of HGF and Met in whole sections of nephrogenic rests and Wilms tumours.
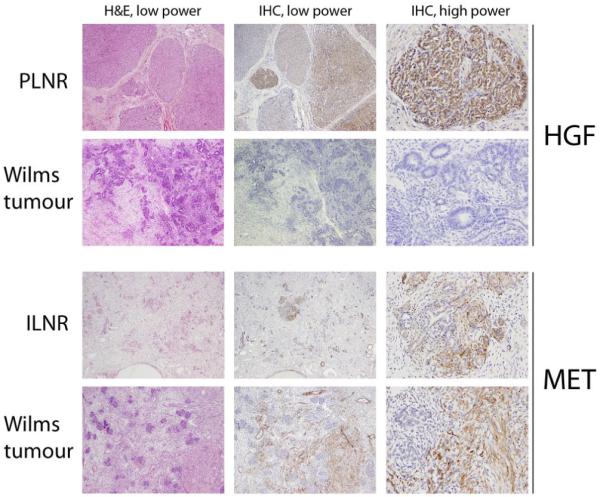
Photomicrographs demonstrating typical patterns of HGF and Met expression by immunohistochemistry in distinct nephrogenic rests and Wilms tumours from different patient samples. First row, a perilobar nephrogenic rest (PLNR) with strong, diffuse HGF staining in blastemal cells. Second row, a Wilms tumour demonstrating no immunoreactivity. Third row, an intralobar nephrogenic rest (ILNR) with strong Met expression in the stromal compartment. Fourth row, a Wilms tumour with strong stromal immunoreactivity. Low power, original magnification ×200; high power, original magnification ×400.
Met expression was seen in 10/46 (22%) nephrogenic rests, restricted to well differentiated epithelial cells or stromal compartments. Similarly, 5/36 (14%) of Wilms tumours were also Met positive, again restricted to either well differentiated tubules or tumorigenic stroma (representative images are shown in Figure 2). In this series, all blastemal components of rests and tumours were negative. The data is summarised in Table 1.
Table 1. Summary of HGF and MET expression in nephrogenic rests and Wilms tumour whole sections.
| HGF | MET | HGF/MET coexpression | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Strong | Weak | Negative | Strong | Weak | Negative | Strong | Weak | Negative | |
| Nephrogenic rest | 12/46 (26%) | 13/46 (28%) | 21/46 (46%) | 8/46 (17%) | 2/46 (4%) | 36/46 (78%) | 3/46 (7%) | 2/46 (4%) | 41/46 (89%) |
| Wilms tumour | 0/36 (0%) | 0/36 (0%) | 36/36(100%) | 3/36 (8%) | 2/36 (6%) | 31/36 (86%) | 0/36 (0%) | 0/36 (0%) | 36/36(100%) |
Expression of HGF and c-Met in Wilms tumours is associated with epithelial and stromal cell types and good prognosis
In order to further probe the possible clinicopathological association of HGF/Met positivity in a larger, independent Wilms tumour cohort, we examined expression on a paediatric renal tumour tissue microarray (TMA). In contrast to our smaller series of whole sections, we observed HGF expression in 15/193 (8%) of favourable histology Wilms tumours. This was not, however, a statistically significant difference to the smaller series (p=0.137, Fisher’s exact test). This was mostly restricted to epithelial and stromal cell types, with only 3/101 (3%) cases exhibiting blastemal cell positivity (Supplementary Table S1). There were no associations with age at diagnosis or tumour stage, and although HGF positivity showed a trend towards an association with better outcome in favourable histology Wilms tumour, regardless of treatment protocol, this failed to reach statistical significance due to the small number of positive cases and events therein (Supplementary Figure S2). Expression in the epithelial cell components was significantly correlated with the predominance of that cell type in the tumour as a whole (p=0.0015, Fishers exact test). Due to the small number of positive cases, it was not possible to analyse the possible impact of HGF expression on outcome independently of histological subtype. Both epithelial and stromal-predominant Wilms tumours are recognised to have an excellent outcome and this may account for the generally better outcome, at least in those cases exposed to pre-operative chemotherapy (22).
An analogous situation was observed for Met expression, with 25/179 (14%) positive cases (Supplementary Table S1). There were few tumours with blastemal cell positivity (3/98, 3%); expression was largely restricted to epithelial and stromal compartments, with a significant association with stromal cell type predominance (p=0.008, Fishers exact test), and a non-significant trend toward better prognosis (Supplementary Figure S2). Once again, there were no association with age, stage or treatment protocol. Coexpression with HGF was observed in only a small proportion of tumours (6/171, 4%), and although the clinical associations did not reach statistical significance, it is notable that there were no relapses or deaths observed in any case with HGF/Met coexpression (Supplementary Figure S2). Multivariate analyses for HGF and/or Met expression with respect to relapse-free and overall survival were not statistically significant.
In addition to the favourable histology Wilms tumours, there was also 1/8 (12.5%) anaplastic Wilms tumour cases with high levels of both HGF and Met. No Met expression was observed in any clear cell sarcomas of the kidney (CCSK), mesoblastic nephroma (MN) or rhabdoid tumours of the kidney (RTK). We did, however, observe HGF expression in 2/12 CCSKs and 1/4 RTKs.
The mouse mesonephric M15 cells as a model for mesenchymal to epithelial transition during nephrogenesis
M15 cells derived from mouse mesonephros have been widely used to model WT1 function during nephrogenesis, due to the constitutive expression of the protein (data not shown). We sought to investigate the roles of HGF and Met in these cells by exploiting their reported ability (23) to demonstrate a mesenchymal to epithelial transition when grown at low and high confluence in vitro. Sparsely grown cells at subconfluence demonstrated a distinct mesenchymal appearance, with spindle shaped cytoplasm, and strong vimentin expression. By contrast, when grown at high confluence, the cells grow as packed sheets with a more cuboidal epithelial-like morphology, and express cytokeratin (Figure 3). Met was consistently expressed at the cell membrane in M15 cells grown under both conditions. HGF, by contrast, whilst expressed in the cytoplasm at all cell densities, showed a clear upregulation when grown at high confluence (Figure 4). This leads to increased Met signalling via MAPK, possibly via autocrine/paracrine activation, with elevated levels of phosphorylated Erk1/2 (data not shown).
Figure 3. Mouse mesonephric M15 cells mimicking the mesenchymal to epithelial transition during nephrogenesis.
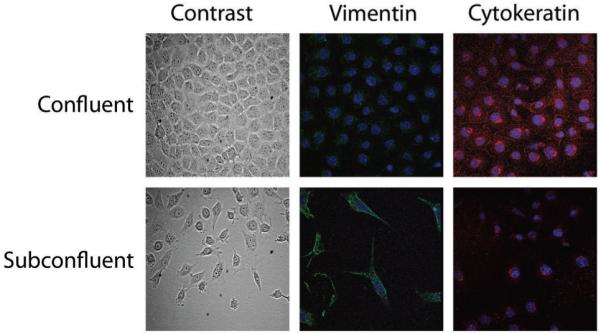
M15 cells grown at different cell densities showing an epithelial morphology (phase contrast microscopy) and cytokeratin expression (red immunofluorescence) at confluence, and a mesenchymal phenotype (spindle shaped morphology and vimentin expression, green) when grown in subconfluent conditions. Nuclei were counterstained with TOPRO-3.
Figure 4. Expression of HGF and Met in M15 cells with epithelial and mesenchymal phenotypes.
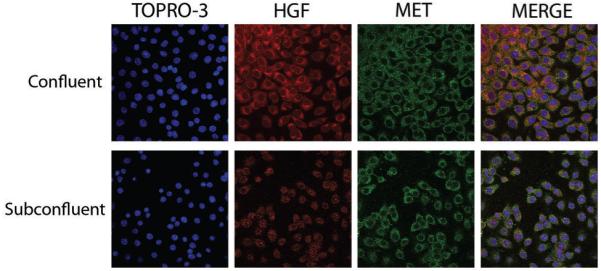
M15 cells grown at confluence and subconfluence stained by immunofluorescence for HGF (red, cytoplasm) and Met (green, cell membrane). Consistent staining is observed for Met, whilst an upregulation of HGF is seen in confluent conditions. Nuclei were counterstained with TOPRO-3. Merge indicates an integrated image of all three channels.
Exogenous exposure to HGF in subconfluent M15 cells drives a more epithelial phenotype
We hypothesised that M15 cells grown at sub-confluence retained a mesenchymal phenotype, which converted to an epithelial one when grown at higher density, and that HGF/Met may play a role in this transition. Addition of recombinant HGF to M15 cultures produced little change to confluent cells, which were seen to already express high levels of the growth factor. Subconfluent cultures, however, demonstrated a clear up-regulation of cytokeratin in the cytoplasm, in addition to the perinuclear expression noted in the absence of HGF, after 1 hour exposure to the growth factor (Figure 5). Mesenchymal cells are known to express keratins in perinuclear aggregates, prior to the development of a filamentous network throughout the cytoplasm, and subsequent attachment to the cell membrane to form the cytoskeletal network of an epithelial cell (24, 25). By 24 hours the cells, continually cultured in low density conditions, showed extensive cytoskeletal filaments reminiscent of epithelial cells, and strong membranous staining, absent from the unstimulated cells (Figure 5). These cytokeratin positive cells retained their spindle shaped morphology and expression of vimentin, even up to 48 hours exposure to HGF (data not shown).
Figure 5. HGF drives the acquisition of an epithelial phenotype in subconfluent M15 cells.
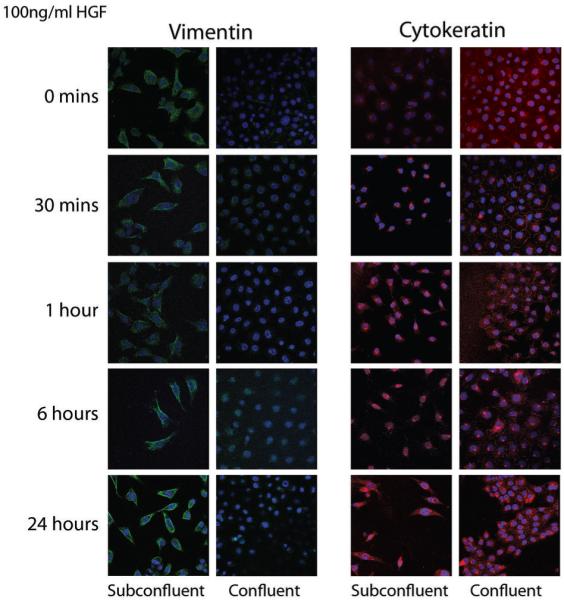
M15 cells grown in confluent and subconfluent conditions were treated with 100ng/ml recombinant HGF and stained for vimentin (green) and cytokeratin (red) by immunofluorescence. Nuclei were counterstained with TOPRO-3. An upregulation of cytokeratin was observed after 1 hour in the subconfluent cultures, demonstrating a distinct epithelial filament expression pattern at 24 hours.
DISCUSSION
In many adult epithelial, and some paediatric cancers, an activated Met pathway is associated with increased invasiveness, metastasis and poor clinical outcome, and is an excellent candidate for novel targetted therapies (15). Despite its discovery in an oncogenic context, the physiological role of HGF signalling through Met is in diverse developmental processes during embryogenesis. In Wilms tumours, a prototype malignancy of differentiation failure, expression of HGF/Met appears to reflect these developmental roles.
During nephrogenesis, HGF and Met form a paracrine loop, the growth factor expressed largely by the mesenchyme and the receptor preferentially in the ureteric bud epithelium. HGF not only stimulates branching morphogenesis, but it also is a potent inducer capable of morphogenic, motogenic (‘scatter’), and mitogenic effects on kidney development (26). Additionally, Met may be coexpressed with HGF in at least a part of the mesenchymal tubulogenic cell population, where it may act either in the rescue from apoptosis or else have a part in the conversion process (1).
As a result of abrogated nephrogenesis, residual embryonal cells may persist in the mature kidney as nephrogenic rests, molecular genetic precursor lesions of Wilms tumours (4). We observed a preferential retention of HGF expression in more than half of our series of nephrogenic rests, whilst such expression was rarely noted in Wilms tumours. In particular, HGF expression in the immature blastemal cells was widespread in rests and almost entirely absent from tumours, suggesting that loss of expression from these primitive embryologic cells may be associated with the acquisition of malignancy.
Where HGF expression was noted in Wilms tumours, it was significantly associated with an enrichment of epithelial cells, consisting of both chemo-naïve ‘epithelial predominant’ and pre-treated ‘epithelial type’ tumours. The role of HGF signalling via Met has been purported to play a key role in the mesenchymal to epithelial transition during nephrogenesis (27). We have utilised the mouse mesonephros M15 model, which although not truly reflecting all of the complex processes of human renal development in vivo, forms a useful system in which to study the epitheliazation process of the mesonephric mesenchyme. In this context, we were able to demonstrate that HGF participates in the upregulation of epithelial cytokeratin filaments in cells with an otherwise mesenchymal phenotype. Thus it is possible that Wilms tumours with a preponderance of malignant epithelial cells are driven by excessive HGF/Met signalling. It is notable that the WiT49 (anaplastic, metastatic) Wilms tumour cell line, known to contain an intact HGF/Met pathway ((28) and unpublished observations), showed extensive vimentin and cytokeratin expression, and a more consistent epithelioid morphology upon continued passages.
In the normal kidney Met expression is limited to epithelial cells in the proximal convoluted tubule, loop of Henle and the collecting duct, with the glomeruli, distal convoluted tubule and stroma consistently negative ((29), and published observations). We observed Met expression in the most well differentiated tubular epithelial cells of both nephrogenic rests and Wilms tumours, but also noted a strong immunoreactivity in stromal cells of both lesions, with a significant association with the predominance of stromal cells in the tumours regardless of treatment protocol.
HGF and Met are know to play a significant role during the development of skeletal muscle, such that signalling through this cascade controls the migration of myogenic precursor cells in the embryo (30). Expression of Met in tumorigenic stromal cells, many with rhabdomyoblastic differentiation, raises the possibility of its role in determining myogenic cell fate during abnormal kidney development and Wilms tumorigenesis. Possible functional links to WT1, mutated forms of which are strongly correlated with stromal histology, remain to be elucidated.
Our data differs from that of the only previous publication of HGF/Met expression in a small series of Wilms tumours, which reported extensive positivity and a correlation with increased cell proliferation (16). Our study differs from this earlier report not only in terms of a considerably larger number of cases examined, but crucially the antibodies and immunohistochemistry protocols used, selected after extensive and careful optimisation from a variety of sources. We further saw no association with an increased proliferation index by Ki67 staining, nor with any other markers previously analysed in our sample cohorts (6, 17-20).
Post-chemotherapy abundance of epithelial or stromal cells is reported to predict for better clinical outcome (22), and it is likely that the trends we observed were a reflection of this histological type, although the numbers of positive tumours in our cohort were too small to allow for robust analyses within treatment groups. Our observations of HGF and Met positive Wilms tumours demonstrating a trend towards better prognosis may be counter-intuitive when considered in the context of oncogenic signalling, however it is likely a reflection of their specific roles in renal cell differentiation, and may yet play an important role in diverse aspects of Wilms tumorigenesis.
Supplementary Material
Acknowledgements
This work is supported by Cancer Research UK and Breakthrough Breast Cancer. We thank the Children's Cancer and Leukaemia Group (CCLG) Tumour Bank, which is funded by Cancer Research UK, as well as contributing pathologists, oncologists and surgeons, for access to samples. We are also grateful to Melissa Little (University of Queensland) for the generous gift of M15 cells.
Footnotes
STATEMENT OF CLINICAL RELEVANCE
HGF and its receptor Met are involved in numerous developmental and oncogenic processes, and dysregulation of this pathway may have adverse effects on patient survival. We noted copy number gains of these loci at the earliest stages of Wilms tumorigenesis, itself a result of abrogated kidney development. HGF was found to be preferentially expressed in the immature blastemal cells of the precursor nephrogenic rests, being largely absent from tumours. Using an in vitro model of developing kidney cells, we demonstrate that HGF/Met is associated with the mesenchymal to epithelial transition. The infrequent retention of both the ligand and the receptor in the Wilms tumours themselves is a reflection of this disrupted organogenesis, rather than a predictor for acquisition of a more malignant phenotype, and this has implications for possible targeted treatment strategies.
REFERENCES
- 1.Horster MF, Braun GS, Huber SM. Embryonic renal epithelia: induction, nephrogenesis, and cell differentiation. Physiol Rev. 1999;79:1157–91. doi: 10.1152/physrev.1999.79.4.1157. [DOI] [PubMed] [Google Scholar]
- 2.Rivera MN, Haber DA. Wilms’ tumour: connecting tumorigenesis and organ development in the kidney. Nat Rev Cancer. 2005;5:699–712. doi: 10.1038/nrc1696. [DOI] [PubMed] [Google Scholar]
- 3.Schedl A. Renal abnormalities and their developmental origin. Nat Rev Genet. 2007;8:791–802. doi: 10.1038/nrg2205. [DOI] [PubMed] [Google Scholar]
- 4.Beckwith JB, Kiviat NB, Bonadio JF. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms’ tumor. Pediatr Pathol. 1990;10:1–36. doi: 10.3109/15513819009067094. [DOI] [PubMed] [Google Scholar]
- 5.Vuononvirta R, Sebire NJ, Dallosso AR, et al. Perilobar nephrogenic rests are non-obligate molecular genetic precursor lesions of IGF2-associated Wilms tumours. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-08-1620. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Natrajan R, Reis-Filho JS, Little SE, et al. Blastemal expression of type I insulin-like growth factor receptor in Wilms’ tumors is driven by increased copy number and correlates with relapse. Cancer Res. 2006;66:11148–55. doi: 10.1158/0008-5472.CAN-06-1931. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123:223–35. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–71. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 9.Uehara Y, Minowa O, Mori C, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–5. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 10.Davies J. Intracellular and extracellular regulation of ureteric bud morphogenesis. J Anat. 2001;198:257–64. doi: 10.1046/j.1469-7580.2000.19830257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peschard P, Park M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene. 2007;26:1276–85. doi: 10.1038/sj.onc.1210201. [DOI] [PubMed] [Google Scholar]
- 12.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 13.Santos OF, Barros EJ, Yang XM, et al. Involvement of hepatocyte growth factor in kidney development. Dev Biol. 1994;163:525–9. doi: 10.1006/dbio.1994.1169. [DOI] [PubMed] [Google Scholar]
- 14.Woolf AS, Kolatsi-Joannou M, Hardman P, et al. Roles of hepatocyte growth factor/scatter factor and the met receptor in the early development of the metanephros. J Cell Biol. 1995;128:171–84. doi: 10.1083/jcb.128.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res. 2006;12:3657–60. doi: 10.1158/1078-0432.CCR-06-0818. [DOI] [PubMed] [Google Scholar]
- 16.Alami J, Williams BR, Yeger H. Expression and localization of HGF and met in Wilms’ tumours. J Pathol. 2002;196:76–84. doi: 10.1002/path.997. [DOI] [PubMed] [Google Scholar]
- 17.Little SE, Bax DA, Rodriguez-Pinilla M, et al. Multifaceted dysregulation of the epidermal growth factor receptor pathway in clear cell sarcoma of the kidney. Clin Cancer Res. 2007;13:4360–4. doi: 10.1158/1078-0432.CCR-07-0398. [DOI] [PubMed] [Google Scholar]
- 18.Natrajan R, Warren W, Messahel B, et al. Complex patterns of chromosome 9 alterations including the p16INK4a locus in Wilms tumours. J Clin Pathol. 2008;61:95–102. doi: 10.1136/jcp.2007.047159. [DOI] [PubMed] [Google Scholar]
- 19.Natrajan R, Little S, Reis Filho JS, et al. Amplification and overexpression of CACNA1E correlates with relapse in favourable histology Wilms tumours. Clin Cancer Res. 2006;12:7284–93. doi: 10.1158/1078-0432.CCR-06-1567. [DOI] [PubMed] [Google Scholar]
- 20.Jones C, Rodriguez-Pinilla M, Lambros M, et al. c-KIT overexpression, without gene amplification and mutation, in paediatric renal tumours. J Clin Pathol. 2007;60:1226–31. doi: 10.1136/jcp.2007.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki K, Cheng J, Watanabe Y. Hepatocyte growth factor and c-Met (HGF/c-Met) in adenoid cystic carcinoma of the human salivary gland. J Oral Pathol Med. 2003;32:84–9. doi: 10.1034/j.1600-0714.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 22.Weirich A, Leuschner I, Harms D, et al. Clinical impact of histologic subtypes in localized non-anaplastic nephroblastoma treated according to the trial and study SIOP-9/GPOH. Ann Oncol. 2001;12:311–9. doi: 10.1023/a:1011167924230. [DOI] [PubMed] [Google Scholar]
- 23.Little MH, Wilkinson L, Brown DL, Piper M, Yamada T, Stow JL. Dual trafficking of Slit3 to mitochondria and cell surface demonstrates novel localization for Slit protein. Am J Physiol Cell Physiol. 2001;281:C486–95. doi: 10.1152/ajpcell.2001.281.2.C486. [DOI] [PubMed] [Google Scholar]
- 24.von Koskull H, Virtanen I. Induction of cytokeratin expression in human mesenchymal cells. J Cell Physiol. 1987;133:321–9. doi: 10.1002/jcp.1041330216. [DOI] [PubMed] [Google Scholar]
- 25.Kartha S, Atkin B, Martin TE, Toback FG. Cytokeratin reorganization induced by adenosine diphosphate in kidney epithelial cells. Exp Cell Res. 1992;200:219–26. doi: 10.1016/0014-4827(92)90167-7. [DOI] [PubMed] [Google Scholar]
- 26.Weidner KM, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–54. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karp SL, Ortiz-Arduan A, Li S, Neilson EG. Epithelial differentiation of metanephric mesenchymal cells after stimulation with hepatocyte growth factor or embryonic spinal cord. Proc Natl Acad Sci U S A. 1994;91:5286–90. doi: 10.1073/pnas.91.12.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alami J, Williams BR, Yeger H. Derivation and characterization of a Wilms’ tumour cell line, WiT 49. Int J Cancer. 2003;107:365–74. doi: 10.1002/ijc.11429. [DOI] [PubMed] [Google Scholar]
- 29.Pisters LL, el-Naggar AK, Luo W, Malpica A, Lin SH. C-met proto-oncogene expression in benign and malignant human renal tissues. J Urol. 1997;158:724–8. doi: 10.1097/00005392-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Dietrich S, Abou-Rebyeh F, Brohmann H, et al. The role of SF/HGF and c-Met in the development of skeletal muscle. Development. 1999;126:1621–9. doi: 10.1242/dev.126.8.1621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.