Abstract
Using HPLC techniques we evaluated the electrical field stimulation-evoked overflow of noradrenaline (NA), adenosine 5′-triphosphate (ATP), and β-nicotinamide adenine dinucleotide (β-NAD) in the presence of low nanomolar concentrations of ω-conotoxin GVIA or ω-agatoxin IVA in the canine mesenteric arteries and veins. ω-conotoxin GVIA abolished the evoked overflow of NA and β-NAD in artery and vein, whereas the evoked overflow of ATP remained unchanged in the presence of ω-conotoxin GVIA. ω-agatoxin IVA significantly reduced the evoked overflow of ATP and β-NAD. The overflow of NA remained largely unaffected by ω-agatoxin IVA, except at 16 Hz in the vein where the overflow of NA was reduced by about 50%. Artery and vein exhibited similar expression levels of the α1B (CaV2.2, N-type) subunit, whereas the vein showed greater levels of the α1A (CaV2.1, P/Q-type) subunit than artery. Therefore, there are at least two release sites for NA, β-NAD and ATP in the canine mesenteric artery and vein: an N-type-associated site releasing primarily NA, β-NAD and some ATP, and a P/Q-type-associated site releasing ATP, β-NAD and some NA. The N-type-mediated mechanisms are equally expressed in artery and vein, whereas the P/Q-type-mediated mechanisms are more pronounced in the vein and may ensure additional neurotransmitter release at higher levels of neural activity. In artery, β-NAD caused a dual effect consisting of vasodilatation or vasoconstriction depending on concentrations, whereas vein responded with vasodilatation only. In contrast, ATP caused vasoconstriction in both vessels. β-NAD and ATP may mediate disparate functions in the canine mesenteric resistive and capacitative circulations.
Keywords: sympathetic, cotransmission, noradrenaline, purines, extracellular β-NAD
1. Introduction
Sympathetic neural control of vascular and visceral smooth muscles involves the release and action of multiple neurotransmitter substances, a phenomenon referred to as co-transmission (Burnstock, 1990). Mechanisms of concomitant release of co-transmitters, including noradrenaline (NA) and adenosine 5′-triphosphate (ATP), have been the subject of studies for several decades. However, there is still controversy around the important question of whether or not synaptic release of NA and ATP is regulated differentially. The answer to this question would forward our knowledge about the existence of common or distinct release sites for neurotransmitter substances and about the possibilities for selective manipulation of release of co-transmitters.
Recent studies have demonstrated that β-nicotinamide adenine dinucleotide (β-NAD) is released along with NA and ATP upon nerve stimulation in a number of vascular and visceral smooth muscles, including the canine mesenteric arteries and veins (Smyth et al., 2004), murine and rat tail arteries, murine, rat, and guinea-pig vas deferens (our unpublished work), urinary bladder detrusor smooth muscle of mouse, rat, guinea-pig, rabbit, dog, monkey and human (Smyth et al., 2004; Breen et al., 2006), and colon of mouse (Mutafova-Yambolieva et al., 2007), non-human and human primates (our unpublished work). The release of β-NAD is not induced by contraction of the smooth muscle per se and is sensitive to inhibition of neural activity or vesicular exocytosis (Smyth et al., 2004; Breen et al., 2006; Smyth et al., 2006a; Mutafova-Yambolieva et al., 2007). Exogenous β-NAD reduces the release of NA in blood vessels (Smyth et al., 2004), inhibits spontaneous mechanical activity in the bladder (Breen et al., 2006), induces membrane hyperpolarization and smooth muscle relaxation in the murine colon (Mutafova-Yambolieva et al., 2007), and causes vasodilatation or vasoconstriction in blood vessels (the present study). Therefore, β-NAD emerges as a candidate neurotransmitter and a neuromodulator at the smooth muscle neuroeffector junction. Recent studies have suggested that the release of ATP and β-NAD may be regulated differentially (Mutafova-Yambolieva et al., 2007). Moreover, in the murine colon β-NAD mimics the effects of the endogenous inhibitory neurotransmitter better than ATP (Mutafova-Yambolieva et al., 2007), and therefore in some systems β-NAD, but not ATP, might be the purine neurotransmitter substance. To our knowledge, detailed characterization of effects of extracellular β-NAD in the peripheral circulation or isoletd blood vessels has not been reported. Likewise, the regulatory mechanisms of β-NAD release in the context of NA/ATP co-transmission remain to be elucidated.
Finally, different smooth muscle systems may require different mechanisms of regulation of co-transmitter release due to their specialized functions in the body. Both mesenteric arteries and veins are densely innervated by sympathetic postganglionic nerves (Smyth et al., 2000; Smyth et al., 2006a) and both release NA, ATP, and β-NAD (Bobalova and Mutafova-Yambolieva, 2001a; Bobalova and Mutafova-Yambolieva, 2001b; Smyth et al., 2004). Arteries and veins differ in the amounts of released co-transmitters upon stimulation of postganglionic nerves (Bobalova and Mutafova-Yambolieva, 2001a). The two vascular networks serve different functions in the peripheral circulation (resistance vs. capacitance) and therefore may require different regulation of co-transmitter release.
It may be assumed that differences in mechanisms underlying Ca2+ entry through neuronal voltage-gated Ca2+ channels (VGCCs) account for the differences in the amounts of released co-transmitters in arteries and veins. The VGCCs mediating Ca2+ entry in the autonomic nerves commonly are N-type and P/Q-type (Waterman, 2000). N-type VGCCs are formed by the α1B (CaV2.2) gene product and are inhibited specifically by ω-conotoxin-GVIA (ω-CTX GVIA) (Olivera et al., 1984). P- and Q-type channels are formed by the α1A (CaV2.1) gene product (Gillard et al., 1997) and represent differential splicing of the α1A subunit (Bourinet et al., 1999) for which they are often referred to as P/Q-type channels; these channels are inhibited selectively by ω-agatoxin IVA (ω-AgaTX IVA) (Mintz et al., 1992). Previous studies have shown that the release of NA and ATP from sympathetic nerves supplying smooth muscles is either differentially (i.e., guinea-pig vas deferens, Westfall et al., 1996) or similarly (i.e., rat tail artery, Brock and Cunnane, 1999) affected by inhibition of Ca2+ entry through VGCCs into the nerve processes. However, comparative information for the arteries and veins of the same vascular region is presently unavailable.
In the present study we investigated the hypothesis that N-type and P/Q-type VGCCs differentially mediate the release of NA, ATP and β-NAD upon activation of postganglionic nerves in blood vessels. We also show that the arterial and venous parts of the splanchnic circulation differ in the expression levels of N-type and P/Q-type VGCCs, which underlies differences in co-transmitter release in arteries and veins. Finally, we provide evidence that extracellular β-NAD and ATP appear to play different roles in control of vascular smooth muscle tone in the splanchnic circulation.
2. Materials and methods
2.1. Tissue preparations
Seventy mongrel dogs of either sex (averaging 15 kg), obtained from vendors licensed by the United States Department of Agriculture, were used for this study. The use of the laboratory animals for these experiments was approved by the University of Nevada’s Animal Care and Use Committee. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). The animals were euthanized with pentobarbitone sodium, 100 mg/kg intravenously. The abdomens were opened and segments of second and third order branches of the superior and inferior mesenteric artery (0.5–1mm in diameter) and vein (1–2 mm in diameter) were dissected out and bathed in oxygenated Krebs solution containing (mM): 118.5 NaCl; 4.2 KCl; 1.2 MgCl2; 23.8 NaHCO3; 1.2 KH2PO4; 11.0 dextrose; 1.8 CaCl2 (pH 7.4). The vessels were denuded of their endothelium by rubbing with a rough-surface needle and slowly perfused with distilled water. This procedure successfully removes endothelium while preserving smooth muscle contractility as shown before in guinea pig blood vessels (Mutafova-Yambolieva et al., 2000). We confirmed that the endothelium was removed in the canine blood vessels by the lack of relaxation to acetylcholine (1 μM) in pre-contracted vessel segments.
2.2. Preparation of membrane protein fraction
Artery and vein (~100 mg wet weight) were pulverized under liquid nitrogen and then homogenized in a sucrose buffer containing (mM): 5.0 HEPES, 0.32 sucrose, 1% Triton X-100 and 1.0 AEBSF. Cell nuclei and large debris were removed by centrifugation at 1500 rpm for 10 min at 4°C. Supernatants were then centrifuged at 100,000 × g for 1.5 h at 4°C, pelleted cell membranes were resuspended in homogenization buffer and subjected to 3 short sonication/vortex cycles to aid solubilization of membrane protein. Total protein concentrations were assayed by the Bradford method and equal amounts of total protein were used for assay of the expression of β-actin, α1B (N-type) and α1A (P/Q-type) proteins by quantitative Western immunoblot analysis. The results were normalized to the β-actin expression levels.
2.3. Western immunoblot analysis
Equal amounts of total protein were resolved by SDS-PAGE (10 % acrylamide) and transferred onto nitrocellulose membranes for 1.5 h at 24 V, 4°C (Genie blotter; Idea Scientific Company, MN). The membranes were blocked for 2 h with 3 % skim milk in PBS. CaV2.1 was probed with a primary rabbit anti-α1A voltage gated calcium channel, P/Q-type antibody (dilution 1:400 in 3 % milk/PBS, at 4°C overnight, Chemicon International, Temecula, CA, Cat. No. AB5152). CaV2.2 was probed with a rabbit anti-α1B voltage gated calcium channel, N-type antibody (dilution 1: 100, Chemicon International, Temecula, CA, Cat. No. AB5154). β-actin was probed with a rabbit polyclonal antibody (Cytoskeleton, Inc., CO). Excess primary antibody was removed by 3 × 5-min washes with TNT buffer (100 mM Tris, pH 7.5, 0.1% Tween-20, 150 M NaCl), and then membranes were incubated for 1 h with a secondary alkaline phosphatase anti-rabbit conjugate, diluted 1:10,000 in 3% milk/PBS. Excess secondary antibody was removed by 3 × 5-min washes with TNT buffer and color was developed with the 5-bromo-4-chloro-3-indolylphosphate/nitro blue tetrazolium (BCIP/NBT) alkaline phosphatase substrate. Immunoblots were scanned to obtain images and the immunoreactive bands of β-actin, α1B (CaV2.2) or α1A (CaV2.1) were analyzed by densitometry, using the Quantity One software (BioRad, Hercules, CA). For quantitation, the immunoreactive band densities of α1B (CaV2.2) or α1A (CaV2.1) were normalized to the densities of the respective β-actin bands, and then expressed relative to the normalized density of the artery. β-actin is often used as a loading control for Western immunoblot assay of neural proteins as shown in previous studies (e.g., Khanna et al., 2007; Anitha et al., 2008). The use of β-actin was based on our previous studies demonstrating that β-actin is equally expressed in the canine mesenteric artery and vein (Yamboliev et al., 2002). β-Actin has both cytosolic and membrane-associated pools (Borbiev et al., 2003) and therefore we utilized the membrane-associated β-actin to verify equal loading of VGCC proteins. In some experiments, pre-absorption with 50-fold higher molar concentration of control antigen (provided by Chemicon International along with the antibodies) for 24 h at 4°C was used to neutralize antibody binding to antigen and to verify that the antibody developed band is specific for each protein.
2.4. Overflow experiments
The tissue segments (68.5±5.5 mg arteries or 53.4±5.4 mg veins) were placed in 200-μl water-jacket BRANDEL superfusion chambers as described previously (Bobalova and Mutafova-Yambolieva, 2001a, Bobalova and Mutafova-Yambolieva, 2001b). Briefly, after 45 min equilibration, the tissues were subjected to a 15-s “conditioning” stimulation with a train of square wave pulses of 0.1 ms duration and a frequency of 4 Hz. Thirty minutes after the conditioning stimulation the preparations were subjected to EFS for 120 s with a train of supra-threshold pulses of 0.3 ms (15 V) at either 4 Hz (low-frequency stimulation) or 16 Hz (high-frequency stimulation). The EFS parameters were chosen after verification that NA overflow is abolished by tetrodotoxin (0.3 μM). Samples of the superfusion Krebs solution were collected before the EFS (resting overflow; PS, pre-stimulation sample) and during the EFS (evoked overflow; ST, stimulation sample) in ice-cold Eppendorf tubes. Samples were split into two parts and processed for assay of NA and ATP/β-NAD content as described below.
In some experiments the tissue segments were superfused with N-type and P/Q-type VGCC blockers for 30 min before EFS. We used 5 nM ω-CTX GVIA to block N-type VGCC (Olivera et al., 1994) and 20 nM ω-AgaTX IVA to block P/Q-type VGCC (Mintz and Bean, 1993). These concentrations exceeded the Kd values for the toxins. Works in Purkinje cells and cerebellar granule neurons has indicated that low nanomolar concentrations of ω-agatoxin IVA block selectively the P-type channels, whereas at high nanomolar concentrations ω-agatoxin VIA also inhibits the Q-type channels (Randall and Tsien, 1995). Since the present study does not discriminate between the two splice variants (i.e., P- and Q-type, Bourinet et al., 1999) we refer to the ω-agatoxin IVA target as P/Q-type channels instead of committing to a specific splice variant. In some experiments the tissue segments were treated with the combination of ω-CTX GVIA and ω-AgaTX IVA to block both N- and P/Q-type VGCC.
2.5. Sample Preparation
Aliquots from the superfusate samples (115 μl) were treated with 3 μl 1 M perchloric acid and filtered through 0.22 μm Cameo 3N syringe filters into HPLC inserts. The samples were then processed for analysis of NA content as described in HPLC assay of NA. Two hundred μl aliquots from the superfusate samples were treated with 10 μl 2-chloroacetaldehyde and 100 μl citrate buffer for 40 min at 80oC in a dry bath incubator to produce 1,N6-etheno-nucleotides and 1,N6-etheno-nucleosides as described previously (Bobalova et al., 2002; Smyth et al., 2004). The samples were then processed for detection of ATP and β-NAD as described in HPLC assay of ATP and β-NAD.
2.6. HPLC assay of NA
Seventy μl samples were injected into an isocratic HP1100 HPLC system equipped with an HP1049A electrochemical detector (Agilent Technologies, Wilmington, DE, USA) and a MD-150 column (ESA Inc., Chelmsford, MA, USA) as described previously (Mutafova-Yambolieva et al., 2003). The mobile phase for separation consisted of the following (mM): 50 NaH2PO4; 0.2 EDTA; 3.0 l-heptanesulfonic acid, 10 LiCl, and methanol 3 % v/v in deionized water (pH 2.6). The electrochemical detector had a glassy carbon target and NA was detected using amperometry mode at 0.5 V. The amounts of NA in each sample were calculated from calibration curves of NA standards run simultaneously with every set of unknown samples. Results were normalized for sample volume and tissue weight and the overflow of NA was expressed in fmol/mg tissue.
2.7. HPLC assay of ATP and β-NAD
The liquid chromatographic system used throughout this study was a binary HP1100 LC module system (Agilent Technologies, Wilmington, DE) as described previously (Smyth et al., 2004). The mobile phase comprised of 0.1 M KH2PO4 (pH 6.0) as eluent A; eluent B consisted of 35 % methanol and 65 % eluent A. Gradient elution was employed according to the following linear program: time 0, 0 % eluent B; 18 min, 100 % eluent B. The fluorescent signals were recorded at an excitation wavelength of 230 nm and emission wavelength of 420 nm, which are optimum parameters for detection of etheno-derivatives of nucleotides and nucleosides (Bobalova et al., 2002). In previous studies we have determined that upon etheno-derivatization at high temperature ATP, ADP, AMP and adenosine generate 1,N6-etheno-ATP, 1,N6-etheno-ADP, 1,N6-etheno-AMP and 1,N6-etheno-ADO, respectively (Bobalova et al., 2002). However, at these experimental conditions β-NAD, ADP-ribose and cyclic ADP-ribose form 1,N6-etheno-ADPR (Smyth et al., 2004). HPLC fraction analysis revealed that the majority of 1,N6-etheno-ADPR in the tissue superfusates of different smooth muscle preparations is formed by β-NAD (Smyth et al., 2004; Breen et al., 2006). Therefore, for clarity, this peak is referred to as β-NAD throughout the study.
2.8. Force measurements
Ring preparations (6 mm long) were mounted in 10-ml vertical organ baths (Myobath 4 System, WPI), by inserting WPI microvessel holders into the lumen. The bottom was stable and the top wire was attached to a strain gauge and changes in the smooth muscle tone were acquired using Biopac System, AcqKnowledge (v. 3.7.1) software. The bath contained Krebs solution, maintained at 37°C. A resting force of 0.75 g and 1.0 g was applied to the vein and the artery, respectively. This was found to stretch vessels to near the optimum length for tension development. In all experiments tissues were initially equilibrated for 1 hour followed with at least 3 alternating 3-min exposures every 15 minutes to KCl (50 mM) and NA (1 μM) in order to establish viability and equilibrate the tissues. Once the response reached a steady state, acetylcholine (1 μM) was added to NA-treated tissue to confirm endothelium denudation. Tissues were again washed and allowed to settle for 45 min before being subjected to the treatments according the protocol. Effects of exogenous nucleotides were evaluated in tissues pre-contracted with either NA (1 μM) or phenylephrine (PE, 1 μM). After reaching a plateau contraction increasing concentrations of nucleotides were applied in a cumulative manner at about 2-min intervals between applications. In some experiments the non-selective P2 receptor antagonists suramin (100 μM) or pyridoxal phosphate 6-azophenyl-2′,4′-disulphonic acid (PPADS, 30 μM) or the non-selective P1 receptor antagonist 8-p-sulfophenyltheophylline (8-SPT, 100 μM) were applied 30 min before PE. Control experiments were performed to determine the consistency in the contractile response to repeated applications of KCl (50 mM) or PE (1 μM) over the duration of time equivalent to the average duration of the protocols. At the end of the experiment the length and the weight (after blotting the tissue on a filter paper) of the tissues were measured and stress (mN/mm2) was calculated (Herlihy and Murphy, 1973; Dillon and Murphy, 1982); the vein and artery differ significantly in lumen diameter and wall thickness and, therefore, stress provides a better means for comparing contractions to PE. Responses to exogenous nucleotides were expressed as a percentage of the contractile response produced by PE.
2.9. Drugs
ω-CTX GVIA, ω-AgaTX IVA, ATP, adenosine, β-NAD, suramin, PPADS, and 8-SPT were purchased from Sigma-Aldrich (St. Louis, MO). The anti-α1A and anti-α1B voltage gated calcium channel antibodies were purchased from Chemicon International (Temecula, CA).
3.0. Statistics
Data are presented as means ± s.e. mean. Means were compared by analysis of variance (one-way ANOVA) (GraphPadPrism v. 3, GraphPad Software, Inc.). A probability value of less than 0.05 was considered significant.
3. Results
3.1. Equal expression levels of CaV2.2 (α1B) subunit of N-type VGCC in artery and vein
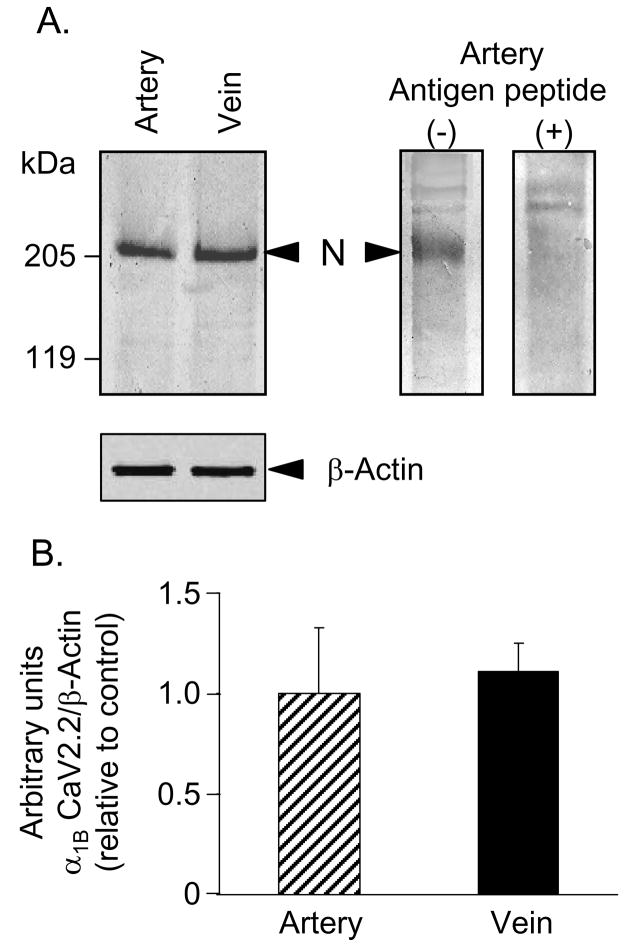
Results from quantitative Western blot analysis of the α1B (CaV2.2, N-type) protein in 100 000 × g membrane fractions are shown in Fig. 1. The predicted molecular size of the α1B subunit of N-type VGCC is 210 kDa, and the observed immunoreactive bands with this size were suggestive of the expression of this protein in both blood vessels (Fig. 1A). To confirm that the immunoreactive signal is specific for the α1B subunit of N-type VGCC, we incubated primary antibody with antigen peptide prior to blotting membranes. This approach largely eliminated the immunoreactive signal at ~210 kDa demonstrating that it was produced by the α1B subunit of N-type VGCC (Fig. 1A). Consistent with our previous results (Yamboliev et al., 2002), the expression of β-actin in artery and vein was equal (Fig. 1A, bottom inset). Densitometry and quantification of the immunoreactive bands indicated that the mesenteric artery and vein express similar amounts of α1B subunit of N-type VGCC (Fig. 1A, top left inset, and 1B, bar graph).
Fig. 1.
Mesenteric artery and vein show similar expression levels of α1B (CaV2.2) subunit of N-type VGCC. (A) Both artery and vein expressed immunoreactive signal at ~210-kDa (top left panel), which was eliminated in blots probed with primary antibody for α1B (CaV2.2) subunit of N-type VGCC, preincubated with antigen peptide (top right panel; artery). Both artery and vein showed equal expression of β-actin (bottom left panel). (B) Relative expression of α1B (CaV2.2) subunit of N-type VGCC in artery and vein normalized to β-actin. Mean ± SEM, n= 5, P>0.05.
3.2. Higher expression levels of CaV2.1 (α1A) subunit of P/Q-type VGCC in vein than artery
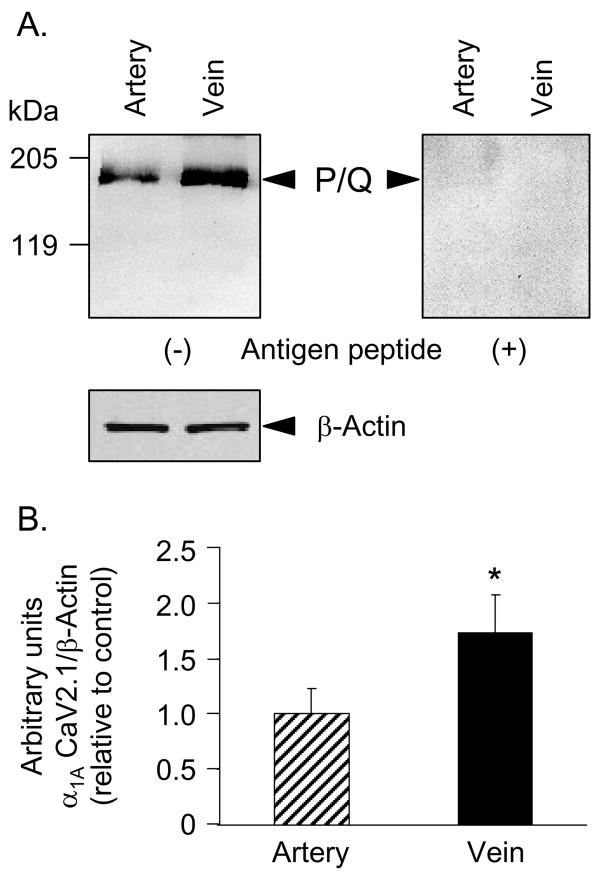
Immune blots using antibody against the α1A subunit of P/Q-type VGCC visualized immunoreactive bands at ~190 kDa, consistent with the predicted molecular size of this subunit (Fig. 2A). Additional proof of the identity of this protein was obtained from immune blots in which probing with primary antibody preincubated with antigen peptide eliminated the immunoreactive bands (Fig. 2A). Unlike the N-type channel, however, the expression of the α1A subunit of P/Q-type was significantly higher in vein than in artery (Fig. 2A,B). These results identify a difference in the expression levels of prejunctional Ca2+ channels and open the possibility for differences in P/Q calcium channel-mediated events in mesenteric artery and vein.
Fig. 2.
The expression levels of α1A (CaV2.1) subunit of P/Q-type VGCC in mesenteric vein exceeds the expression levels in mesenteric artery (top left panel). (A) The immunoreactive band of α1A (CaV2.1) subunit of P/Q-type VGCC (~190 kDa) was eliminated by preincubation of the primary antibody with antigen peptide (top right panel). Both artery and vein showed equal expression of β-actin (bottom left panel). (B) Relative expression of α1A (CaV2.1) subunit of P/Q-type VGCC in artery and vein normalized to β-actin and control artery. Mean ± SEM, n= 5. The asterisk denotes significant difference from artery, P<0.05.
3.3. The overflow of NA is mediated by N-type VGCC in the artery and by both N- and P/Q-types VGCCs in the vein
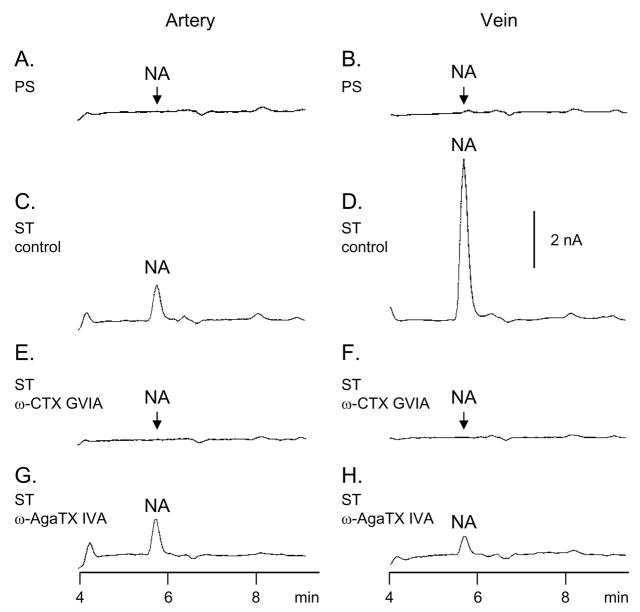
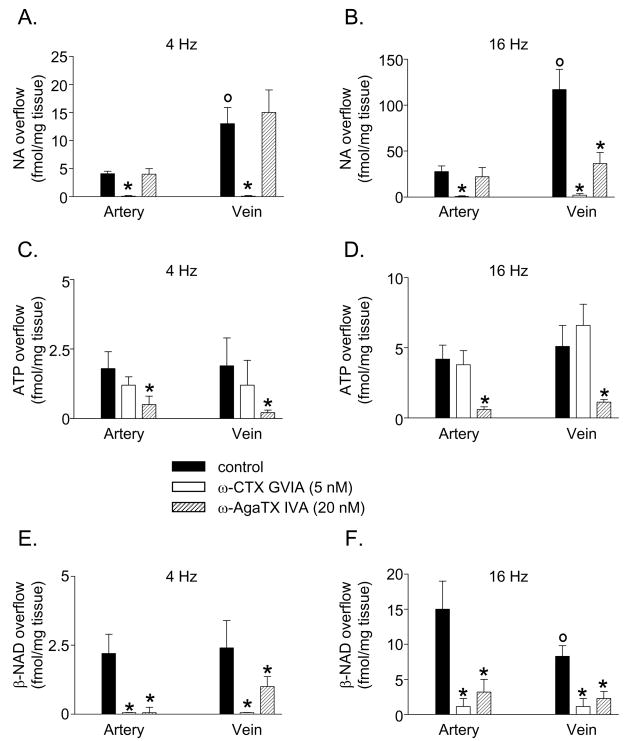
In agreement with our previous studies (Bobalova and Mutafova-Yambolieva, 2001a, Bobalova and Mutafova-Yambolieva, 2001b) no NA was detected in the pre-stimulation (PS) samples of either artery or vein (Figs. 3A,B), whereas upon EFS both blood vessels released NA (Fig. 3C,D; Fig. 5A,B). The release of NA in the vein exceeded the release of NA in the artery (Fig. 3C,D) at both low (4 Hz) and high (16 Hz) frequencies of stimulation (Fig. 5A,B). At 4 Hz the effects of ω-CTX GVIA and ω-AgaTX IVA were qualitatively similar in the artery and vein (Fig. 5A). At 16 Hz the overflow of NA in the artery was inhibited by ω-CTX GVIA, but not by ω-AgaTX IVA, whereas in the vein the evoked overflow of NA was reduced by both ω-CTX GVIA and ω-AgaTX IVA (Fig. 5B). The ω-AgaTX IVA-insensitive component of NA release was abolished by the addition of ω-CTX GVIA (5 nM) to the ω-AgaTX IVA solution (data not shown).
Fig. 3.
HPLC-electrochemical detection analysis of NA release upon EFS (16 Hz, 0.3 ms, 120 s) in canine isolated mesenteric artery (left column) and vein (right column). Original chromatograms are shown from tissue superfusate samples collected before (pre-stimulation, PS, A and B) and during EFS (ST) in the absence (control – C, D) and presence of ω-CTX GVIA (5 nM – E, F), and ω-AgaTX IVA (20 nM – G, H). The amount of vascular segments loaded per chamber was 62.0 ± 1.5 mg wet weight for the artery and 58.0 ± 1.0 mg wet weight for the vein (P>0.05). Scales apply to all chromatograms.
Fig. 5.
The EFS (4 Hz or 16 Hz, 0.3 ms, 120 s)-evoked release of NA (A, B), ATP (C, D), and β-NAD (E, F) is affected differently by ω-CTX GVIA (5 nM) or ω-AgaTX IVA (20 nM) in canine isolated mesenteric artery and vein. Data represent mean±SEM from 5–10 experiments. The asterisks denote significant difference from controls, P<0.05. The open circles denote significant difference from artery control, P<0.05.
3.4. The overflow of ATP is mediated primarily by P/Q-type VGCC
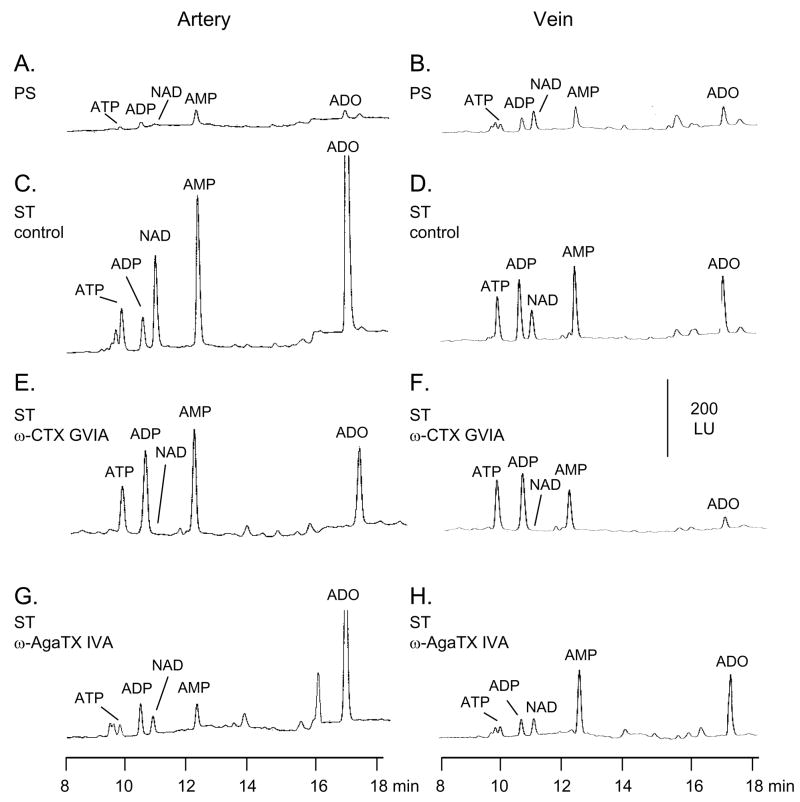
Small amounts of ATP were present in the PS samples of both artery and vein (Fig. 4A, B). Larger amounts of ATP were seen in the samples collected during EFS (Fig. 4C, D); greater amounts of ADP, AMP, and ADO were also seen in the samples collected during stimulation (ST samples) due to the degradation of both ATP and β-NAD (discussed below). In agreement with previous studies (Bobalova and Mutafova-Yambolieva, 2001a, Bobalova and Mutafova-Yambolieva, 2001b) the overflow of ATP did not differ significantly between the artery and vein (Fig. 5C,D). In contrast to the overflow of NA the overflow of ATP was not significantly affected by ω-CTX GVIA in either artery or vein (Fig. 4E, F; Fig. 5C,D). However, unlike ATP the peaks of AMP and ADO were reduced by ω-CTX GVIA (Fig. 4E, F). In the presence of ω-AgaTX IVA (20 nM) the evoked overflow of ATP was reduced in the two blood vessels (Fig. 4G, H; Fig. 5C,D). Similar patterns of ω-CTX GVIA and ω-AgaTX IVA effects were observed at low (4 Hz) and high (16 Hz) frequencies of stimulation (Fig. 5C,D). Once released ATP is subject to rapid degradation by ecto-nucleotidases (Zimmermann, 2000) and possibly releasable enzymes (Todorov et al., 1997) to ADP, AMP and ADO. However, part of the AMP and ADO in the tissue superfusates is formed by the degradation of β-NAD (discussed below). Therefore, ADP is the only compound in the superfusate sample that is an immediate metabolite of ATP and hence the overflow of ATP plus ADP would reflect closely the release of ATP. Indeed, ω-CTX GVIA and ω-AgaTX IVA exerted similar effects on the overflow of ATP alone and on the combined overflow of ATP and ADP. For example, in the artery the combined overflow of ATP and ADP was 8.8±1.2 fmol/mg tissue (n=10) in controls, 8.5±1.6 fmol/mg tissue (n=5) in the presence of ω-CTX GVIA, and 6.3±0.2 fmol/mg tissue (n=5) in the presence of ω-AgaTX IVA. In the vein the overflow of ATP plus ADP was 11.5±2.2 fmol/mg tissue (n=10) in controls and 12.7±1.2 fmol/mg tissue (n=5) and 6.2±0.7 fmol/mg tissue (n=5) in the presence of ω-CTX GVIA and ω-AgaTX IVA, respectively. Thus, in both blood vessels ω-CTX GVIA had no effect on the combined overflow of ATP and ADP, whereas ω-AgaTX IVA significantly reduced the ATP plus ADP overflow, suggesting again that the overflow of ATP is regulated primarily by the ω-AgaTX IVA-sensitive P/Q type VGCCs.
Fig. 4.
HPLC-fluorescence detection analysis of purine release upon EFS (16 Hz, 0.3 ms, 120 s) in canine isolated mesenteric artery (left column) and vein (right column). Original chromatograms are shown from tissue superfusate samples collected before (pre-stimulation, PS, A and B) and during EFS (stimulation, ST) in the absence (control - C, D) and presence of ω-CTX GVIA (5 nM – E, F), and ω-AgaTX IVA (20 nM – G, H). The amount of vascular segments loaded per chamber was 62.0 ± 0.5 mg wet weight for the artery and 58.0 ± 1.0 mg wet weight for the vein (P>0.05). Scales apply to all chromatograms.
3.5. The overflow of β-NAD is mediated by both N-type and P/Q-type VGCC
Small amounts of β-NAD were present in the PS samples (Fig. 4A, B): 0.42±0.16 fmol/mg tissue (n=16) in the artery and 0.68±0.20 fmol/mg tissue (n=14) in the vein (P>0.05). Larger amounts of β-NAD were detected in the samples collected during ST (Fig. 4C, D, results at 16 Hz EFS shown). At 4 Hz of stimulation the overflow of β-NAD was similar in the artery and vein, whereas at 16 Hz EFS the evoked overflow of β-NAD in the artery exceeded the release of β-NAD in the vein (Fig. 5E, F) as demonstrated previously (Smyth et al., 2004). Similarly to NA, the overflow of β-NAD was almost abolished in the presence of ω-CTX GVIA in both blood vessels (Fig. 4G, H; Fig. 5E, F). In the presence of ω-AgaTX IVA (20 nM) the evoked overflow of β-NAD was reduced in both artery and vein (Fig. 4G, H; Fig. 5E, F).
3.6. Exogenous β-NAD, ATP and adenosine have disparate effects on the vascular smooth muscle tone
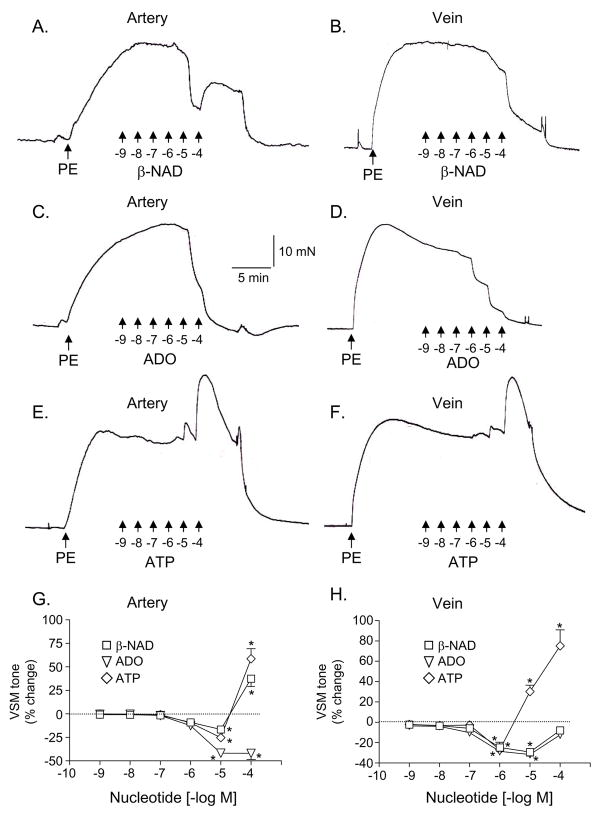
PE (1 μM) caused robust contractions in both the artery (154.79 ± 13.74 mN/mm2, n = 16) and vein (180.28±17.07 mN/mm2, n=18). As shown in Fig. 6, exogenous β-NAD at low micromolar concentrations caused relaxations in both the artery and vein, whereas at high micromolar concentrations β-NAD caused vasoconstriction in the artery but not in the vein (Fig. 6A,B,G,H). Exogenous adenosine (ADO) caused concentration-dependent vasorelaxation in both artery and vein (Fig. 6C,D,G,H), whereas ATP caused a weak relaxation at low micromolar concentrations (if any) and a pronounced contraction at high micromolar concentrations in both artery and vein (Fig. 6E–H).
Fig. 6.
In vascular segments pre-contracted with phenylephrine (PE, 1 μM) cumulatively applied β-NAD (A, B), adenosine (ADO, C, D), and ATP (E, F) induced changes in the smooth muscle tone of canine isolated mesenteric artery (left column) and vein (right column); panels A–F represent original traces from mechanical experiments. Panels G and H show average changes in smooth muscle tone expressed as percentage from the PE contraction. Data represent mean±SEM from 6–14 experiments. The asterisks denote significant difference from control PE contractions, P<0.05.
3.7. Different purine receptors appear to mediate the effects of β-NAD, ATP, and adenosine on vascular smooth muscle tone
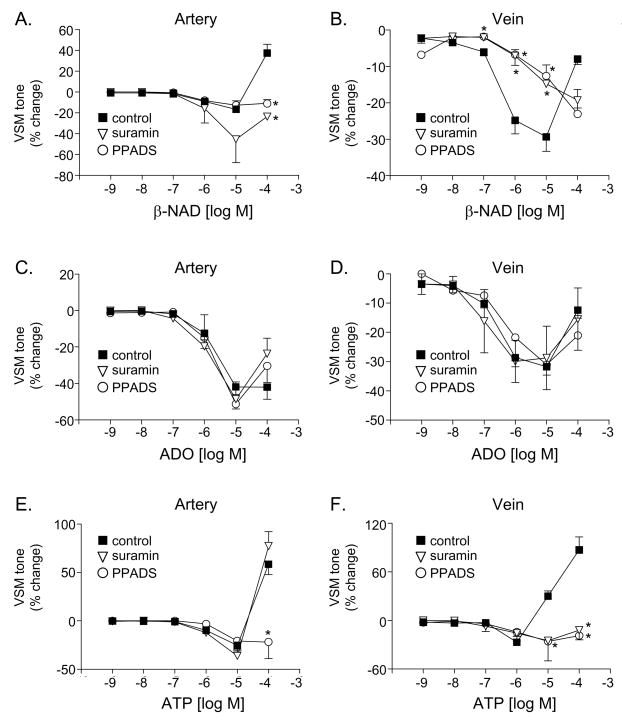
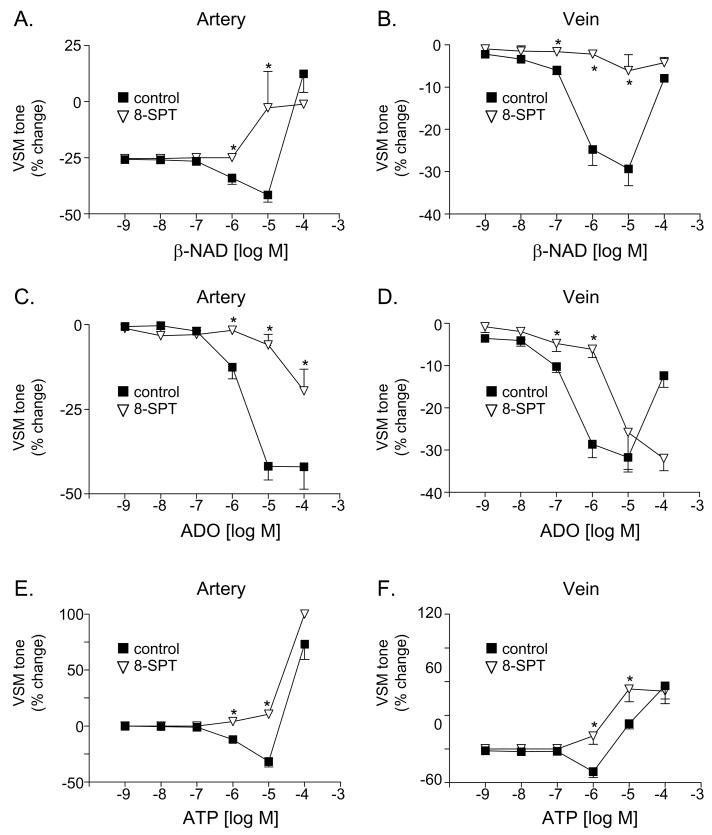
The non-selective P2 receptor antagonist PPADS attenuated the vasorelaxation to β-NAD in the vein (Fig. 7B) as well as the vasoconstriction to β-NAD in the artery (Fig. 7A) and ATP in the artery and vein (Fig. 7E,F). Suramin, another non-specific P2 receptor antagonists, had similar effects to PPADS except for that suramin did not affect significantly the contractile response to ATP in the artery (Fig. 7E). As anticipated, the vasorelaxation responses to ADO were insensitive to the P2 receptor antagonists (Fig. 7C,D). In contrast, the non-selective P1 receptor antagonist 8-SPT reduced the vasorelaxation to ADO in both artery and vein (Fig. 8C,D). Interestingly the vasorelaxation to ATP was abolished in both blood vessels in the presence of 8-SPT (Fig. 8E,F), suggesting that the vasorelaxation to ATP is mediated by its metabolite adenosine. Likewise, 8-SPT-sensitive receptors appear to mediate the vasorelaxation in response to exogenous β-NAD (Fig. 8A, B).
Fig. 7.
The effects of β-NAD (A, B), adenosine (ADO, C, D) and ATP (E, F) in the absence (closed squares) and presence of the P2 receptor antagonists suramin (100 μM) or PPADS (30 μM) in the canine mesenteric artery (left column) and vein (right column). Data represent mean±SEM from 2–6 experiments. The asterisks denote significant difference from controls in the absence of P2 receptor antagonists, P<0.05.
Fig. 8.
The effects of β-NAD (A, B), adenosine (ADO, C, D) and ATP (E, F) in the absence (closed squares) and presence of the P1 receptor antagonist 8-p-sulfophenyltheophylline (8-SPT, 100 μM) in the canine mesenteric artery (left column) and vein (right column). Data represent mean±SEM from 2–6 experiments. The asterisks denote significant difference from controls in the absence of 8-SPT, P<0.05.
4. Discussion
In the present study we assayed the contribution of N- and P/Q-types VGCCs in the simultaneous release of NA, ATP, and β-NAD upon activation of perivascular postganglionic nerves in canine mesenteric arteries and veins. We found that N-type VGCCs primarily regulated the evoked overflow of NA, whereas the overflow of ATP was regulated mainly by P/Q-type channels. The evoked overflow of β-NAD was mediated by both N-type and P/Q-type VGCCs. The N-type-mediated mechanisms appear equally expressed in the artery and vein, whereas the P/Q-type-regulated mechanisms are more pronounced in the vein.
A key event in exocytosis of neurotransmitters from synaptic vesicles is the entry of Ca2+ through VGCCs (Waterman, 2000). Release of co-transmitters can be mediated by entry of Ca2+ through different channels into the same autonomic neuron (Westfall et al., 1996; Waterman, 1996), or in other cases, release of co-transmitters involves the same channel subtypes (Brock and Cunnane, 1999; Waterman, 1997). In the autonomic nerves N-type and P/Q-type VGCCs seem to play the central role in these mechanisms. The α1 pore-forming subunit is a key determinant of the properties of Ca2+ channel subtypes (Catterall, 2000), with P/Q-type channels containing α1A subunits (CaV2.1) and N-type channels containing α1B subunits (CaV2.2) (Ertel et al., 2000). Both α1B and α1A subunits were expressed in the canine mesenteric artery and vein. The expression levels of the α1B (N-type) protein were similar in the two vascular networks; however, the expression level of the α1A (P/Q-type) protein was greater in the vein than in artery. These differences in the protein expression were associated with different responses to VGCC blockers, which is suggestive of different functional activities of the VGCC in the neurotransmitter release. For example, the release of NA and β-NAD was almost abolished by ω-CTX GVIA in both blood vessels indicating that the release of the two substances was primarily mediated by Ca2+ entry through N-type channels. The release of ATP, however, was largely insensitive to inhibition of N-type VGCC but was significantly reduced by inhibition of P/Q-type VGCC. A role of neuronal non-N-type (presumably P/Q- type) VGCC in sympathetic transmission in mesenteric arteries has previously been suggested (Tanaka et al., 1999). While N-type VGCCs show exclusive neural localization, P/Q-type channels have been reported localized in neurons (e.g., Catterall, 1999) and in vascular smooth muscle cells (e.g., Andreasen et al., 2006). Therefore, one might argue that the evoked overflow of ATP reflects the release of ATP from smooth muscle cells but not from nerve endings. However, it is well-established that veins contain lower amounts of smooth muscle cells than the parallel arteries due to thinner vascular walls. In particular, the canine mesenteric artery contains on average 24 smooth muscle cells in cross section, whereas the parallel vein consists of 3 smooth muscle cells (Yamboliev et al., 2002). Nonetheless, veins showed higher expression levels of P/Q-type channels than the parallel arteries. We assume therefore that the higher expression levels of P/Q channels in the vein are not attributable to higher expression levels of these channels in the smooth muscle cells but are rather due to higher expression levels in nerve processes.
Furthermore, the evoked overflow of NA (which is known to be exclusively released from nerves and not from smooth muscle cells) was significantly reduced by the P/Q-type channel inhibitor in the vein at 16 Hz of EFS, suggesting that P/Q-type channels are indeed present in postganglionic nerves in the canine mesenteric blood vessels and mediate release of neurotransmitters. Also, about 80 % of the overflow of ATP evoked by 16 Hz of stimulation in the canine mesenteric blood vessels was significantly reduced by tetrodotoxin (Bobalova and Mutafova-Yambolieva, 2001b). Yet, the overflow of ATP was entirely unchanged by inhibition of N-type VGCCs, but was diminished by inhibition of P/Q-type channels, suggesting that neural P/Q-type channels (and not N-type VGCCs) likely participate in the EFS-evoked overflow of ATP. Besides postganglionic sympathetic nerves, agatoxin-sensitive Ca2+ channels seem to be functionally important in visceral sensory nerves (Wang et al., 2001). Furthermore, sensory nerves appear to contain tetrodotoxin-insensitive Na+ channels (Campbell, 1992). Thus, both sympathetic nerves and sensory nerves may be a source of EFS-evoked overflow of ATP. It is unlikely that cell bodies present a major location of P/Q-type channels, since in previous immunohistochenmistry studies no cell bodies were observed in mesenteric artery and vein preparations from different species including dog (e.g., Smyth et al., 2000, Smyth et al., 2006b).
The observations that the EFS-evoked overflow of ATP is differentially regulated by ω-conotoxin GVIA and agatoxin IVA are agreement with previous reports in the guinea-pig vas deferens, which by utilizing similar overflow techniques showed that the EFS-evoked overflow of ATP was mediated by P/Q-type calcium channels but not by N-type VGCCs (Westfall et al., 1996). On the other hand, the present results are in contrast to studies reporting that the release of ATP in rat tail artery (Brock and Cunnane, 1999), rat mesenteric artery (Xi et al., 2002), determined by changes in the EFS-elicited excitatory junction potentials, or cultured sympathetic neurons (von Kugelgen et al., 1994) is significantly reduced by ω-CTX GVIA. One might argue that sources other than sympathetic nerves may be involved in the release of ATP in the overflow experiments. However, still about 80% of the evoked overflow of ATP in the canine mesenteric circulation was sensitive to TTX or guanethidine (Bobalova and Mutafova-Yambolieva, 2001b) and hence originated from sympathetic nerves. Furthermore, in most studies reporting reduced release of ATP by ω-CTX GVIA, the toxin was used in concentrations equal to or higher than 100 nM at which concentrations ω-CTX GVIA most likely is losing its selectivity for the N-type VGCCs. Most studies agree that the release of NA from sympathetic nerves is mediated by Ca2+ influx through N-type VGCCs. An NA-mediated component that is not sensitive to N-type VGCCs inhibition is revealed at high frequencies of stimulation or longer stimulation trains (Waterman, 2000). In the present study we fond that N-type VGCCs mediate NA and β-NAD overflow at both low and high frequencies of stimulation. A P/Q-type VGCC-mediated component in NA release is revealed at higher frequency of stimulation in the vein, which may be required by specific functions of the systemic veins at higher levels of neural activity (discussed below).
While NA and ATP are relatively well studied sympathetic co-transmitters in perivascular nerve networks and in mesenteric artery and vein in particular (i.e., Bobalova and Mutafova-Yambolieva, 2001a, Bobalova and Mutafova-Yambolieva, 2001b), β-NAD is a newly identified extracellular substance released upon action potential with the aptitude to be a novel neurotransmitter and a neuromodulator at the vascular and visceral smooth muscle neuroeffector junction (Smyth et al., 2004; Breen et al., 2006; Mutafova-Yambolieva et al., 2007). Initial characterization in the canine mesenteric artery revealed that in many aspects the release of β-NAD parallels the release of NA (Smyth et al., 2004; Smyth et al., 2006a), although NA itself does not induce β-NAD overflow (Smyth et al., 2006a). Thus, the evoked release of both substances was significantly reduced by the fast Na+ channel inhibitor tetrodotoxin, by the inhibitor of neurotransmitter release from sympathetic nerves guanethidine, by the adrenergic neurotoxin 6-hydroxydopamine (Smyth et al., 2004), by disruption of SNAP-25-mediated vesicular exocytosis by botulinum neurotoxin A (Smyth et al., 2006a), by activation of the adenylyl cyclase-mediated pathway (Bobalova and Mutafova-Yambolieva, 2006; Mutafova-Yambolieva et al., 2003), and by the specific inhibitor of N-type VGCCs ω-CTX GVIA (Smyth et al., 2004; the present study). The evoked overflow of ATP (Bobalova and Mutafova-Yambolieva, 2001a) and of β-NAD (Smyth et al., 2004) in the canine blood vessels is also significantly reduced by tetrodotoxin and guanethidine. However, artery and vein of the mesenteric circulation differ in the amounts of NA, ATP, and β-NAD released upon EFS, suggesting that the three compounds may not be released in constant ratios and, hence, may not be stored in and released from the same synaptic vesicles. For example, at 16 Hz of stimulation the evoked overflow of NA in the vein exceeds the overflow of NA in the artery (Bobalova and Mutafova-Yambolieva, 2001a, Bobalova and Mutafova-Yambolieva, 2001b; the present study). However, the evoked overflow of ATP alone or ATP plus ADP (which should faithfully reflect the release of ATP, discussed below) was equal in the two blood vessels. Unlike NA or ATP, the evoked overflow of β-NAD was greater in artery than in vein, suggesting that the artery may have greater amounts of β-NAD available for release upon nerve stimulation, although further work needs to be done to clarify this issue. These are intriguing observations emphasizing the importance of simultaneous evaluation of EFS-evoked overflow of co-transmitters released under identical experimental conditions. Further studies are warranted to define the precise conditions of VGCC activation in these blood vessels, which might reveal different thresholds for exocytosis of different populations of synaptic vesicles.
In addition to differences in VGCC-mediated regulation of evoked overflow of NA, ATP and β-NAD, the present study provides evidence that arteries and veins from the mesenteric circulation differ in their responses to VGCC inhibitors. While the release of NA in the artery was insensitive to inhibition of P/Q-type channels, the release of NA in the vein was significantly reduced by inhibitors of P/Q-type VGCCs. The similar expression levels of the α1B protein in the artery and vein, as well as the similar effects of ω-CTX GVIA in both blood vessels, cannot explain why the evoked release of NA in the vein exceeded the release of NA in the artery. It is unclear whether the greater expression of the α1A subunit of the P/Q-type channel in the vein contributes to the greater release of NA in the vein, especially at higher levels of neural activity. Such reasoning appears logical, bearing in mind that veins are particularly important during sympathetic hyperactivity (e.g., hemorrhage, strenuous exercise, and stress) commonly accompanied by pronounced venoconstriction (Rothe, 1983; Kreulen, 2003).
Another interesting aspect of the present study is presented by the observation that although the overflow of ATP was not affected by ω-CTX GVIA, the overflow of both AMP and ADO was reduced concomitantly with β-NAD overflow. Once released, ATP is rapidly degraded to ADP and AMP, which in turn is degraded to ADO (Zimmermann, 2000). β-NAD, on the other hand, is degraded to ADP-ribose (Lee, 2001), which in turn is degraded to AMP (Di Girolamo et al., 1997) and ADO. Therefore, AMP and ADO could be formed by the degradation of both β-NAD and ATP. Hence, the overflow of AMP and ADO can be reduced when either the overflow of β-NAD, of ATP, or both, is reduced. Thus, our previous (Smyth et al., 2004; Breen et al., 2006) and present data demonstrating release of β-NAD indicate that ATP, contraty to previous suggestions (Todorov et al., 1999), can no longer be considered the only source of AMP and ADO in the tissue superfusates. In other words, the combined release of ATP, ADP, AMP, and ADO reflects the release of not only ATP but also of β-NAD. The EFS-evoked overflow of endogenous nucleotides and nucleosides is a result of both the release and degradation of neurotransmitter substances (i.e., ATP and β-NAD). Therefore differences in enzyme activities between the artery and vein (Bobalova and Mutafova-Yambolieva, 2003) should be taken into account. In the present study differences in nucleotide metabolism do not appear to underlie the differences in the effects of VGCC inhibitors. For example, the effects of VGCC inhibitors on the combined overflow of ATP and ADP paralleled quite accurately the effects of the VGCC blockers on ATP overflow. Moreover, the effects of the VGCC inhibitors were compared with controls (which also exhibit nucleotide degradation), suggesting that difference in the degradation of ATP or β-NAD in artery and vein did not account much for the differential effects of the VGCC inhibitors in the two blood vessels.
The present study examines the role of two types of neural VGCC in the EFS-evoked overflow of NA, ATP, and β-NAD. Roles of extracellular ATP have been established for the last 40 years: among other functions ATP has been proposed to be a co-transmitter with NA in the sympathetic nervous system (Burnstock, 2004). In recent years β-NAD has also been recognized as an extracellular signaling molecule with diverse functions (Ziegler and Niere, 2004): Extracellular β-NAD induces Ca2+ signaling and apoptosis in human osteoclastic cells (Romanello et al., 2001), activates P2Y11 receptors in human granulocytes (Moreschi et al., 2006) and activates Ca2+ influx in human monocytes through plasma membrane Ca2+ uptake (Gerth et al., 2004). As discussed in Introduction, β-NAD affects the release of neurotransmitters in blood vessels (Smyth et al., 2004), inhibits the smooth muscle tone in the bladder (Breen et al., 2006), and mimics the effects of the purine inhibitory neurotransmitter in the murine colon better than ATP (Mutafova-Yambolieva et al., 2007). The role(s) of extracellular β-NAD in the peripheral circulation remain to be determined. Here, we show that the canine mesenteric artery responds similarly to exogenous β-NAD and ATP: at low micromolar concentrations both purines cause weak vasodilation that is mediated by 8-SPT-sensitive, presumably P1 receptors. Likewise, exogenous adenosine produced vasodilatation, which was reduced by 8-SPT. Therefore, the vasodilatation to β-NAD or ATP may be caused by their metabolite adenosine. The vasoconstriction in response to high micromolar concentrations of β-NAD or ATP appears mediated by PPADS-sensitive P2 receptors. Interestingly, suramin was able to abolish the vasoconstriction to β-NAD but not to ATP, suggesting that different receptors may mediate the effects of the two purines in the artery. In the vein, β-NAD and ATP caused different effects: while ATP had a similar dual effect as in the artery (i.e., vasdilation at low micromolar concentrations and vasoconstriction at high micromolar concentrations), β-NAD caused only relaxation of the venous smooth muscle. The contractile response to ATP was abolished by both suramin and PPADS, suggesting that P2 receptors mediate the vasoconstriction to ATP in both the artery and vein. The relaxation induced by β-NAD in the vein was abolished by 8-SPT; still no vasoconstriction was revealed in the presence of 8-SPT. It is unclear why β-NAD did not cause any vasoconstriction despite that receptors mediating such responses appear present in the vein. One possible explanation is that the vasoconstriction to β-NAD in the artery is mediated by receptors specific for β-NAD but insensitive to ATP; these receptors appear sensitive to suramine and PPADS, but seem absent in the vein. It is interesting that β-NAD produced different effect in the artery and vein, which may be related to the different functions of the two parts of the splanchnic circulation. In other words, the arterial vasoconstriction caused by β-NAD may be associated with the resistive functions of the arteries and the venodilation produced by β-NAD might be related to the capacitative functions of the veins. It is also possible that β-NAD, simultaneously released with NA in the vein (especially at high levels of neural activity), would counteract the vasoconstrictive responses to NA and thus could prevent the veins from excessive vasoconstriction.
Similar mechanisms may be considered in regard to the actions of β-NAD simultaneously released with ATP. Our results suggest that ATP and β-NAD have disparate functions in the mesenteric circulation; it remains unclear what exact functions are associated with these purines (i.e., neurotransmission and/or neuromodulation). Further studies are needed to determine the exact mechanisms of changes of the vascular smooth muscle tone induced by β-NAD released upon action potential.
In conclusion, the release of NA, ATP and β-NAD from sympathetic nerves supplying the canine mesenteric artery and vein is affected differentially by inhibitors of Ca2+ entry through N-type and P/Q-type VGCC. Therefore, at least two pools for release of NA, β-NAD and ATP are present in the canine mesenteric circulation: an N-type-associated pool releasing primarily NA, β-NAD and some ATP, and a P/Q-type-associated pool releasing ATP, β-NAD and some NA. The N-type-mediated mechanisms are equally expressed in the artery and vein, whereas the P/Q-type-regulated mechanisms are more pronounced in the vein. The differential release of neurotransmitters may provide means for fine tuning of the vascular neuroeffector transmission in the resistive and capacitative parts of the mesenteric circulation.
Acknowledgments
We thank Ms. Christy Lew for her excellent technical assistance. This work was supported by the National Institutes of Health Grant HL60031.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen D, Friis UG, Uhrenholt TR, Jensen BL, Skott O, Hansen PB. Coexpression of Voltage-Dependent Calcium Channels Cav1.2, 2.1a, and 2.1b in Vascular Myocytes. Hypertension. 2006;47:735–741. doi: 10.1161/01.HYP.0000203160.80972.47. [DOI] [PubMed] [Google Scholar]
- Anitha M, Joseph I, Ding X, Torre ER, Sawchuk MA, Mwangi S, Hochman S, Sitaraman S, Anania F, Srinivasan S. Characterization of Fetal and Postnatal Enteric Neuronal Cell Lines With Improvement in Intestinal Neural Function. Gastroenterology. 2008;134:1424–1435. doi: 10.1053/j.gastro.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobalova J, Bobal P, Mutafova-Yambolieva VN. High-Performance Liquid Chromatographic Technique for Detection of a Fluorescent Analogue of ADP-Ribose in Isolated Blood Vessel Preparations. Anal Biochem. 2002;305:269–276. doi: 10.1006/abio.2002.5667. [DOI] [PubMed] [Google Scholar]
- Bobalova J, Mutafova-Yambolieva VN. Co-release of endogenous ATP and noradrenaline from guinea-pig mesenteric veins exceeds co-release from mesenteric arteries. Clin Exp Pharmacol Physiol. 2001a;28:397–401. doi: 10.1046/j.1440-1681.2001.03460.x. [DOI] [PubMed] [Google Scholar]
- Bobalova J, Mutafova-Yambolieva VN. Presynaptic alpha2-adrenoceptor-mediated modulation of adenosine 5′ triphosphate and noradrenaline corelease: differences in canine mesenteric artery and vein. J Auton Pharmacol. 2001b;21:47–55. doi: 10.1046/j.1365-2680.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- Bobalova J, Mutafova-Yambolieva VN. Membrane-bound and releasable nucleotidase activities: differences in canine mesenteric artery and vein. Clin Exp Pharmacol Physiol. 2003;30:194–202. doi: 10.1046/j.1440-1681.2003.03808.x. [DOI] [PubMed] [Google Scholar]
- Bobalova J, Mutafova-Yambolieva VN. Activation of the adenylyl cyclase/protein kinase A pathway facilitates neural release of beta-nicotinamide adenine dinucleotide in canine mesenteric artery. Eur J Pharmacol. 2006;536:128–132. doi: 10.1016/j.ejphar.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Borbiev T, Verin AD, Birukova A, Liu F, Crow MT, Garcia JGN. Role of CaM kinase II and ERK activation in thrombin-induced endothelial cell barrier dysfunction. AJP - Lung Cellular and Molecular Physiology. 2003;285:L43–L54. doi: 10.1152/ajplung.00460.2001. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2:407–415. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- Breen LT, Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. {beta}-NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle. Am J Physiol Renal Physiol. 2006;290:F486–F495. doi: 10.1152/ajprenal.00314.2005. [DOI] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC. Effects of Ca2+ concentration and Ca2+ channel blockers on noradrenaline release and purinergic neuroeffector transmission in rat tail artery. Br J Pharmacol. 1999;126:11–18. doi: 10.1038/sj.bjp.0702256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. The fifth Heymans memorial lecture-Ghent, February 17, 1990. Co- transmission. Arch Int Pharmacodyn Ther. 1990;304:7–33. [PubMed] [Google Scholar]
- Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Campbell DT. Large and small vertebrate sensory neurons express different Na and K channel subtypes. Proc Natl Acad Sci U S A. 1992;89:9569–9573. doi: 10.1073/pnas.89.20.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Interactions of Presynaptic Ca2+ Channels and Snare Proteins in Neurotransmitter Release. Annals of the New York Academy of Sciences. 1999;868:144–159. doi: 10.1111/j.1749-6632.1999.tb11284.x. [DOI] [PubMed] [Google Scholar]
- Catterall WA. STRUCTURE AND REGULATION OF VOLTAGE-GATED Ca2+ CHANNELS. Annual Review of Cell and Developmental Biology. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M, Lupi R, Silletta MG, Turacchio S, Iurisci C, Luini A, Corda D. Modulatory role of GTP-binding proteins in the endogenous ADP-ribosylation of cytosolic proteins. Adv Exp Med Biol. 1997;419:343–347. doi: 10.1007/978-1-4419-8632-0_45. [DOI] [PubMed] [Google Scholar]
- Dillon PF, Murphy RA. High force development and crossbridge attachment in smooth muscle from swine carotid arteries. Circ Res. 1982;50:799–804. doi: 10.1161/01.res.50.6.799. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of Voltage-Gated Calcium Channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Gerth A, Nieber K, Oppenheimer NJ, Hauschildt S. Extracellular NAD+ regulates intracellular free calcium concentration in human monocytes. Biochemical Journal. 2004;382:849–856. doi: 10.1042/BJ20040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard SE, Volsen SG, Smith W, Beattie RE, Bleakman D, Lodge D. Identification of pore-forming subunit of P-type calcium channels: an antisense study on rat cerebellar Purkinje cells in culture. Neuropharmacology. 1997;36:405–409. doi: 10.1016/s0028-3908(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Herlihy JT, Murphy RA. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res. 1973;33:275–283. doi: 10.1161/01.res.33.3.275. [DOI] [PubMed] [Google Scholar]
- Khanna R, Zougman A, Stanley EF. A proteomic screen for presynaptic terminal N-type calcium channel (CaV2.2) binding partners. J Biochem Mol Biol. 2007;40:302–314. doi: 10.5483/bmbrep.2007.40.3.302. [DOI] [PubMed] [Google Scholar]
- Kreulen DL. Properties of the venous and arterial innervation in the mesentery. J Smooth Muscle Res. 2003;39:269–279. doi: 10.1540/jsmr.39.269. [DOI] [PubMed] [Google Scholar]
- Lee HC. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Bean BP. Block of calcium channels in rat neurons by synthetic omega-Aga-IVA. Neuropharmacology. 1993;32:1161–1169. doi: 10.1016/0028-3908(93)90010-z. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Venema VJ, Swiderek KM, Lee TD, Bean BP, Adams ME. P-type calcium channels blocked by the spider toxin [omega]-Aga-IVA. Nature. 1992;355:827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Moreschi I, Bruzzone S, Nicholas RA, Fruscione F, Sturla L, Benvenuto F, Usai C, Meis S, Kassack MU, Zocchi E, De Flora A. Extracellular NAD+ Is an Agonist of the Human P2Y11 Purinergic Receptor in Human Granulocytes. Journal of Biological Chemistry. 2006;281:31419–31429. doi: 10.1074/jbc.M606625200. [DOI] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Carolan BM, Harden TK, Keef KD. Multiple P2Y receptors mediate contraction in guinea pig mesenteric vein. Gen Pharmacol. 2000;34:127–136. doi: 10.1016/s0306-3623(00)00054-9. [DOI] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. beta-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proceedings of the National Academy of Sciences. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Smyth L, Bobalova J. Involvement of cyclic AMP-mediated pathway in neural release of noradrenaline in canine isolated mesenteric artery and vein. Cardiovascular Research. 2003;57:217–224. doi: 10.1016/s0008-6363(02)00648-x. [DOI] [PubMed] [Google Scholar]
- Olivera BM, McIntosh JM, Cruz LJ, Luque FA, Gray WR. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984;23:5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Miljanich GP, Ramachandran J, Adams ME. Calcium Channel Diversity and Neurotransmitter Release: The -Conotoxins and -Agatoxins. Annual Review of Biochemistry. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. Journal of Neuroscience. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanello M, Padoan M, Franco L, Veronesi V, Moro L, D’Andrea P. Extracellular NAD(+) induces calcium signaling and apoptosis in human osteoblastic cells. Biochem Biophys Res Commun. 2001;285:1226–1231. doi: 10.1006/bbrc.2001.5325. [DOI] [PubMed] [Google Scholar]
- Rothe CF. Reflex control of veins and vascular capacitance. Physiological Reviews. 1983;63:1281–1342. doi: 10.1152/physrev.1983.63.4.1281. [DOI] [PubMed] [Google Scholar]
- Smyth L, Bobalova J, Ward SM, Keef KD, Mutafova-Yambolieva VN. Cotransmission from sympathetic vasoconstrictor neurons: differences in guinea-pig mesenteric artery and vein. Auton Neurosci. 2000;86:18–29. doi: 10.1016/S1566-0702(00)00203-4. [DOI] [PubMed] [Google Scholar]
- Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. Release of {beta}-Nicotinamide Adenine Dinucleotide upon Stimulation of Postganglionic Nerve Terminals in Blood Vessels and Urinary Bladder. Journal of Biological Chemistry. 2004;279:48893–48903. doi: 10.1074/jbc.M407266200. [DOI] [PubMed] [Google Scholar]
- Smyth LM, Breen LT, Mutafova-Yambolieva VN. Nicotinamide adenine dinucleotide (NAD) is released from sympathetic nerve terminals via a botulinum neurotoxin A mediated mechanism in canine mesenteric artery. Am J Physio - Heart Circ Physiol. 2006a;290:H1818–H1825. doi: 10.1152/ajpheart.01062.2005. [DOI] [PubMed] [Google Scholar]
- Smyth LM, Breen LT, Yamboliev IA, Mutafova-Yambolieva VN. Novel localization of CD38 in perivascular sympathetic nerve terminals. Neuroscience. 2006b;139:1467–1477. doi: 10.1016/j.neuroscience.2006.01.043. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Mochizuki Y, Tanaka H, Shigenobu K. Significant role of neuronal non-N-type calcium channels in the sympathetic neurogenic contraction of rat mesenteric artery. Br J Pharmacol. 1999;128:1602–1608. doi: 10.1038/sj.bjp.0702954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov LD, Mihaylova-Todorova S, Westfall TD, Sneddon P, Kennedy C, Bjur RA, Westfall DP. Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature. 1997;387:76–79. doi: 10.1038/387076a0. [DOI] [PubMed] [Google Scholar]
- Todorov LD, Mihaylova-Todorova ST, Bjur RA, Westfall DP. Differential cotransmission in sympathetic nerves: role of frequency of stimulation and prejunctional autoreceptors. J Pharmacol Exp Ther. 1999;290:241–246. [PubMed] [Google Scholar]
- von Kugelgen I, Allgaier C, Schobert A, Starke K. Co-release of noradrenaline and ATP from cultured sympathetic neurons. Neuroscience. 1994;61:199–202. doi: 10.1016/0306-4522(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Mendelowitz D. Agatoxin-IVA-Sensitive Calcium Channels Mediate the Presynaptic and Postsynaptic Nicotinic Activation of Cardiac Vagal Neurons. J Neurophysiol. 2001;85:164–168. doi: 10.1152/jn.2001.85.1.164. [DOI] [PubMed] [Google Scholar]
- Waterman SA. Role of N-, P- and Q-type voltage-gated calcium channels in transmitter release from sympathetic neurones in the mouse isolated vas deferens. Br J Pharmacol. 1997;120:393–398. doi: 10.1038/sj.bjp.0700948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SA. Voltage-gated calcium channels in autonomic neuroeffector transmission. Prog Neurobiol. 2000;60:181–210. doi: 10.1016/s0301-0082(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Waterman SA. Multiple Subtypes of Voltage-Gated Calcium Channel Mediate Transmitter Release from Parasympathetic Neurons in the Mouse Bladder. Journal of Neuroscience. 1996;16:4155–4161. doi: 10.1523/JNEUROSCI.16-13-04155.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall DP, Todorov LD, Mihaylova-Todorova ST, Bjur RA. Differences between the regulation of noradrenaline and ATP release. J Auton Pharmacol. 1996;16:393–395. doi: 10.1111/j.1474-8673.1996.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Xi Q, Ziogas J, Roberts JA, Evans RJ, Angus JA. Involvement of T-type calcium channels in excitatory junction potentials in rat resistance mesenteric arteries. Br J Pharmacol. 2002;137:805–812. doi: 10.1038/sj.bjp.0704943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamboliev I, Ward S, Mutafova-Yambolieva V. Canine mesenteric artery and vein convey no difference in the content of major contractile proteins. BMC Physiology. 2002;2:17. doi: 10.1186/1472-6793-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M, Niere M. NAD+ surfaces again. Biochemical Journal. 2004;382:e5–e6. doi: 10.1042/BJ20041217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]