Summary
FLK1 expressing (FLK1+) mesoderm generates blood and vessels. Here, we show that combined BMP, Notch and Wnt signaling is necessary for efficient FLK1+ mesoderm formation from embryonic stem (ES) cells. Inhibition of BMP, Notch and Wnt signaling pathways greatly decreased the generation of FLK1+ mesoderm and expression of the Ets transcription factor Er71. Enforced expression of ER71 in ES cells resulted in a robust induction of FLK1+ mesoderm, rescued the generation of FLK1+ mesoderm when blocked by BMP, Notch and Wnt inhibition, and enhanced hematopoietic and endothelial cell generation. Er71 deficient mice had greatly reduced FLK1 expression, died early in gestation and displayed severe blood and vessel defects that are highly reminiscent of the Flk1 null mouse phenotype. Collectively, we provide compelling evidence that ER71 functions downstream of BMP, Notch and Wnt signals and regulates FLK1+ mesoderm, blood and vessel development.
Keywords: Embryonic stem cells, knockout, FLK1, SCL, hematopoietic, vascular, BMP4, Notch, Wnt, Ets, ER71
Introduction
Successful derivation of blood and vessels from embryonic stem (ES) cells for tissue engineering will require a comprehensive understanding of inductive signals and downstream effectors involved in blood and vessel lineage development. Ideally, identification of factors/signals that regulate blood and vessel specification could facilitate the generation of blood and vessel cells from ES cells for the treatment of disease and injury. The VEGF/FLK1 axis is critical for development as well as postnatal and pathological angiogenesis. In primitive streak stage embryos, Flk1 is expressed in the extraembryonic and the paraxial-lateral embryonic mesoderm (Dumont et al., 1995; Ema et al., 2006). Subsequently, its expression is confined to endothelial cells of the yolk sac blood islands and the developing endocardial tube. Mice deficient in Flk1 display defects in the yolk sac blood islands, blood vessels and endocardium and die around embryonic day (E) 9.5 (Shalaby et al., 1995). Vegf+/− embryos also display blood and vessel defects which phenocopy deficiencies observed in the Flk1−/− embryos (Carmeliet et al., 1996; Ferrara et al., 1996). This indicates that VEGF is an exclusive ligand for FLK1 in hematopoiesis and angiogenesis during development and that VEGF/FLK1 signaling is indispensable for establishment of the hematopoietic and vascular systems.
FLK1+ mesoderm is developmentally derived from Brachyury/T expressing mesoderm and contributes to cells of the circulatory system, including blood, vessel, cardiac, smooth, and skeletal muscle (Faloon et al., 2000; Chung et al., 2002; Ema et al., 2003; Fehling et al., 2003; Motoike et al., 2003). Despite the crucial role of FLK1+ mesoderm in the generation of the circulatory system, the mechanisms that regulate FLK1+ mesoderm formation and differentiation are poorly understood. Members of the Ets family of transcription factors have been implicated in vasculogenesis, angiogenesis and hematopoiesis (reviewed in Oikawa and Yamada, 2003). Genetic studies have revealed distinct roles for each Ets transcription factor in various developmental stages of hematopoiesis and angiogenesis (reviewed in Bartel et al., 2000). Recently, etsrp, an Ets transcription factor, was identified as a potent inducer of Flk1+ cells in zebrafish (Sumanas and Lin, 2006). Injection of etsrp mRNA at the 1 cell stage was sufficient to ectopically induce hemangioblast and/or early hematopoietic and endothelial markers, such as scl and flk1, in a variety of different cell types throughout the embryo. Conversely, morpholino knockdown of etsrp in zebrafish embryos resulted in the complete absence of circulation due to the failure of angioblasts to migrate, differentiate, and coalesce into functional vessels. etsrp was down regulated in zebrafish cloche (clo) mutant embryos, which display defective hematopoietic and endothelial cell formation. Importantly, etsrp RNA restored expression of vascular markers in clo zebrafish mutants (Sumanas et al., 2005).
We previously demonstrated that BMP4 was sufficient to generate FLK1+ mesoderm from differentiating ES cells in serum free conditions. Yet, Noggin, a potent inhibitor of BMPs, could not completely block FLK1+ cell production from ES cells in serum (about 40–50% of the control level) (Park et al., 2004). To better understand the pathways and molecular mechanisms involved in the regulation of FLK1+ mesoderm formation and differentiation, we tested additional factors apart from BMP4 by utilizing pharmacologic inhibitors of various pathways in the in vitro differentiation model of ES cells. We also investigated the role of the Ets transcription factor Er71, a mouse ortholog of etsrp (Sumanas et al., 2008) and a gene that is up regulated in the hemangioblast compared to its progeny (Lugus et al., 2007). We demonstrate that combined BMP, Notch and Wnt signaling is necessary for FLK1+ mesoderm formation and that ER71 functions downstream of these signals to regulate FLK1+ mesoderm generation. We also demonstrate that Er71 deficient mice die around E9.5 due to the failure to develop blood, vessels and endocardium. FLK1 expression was greatly diminished in these mutant embryos. Collectively, we provide strong evidence that the BMP/Notch/Wnt-ER71 axis is necessary for blood and vessel formation.
Results
Combined BMP, Notch and Wnt signals are critical for the generation of FLK1+ mesoderm
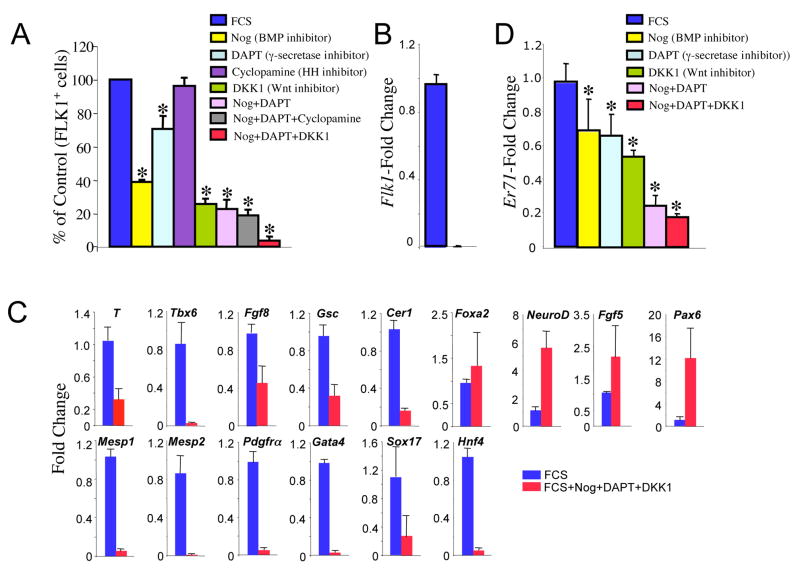
To identify additional factor(s) that may cooperate with BMP4 in FLK1+ mesoderm generation, we assessed pharmacologic inhibitors of various signaling pathways, including BMP, Notch, Hedgehog, and Wnt in differentiating ES cells. Specifically, individual or combinations of inhibitors were added on day 1 to differentiating ES cells and FLK1+ cells were assessed on day 3, when Brachyury expression peaks. Thus, FLK1+ cells present at this stage represent mesodermal cells as they still express Brachyury and generate both hematopoietic and endothelial cells in culture (Faloon et al., 2000; Chung et al., 2002; Fehling et al., 2003). Noggin, the Notch inhibitor DAPT (gamma-secretase inhibitor N-S-phenyl-glycine-t-butyl ester) or the Wnt inhibitor DKK1 (dickkopf-1) reduced, to a varying degree, FLK1+ cells, whereas cyclopamine, a small-molecule antagonist of the Hedgehog pathway, did not (Figure 1A). Treatment of both Noggin and DAPT reduced FLK1+ cells even greater, by more than 80%. Importantly, the addition of all three inhibitors, Noggin + DAPT + DKK1, nearly completely abolished FLK1+ cells. Notably, Flk1 expression was also completely blocked by Noggin + DAPT + DKK1 (Figure 1B). In the presence of Noggin, DAPT, or DKK1, the expression of Id genes/Gata2, Hey1, or Lef1, respectively, was reduced, indicating that Noggin, DAPT, or DKK1 successfully inhibited the respective signaling pathway (not shown). Moreover, the total cell number after inhibitor treatment did not change significantly.
Figure 1. BMP, Notch and Wnt inhibition blocks the generation of FLK1+ cells and abrogates Er71 expression during ES cell differentiation.
ES cells were differentiated in serum (FCS), inhibitors were added on day 1, and day 3 EB cells were analyzed for FLK1 expression (A) and subjected to gene expression (B–D). *P<0.05 versus FCS. Genes were normalized against Gapdh and then the ratio of the gene quantity (FCS) to gene quantity (FCS + inhibitors) was determined to yield fold change shown on the Y-axis.
To better understand the genetic program mediated by BMP, Notch and Wnt signaling, we examined genes that are expressed during germ layer formation and patterning (Figure 1C) (Gadue et al., 2006; Lindsley et al., 2006). Noggin + DAPT +DKK1 treatment significantly inhibited the expression of posterior primitive streak genes, including Brachyury (T), Tbx6 and Fgf8, and the anterior primitive streak genes, Gsc and Cer1. Intriguingly, the expression of another anterior primitive streak gene, Foxa2, was not changed considerably by these inhibitors. Genes that are expressed in mesoderm and endoderm, such as Mesp1, Mesp2, Pdgfrα, Gata4, Sox17 and Hnf4, were almost completely suppressed by these inhibitors. Conversely, the expression of ectoderm genes, such as NeuroD, Fgf5 and Pax6, was upregulated by Noggin + DAPT + DKK1 treatment. Thus, consistent with previous studies (Lai, 2004; Tam and Loebel, 2007), BMP, Notch and Wnt pathways are ciritical for mesoderm and endoderm formation and patterning.
Er71 expression pattern in developing embryo and differentiating ES cells
We previously reported that the expression of the Ets transcription factor Er71 was greatly enriched in the hemangioblast population (FLK1+SCL+ cells from day 2.75 embryoid bodies, EBs, differentiated ES cells) compared to their progeny, blast cells (Lugus et al., 2007). As Er71 is a mouse ortholog of zebrafish etsrp (Sumanas et al., 2008), we assessed the possbility that ER71 could modulate FLK1+ mesoderm development in the mouse. The expression of Er71 was decreased in differentiating ES cells when treated with Noggin, DAPT, or DKK1 (Figure 1D). Importantly, Er71 was diminished by more than 80%, when Noggin + DAPT + DKK1 were included in the differentiation media. This suggested that Er71 is downstream of BMP, Notch and Wnt signaling and could regulate FLK1+ mesoderm generation.
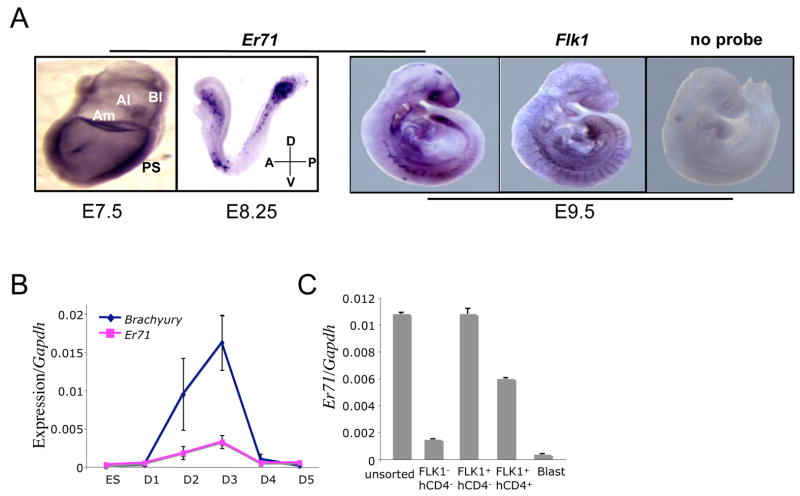
Er71 has been shown to be mainly expressed in the adult testis (Brown and McKnight, 1992). As its embryonic expression and its role in development is not known, we first evaluated the expression pattern of Er71 by in situ hybridization. Er71 was expressed in the primitive streak, embryonic mesoderm, amnion, allantois, and yolk sac blood islands in E7.5 embryos (Figure 2A). At later embryonic stages (E8.25–E9.5), Er71 expression was mainly detected within major vessels including the dorsal aorta and branchial arch (Figure 2A). Next, we examined the expression kinetics of Er71 in differentiating ES cells in relationship to Brachyury. The onset of Er71 expression in differentiating ES cells was detectable at the time of Brachyury expression and preceeded that of Flk1 (Figure 2B) (Lugus et al., 2007, not shown). Similar to Brachyury expression, Er71 expression within differentiating ES cells was transient and became undetectable 4 days after differentiation. More importantly, when day 2.75 EB cells were sorted as FLK1+ or FLK1−, Er71 was mainly expressed within FLK1+ cells, not FLK1−, suggesting that ER71 potentially regulates Flk1 expression (Figure 2C). As we previously reported (Lugus et al. 2007), Er71 was greatly down regulated within blast cells compared to a cell population enriched for hemangioblasts (FLK1+hCD4+). Collectively, these data indicate that Er71 is expressed transiently in developing embryos and differentiating ES cells, and that its expression is mainly restricted to FLK1+ mesoderm and its derivatives.
Figure 2. Er71 is expressed transiently in early embryos and differentiating ES cells.
(A) In situ hybridization analysis of Er71. PS, primitive streak; Bl, blood islands; Al, allantois; Am, amnion; A, anterior; P, posterior; D, dorsal; V, ventral (B, C) Er71 expression analysis. RNA obtained from differentiating ES cells (B) or various cell populations sorted from day 2.75 EBs (C) was used to examine Er71 expression. The value normalized against Gapdh is shown on the Y-axis.
ER71 is a potent inducer of FLK1+ mesoderm
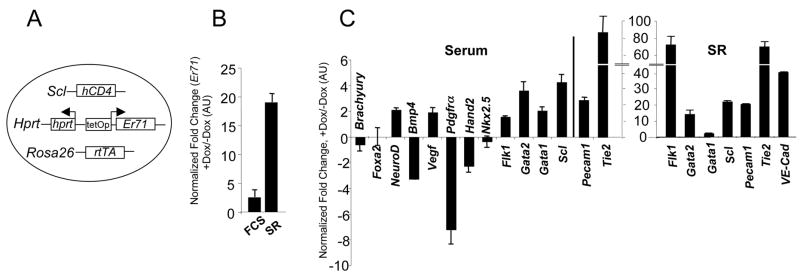
We assessed the role of ER71 in FLK1+ mesoderm generation and differentiation in the ES/EB system. To this end, we generated an inducible ER71 (iER71) ES cells by targeting the tet-responsive locus of A2Lox ES cells (an E14Tg2a based ES cell line) with the Er71 cDNA, fused to a V5 tag at the 3′ end (Figure 3A). Similar to Ainv15 (Kyba et al., 2002), A2Lox cells were engineered to constitutively express the reverse tetracycline transactivator (rtTA) from the Rosa26 locus (Kyba, in preparation). In addition, these ES cells contain a targeting site upstream of the HPRT locus, such that a transgene is under the regulation of the tetracycline operator after site-specific recombination. To track SCL expressing cells, we generated an A2Lox derivative, in which non-functional human CD4 (hCD4) was knocked-in into the Scl locus (Lugus et al., 2007). After the correct targeting event was confirmed by a tet-responsive locus/cDNA vector specific PCR (not shown), inducible Er71 expression was verified by RNA analyses. The addition of doxycycline (Dox, 1 μg/ml, at which concentration no cytotoxicity was observed, not shown) resulted in about three-fold induction of Er71 RNA expression in differentiating iER71 ES cells compared to non-induced cells in serum (Figure 3B). The fold change in Er71 expression was even greater (~18 fold) when Er71 was induced in serum free conditions (i.e. SR media). Two independent iER71 ES cell lines showed similar phenotypes.
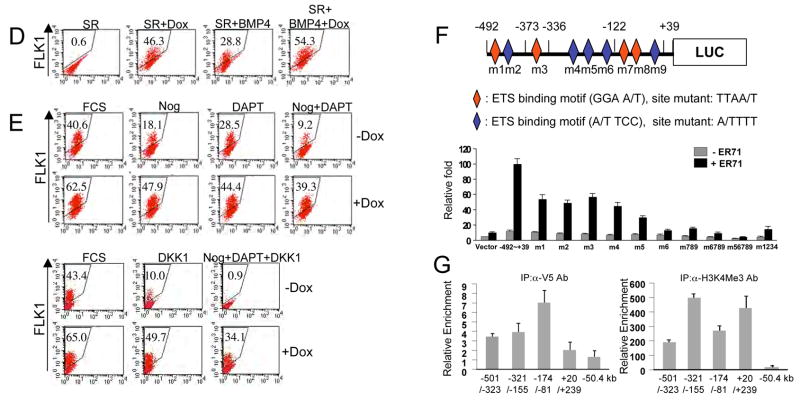
Figure 3. ER71 can induce FLK1+ mesoderm by activating Flk1 expression.
(A) Schematic diagram of the inducible ER71 (iER71) ES cells used, with indicated loci carrying alterations allowing for production of the rtTA, expression of the Er71 cDNA and generation of hCD4 as a surrogate marker for Scl. (B, C) Er71 induction upon Dox treatment and the ER71-mediated transcriptional program. iER71 ES cells were differentiated for 1 day in serum or for 2 days in SR and then treated with 1 μg/ml Dox for an additional 2 days. RNA was prepared and used for qRT-PCR. Genes were normalized against Gapdh and the ratio of the gene quantity (+Dox) to gene quantity (−Dox) was determined to yield normalized fold change. (D) iER71 ES cells were differentiated as described. BMP4 was added on day 2. The resulting cells were FACS analyzed for FLK1. Numbers in insets indicate the percentage of FLK1+ cells. (E) iER71 ES cells were differentiated in serum as described. Noggin, DAPT and DKK1, singularly or in combination, were added on day 1 of differentiation. Dox was added on day 1 and FLK1+ cells were analyzed on day 3. Numbers in insets indicate the percentage of FLK1+ cells. (F) (upper) Schematic diagram of the Flk1 promoter used for luciferase assay. Diamonds indicate potential Ets binding sites (red in sense and blue in anti-sense). For mutagenesis, potential Ets sites GGAA/T (sense) and A/TTCC (anti-sense) were mutated to TTAA/T and A/TTTT, respectively. (lower) 293T cells were transfected with pGL3 or pGL3-Flk1 promoter-luciferase reporter plasmid with or without pCS3-Myc-Er71(WT or MT). Fire fly luciferase activity was normalized by Renilla luciferase activity. (G) iER71 ES cells differentiated in serum in the presence of Dox were cross-linked, sonicated and subjected to ChIP assay. Numbers on the X-axis indicate the locations of amplicons of each qPCR primer set on Flk1. The value normalized against IgG IP values is shown on the Y-axis.
We first investigated the ER71 mediated transcriptional program. To this end, iER71 ES cells were differentiated in serum or SR, ER71 was induced at day 1 (day 2 for SR) and the gene expression profile was analyzed 2 days later. The expression of Brachyury (mesoderm), Foxa2 (endoderm) or NeuroD (ectoderm) was similar in ER71 induced cells as compared to non-induced (<2 fold change) (Figure 3C). This indicates that enforced Er71 expression did not significantly alter the expression of genes involved in germ layer formation. Importantly, genes expressed in hematopoietic as well as endothelial cells were increased. The fold increase of these genes was greater in serum free conditions compared to serum, potentially due to the higher fold of Er71 induction. Consistent with the gene expression studies, ER71 induction in serum free conditions led to a de novo generation of FLK1+ cells (Figure 3D, 47%±4% vs. 0.75%±0.15%, p<0.0001, n=3). ER71 and BMP4 together generated slightly more FLK1+ cells compared to ER71 alone (56.5%±2% vs. 46.6%± 3.7%, p<0.05, n=3). ER71 also enhanced FLK1+ cell generation in serum (Figure 3E, 68%± 7% vs. 45%±3%, p<0.05, n=3).
To determine if ER71 truly functions downstream of BMP, Notch and Wnt pathways to regulate FLK1+ mesoderm generation, we determined if ER71 could rescue the generation of FLK1+ cells when the respective pathways were blocked. To this end, iER71 ES cells were differentiated in serum; Noggin, DAPT and/or DKK1 was added on day 1, ER71 was induced from day 1, and FLK1+ cells were analyzed on day 3. As shown, ER71 could rescue the generation of FLK1+ cells inhibited by Noggin, DAPT or DKK1. More importantly, ER71 was sufficient to rescue the generation of FLK1+ cells inhibited by Noggin + DAPT + DKK1 (Figure 3E). This argues that ER71 acts downstream of BMP, Notch and Wnt and regulates FLK1+ mesoderm generation.
ER71 directly activates Flk1 expression
Based on the findings that ER71 induced FLK1+ mesoderm (Figure 3D, E), is a potent transcriptional activator (De Haro and Janknecht, 2002; Knebel et al., 2006), and that the Flk1 promoter/enhancer region contains putative Ets binding sites (Kappel et al., 2000; Elvert et al., 2003), we tested if ER71 could directly induce Flk1 expression. To this end, Flk1 promoter-luciferase constructs were transiently transfected in 293T cells with or without a construct expressing ER71, and luciferase activity was assayed. As shown, ER71 robustly activated the −492 to +39 Flk1 promoter, which contains 9 putative Ets binding sites (Figure 3F, Supplementary Figure 1A). ER71 could also induce Flk1 expression in endothelial cells (Supplementary Figure 1B). Individual mutations of the 9 putative sites in the Flk1 promoter reduced its response to ER71 (Figure 3F). More importantly, multiple mutations including sites 1–4 or 5–9 in the Flk1 promoter effectively abolished its response to ER71.
To examine whether these conserved sites were relevant in vivo, we used chromatin immunoprecipitation (ChIP) to measure DNA binding by ER71. As shown in Figure 3G, the Flk1 promoter region was enriched for H3K4Me3, indicating that this region of DNA is actively being transcribed in day 3 EBs. Additionally, we found ER71 binding to this region of the promoter. The enrichment of ER71 occupancy at the −174 to −81 region was greater compared to other promoter regions. As expected, the −50.4kb region, which is outside of the Flk1 locus, was not occupied by ER71. The +20 to +239bp region generated a similar result to that of the −50.4kb region, indicating that ER71 mainly occupies the −492 to +39bp region of the promoter. Collectively, we conclude that ER71 promotes FLK1+ mesoderm formation by directly activating Flk1 gene expression.
ER71 is sufficient to induce blood and endothelial cell differentiation from ES cells
Enforced ER71 expression during mesoderm formation resulted in an increase of genes expressed in the endothelial lineage (Pecam1, Tie2 and VE-cadherin) as well as hemangioblast and/or hematopoietic genes (Gata1, Gata2, and Scl) (Figure 3C). The increased expression of these genes could be due to enhanced hematopoietic and/or endothelial differentiaion by ER71. To determine if ER71 additionally plays a role in blood and endothelial cell differentiation from FLK1+ mesoderm, iER71 ES cells were differentiated, ER71 was induced from day 3 and hCD4+ (SCL+ hematopoietic progenitors) as well as FLK1+ or VE-cadherin+ cells (endothelial cells) were assessed on day 5 and onward by FACS analyses (Figure 4, not shown). As shown, ER71 induction alone in serum free conditions was sufficient to generate SCL+ hematopoietic (76%±3% vs. 7%±1%), FLK1+ (33%±1% vs. 8%±3%) or VE-Cadherin+ (32%±7.5% vs. 2.6%±1%) endothelial cells (Figure 4A, upper panels). The generation of functional hematopoietic cells by ER71 in serum free conditions was confirmed by replating EB cells after Dox treatment (Figure 4B). Previously, we reported that BMP and VEGF were sufficient for blood generation in serum free conditions (Park et al., 2004). Importantly, the level of SCL+ cells or the actual hematopoietic progenitor number generated by ER71 was similar to that by BMP and VEGF (Figure 4A and B). In serum, ER71 induction led to a greater increase in FLK1+ or VE-Cadherin+ endothelial cells (64%±1% vs. 11%±1%; 70%±5% vs. 10%±2%, p<0.001, n=3, respectively), with a concurrent decrease in SCL+ hematopoietic cells (44%±11% vs. 72%±1%, p<0.05, n=3) (Figure 4A, lower panels). Factor(s) present in serum could have contributed to this outcome. Intriguingly, an increase in primitive hematopoiesis was seen with a pulse induction, from day 2–3 (Figure 4C). Continuous expression of ER71 from day 2 or 3 of EB culture inhibited primitive hematopoiesis. Potentially, this reflects an enhancement of FLK1+ mesoderm and/or hemangioblast cell population from the pulse of Er71 induction.
Figure 4. ER71 can induce hematopoietic and endothelial cell generation.
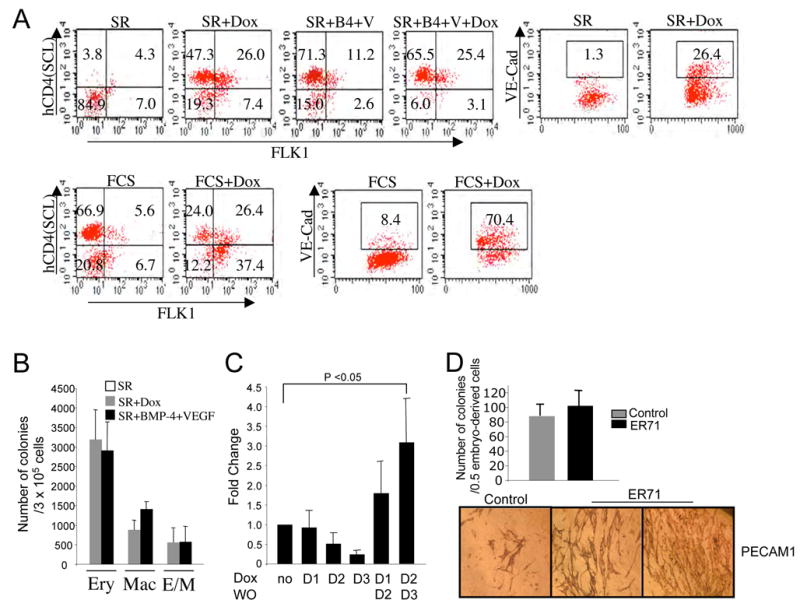
(A) iER71 ES cells were differentiated in SR or serum and Dox (1 μg/ml) was added on day 3. BMP4 and VEGFA165 were added on day 2 and day 3, respectively. In all cases, cells were FACS analyzed for FLK1, hCD4 or VE-Cadherin (VE-Cad) expression on day 5 (FCS) or day 6 (SR). (left panels) Numbers in a given box indicate the percentage of cells that are FLK1−hCD4− (lower left) FLK1+hCD4− (lower right), FLK1+hCD4+ (upper right), or FLK1−hCD4+ (upper left). (right panels) Numbers in a given box indicate the percentage of VE-Cad+ cells. (B) iER71 cells differentiated for 6 days in serum free conditions with indicated factors were replated in methylcellulose medium containing hematopoietic cytokines. Colonies were counted 5–7 days after replating. EB cells generated in SR alone gave rise to few erythoid colonies (average=10.7) with no Mac or E/M colonies. Error bars indicate standard deviations from three independent experiments. Ery; Erythrocytes, Mac; Macrophages, E/M; Erythroid/Macrophage colonies. (C) Transient expression of ER71 increases the generation of primitive erythroid progenitors. Dox was added to the culture at the indicated day and kept until day 4 or washed out (WO) the next day. On day 4, iER71 EB cells were replated for primitive erythroid colonies. Colonies were counted 4 days later. Data represent four independent experiments. (D) Hematopoietic and endothelial cell development upon ER71 overexpression using embryo-derived cell culture. Pooled embryos between E8.0–E8.5 were dissociated into single cells and infected with MSCV or MSCV-ER71-IRES-GFP virus. Subsequently, cells cultured on OP9 cells were subjected to hematopoietic colony replating (upper, values are mean±s.e.m. from two independent experiments) or PECAM1 staining (lower, representative images are shown).
Next, we utilized an embryo-derived cell culture (Zeigler et al., 2006) to further determine the role that ER71 might have in hematopoietic and endothelial cell generation. As shown, embryo cells that were infected with MSCV-ER71 virus displayed a robust endothelial cell generation compared to control retroviruses as judged by PECAM1 antibody staining (Figure 4D). The number of hematopoietic colonies that were generated did not show significant change. This indicates that ER71 can additionally promote endothelial cell progenitor proliferation and/or differentiation.
Generation of Er71−/− mice
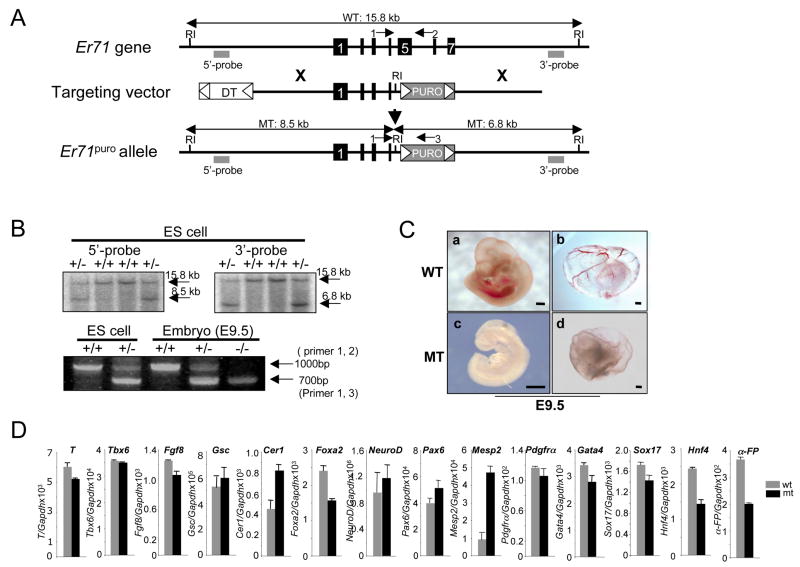
Our data demonstrate that ER71 is sufficient to generate FLK1+ mesoderm, blood and endothelial cells. To assess the in vivo role of ER71, we generated mice lacking Er71 and examined blood and vessel development. The targeted mutation deleted exons 5 to 7, which encompasses the ETS binding domain of the protein (Figure 5A). After electroporation, puromycin selection, and screening, 9 correctly targeted clones were identified (Figure 5B). The mutant allele from two independent clones was transmitted through the germline after crossing chimeric mice with C57BL/6 females.
Figure 5. Disruption of the murine Er71 locus.
(A) Structures of the mouse Er71 gene, the pGKpuro targeting vector, and the targeted allele after homologous recombination. Black boxes depict exons. The flanking genomic probes used for Southern blot analysis are indicated with gray squares. Only the EcoRI restriction enzyme site relevant to the targeting construct and screening strategies is shown. (B) Southern blot analyses of representative Er71+/− ES cell clones and genotyping of ES cells and E9.5 embryos by PCR. The wild type (15.8kb) and the mutant allele (8.5kb) detected with the 5′ external probe as well as the wild type (15.8kb) and the mutant allele (6.8kb) detected with the 3′ external probe after EcoRI digest are shown. PCR products of the wild type Er71 allele (primer 1, 2: 1000 bp) and the mutant allele (primer 1, 3: 700 bp) are shown. (C) Gross morphology of Er71−/− embryos at E9.5. (a, c, embryo proper; b, d, yolk sac). Scale bars; 200 μm. (D) Gene expression analysis. RNA was prepared from E8.5 (n=2) embryos and subjected to qRT-PCR. The value normalized against Gapdh is shown on the Y-axis.
Mice heterozygous for the Er71 mutation were viable, fertile, and phenotypically indistinguishable from wild type mice. However, no homozygotes were obtained from heterozygous intercrosses at weaning, suggesting that Er71−/− mice were embryonic lethal. To identify the developmental stage at which lethality occurs, embryos from heterozygote intercrosses were examined at different gestational stages. The Mendelian distribution of Er71−/− embryos was normal at E8.5 and there were no noticeable differences between the wild type and the mutant embryos. Homozygous embryos were still obtained with the expected Mendelian frequency at E9.0 (supplementary Table 1). However, Er71−/− embryos at E9.0 were significantly smaller in size than their wild-type littermates (data not shown). At E9.5, the Mendelian frequency of Er71−/− embryos was reduced significantly, and all mutant embryos were notably pale and showed growth retardation (Figure 5C). Although large vitelline blood vessels were normally seen in the wild type yolk sacs, Er71−/− yolk sacs were pale and no blood vessels were seen. No Er71−/− embryos were recovered beyond E11.0.
Mice lacking Er71 display hematopoietic and vascular defects
Histological analyses revealed that E7.5–E8.5 mutants were morphologically normal and gastrulation occurred normally, generating all three germ layers (Supplementary Figure 2A, data not shown). Consistent with these observations, the expression level of genes expressed in primitive streak, mesoderm, endoderm or ectoderm was not greatly changed between the wild type and mutant embryos at E8.5 (Figure 5D). We further examined Brachyury expression by in situ hybridization, which was expressed normally in the axial mesoderm of mutant embryos (Figure 5D, data not shown). Moreover, sagittal sections showed that somites were present in E9.0 mutants (supplementary Figure 2B). Thus, the embryonic lethality of Er71−/− mice is not due to general defects in mesoderm formation and differentiation or axial patterning.
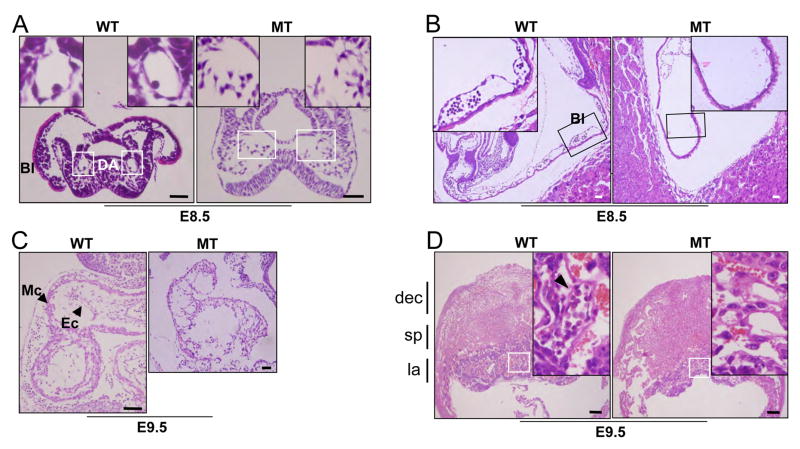
Histological examination demonstrated that although yolk sac blood islands were readily detectable in the wild type, no such structures were seen in the mutant yolk sac, nor did the dorsal aorta or endocardium form in the mutant embryos (Figure 6, Supplementary Figure 2). The immunohistochemical staining pattern of smooth muscle α-actin was similar between the wild type and mutant embryos (Supplementary Figure 2C), suggesting that cardiomyocytes developed relatively normally. Similar expression levels of smooth muscle α-actin and myosin heavy chain (MHC) genes, as judged by quantitative RT-PCR, from E9.0 hearts further support this notion (Supplementary Figure 3B). Since blood vessels could not be detected in the mutant embryo proper or their yolk sacs, we also examined the placenta. During development, the allantois attaches to the chorionic plate in the formation of a functional placenta, followed by vascular invasion of the labyrinth layer. In wild type embryos, blood vessels containing many nucleated erythrocytes normally invaded and integrated into the labyrinth layer of the developing placenta (arrowhead in Figure 6D). In contrast, the labyrinth formation in Er71−/− embryos was retarded and vascularization had not started in the placenta. Consequently, the labyrinth layer of the mutant placenta is significantly decreased in thickness (supplementary Figure 2D). No nucleated erythrocytes were detected in the mutant placenta, although enucleated erythrocytes of maternal origin were readily detectable. There was a higher level of TUNEL staining in Er71 mutants as compared to wild type embryos at E9.5, indicating that without proper blood and vessels, the embryos cease to develop and undergo apoptosis (Supplementary Figure 2E).
Figure 6. Histology of Er71 mutants.
(A, B) H & E staining of E8.5 embryos. The boxed area is shown at higher magnification. Bl, blood island; DA, dorsal aorta. Scale bars; 50μm. (C) Sagittal sections of wild type and Er71−/− hearts from E9.5 embryos. Ec, endocardium; Mc, myocardium. Scale bars; 50 μm. (D) Sagittal sections of the placenta of E9.5 embryos. Higher magnification of the labyrinth layers of the wild type and Er71−/− placentas is shown in the box. dec, maternal decidual tissue; sp, spongiotrophoblast layer; la, labyrinth layer. Scale bars; 200 μm.
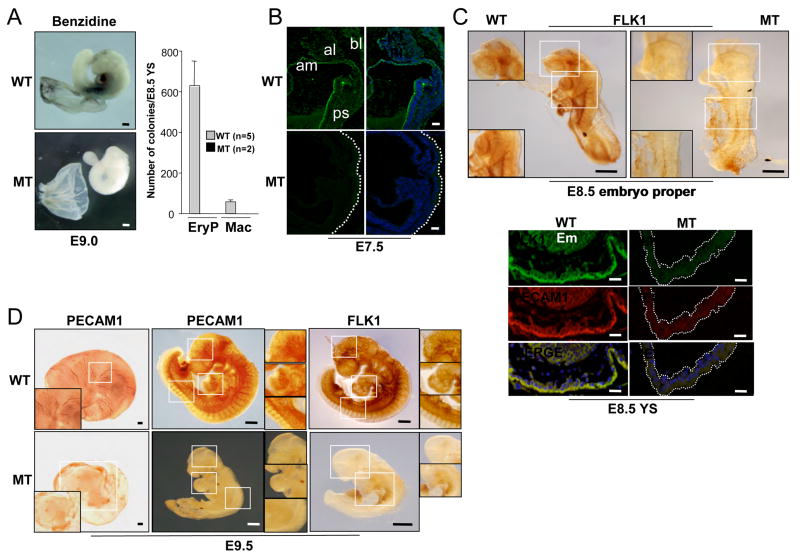
To further characterize blood defects in Er71−/− embryos, we subjected E9.0 embryos to benzidine staining. As shown, the presence of erythrocytes in the E9.0 wild type embryos was readily confirmed by benzidine staining, although no such staining was found in the mutant embryos (Figure 7A). No primitive erythroid colonies formed when E8.5 Er71−/− yolk sacs were subjected to hematopoietic replating. (Figure 7A). These results demonstrate the absence of hematopoietic progenitors in the mutant yolk sacs.
Figure 7. Er71−/− embryos show defects in blood and vessel development.
(A) (left) Benzidine staining. Scale bars; 200 μm. (right) Hematopoietic progenitor assay. Cells prepared from E8.5 YS were replated into methycellulose containing hematopoietic cytokines. (B) FLK1 expression in E7.5 embryos. FLK1 is expressed in primitive streak (PS), blood islands (Bl), allantois (Al) and amnion (Am) in the wild type but not in the mutants. Scale bars; 50 μm. (C) FLK1 expression in E8.5 embryos. (upper) Whole mount FLK1 staining of the embryo proper. Scale bars; 200 μm. (lower) Immunofluorescent FLK1 (green) and PECAM1 (red) staining of the yolk sac. Nuclei were stained with DAPI (blue). Em, embryo proper. Scale bars; 50 μm. (D) Whole-mount PECAM1 or FLK1 staining of E9.5 embryos. The boxed area is the vasculature of the yolk sac, brain, heart and intersomitic regions at higher magnification. Scale bars; 200 μm.
We next confirmed the complete absence of the vasculature in Er71−/− embryos by immunohistochemistry. At E7.5, FLK1 staining was readily detectable in the primitive streak, blood islands, allantois and amnion. However, no such staining was found in the mutant embryos (Figure 7B). At E8.5, FLK1 staining was detectable in the vasculature of the head, trunk, heart and intersomitic regions. No FLK1 staining was detectable in the mutant embryos (Figure 7C). Immunofluorescent staining with FLK1 and PECAM1 antibodies also confirmed the absence of vasculature in the mutant yolk sac. At E9.5, large, well-branched vitelline blood vessels, which are lined with PECAM1-positive endothelial cells, were normally seen in wild-type yolk sacs, but not in mutant yolk sacs (Figure 7D). Well-organized blood vessels were also detectable, as judged by PECAM1 and FLK1 staining in the wild-type brain, heart and intersomitic regions, but not in Er71−/− embryos. Collectively, we conclude that ER71 is essential for FLK1+ cell formation, blood and endothelial cell development.
Discussion
Flk1, a receptor tyrosine kinase, is a critical gene involved in blood and vessel development as evidenced by knockout studies. Fate mapping studies have demonstrated that FLK1+ mesoderm contributes to hematopoietic, endothelial, skeletal and smooth muscle cells. FLK1 continues to play a critical role in endothelial cells in the adult. Therefore, understanding the upstream signals and molecular mechanisms that regulate Flk1 expression is essential for delineating the pathways involved in blood and vessel differentiation during embryogenesis as well as postnatal angiogenesis. We provide evidence that inhibition of BMP, Notch, or Wnt pathways individually could partially block the generation of FLK1+ cells, while inhibition of all three pathways completely blocked the generation of FLK1+ cells. Our studies are supported by recent studies that Wnt signaling is important for ES-derived mesoderm and hematopoietic development (Lindsley et al., 2006; Lengerke et al., 2008; Nostro et al., 2008). Intriguingly, Lindsley et al. (2006) showed that DKK1 addition to differentiating ES cells could almost completely block Brachyury expression and formation of FLK1+ mesoderm. In our hands, purified recombinant DKK1 could not completely abrogate generation of FLK1+ mesoderm. It is likely that the undefined composition of the conditioned media used as a source for DKK1 by Lindsley et al. could have contributed to this discrepancy. In examining Notch signaling, the expression of tamoxifen-inducible, constitutively active Notch1 in ES cells could inhibit generation of FLK1+ mesoderm (Schroeder et al., 2006). Potentially, the prolonged, constitutive Notch activity, which is known to block differentiation of stem cells, could have contributed to this observation. As Er71 is expressed at low levels in adult endothelial cells (not shown), it will be exciting to determine if Er71 expression can be reactivated, potentially by BMP, Notch and Wnt signaling during pathologic angiogenesis.
Previous studies indicate that Ets1 could transactivate the Flk1 promoter and mutation of either of two Ets binding sites, -141 to -136 bp and -71 to -66 bp, impaired Flk1 promoter activity by Ets1 in vitro (Kappel et al., 2000; Elvert et al., 2003). Moreover, the mutation of the -141 to -136 Ets site resulted in a reduced reporter activity in the vasculature of transgenic embryos. Intriguingly, this site (# 6 in Figure 3F) was also critical for ER71 mediated transactivation of Flk1. ER71 is a unique Ets factor within the Ets family of transcription factors, as it shares no homology with any other Ets members other than its DNA-binding domain (Brown and McKnight, 1992; De Haro and Janknecht 2005). Our analyses of Er71 null embryos and ES cell differentiation demonstrate that ER71 is critical for Flk1 expression as well as hematopoietic and vascular development. As such, the hematopoietic and vascular defects seen in Er71 null mice are most similar to those of Flk1 deficient mice. As no defects in hematopoietic and vascular development were observed in Ets1 null mice, we propose that ER71 is the genuine Ets transcription factor that regulates Flk1 expression during early embryonic development. To our knowledge, ER71 is the first transcription factor that shows a non-redundant, indispensable function in the regulation of blood and vessel development in the mouse.
Pham et al. (2007) reported that the ENU-generated y11 zebrafish mutants have a mutation in the etsrp gene. etsrpy11 mutants display defects in vascular morphogenesis and angiogenesis. Specification of angioblasts during initial vasculogenesis occurs normally in these mutants. Importantly, expression of endothelial and hematopoietic genes, including flk1, flt4, gata1 and gata2, were all reduced in etsrpy11 mutants. In the developing zebrafish vasculature, the Ets factors etsrp, fli1, fli1b, and ets1 are all expressed in an overlapping manner spatially and temporally. Morpholino knockdown of individual ets genes resulted in partial or no defects in endothelial differentiation, but combined reduction of all four ets genes, etsrp, fli1, fli1b, and ets1, led to dramatic decrease in endothelial cells. These studies suggest that Ets factors perform overlapping function in zebrafish vascular development. This is in sharp contrast to our findings that ER71 inactivation results in a complete block of hematopoietic and vascular development in mice. Whether etsrp function is duplicated in zebrafish or whether zebrafish vascular development also requires the new fourth VEGF receptor (VEGFR4) that is lost in mammals (Bussmann et al., 2007) should be investigated in the future.
Recent studies (Schoenebeck et al., 2007) suggest a critical regulatory relationship between the nascent blood/vessel vs. cardiac program. Specifically, scl and etsrp injection into zebrafish embryos resulted in the expansion of hematopoietic and endothelial specification with a concurrent reduction of the myocardial field. Conversely, knockdown of scl and etsrp resulted in an expansion of the boundaries of hand1 expression, suggesting reciprocal regulation between the hematopoietic/vascular and cardiovascular systems. Intriguingly, enforced ER71 expression in ES cells resulted in an increase in genes expressed in the blood and endothelial cell lineages, and a decrease in genes expressed in the cardiac lineage (Figure 3E). Conversely, genes expressed in hematopoietic as well as endothelial cells were down regulated, and cardiogenic genes were upregulated in the Er71 mutant embryos (supplementary Figure 3). Quantitative RT-PCR between wild type and mutant E9.0 hearts confirmed that some of these genes were upregulated in the mutant hearts, although the fold difference was not significant. Future studies on downstream mediators of ER71 in the regulation of hematopoietic/vessel vs. cardiac differentiation are warranted.
Experimental Procedures
Cell culture and Flow cytometry
ES cell culture, in vitro differentiation, hematopoietic replating and flow cytometry were performed as described previously (Park et al., 2004; Lugus et al., 2007). Factors were used as follows: 5 ng/ml BMP4, 10 ng/ml VEGF165, 11 nM DKK1, 1 nM Noggin, 2 μM DAPT (R & D systems), 3 μM Cyclopamine (TRC-Canada) and 1 μg/ml Doxcycline (Sigma).
Generation of the ER71 knockout mice and genotyping
Cloning of a 12kb genomic XbaI fragment encompassing all exons of Er71 has been described previously (De Haro and Janknecht, 2005). We then subcloned 6.8kb of the XbaI-SalI genomic fragment (5′ homologous arm) and 3.1 kb of the SpeI-XbaI fragment (3′ fragment) into pBluescript(+) KS vector and then inserted puro, phosphoglycerokinase (pgk)-puromycin poly(A) resistance cassettes and DT-A genes, leading to disruption of exons 5, 6 and 7 of mouse Er71. The Er71-pGK-puro vector was linearized with NotI, and introduced into ES cells by electroporation using a Gene Pulser (1 pulse of 0.25 kV and 500 μF; Bio-Rad). Resistant ES cell clones grown in conditioned medium were isolated after 8 days of culture. Homologous recombination was confirmed by Southern blot analysis of the genomic DNA prepared from ES cell clones using external probes, as indicated in Figure 5A and 5B. Genotyping of embryos and mice was performed by PCR using primers Er71-L(ACGTAGAAGGCTGCTGGAAA), Er71-R(GCAGTTTGCAGCCTCTTACC) and pGK-3(GCACGAGACTAGTGAGACGTGCTAC). The products are 1000bp (wild type allele) and 700bp (puro allele).
Whole mount In situ hybridization
Whole mount in situ hybridization was performed as described previously (Manley and Capecchi, 1995). The mouse T probe was a gift from Dr. Y. Kong (Pohang University of Science and Technology, Korea). The mouse Flk1 or Er71 in pBS SK(−) was linearized, and transcribed with T7 (Ambion). Probes were labeled with digoxigenin and signals were detected by alkaline phosphatase-conjugated anti-digoxigenin antibody.
Histology and Immunohistochemistry
Immunostaining of embryos was performed as described previously (Li et al., 2003). Briefly, embryos were fixed in 4% paraformaldehyde, bleached with 5% H2O2, blocked in 2% bovine serum albumin, incubated with primary antibodies and second antibody sequentially, and stained with the DAB kit (DAKO). Antibodies used were anti- PECAM1 (BD), anti-FLK1 (BD) and HRP-conjugated goat anti-rat IgG (Jackson ImmunoResearch).
Retrovirus Production
Phoenix packaging cells were maintained in DMEM/10% FCS/1% L-Glutamine. On day 0, 1.25×106 cells were plated on gelatinized 6 cm dishes. On day 1, 3 μg of MSCVpac (control) or MSCV-ER71-IRES-GFP was used to transfect cells with FuGene 6 (Roche) and 18–24 hours later media was changed. Cells were then grown at 32°C for 24 hours and media was recovered and filtered with a 0.45 μm filter before being used to infect embryo-derived cells.
Co-culture of embryonic cells with OP-9 cells
Co-culture of embryonic cells with OP-9 cells was previously described (Zeigler et al., 2006). Briefly, embryos between E8.0 and E8.5 were dissected, dissociated and infected with MSCV or MSCV-ER71-IRES-GFP viral supernatant in the presence of protamine (10μg/ml). After ER71 retroviral infection, duplicate of one half equivalent embryo cells were cultured on mitomycin C-treated OP9 cells in α-MEM containing 10% FCS supplemented with 1% of IL-3, 1% of KL, 10 ng/ml of human thrombopoietin (TPO), 2 unit/ml of EPO, 5ng/ml of G-CSF, 20 unit/ml of GM-CSF and 10 ng/ml of VEGFA165. On day 4 of culture, cells from one-half duplicate were replated into methylcelluose containing hematopoietic cytokines. On day 7 of culture, cells from another one-half duplicate were stained with α-PECAM1 (CD31) antibody (1:30, Pharmingen) overnight at 4°C. The following day, cells were incubated with alkaline phosphatase-conjugated anti-Rat IgG (1:250, Zymed), signals were visualized with the NBT/BCIP kit (Promega).
Quantitative real time PCR (qRT-PCR)
RNA preparation and cDNA synthesis were previously described (Park et al., 2006; Lugus et al., 2007). Primer sequences used in this study are provided in supplementary Table 2.
Chromatin Immunoprecipitation (ChIP)
ChIP assays were performed as previously described (Im et al., 2004). Briefly, iER71-V5 ES cells differentiated in serum, Dox was added on day 1, EBs were collected on day 3 and crosslinked with 1% formaldehyde. Cells were lysed and chromatin was fragmented to ~500bp by sonication. The chromatin slurry was incubated with anti-mouse V5 or anti-rabbit H3K4Me3 antibodies. Anti-mouse IgG or anti-rabbit IgG, respectively, was used as a control. The immunoprecipitated DNA fragments were recovered and subjected to qPCR using the primers listed in supplementary Table 3.
Cloning of mouse Flk1 promoter and Reporter assays
The PCR-amplified murine Flk1 promoter fragment from 129/Sv mouse ES cell genomic DNA was inserted into the XhoI and HindIII sites of pGL3-Basic, and verified by sequencing. 293T cells (1×105/well/6-well plate) were transfected with 250ng Er71 expression plasmid (pCS3-Myc-ER71), 50ng pGL3-mFlk1 promoter constructs and 25ng pRL-CMV by CaCl2 precipitation. Forty-eight hours later, cells were harvested and luciferase activity was measured using the Dual-Luciferase reporter assay system (Promega) according to the manufacturer’s instructions. Firefly luciferase values were divided by Renilla luciferase values to calculate transfection efficiency. Primers used for the construct of the −492 ~ +39 Flk1 promoter were listed in the supplementary Table 3.
Statistics
The results of qRT-PCR, FACS analysis and hematopoietic replating were analyzed by Student’s t-test. P<0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Drs. G.Y. Koh, Y. Y. Kong and Derek Persons for valuable materials and F. Long for helpful discussion. We also thank Drs. P. Jay and J. M. Kim for histological examinations. This work was supported by grants from the National Institutes of Health, NHLBI, HL63736 and HL55337 (K.C.), the 21st Century Frontier Functional Human Genome Project, NRL, Nuclear Research Programs of Korea (D.S. L.), Korea National Cancer Center Control Program (H. L.), NIH, NIDDK, DK54508 (S.L.), the Mayo Foundation (R.J.) and by a pre-doctoral grant from the American Heart Association (J.J.L.). J.J.L. is a fellow of the Lucille P. Markey Pathway in Human Pathobiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartel FO, Higuchi T, Spyropoulos DD. Mouse models in the study of the Ets family of transcription factors. Oncogene. 2000;19:6443–6454. doi: 10.1038/sj.onc.1204038. [DOI] [PubMed] [Google Scholar]
- Brown TA, McKnight SL. Specificities of protein-protein and protein-DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 1992;6:2502–2512. doi: 10.1101/gad.6.12b.2502. [DOI] [PubMed] [Google Scholar]
- Bussmann J, Bakkers J, Schulte-Merker S. Early Endocardial Morphogenesis Requires Scl/Tal1. PLoS Genet. 2007;24:e140. doi: 10.1371/journal.pgen.0030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chung YS, Zhang WJ, Arentson E, Kingsley PD, Palis J, Choi K. Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression. Development. 2002;129:5511–5520. doi: 10.1242/dev.00149. [DOI] [PubMed] [Google Scholar]
- De Haro L, Janknecht R. Functional analysis of the transcription factor ER71 and its activation of the matrix metalloproteinase-1 promoter. Nucleic Acids Res. 2002;30:2972–2979. doi: 10.1093/nar/gkf390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haro L, Janknecht R. Cloning of the murine ER71 gene (Etsrp71) and initial characterization of its promoter. Genomics. 2005;85:493–502. doi: 10.1016/j.ygeno.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Fon GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn. 1995;203:80–92. doi: 10.1002/aja.1002030109. [DOI] [PubMed] [Google Scholar]
- Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, Flamme I. Cooperative interaction of hypoxia-inducible factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1) J Biol Chem. 2003;278:7520–7530. doi: 10.1074/jbc.M211298200. [DOI] [PubMed] [Google Scholar]
- Ema M, Faloon P, Zhang WJ, Hirashima M, Redi T, Stanford W, Choi K, Rossant J. Combinatorial effects of Flk-1 and Tal1 (SCL) on vascular and hematopoietic development in the mouse. Genes Dev. 2003;17:380–393. doi: 10.1101/gad.1049803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107:111–117. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- Faloon P, Arentson E, Kazarov A, Deng CX, Porcher C, Orkin S, Choi K. Basic fibroblast growth factor positively regulates hematopoietic development. Development. 2000;127:1931–1941. doi: 10.1242/dev.127.9.1931. [DOI] [PubMed] [Google Scholar]
- Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im H, Grass JA, Johnson KD, Boyer ME, Wu J, Bresnick EH. Measurement of protein-DNA interactions in vivo by chromatin immunoprecipitation. Methods Mol Biol. 2004;284:129–146. doi: 10.1385/1-59259-816-1:129. [DOI] [PubMed] [Google Scholar]
- Kappel A, Schlaeger TM, Flamme I, Orkin SH, Risau W, Breier G. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of flk-1 expression during murine vascular development. Blood. 2000;96:3078–3085. [PubMed] [Google Scholar]
- Knebel J, De Haro L, Janknecht R. Repression of transcription by TSGA/Jmjd1a, a novel interaction partner of the ETS protein ER71. J Cell Biochem. 2006;99:319–329. doi: 10.1002/jcb.20945. [DOI] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Lai EC. Notch signaling: Control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lengerke C, Schmitt S, Bowman TV, Jang I, Maouche-Chretien L, McKinney-Freeman S, Davidson AJ, Hammerschmidt M, Rentzsch F, Green JBA, Zon LI, Daley GQ. BMP and Wnt Specify Hematopoietic Fate by Activation of the Cdx-Hox Pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci USA. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- Lugus JJ, Chung YS, Mills J, Kim S, Grass F, Kyba M, Doherty J, Bresnick EH, Choi K. GATA-2 functions at multiple steps in hemangioblast development and differentiation. Development. 2007;134:393–405. doi: 10.1242/dev.02731. [DOI] [PubMed] [Google Scholar]
- Manley NR, Capecchi MR. The role of Hoxa-3 in mouse thymus and thyroid development. Development. 1995;121:1989–2003. doi: 10.1242/dev.121.7.1989. [DOI] [PubMed] [Google Scholar]
- Motoike T, Markham DW, Rossant J, Sato TN. Evidence for novel fate of Flk1+ progenitor: contribution to muscle lineage. Genesis. 2003;35:153–159. doi: 10.1002/gene.10175. [DOI] [PubMed] [Google Scholar]
- Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, Activin, and BMP Signaling Regulate Distinct Stages in the Developmental Pathway from Embryonic Stem Cells to Blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- Park C, Afrikanova I, Chung YS, Zhang WJ, Arentson E, Fong GHG, Rosendahl A, Choi K. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development. 2006;133:3473–3484. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- Pham VN, Lawson ND, Mugford JW, Dye L, Castranova D, Lo B, Weinstein BM. Combinatorial function of ETS transcription factors in the developing vasculature. Dev Biol. 2007;303:772–783. doi: 10.1016/j.ydbio.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenebeck JJ, Keegan BR, Yelon D. Vessel and Blood Specification Override Cardiac Potential in Anterior Mesoderm. Dev Cell. 2007;13:254–267. doi: 10.1016/j.devcel.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Schroeder T, Meier-Stiegen F, Schwanbeck R, Eilken H, Nishikawa S, Häsler R, Schreiber S, Bornkamm GW, Nishikawa S, Just U. Activated Notch1 alters differentiation of embryonic stem cells into mesodermal cell lineages at multiple stages of development. Mech Dev. 2006;123:570–579. doi: 10.1016/j.mod.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Jorniak T, Lin S. Identification of novel vascular endothelial-specific genes by the microarray analysis of the zebrafish cloche mutants. Blood. 2005;106:534–541. doi: 10.1182/blood-2004-12-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumanas S, Gomez G, Zhao Y, Park C, Choi K, Lin S. Interplay between Etsrp/ER71, scl and alk8 signaling controls endothelial and myeloid cell formation. Blood. 2008 Feb 12; doi: 10.1182/blood-2007-09-110569. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP, Loebel DA. Gene function in mouse embryogenesis: get set for gastrulation. Nat Rev Genet. 2007;8:368–381. doi: 10.1038/nrg2084. [DOI] [PubMed] [Google Scholar]
- Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM, Speck NA. The allantois and chorion, when isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development. 2006;133:4183–4192. doi: 10.1242/dev.02596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.