Introduction
There is growing appreciation for functional interrelationships between nuclear structure and gene expression. Equally important, it is well documented that modifications in nuclear architecture are a hallmark of tumor cells, and there is growing evidence for a functionally compartmentalized assembly and organization of regulatory machinery for combinatorial control of gene expression in nuclear microenvironments (Figure 1). Further understanding of mechanisms modulating the regulation and regulatory parameters of nuclear architecture can provide necessary insight into: 1) proliferation and tissue-specific gene expression, and 2) aberrant gene expression that is linked to the onset and progression of cancer. Such understanding can serve as a platform for novel approaches to cancer diagnosis and therapy. We are characterizing cell and tissue microenvironments that contribute to transformation and tumor progression. Our approach is to functionally define intranuclear microenvironments that require structural and functional fidelity for biological control and exhibit striking aberrations with the onset and progression of tumorigenesis.
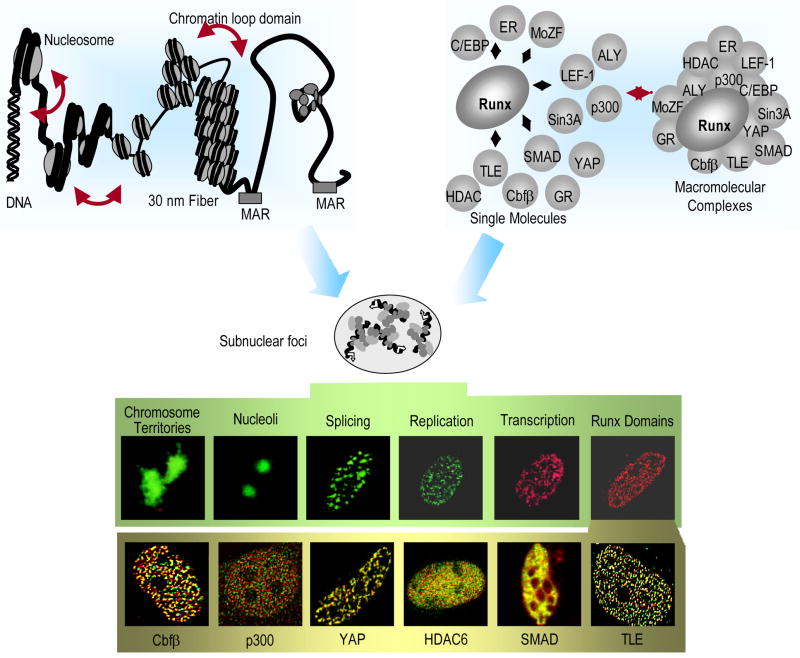
Fig. 1. Levels of nuclear organization.
The linear placement of DNA-regulatory elements in gene promoters constitutes the primary level of nuclear organization. The distance between these regulatory sites is intricately regulated by the packaging of DNA into nucleosomes and higher order chromatin structures (left, upper panel). Scaffolding nuclear proteins, such as RUNX, provide structural platforms for the assembly of multiprotein supercomplexes to facilitate the combinatorial control of gene expression (left, bottom panel). Genes and macromolecular regulatory complexes together give rise to dynamic nuclear microenvironments in the nucleus. RUNX bodies are nuclear microenvironments that contain various co-regulatory proteins that are involved in gene activation, as well as repression, chromatin remodeling and cellular signaling (immunofluorescence images on the right, shaded yellow). RUNX was visualized using the Alexa 488 secondary antibody in all images and the proteins were detected using Alexa 568 fluorochrome-conjugated secondary antibodies, as indicated.
Focused on obligatory relationships of nuclear structure with gene expression we are experimentally addressing components of nuclear organization that are causally linked to modified transcriptional control in transformed and tumor cells. In this chapter we will provide an overview of our strategies to explore the working hypothesis that parameters of nuclear structure support cell growth and phenotypic properties of normal and tumor cells by facilitating the organization of chromosomes, chromatin, genes, transcripts and regulatory complexes within the dynamic three-dimensional context of nuclear architecture.
Nuclear architecture and subnuclear organization of regulatory functions
The nucleus is highly compartmentalized and contains a multiplicity of specialized functional domains involved in gene expression, DNA replication and DNA repair (reviewed in (Zaidi et al., 2004)) (Figure 1). A specific repertoire of proteins and nucleic acids is associated with each domain (e.g., nucleoli/rDNA, splicing speckles/RNA, cajal bodies/U7snRNP/histone genes, Barr body/X chromosome). The nuclear matrix (Berezney and Coffey, 1975) is a principal component of nuclear architecture that has been functionally associated with DNA replication (Vaughn et al., 1990, Jackson and Cook, 1986); gene localization (Robinson et al., 1982); imposition of physical constraints of chromatin structure which support formation of loop domains (Nelkin et al., 1980, Ciejek et al., 1983, Mirkovitch et al., 1984, Cockerill and Garrard, 1986); concentration and targeting of transcription factors (Merriman et al., 1995, van Wijnen et al., 1993, Bidwell et al., 1993, Dworetzky et al., 1992, Dickinson et al., 1992); RNA processing and transport of gene transcripts (van Eekelen and van Venrooij, 1981, Xing et al., 1993, Jackson et al., 1981, Herman et al., 1978, Blencowe et al., 1994, Mortillaro et al., 1996, Grande et al., 1996); post-translational modifications of chromosomal proteins (Hendzel et al., 1994); as well as imprinting and modifications of chromatin organization (Brown et al., 1992) and chromatin remodeling (Reyes et al., 1997). Our studies have been focused on the nuclear matrix as a specialized component of nuclear architecture that facilitates localization of genes and cognate gene regulatory factors at specific subnuclear sites (Zaidi et al., 2004).
The classical view of the nuclear matrix, which is based on seminal ultra-structural studies using electron microscopy (Berezney and Coffey, 1975, Nickerson et al., 1989), indicates that the nuclear matrix is a heterogeneous internal fibrogranular network surrounded by the nuclear lamina-pore complex. The dynamic properties of the nuclear matrix are illustrated by the rapid disassembly and assembly of the nucleus during mitosis. Furthermore, data from our group and others that were obtained by in situ immunofluorescence microscopy and biochemical approaches have shown that the nuclear matrix during interphase is a dynamic structure that reversibly associates with gene regulatory factors under different physiological circumstances (reviewed in (Zaidi et al., 2004; Stein et al., 2003; Zaidi et al., 2005; Lian et al., 2004)). While it is recognized that the nuclear matrix is an organized composite of nuclear regulatory machineries with modifications in composition and organization that reflect cell function, the extent to which the nuclear matrix is architecturally or activity driven is unresolved.
Molecular and structural determinants of subnuclear targeting of Runx(AML) transcription factors
The overall protein composition of the nuclear matrix is tissue-specific, modified during neoplastic transformation, and developmentally regulated (Stein et al., 1995). Furthermore, specific proteins are differentially associated with the nuclear matrix in distinct cell types (van Wijnen et al., 1993) and during differentiation (Lindenmuth et al. 1997; Choi et al., 1998). We have demonstrated that the Runx(AML) class of transcription factors, which are key regulators of cell growth and differentiation during myeloid lineage maturation and mesenchymal tissue development, is associated with the nuclear matrix. We have defined the molecular basis for Runx(AML)/nuclear matrix interactions by identifying a specific (C terminal) subnuclear targeting signal (nuclear matrix targeting signal, NMTS). We showed that the NMTS is a unique and autonomous 30–35 amino acid protein motif that is conserved in Runx(AML) proteins and is both necessary and sufficient for localizing Runx(AML) proteins to nuclear matrix associated subnuclear foci. We determined the crystal structure of the NMTS, which is composed of two loops connected by a flexible linker (Tang et al., 1999). This structural model was used as the foundation for mutagenesis studies that targeted a putative protein/protein interface composed of basic and aromatic residues in the two loops to define subnuclear trafficking specificity at single amino acid resolution (Zaidi et al., 2006). We observed that a highly conserved Y motif in the C-terminal loop is critical for subnuclear targeting of Runx(AML) and transcriptional control (Li et al., 2005; Zaidi et al., 2006, 2001).
Runx1(AML1) related subnuclear targeting defects in Acute Myelogenous Leukemia
The NMTS is located in the C-terminus of Runx1(AML1) and this segment is deleted in the t(8;21) related Runx1(AML1)/ETO fusion protein which results from one of the most prevalent chromosomal aberrations in human acute myelogenous leukemia. Indeed, we have demonstrated that Runx1(AML1)/ETO is misrouted to intranuclear locations that are distinct from normal Runx(AML) foci (McNeil et al., 1999) through two ETO-specific targeting signals we discovered (Barseguian et al., 2002). Expression of Runx1(AML1)/ETO also alters the subnuclear organization of PML domains (McNeil et al., 2000), and changes in subnuclear organization appear to be reversible in leukemia patients that are in remission (Gordon et al., 2000). The expression of the Runx1(AML1)/ETO fusion protein may cause a pathological phenotype because (i) the C-terminus of Runx1(AML1) is removed (loss-of-function), (ii) the DNA binding domain of Runx1(AML1) is fused to the unrelated ETO protein (gain-of-function), or (iii) the normal function of Runx(AML) foci is perturbed due to the subnuclear targeting defect (dominant negative effect). We have recently shown that expression of mutant Runx(AML) proteins with a subnuclear targeting defect (Runx(AML) std point-mutants) causes a profound alteration of cellular phenotypes in both myeloid progenitor cells and metastatic breast cancer cells (Javed et al., 2005, Vradii et al., 2005). Significantly, the co-expression of a Runx1(AML1) std mutant during myeloid differentiation in the presence of the endogenous Runx1(AML1) protein results in a maturation arrest (Vradii et al., 2005). These data demonstrate that subnuclear targeting defects of Runx1(AML1) may have dominant negative effects that contribute to the pathology of acute myelogenous leukemias.
Dynamics and mechanisms of subnuclear targeting of Runx(AML) proteins in live cells
Based on the major functional defects that arise from compromised subnuclear targeting of Runx(AML) proteins we have observed in two distinct biological models (Javed et al., 2005, Vradii et al., 2005), it is now necessary to define the mechanism by which abrogation of subnuclear targeting causes gene regulatory defects and pathological phenotypes. Our working model is that Runx(AML) factors are architectural proteins that provide DNA-bound scaffolds for the nucleation of multiple co-regulatory proteins into macromolecular complexes. Runx(AML) proteins are known to be associated with many co-regulators, some of which have been shown to be nuclear matrix proteins themselves (Durst and Hiebert, 2004; Westendorf, 2006). Interestingly, we have shown that Runx(AML) proteins are required for chromatin remodeling of tissue-restricted genes (Javed et al., 1999). Data from our group and others indicate that many Runx(AML) co-factors are components of large macromolecular complexes capable of modifying chromatin structure (e.g., histone/lysine deacetylases [HDACs] and acetyl transferases [HATs], as well as SWI/SNF components) (reviewed in (Durst and Hiebert, 2004; Zaidi et al., 2005), and unpublished data). We have observed that Runx(AML) recognition motifs are frequently clustered in target promoters, and that Runx(AML) responsive genes are clustered in specific genomic regions. We postulate that the super-clustering of multiple Runx(AML) proteins with macro-molecular co-factor complexes at many loci results in the formation of the subnuclear target sites we refer to as Runx(AML) foci.
Our live cell studies with Runx(AML)/GFP fusion proteins have shown that Runx(AML) foci remain in a relatively steady location (> 30 minutes based on time-lapse fluorescence microscopy). Thus, Runx(AML) foci are positionally stable (Harrington et al., 2002). However, photo-bleaching results clearly establish that Runx(AML) foci are dynamic entities and that Runx(AML) proteins are rapidly discharged and recruited (half-time of recovery ~5 to 10 sec). Runx(AML) proteins that are defective for subnuclear targeting, yet competent for DNA binding, exhibit a much faster exchange rate that is comparable to that of GFP (half-time of recovery <500 milliseconds) (Harrington et al., 2002).
The alteration in exchange rates of Runx(AML) mutant proteins in live cells is reflected by differences in the detection of wild type and std mutant Runx(AML) in subcellular fractions by Western blot analysis. The biochemical phenotype of all std mutants is decreased detection (or absence) in the nuclear matrix fraction and increased detection in non-matrix fractions. Most Runx(AML) std mutants exhibit a punctate subnuclear distribution in whole cells but are typically not detected in the nuclear matrix as examined by immunofluorescence microscopy. Therefore, we postulate that Runx(AML) std mutants form meta-stable complexes and that the recruitment of these mutant complexes at subnuclear foci containing wild type Runx1(AML1) alters the micro-environment and abrogates fidelity of gene regulation.
Mitotic control by Runx(AML) proteins and association with microtubules
We recently demonstrated that Runx(AML) proteins are associated with metaphase chromosomes at multiple distinct foci (Zaidi et al., 2003). This key finding is a paradigm shifting observation that indicates a regulatory function for sequence-specific transcription factors at genomic loci in otherwise massively condensed metaphase chromosomes. Our data provide compelling support for the hypothesis, which has been discussed by Workman and colleagues (John and Workman, 1998), that specific genomic regions may maintain a locally active chromatin conformation during mitosis through proteins that support the bookmarking of genes (Young et al., 2005a, 2005b) and mediate their post-mitotic transcriptional regulation. We have observed that Runx(AML) proteins interact with microtubules.
Runx(AML)/microtubule interactions may regulate the cytoplasmic/nuclear equilibrium of Runx(AML) proteins, and nuclear compartmentalization is a prerequisite for subnuclear targeting during interphase. The association with microtubules may also facilitate the interaction of Runx(AML) proteins with the mitotic apparatus during metaphase. Our quantitative microscopic analysis has revealed that Runx(AML) proteins partition equivalently into progeny cells during cell division (Zaidi et al., 2003). Therefore, we postulate that the association of Runx(AML) proteins with metaphase chromosomes and the mitotic apparatus may secure the appropriate mitotic distribution of this regulatory protein. We are further assessing the biological significance of the association of Runx(AML) proteins with microtubule-related cytoarchitecture.
Active retention of phenotype during cell division: Runx(AML) transcription factors remain associated with gene promoters within the condensed mitotic chromosomes
During cell division there is a cessation of transcription that is coupled with chromosome condensation. Resumption of gene expression post-mitotically requires restoration of nuclear organization and assembly of regulatory complexes. We have found that transcription factor stability during mitosis and biochemical association with chromatin in mitotic cells suggest that association of regulatory factors with metaphase chromosomes may be a biologically relevant component of gene regulatory functions following mitosis (Young et al., 2005a). We propose that transcription factors that include the Runx(AML) proteins have an active role in retaining phenotype during cell division to support lineage-specific gene expression in progeny cells. Such factor-dependent mitotic control provides an epigenetic mechanism for the retention of gene expression patterns and lineage commitment during cell division.
From a fundamental regulatory perspective, the contributions of nuclear organization in control of replication and transcription are evident despite the gaps in our understanding of the rules that govern gene expression. Equally important, insight into regulatory parameters of organization and assembly of machinery for transcription, replication and repair in nuclear microenvironments provide a new dimension to cancer diagnosis and targeted therapy.
Functional implications of nuclear organization for biological control and cancer
Biochemical, in situ microscopic, and in vivo genetic evidence demonstrate the requirement for intranuclear placement of regulatory complexes, which is directly linked with cellular response to physiological cues and is essential for combinatorial control of gene expression. Subnuclear targeting of transcription factors and regulatory proteins provides a mechanistic link between the temporal-spatial regulation of gene expression and architectural organization of regulatory complexes within the nucleus. It also establishes the requirement for delivery of regulatory proteins to the right place at the right time.
Summary
The nucleus is highly compartmentalized and contains a multiplicity of specialized functional microenvironments involved in gene expression, DNA replication and DNA repair. We have demonstrated that the Runx(AML) class of transcription factors, which are key regulators of cell growth and differentiation during myeloid lineage maturation and mesenchymal tissue development, is associated with the nuclear matrix by a specific C terminal subnuclear targeting signal (Nuclear Matrix Targeting Signal, NMTS). We have shown that expression of mutant Runx(AML) proteins with a subnuclear targeting defect causes a profound alteration of cellular phenotypes in both myeloid progenitor cells and metastatic breast cancer cells. We have established that Runx(AML) proteins are associated with metaphase chromosomes at multiple distinct foci indicating a regulatory function for sequence-specific transcription factors at genomic loci and a mechanism for mitotic distribution of regulatory factors. We propose that transcription factors that include the Runx(AML) proteins have an active role in epigenetically retaining phenotype during cell division to support lineage-specific gene expression and cell fate determination in progeny cells. The contributions of nuclear organization in control of replication and transcription are evident despite gaps in our understanding of the rules that govern gene expression. Insight into regulatory parameters of organization and assembly of machinery for transcription, replication and repair in nuclear microenvironments provide a new dimension to cancer diagnosis and targeted therapy.
Acknowledgments
Studies from our laboratory presented in this review were in part supported by grants PO1 AR48818, PO1 CA82834, and 5 P30 DK32520 from the National Institutes of Health. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Authors thank Ms Elizabeth Bronstein for editorial assistance in preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barseguian K, Lutterbach B, Hiebert SW, Nickerson J, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Multiple subnuclear targeting signals of the leukemia-related AML1/ETO and ETO repressor proteins. Proc Natl Acad Sci U S A. 2002;99:15434–9. doi: 10.1073/pnas.242588499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R, Coffey DS. Nuclear protein matrix: association with newly synthesized DNA. Science. 1975;189:291–3. doi: 10.1126/science.1145202. [DOI] [PubMed] [Google Scholar]
- Bidwell JP, van Wijnen AJ, Fey EG, Dworetzky S, Penman S, Stein JL, Lian JB, Stein GS. Osteocalcin gene promoter-binding factors are tissue-specific nuclear matrix components. Proc Natl Acad Sci USA. 1993;90:3162–6. doi: 10.1073/pnas.90.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe BJ, Nickerson JA, Issner R, Penman S, Sharp PA. Association of nuclear matrix antigens with exon-containing splicing complexes. J Cell Biol. 1994;127:593–607. doi: 10.1083/jcb.127.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Hendrich BD, Rupert JL, Lafreniere RG, Xing Y, Lawrence J, Willard HF. The human XIST gene: analysis of a 17kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71:527–42. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Choi J-Y, van Wijnen AJ, Aslam F, Leszyk JD, Stein JL, Stein GS, Lian JB, Penman S. Developmental association of the β-galactoside-binding protein galectin-1 with the nuclear matrix of rat calvarial osteoblasts. J Cell Sci. 1998;111:3035–43. doi: 10.1242/jcs.111.20.3035. [DOI] [PubMed] [Google Scholar]
- Ciejek EM, Tsai MJ, O’Malley BW. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983;306:607–9. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Cockerill PN, Garrard WT. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986;44:273–82. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Dickinson LA, Joh T, Kohwi Y, Kohwi-Shigematsu T. A tissue-specific MAR/SAR DNA-binding protein with unusual binding site recognition. Cell. 1992;70:631–45. doi: 10.1016/0092-8674(92)90432-c. [DOI] [PubMed] [Google Scholar]
- Durst KL, Hiebert SW. Role of RUNX family members in transcriptional repression and gene silencing. Oncogene. 2004;23:4220–4. doi: 10.1038/sj.onc.1207122. [DOI] [PubMed] [Google Scholar]
- Dworetzky SI, Wright KL, Fey EG, Penman S, Lian JB, Stein JL, Stein GS. Sequence-specific DNA-binding proteins are components of a nuclear matrix-attachment site. Proc Natl Acad Sci USA. 1992;89:4178–82. doi: 10.1073/pnas.89.9.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Pockwinse SM, Stewart FM, Quesenberry PJ, Nakamura T, Croce CM, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Modified intranuclear organization of regulatory factors in human acute leukemias: reversal after treatment. J Cell Biochem. 2000;77:30–43. [PubMed] [Google Scholar]
- Grande MA, van der Kraan I, van Steensel B, Schul W, de The H, van der Voor HT, de Jong L, van Driel R. PML-containing nuclear bodies: their spatial distribution in relation to other nuclear components. J Cell Biochem. 1996;63:280–91. doi: 10.1002/(sici)1097-4644(19961201)63:3<280::aid-jcb3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Harrington KS, Javed A, Drissi H, McNeil S, Lian JB, Stein JL, van Wijnen AJ, Wang Y-L, Stein GS. Transcription factors RUNX1/AML1 and RUNX2/Cbfa1 dynamically associate with stationary subnuclear domains. J Cell Sci. 2002;115:4167–76. doi: 10.1242/jcs.00095. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Sun JM, Chen HY, Rattner JB, Davie JR. Histone acetyltransferase is associated with the nuclear matrix. J Biol Chem. 1994;269:22894–901. [PubMed] [Google Scholar]
- Herman R, Weymouth L, Penman S. Heterogeneous nuclear RNA-protein fibers in chromatin-depleted nuclei. J Cell Biol. 1978;78:663–74. doi: 10.1083/jcb.78.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DA, Cook PR. Replication occurs at a nucleoskeleton. EMBO J. 1986;5:1403–10. doi: 10.1002/j.1460-2075.1986.tb04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DA, McCready SJ, Cook PR. RNA is synthesized at the nuclear cage. Nature. 1981;292:552–5. doi: 10.1038/292552a0. [DOI] [PubMed] [Google Scholar]
- Javed A, Barnes GL, Pratap J, Antkowiak T, Gerstenfeld LC, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Impaired intranuclear trafficking of Runx2 (AML3/CBFA1) transcription factors in breast cancer cells inhibits osteolysis in vivo. Proc Natl Acad Sci, USA. 2005;102:1454–9. doi: 10.1073/pnas.0409121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed A, Gutierrez S, Montecino M, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Multiple Cbfa/AML sites in the rat osteocalcin promoter are required for basal and vitamin D responsive transcription and contribute to chromatin organization. Mol Cell Biol. 1999;19:7491–500. doi: 10.1128/mcb.19.11.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Workman JL. Bookmarking genes for activation in condensed mitotic chromosomes. BioEssays. 1998;20:275–9. doi: 10.1002/(SICI)1521-1878(199804)20:4<275::AID-BIES1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Li X, Lian JB, Nickerson JA, Stein JL, van Wijnen AJ, Stein GS, Javed A. Subnuclear targeting of Runx1 is required for synergistic activation of the myeloid specific M-CSF receptor promoter by PU.1. J Cell Biochem. 2005;96:795–809. doi: 10.1002/jcb.20548. [DOI] [PubMed] [Google Scholar]
- Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- Lindenmuth DM, van Wijnen AJ, Hiebert S, Stein JL, Lian JB, Stein GS. Subcellular partitioning of transcription factors during osteoblast differentiation: developmental association of the AML/CBFα/PEBP2α-related transcription factor-NMP-2 with the nuclear matrix. J Cell Biochem. 1997;66:123–32. [PubMed] [Google Scholar]
- McNeil S, Javed A, Harrington KS, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Leukemia-associated AML1/ETO (8;21) chromosomal translocation protein increases the cellular representation of PML bodies. J Cell Biochem. 2000;79:103–12. [PubMed] [Google Scholar]
- McNeil S, Zeng C, Harrington KS, Hiebert S, Lian JB, Stein JL, van Wijnen AJ, Stein GS. The t(8;21) chromosomal translocation in acute myelogenous leukemia modifies intranuclear targeting of the AML1/CBFalpha2 transcription factor. Proc Natl Acad Sci U S A. 1999;96:14882–7. doi: 10.1073/pnas.96.26.14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman HL, van Wijnen AJ, Hiebert S, Bidwell JP, Fey E, Lian J, Stein J, Stein GS. The tissue-specific nuclear matrix protein, NMP-2, is a member of the AML/CBF/PEBP2/runt domain transcription factor family: interactions with the osteocalcin gene promoter. Biochemistry. 1995;34:13125–32. doi: 10.1021/bi00040a025. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J, Mirault ME, Laemmli UK. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984;39:223–32. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Mortillaro MJ, Blencowe BJ, Wei X, Nakayasu H, Du L, Warren SL, Sharp PA, Berezney R. A hyperphosphorylated form of the large subunit of RNA polymerase II is associated with splicing complexes and the nuclear matrix. Proc Natl Acad Sci USA. 1996;93:8253–7. doi: 10.1073/pnas.93.16.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelkin BD, Pardoll DM, Vogelstein B. Localization of SV40 genes within supercoiled loop domains. Nucl Acids Res. 1980;8:5623–33. doi: 10.1093/nar/8.23.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson JA, Krochmalnic G, Wan KM, Penman S. Chromatin architecture and nuclear RNA. Proc Natl Acad Sci U S A. 1989;86:177–81. doi: 10.1073/pnas.86.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes J-C, Muchardt C, Yaniv M. Components of the human SWI/SNF complex are enriched in active chromatin and are associated with the nuclear matrix. J Cell Biol. 1997;137:263–74. doi: 10.1083/jcb.137.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SI, Nelkin BD, Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982;28:99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Stein GS, van Wijnen AJ, Stein JL, Lian JB, Montecino M. Contributions of nuclear architecture to transcriptional control. In: Berezney R, Jeon KW, editors. Structural and Functional Organization of the Nuclear Matrix. San Diego: Academic Press; 1995. pp. 251–78. [DOI] [PubMed] [Google Scholar]
- Stein GS, Zaidi SK, Braastad CD, Montecino M, van Wijnen AJ, Choi J-Y, Stein JL, Lian JB, Javed A. Functional architecture of the nucleus: organizing the regulatory machinery for gene expression, replication and repair. Trends Cell Biol. 2003;13:584–92. doi: 10.1016/j.tcb.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Tang L, Guo B, Javed A, Choi J-Y, Hiebert S, Lian JB, van Wijnen AJ, Stein JL, Stein GS, Zhou GW. Crystal structure of the nuclear matrix targeting signal of the transcription factor AML-1/PEBP2αB/CBFα2. J Biol Chem. 1999;274:33580–6. doi: 10.1074/jbc.274.47.33580. [DOI] [PubMed] [Google Scholar]
- van Eekelen CA, van Venrooij WJ. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981;88:554–63. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijnen AJ, Bidwell JP, Fey EG, Penman S, Lian JB, Stein JL, Stein GS. Nuclear matrix association of multiple sequence-specific DNA binding activities related to SP-1, ATF, CCAAT, C/EBP, OCT-1, and AP-1. Biochemistry. 1993;32:8397–402. doi: 10.1021/bi00084a003. [DOI] [PubMed] [Google Scholar]
- Vaughn JP, Dijkwel PA, Mullenders LH, Hamlin JL. Replication forks are associated with the nuclear matrix. Nucl Acids Res. 1990;18:1965–9. doi: 10.1093/nar/18.8.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vradii D, Zaidi SK, Lian JB, van Wijnen AJ, Stein JL, Stein GS. A point mutation in AML1 disrupts subnuclear targeting, prevents myeloid differentiation, and results in a transformation-like phenotype. Proc Natl Acad Sci, USA. 2005;102:7174–9. doi: 10.1073/pnas.0502130102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf JJ. Transcriptional co-repressors of Runx2. J Cell Biochem. 2006;98:54–64. doi: 10.1002/jcb.20805. [DOI] [PubMed] [Google Scholar]
- Xing Y, Johnson CV, Dobner PR, Lawrence JB. Higher level organization of individual gene transcription and splicing. Science. 1993;259:1326–30. doi: 10.1126/science.8446901. [DOI] [PubMed] [Google Scholar]
- Young DW, Galindo M, Doxsey S, Imbalzano AN, Stein JL, Lian JB, van Wijnen AJ, Stein GS. Active retention of phenotype during cell division: AML/Runx transcription factors remain associated with gene promoters within the condensed mitotic chromosomes. 2005a Ref Type: Unpublished Work. [Google Scholar]
- Young DW, Nickerson JA, Doxsey S, Imbalzano AN, Stein JL, Lian JB, van Wijnen AJ, Stein GS. AML/Runx transcription factors provide lineage specific and cell cycle dependent regulation of ribosomal RNA transcription. 2005b Ref Type: Unpublished Work. [Google Scholar]
- Zaidi SK, Javed A, Choi J-Y, van Wijnen AJ, Stein JL, Lian JB, Stein GS. A specific targeting signal directs Runx2/Cbfa1 to subnuclear domains and contributes to transactivation of the osteocalcin gene. J Cell Sci. 2001;114:3093–102. doi: 10.1242/jcs.114.17.3093. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Javed A, Pratap J, Schroeder TM, Westendorf J, Lian JB, van Wijnen AJ, Stein GS, Stein JL. Alterations in intranuclear localization of Runx2 affect biological activity. J Cell Physiol. 2006;209:935–42. doi: 10.1002/jcp.20791. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Young DW, Choi JY, Pratap J, Javed A, Montecino M, Stein JL, Lian JB, van Wijnen AJ, Stein GS. Intranuclear trafficking: organization and assembly of regulatory machinery for combinatorial biological control. J Biol Chem. 2004;279:43363–6. doi: 10.1074/jbc.R400020200. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Young DW, Choi JY, Pratap J, Javed A, Montecino M, Stein JL, van Wijnen AJ, Lian JB, Stein GS. The dynamic organization of gene-regulatory machinery in nuclear microenvironments. EMBO Rep. 2005;6:128–133. doi: 10.1038/sj.embor.7400337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SK, Young DW, Pockwinse SH, Javed A, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Mitotic partitioning and selective reorganization of tissue specific transcription factors in progeny cells. Proc Natl Acad Sci, USA. 2003;100:14852–7. doi: 10.1073/pnas.2533076100. [DOI] [PMC free article] [PubMed] [Google Scholar]