Abstract
The protein kinase Mps1 is, among others, essential for the spindle assembly checkpoint (SAC). We found that Saccharomyces cerevisiae Mps1 interacts physically with the N-terminal domain of Ndc80 (Ndc801−257), a constituent of the Ndc80 kinetochore complex. Furthermore, Mps1 effectively phosphorylates Ndc801−257 in vitro and facilitates Ndc80 phosphorylation in vivo. Mutating 14 of the phosphorylation sites to alanine results in compromised checkpoint signalling upon nocodazole treatment of mutants. Mutating the identical sites to aspartate (to simulate constitutive phosphorylation) causes a metaphase arrest with wild-type-like bipolar kinetochore–microtubule attachment. This arrest is due to a constitutively active SAC and consequently the inviable aspartate mutant can be rescued by disrupting SAC signalling. Therefore, we conclude that a putative Mps1-dependent phosphorylation of Ndc80 is important for SAC activation at kinetochores.
Keywords: centromere, kinetochore, Mps1, Ndc80, spindle assembly checkpoint
Introduction
Accurate chromosome segregation requires that sister kinetochores attach to microtubules emanating from opposing poles (bipolar attachment) before sister chromatides separate. This mitotic dependency is supervised by the spindle assembly checkpoint (SAC) in eukaryotic cells (Musacchio and Salmon, 2007). In Saccharomyces cerevisiae, the SAC signal is executed by the SAC components Mad1, Mad2, Mad3, Bub1, Bub3 and Mps1 that localize to kinetochores lacking bipolar attachment (Gillett et al, 2004; Vigneron et al, 2004; Chan et al, 2005) and cooperate in inactivating the anaphase promoting complex (APC) through its regulatory subunit Cdc20 (Musacchio and Salmon, 2007). As APC is required to mark Pds1/Securin, an inhibitor of the cohesion-cutting protease Esp1/Separin, for degradation, an activated SAC prevents sister chromatid separation until all sister kinetochores have obtained a bipolar attachment. The SAC machinery recognizes kinetochores that have not achieved bipolar attachment either because these kinetochores are not attached to microtubules and/or because they lack the appropriate tension. In S. cerevisiae, the Ipl1/Sli15/Bir1 complex has been proposed to serve as the tension sensor (Sandall et al, 2006). Also, Ipl1 has been suggested to translate missing tension into nonattached kinetochores (Biggins and Murray, 2001; Tanaka et al, 2002) possibly by phosphorylating subunits of the Dam1 complex (Cheeseman et al, 2002), which contributes to the kinetochore–microtubule interface in S. cerevisiae. This would make nonattached kinetochores the predominant direct origin of SAC activation.
Mps1 is the only essential SAC protein in S. cerevisiae because Mps1 has additional cellular roles, besides SAC function. In S. cerevisiae, these include spindle pole body duplication (Winey et al, 1991) and the regulation of kinetochore–microtubule interaction (Jones et al, 2005; Shimogawa et al, 2006). SAC function of Mps1 has been demonstrated in S. cerevisiae by mps1 mutant analysis (Weiss and Winey, 1996) and by Mps1 overexpression (Hardwick et al, 1996). The latter induces a constitutive checkpoint arrest that is dependent on the remaining SAC components. Beside its general SAC function, Mps1 facilitates SAC activation as a result of lacking tension at the kinetochores (Maure et al, 2007; Jelluma et al, 2008). In Xenopus, kinetochore localization of Mps1 depends on the Ipl1/AuroraB-Sli15/Survivin-Bir1/INCENP complex and Bub1 (Vigneron et al, 2004). Conversely, the kinetochore localization of Bub1 as well as Mad1 and Mad2 depends on Mps1 in this system, which may indicate that Mps1 is targeting SAC components to kinetochores. However, with the exception of Mad1 (Hardwick et al, 1996), and Borealin in mammals (Jelluma et al, 2008), no substrate of the Mps1 kinase that functions in the context of SAC activation has been described to date.
How kinetochores contribute to SAC activation is poorly understood. The presence of polo kinase-dependent phosphoepitopes (3F3/2 epitopes) at vertebrate kinetochores strongly correlates with the absence of tension (Nicklas et al, 1995; Ahonen et al, 2005; Wong and Fang, 2005). Furthermore, polo kinase targets Mad2 and BubR1 (the vertebrate Mad3 homologue) to Xenopus kinetochores and may contribute to target Mad2 to mammalian kinetochores (Wong and Fang, 2005). However, the kinetochore components that harbour the 3F3/2 epitopes have not been identified to date. Also, the kinetochore proteins that directly contribute to the kinetochore binding of the SAC components have not been elucidated yet. On the other side, it has been shown that SAC function and kinetochore targeting of SAC components is dependent on the Ndc80 complex (Janke et al, 2001; Martin-Lluesma et al, 2002; DeLuca et al, 2003; McCleland et al, 2003; Gillett et al, 2004), a conserved kinetochore component that contributes to the microtubule-binding interface of kinetochores (Cheeseman et al, 2006; DeLuca et al, 2006). In S. cerevisiae, similar to mammalian cells, the subunits Ndc80 and Nuf2 as well as Spc24 and Spc25 form heterodimers, respectively (Ciferri et al, 2005; Wei et al, 2005, 2007). These dimers are held together by coiled-coil interactions and associate with each other tail-to-tail to form a heterotetramer. The Ndc80 complex thus appears as a long rod with two globular domains on each side (one for each subunit). The globular domains of Spc24 and Spc25 are oriented to the inner kinetochore, and the globular domains of Ndc80 and Nuf2 are oriented to the outer kinetochore (Wei et al, 2005). The latter two represent calponin-homology domains (Wei et al, 2007; Ciferri et al, 2008). Basic residues within these domains and possibly within the N-terminal unstructured region of Ndc80 are important for microtubule binding of the Ndc80 complex (Ciferri et al, 2008).
Here, we show that Mps1 phosphorylates Ndc80 in vitro and facilitates Ndc80 phosphorylation in vivo. Mutating sufficient phosphorylation sites to alanine compromises SAC function. Mutating the same sites to aspartate causes a constitutive SAC activation although kinetochore-spindle attachment is stable enough to produce tension at the kinetochores and to support cell viability when SAC signalling is disrupted.
Results
Mps1 physically interacts with the N-terminal domain of Ndc80
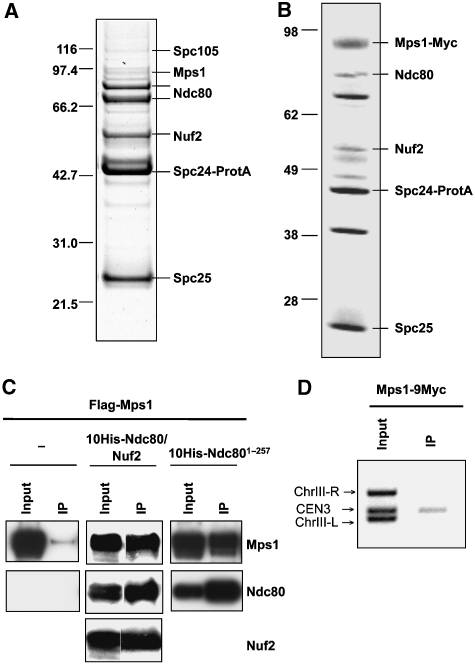
When we isolated the Ndc80 complex using Protein A-tagged Spc24, we found small amounts of co-purifying Mps1 (Figure 1A), and upon Mps1 overexpression, the amount of co-purifying Mps1 markedly increased (Figure 1B). We speculated that Mps1 might interact with Ndc80 complex components that contribute to the microtubule interface and therefore co-expressed a 10His-Ndc80/Nuf2 heterodimer together with Mps1 in Escherichia coli. Affinity purification of 10His-Ndc80 revealed a physical interaction of Mps1 with the 10His-Ndc80/Nuf2 heterodimer and/or 10His-Ndc80 per se (Figure 1C). As mentioned earlier, Ndc80 and Nuf2 consist of C-terminal coiled-coil regions that confer interactions within the Ndc80 complex and of N-terminal globular domains. We speculated that the latter might interact with Mps1. When Mps1 was co-expressed with the His-tagged N-terminal domain of Ndc80 (Ndc801−257) or Nuf2 (Nuf21−219), only interaction of Mps1 with the former was detected (Figure 1C and not shown). Therefore, we conclude that Mps1 interacts specifically with the N-terminal domain of Ndc80. Mps1 localization at metazoan kinetochores has been described (see ‘Introduction'). Also, evidence for the localization at S. cerevisiae kinetochores exists (Castillo et al, 2002; Maure et al, 2007). We confirmed this point by chromatin immunoprecipitation (ChIP) with Mps1-9Myc (Figure 1D). Therefore, Mps1 may localize to the S. cerevisiae kinetochore through the N-terminal domain of Ndc80.
Figure 1.
Mps1 physically interacts with Ndc80 and localizes to the kinetochore. (A) The Ndc80 complex was purified through protein A-tagged Spc24 by affinity chromatography with IgG-sepharose and fractionated by SDS–PAGE. Proteins were identified from Coomassie-stained bands by peptide mass fingerprinting (MALDI-TOF). Bands that are not labelled represent contaminants. (B) As in (A) with the exception that the Ndc80 complex was purified from cells overexpressing Mps1 (strain YSK632). (C) Mps1 and Ndc80 interact in vitro. Flag-Mps1, 10His-Ndc801−257 or 10His-Ndc80 and Nuf2 were co-expressed in E. coli as indicated. 10His-Ndc80 or 10His-Ndc801−257 was affinity purified with NTA-agarose (IP). Input (0.01% of total) and IP (1% of total) were subjected to western analysis with anti-Flag, anti-His and anti-Nuf2 antibodies. (D) ChIP analysis. Mps1-9Myc was immunoprecipitated with anti-Myc antibody. The presence of CEN3 DNA and two flanking DNA regions (ChIII-R and ChIII-L) was analysed by PCR in the input and the immunoprecipitate (IP).
Mps1 phosphorylates Ndc80 in vitro and supports Ndc80 phosphorylation in vivo
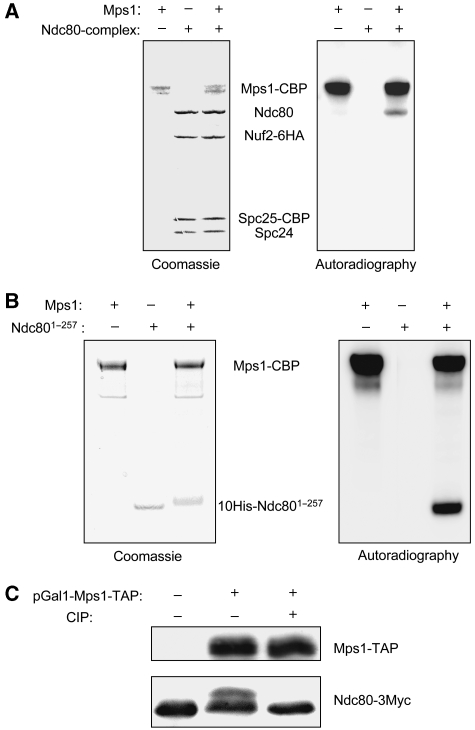
As Mps1 interacts with the Ndc80 complex, we tested whether the Ndc80 complex is a substrate of Mps1. In vitro kinase assays with the purified Ndc80 complex and Mps1 purified from S. cerevisiae revealed strong autophosphorylation of Mps1 and that Mps1 phosphorylates Ndc80 efficiently (Figure 2A). Nuf2, Spc24 and Spc25 phosphorylation was considerably less prominent. Furthermore, we found that Ndc801−257 purified from E. coli serves as a very good Mps1 substrate in the in vitro assay (Figure 2B). This is in agreement with the finding that Mps1 interacts with Ndc801−257 in vitro. As mentioned earlier, Mps1 overexpression results in a constitutive activation of the SAC in S. cerevisiae. Therefore, we asked whether Mps1 overexpression results in hyperphosphorylated Ndc80. Indeed, after Mps1 overexpression, a lower-mobility form of Ndc80 could be detected by SDS–PAGE (Figure 2C). Phosphatase treatment reverted this form's mobility back to the original. Thus, Mps1 overexpression results in Ndc80 hyperphosphorylation.
Figure 2.
Mps1 phosphorylates Ndc80. (A) In vitro kinase assay. The Ndc80 complex and Mps1 were individually purified from S. cerevisiae by tandem affinity purification and incubated with γ-32P-ATP as indicated. Samples were fractionated by SDS–PAGE and visualized by Coomassie staining plus autoradiography as indicated. CBP, calmodulin-binding peptide. (B) As in (A) with the exception that the His-tagged N-terminal domain of Ndc80 (10His-Ndc801−257) purified from E. coli was used instead of the Ndc80 complex. (C) Mps1 facilitates Ndc80 phosphorylation in vivo. Whole cell extracts were subjected to western analysis with peroxidase-anti-peroxidase (to detect Mps1-TAP) and anti-Myc antibody (to detect Ndc80-3Myc). As shown, control cells (YSK658: NDC80-3MYC) or cells overexpressing Mps1-TAP (YSK658 harbouring plasmid pSK950: pGAL1-MPS1-TAP, 2 μ) were analysed. Extracts were treated with 10 U calf intestinal phosphatase (CIP) for 1 h at 30°C as indicated.
Analysis of Ndc80 phosphorylation sites
To identify Mps1-dependent phosphorylation sites, we performed in vitro phosphorylation of purified Ndc801−257, subjected it to in-gel digestion with trypsin and analysed phosphopeptides by LC-ESI-MS/MS (see Materials and methods). This identified at least 25 phosphorylated sites in Ndc801−257 (Table I). To compare in vivo and in vitro Mps1-dependent phosphorylation of Ndc80, we purified the Ndc80 complex from cells that overexpressed Mps1 for 3 h, subjected Ndc80 to in-gel digestion with trypsin and analysed phosphopeptides by LC-ESI-MS/MS. Ten phosphorylated sites were detected in the N-terminal domain of Ndc80 (Table I). All of these sites had been identified by the in vitro approach. In addition, one site at the very C-terminus (T690) was detected. The fact that several sites found in vitro were not detected in vivo might reflect altered accessibility of sites by Mps1 in vivo versus in vitro. Alternatively, some phosphorylation sites were not detectable, because we subjected about five times less in vivo material than in vitro material to mass spectrometry. Two of the phosphorylated residues (S100 and T248) were also detected when Ndc80 from control cells (without Mps1 overexpression) was analysed. This may be the result of endogenous Mps1 activity. For S100, it may be also due to Ipl1 activity, as this residue is part of an Ipl1 consensus site (Cheeseman et al, 2002). All phosphorylated amino acids were either serines or threonines. No phosphorylated tyrosine was detected, although Mps1 has been described as a dual specific kinase (Lauze et al, 1995). In some cases, the MS/MS fragmentation pattern only allowed to attribute a phosphate group to either of two or more neighbouring serines and threonines (Table I). Frequently multiple phosphorylated peptides were observed (Table I), indicating that many of the identified phosphorylated residues occur together on the same Ndc80 molecule. According to the structural data provided recently for the human Ndc80 N-terminal domain (Wei et al, 2007) and for the human Ndc80-Nuf2 heterodimer (Ciferri et al, 2008), the Ndc80 N-terminus consists of an unstructured N-terminal tail followed by a six-helix calponin-homology domain. On the basis of sequence and structure alignments between human and S. cerevisiae Ndc80 (Wei et al, 2007), 18 of the identified phosphorylation sites reside in the unstructured N-terminal tail of S. cerevisiae Ndc80 (amino acids 1–113), one site (S126) corresponds to α helix 1, one (S205) to α helix 5, two to loop 1/2, one to loop 3/4 and two are in the sequence that links α helix 6 with the coiled-coil region (Supplementary Figure S1). With the exception of S205 and to a lesser extent S150 and maybe S4 and T5, the identified sites are not conserved between S. cerevisiae and higher eukaryotes.
Table 1.
Ndc801−257 phosphorylation sites
| Amino acids | Peptides identified | Maximal number of phosphates detected together on the peptide | |
|---|---|---|---|
| In vitro | In vivo | ||
| 1–19 | MQSSTSTDQHVLHHMDPHR | 2 | 2 |
| 20–33 | FTSQIPTATSSQLR | 3 | 1 |
| 36–52 | NSTNQGLTDMINKSIAR | 3 | 2 |
| 53–67 | NTISGTGIPTGGINK | 3 | 2 |
| 73–89 | STVAGGTNGTALALNDKSNSR | 3 | — |
| 94–114 | NSVSRLSINQLGSLQQHLSNR | 3 | 1 |
| 121–137 | DKNFQSAIQEEIYDYLK | 1 | — |
| 141–152 | FDIETNHPISIK | 2 | — |
| 173–181 | LDPGYGFTK | 1 | — |
| 205–217 | SQISAVGGSNWHK | 1 | — |
| 242–257 | SLINQNTQEITILSQP | 2 | 2 |
| Sites were identified by mass spectrometry as described in the text. | |||
| Sites detected in vitro are indicated in bold. | |||
| Sites detected in vitro and in vivo are bold underlined. | |||
| Unresolved which of the neighbouring sites is phosphorylated are indicated in italics. | |||
Mutating Ndc80 phosphorylation sites to alanine results in a compromised SAC
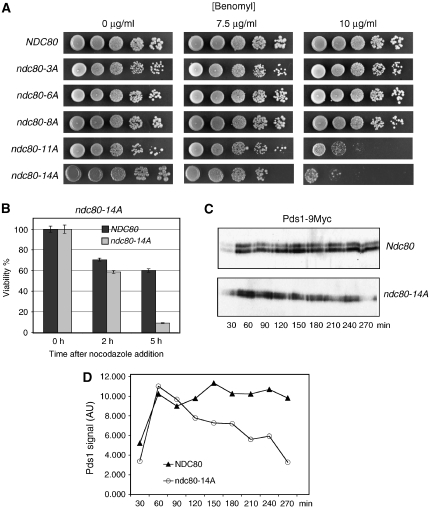
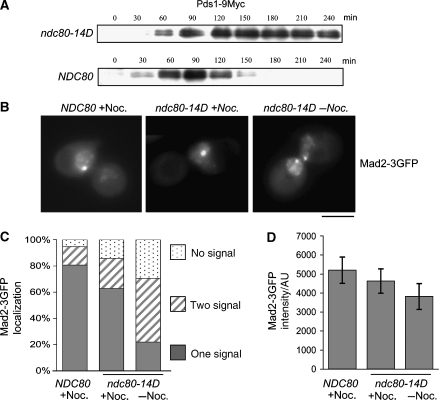
The high number of phosphorylation sites identified made a complete analysis of all possible mutant combinations not feasible. Thus, our initial strategy was to mutate increasing numbers of corresponding serine and threonine residues to alanine and test for benomyl sensitivity as a first indicator for a SAC defect. For technical reasons, we mutated sites in close proximity together. Also, in two cases where it was unclear, which of two neighbouring sites (T21 versus S22 and S37 versus T38) was the phosphorylated one (see above), we mutated both. In the region S2–S6, where two phosphate residues had been identified, we mutated three sites. Of the mutant combinations tested, strains with ⩽8 mutations were not benomyl sensitive (Figure 3A; Table II). However, a strain with 11 mutations (ndc80-11A) exhibited clear benomyl sensitivity (Figure 3A; Table II). Notably, strain ndc80-11A* that also carries 11 mutations but has T145A and S150A instead of T248A and T252A (as in ndc80-11A) was not benomyl sensitive. Thus, the T248A and T252A mutations contributed strongly to the benomyl sensitive phenotype. Mutating 14 phosphorylation sites to alanine (ndc80-14A) also caused benomyl sensitivity (Figure 3A). Furthermore, it severely decreased cell viability when cells were incubated with nocodazole (Figure 3B; Table II), providing further evidence that eliminating sufficient phosphorylation sites can compromise SAC function. To support this, we released α factor arrested wild-type and ndc80-14A cells into nocodazole and analysed Pds1 degradation kinetics. As expected, both strains increased Pds1 levels initially. However, whereas wild-type cells maintain high Pds1 levels due to the APC inactivation by SAC, the Pds1 level in ndc80-14A decreased markedly throughout the nocodazole arrest (Figure 3C and D). This is in agreement with a compromised SAC function in ndc80-14A cells.
Figure 3.
Ndc80 serine and threonine to alanine mutants exhibit a SAC defect. (A) ndc80-11A and ndc80-14A are benomyl sensitive. Serial dilutions of cells with increasing numbers of phosphorylation sites mutated to alanine (see Table II for exact specification of sites mutated) were tested for growth on YPD plates containing the indicated concentration of benomyl. (B) Survival assay. ndc80-14A cells were exposed to nocodazole for the indicated times and then plated onto YPD plates lacking the drug. Viability was calculated by normalizing the number of colonies formed by cells treated with nocodazole to the number of colonies formed by untreated cells (0 h). (C) ndc80-14A fails to maintain long-term Pds1 stability upon nocodazole treatment. α Factor arrested PDS1-9MYC cells were released into medium containing nocodazole. For the indicated time points after the release, Pds1 levels in whole cell extracts were determined by western analysis using anti-Myc antibody. (D) Quantification of (C). AU, arbitrary units.
Table 2.
Ndc80 phosphorylation sites mutated to alanine
| Mutations | Druga sensitive | Mutant |
|---|---|---|
| S205A | − | |
| S205A T248A T252A | − | ndc80-3A |
| S37A T38A T43A S205A T248A T252A | − | ndc80-6A |
| T21A S22A S37A T38A T43A S205A T248A T252A | − | ndc80-8A |
| S4A T5A S6A T21A S22A S37A T38A T43A S205A T248A T252A | + | ndc80-11A |
| S4A T5A S6A T21A S22A S37A T38A T43A T74 T79 T82 S205A T248A T252A | + | ndc80-14A |
| S4A T5A S6A T21A S22A S37A T38A T43A | − | |
| S4A T5A S6A T21A S22A S37A T38A T43A T145A S150A S205A | − | ndc80-11A* |
| T145A S150A S205A | − | |
| T145A S150A S205A T248A T252A T255A | − | |
| aBenomyl or nocodazole. | ||
Mutating Ndc80 phosphorylation sites to aspartate causes constitutive SAC activation
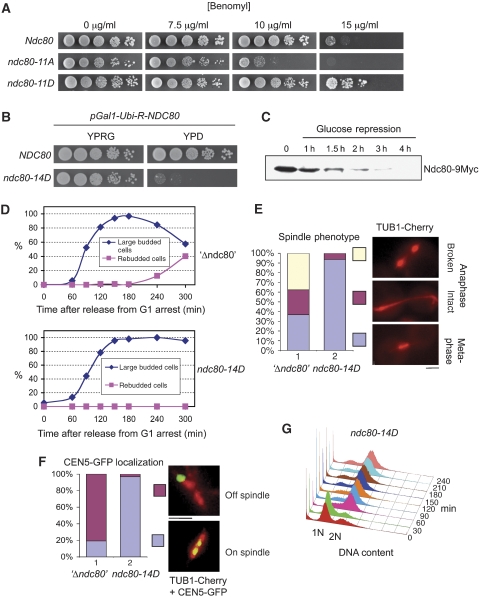
If elimination of phosphorylation sites results in SAC defects, simulating constitutive phosphorylation by serine/threonine to aspartate mutations might cause aberrant SAC activation. Corresponding to ndc80-11A and ndc80-14A, we constructed the aspartate mutants ndc80-11D and ndc80-14D. When tested for growth on benomyl plates, ndc80-11D surprisingly not only lacked the benomyl sensitivity of ndc80-11A but was considerably more resistant to benomyl than wild-type cells (Figure 4A). This indicated that simulating constitutive phosphorylation as in ndc80-11D might enhance SAC activation and thus allow the cells to cope with microtubule stability interferences better than wild-type cells. If the introduction of 11 aspartates enhances SAC activation, then mutating further sites to aspartate might cause permanent SAC activation and consequently cell death. Indeed, ndc80-14D, in contrast to ndc80-14A, was dead (Figure 7A, lane 2). Already the overexpression of wild-type Ndc80 resulted in very sick cells. Therefore, overexpression of Ndc80-14D was not a feasible option to determine the arrest phenotype of ndc80-14D. Instead, we constructed a strain that expressed a destabilized wild-type Ndc80 under the control of the Gal1 promoter in the background of ndc80-14D and, thus, was unable to grow when Ndc80 expression was repressed by glucose (Figure 4B). Furthermore, when cells grown in 0.1% galactose to allow Ndc80 expression just above wild-type levels were shifted to glucose, the amount of Ndc80 in these cells dropped to levels undetectable by western analysis (Figure 4C). When released from an α factor arrest after Ndc80 depletion, the ndc80-14D mutants arrested as large budded cells (Figure 4D) with a 2N DNA content (Figure 4G) short (<2 μm) spindles (Figure 4E) and bipolar attached kinetochores (Figures 4F and 6E). Furthermore, Pds1 levels remained highly stable throughout the ndc80-14D arrest (Figure 5A). Thus, these data are consistent with the idea that ndc80-14D cells arrest in metaphase due to long-lasting SAC activation. In contrast to this, cells depleted of wild-type Ndc80 in the absence of Ndc80-14D, exhibited 2 h after the release from a G1 arrest long, partially broken spindles (Figure 4E) and predominately kinetochores that did not localize to the spindle (Figure 4F). This shows a severe defect in kinetochore–microtubule attachment. Furthermore, these cells accomplished re-budding (Figure 4D) and DNA re-replication to 4N (Supplementary Figure S2) indicative of cells that fail to maintain SAC activation. Thus, the ndc80-14D phenotype is not a consequence of Ndc80 depletion per se. Mad2 localization at kinetochores is a hallmark of cells with an activated SAC (see ‘Introduction'). To confirm that the ndc80-14D mutation results in SAC activation, we therefore investigated Mad2 localization in ndc80-14D cells released from an α factor arrest after Ndc80 depletion and compared the results with Mad2 localization in cells released into medium containing nocodazole. About 145 min after the release, 71% of untreated ndc80-14D cells, 86% of nocodazole treated ndc80-14D cells and 96% of nocodazole treated wild-type cells revealed focal Mad2 signals indicative of kinetochore localization (Figure 5B and C). The remaining cells predominately eluded analysis because of a too high Mad2-3GFP background signal. Notably, 69% of the Mad2 signals in untreated ndc80-14D cells were observed in two lobes most likely reflecting clusters of sister kinetochores in metaphase, whereas 73 or 80% of the Mad2 signals in the nocodazole-treated cells were observed as one cluster reflecting the fact that sister kinetochores remain in close proximity in the absence of microtubules. Furthermore, the average signal intensity of Mad2-3GFP in untreated ndc80-14D cells was similar to that of nocodazole-treated cells (Figure 5D), indicating that the ndc80-14D mutation promotes the efficient binding of Mad2 to the majority of kinetochores within a cell. In summary, we therefore conclude that simulating constitutive Ndc80 phosphorylation, as in ndc80-14D, results in constitutive SAC activation.
Figure 4.
Phenotype of Ndc80 serine and threonine to aspartate mutants. (A) ndc80-11D is hypertolerant to benomyl. Serial dilutions of cells were tested for growth on YPD plates containing the indicated concentrations of benomyl. (B–F) ndc80-14D arrests in metaphase. (B) Serial dilutions of strain YSK1173 (ndc80-14D; PGAL1-Ubi-R-NDC80-9Myc; TUB1-Cherry; CEN5-GFP) and YSK1128 (NDC80; PGAL1-Ubi-R-NDC80-9myc) were tested on YPRG (2% raffinose, 0.1% galactose) or YPD (2% glucose) plates for growth. (C) Ndc80 depletion. Strain YSK1173 was grown to mid-log phase in raffinose medium supplemented with 0.1% galactose. The medium was exchanged for YPD (time point 0), whole cell extracts were derived at the indicated time points and subjected to western analysis with anti-Myc antibody. (D–G) YSK1173 and YSK1155 (PGAL1-Ubi-R-NDC80-9Myc; TUB1-Cherry; CEN5-GFP) (‘Δndc80') were synchronized by α factor treatment in YPD for 3 h and released into YPD medium. (D) Cells with large buds (>2/3 of mother) and cells that exhibited rebudding were counted at the indicated time points (n>100). (E) Spindles were visualized 120 min after the release by fluorescent microscopy (TUB1-Cherry) and quantified as indicated (n>100). Bar: 2 μm. (F) Spindles (TUB1-Cherry) and kinetochores of chromosome V (CEN5-GFP) were visualized 120 min after the release by fluorescent microscopy and quantified as indicated. Bar: 2 μm. (G) The DNA content of YSK1173 was determined by FACS analysis at the indicated time points after the release. α Factor was re-added to the cultures 1 h after the release to arrest cells that executed mitosis in G1 of the next cell cycle. For FACS, data concerning YSK1173 and YSK1155 in the absence of α factor see Supplementary Figure S2.
Figure 5.
SAC activation causes the ndc80-14D arrest in metaphase. (A–D) Cells were synchronized by α factor treatment and depleted of Ndc80 as in Figure 4D–F. (A) Pds1 levels. At the indicated time points after the release, whole cell extracts of strains YSK1173 (ndc80-14D; PGAL1-Ubi-R-NDC80; PDS1-9MYC) and YSK1128 (NDC80; PGAL1-Ubi-R-NDC80; PDS1-9MYC) were obtained. Pds1 levels were determined by western analysis using anti-Myc antibody. (B) Mad2 localization. Strains YSK1226 (ndc80-14D; PGAL1-Ubi-R-NDC80; MAD2-3GFP) and YMS739 (NDC80; MAD2-3GFP) were analysed by fluorescence microscopy 145 min after the release into YPD with or without nocodazole (Noc.) as indicated. Bar: 5 μm. (C) Quantification of Mad2-3GFP localization. n>100 for each sample. (D) Quantification of Mad2-3GFP signal intensity. The data represent the intensity average of 60 cells (for each sample). When two signals per cell were detected, the values were combined. Error bars represent the standard deviation of the measurements. AU, arbitrary units.
ndc80-14D kinetochores support stable bipolar microtubule interaction
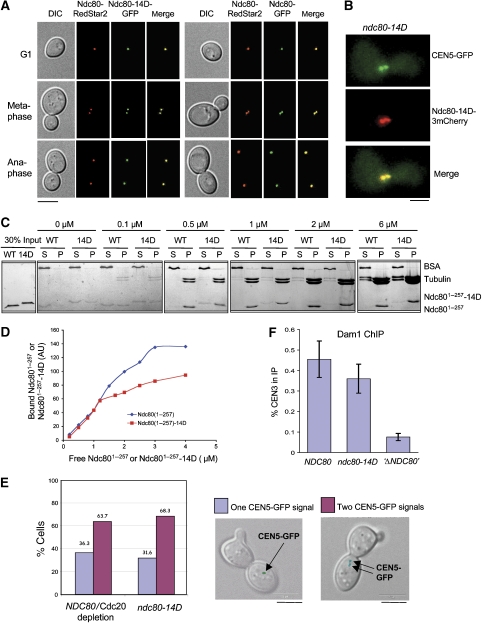
SAC activation can be a result of defect kinetochores if the defect interferes with kinetochore–microtubule interaction but still allows kinetochore-dependent SAC signalling. As positively charged amino acids (lysines) residing in the N-terminal domains of human Ndc80 and Nuf2 are important for in vitro interaction between the human Ndc80 complex and microtubules (Ciferri et al, 2008), the introduction of 14 negative charges might severely affect kinetochore–microtubule interaction in ndc80-14D cells. Therefore, we tested the functionality of Ndc80-14D. Cellular levels of Ndc80-14D were very similar to wild-type Ndc80 levels (not shown), demonstrating that the mutations did not produce an unstable protein. Furthermore, Ndc80-14D clearly is assembled into kinetochores in the presence (Figure 6A) or absence (Figure 6B) of wild-type Ndc80. Next, we analysed in vitro microtubule binding of purified Ndc801−257 and Ndc801−257-14D. As shown in Figure 6C and D, at high tubulin to Ndc801−257 ratios, the N-terminal domains of Ndc80 and Ndc801−257-14D interact with microtubules in vitro (even in the absence of Nuf2) with similar efficiency. When approaching saturation (at low tubulin to Ndc801−257 ratios), the binding of Ndc801−257-14D was about 1.5-fold less efficient than the wild type (Figure 6D). The in vitro interaction assay predominantly monitors lateral microtubule attachment. In vivo, however, kinetochores finally achieve a bipolar end on attachment that has to withstand the force generated under this condition. As this force distances sister kinetochores from one another (Goshima and Yanagida, 2000) the separation of sister kinetochores at metaphase is a readout for the stability of kinetochore–microtubule attachment in vivo. Sister kinetochore separation and rejoining is a dynamic process (kinetochore breathing). At a given time point about 2/3 of the sister kinetochores appear separated when wild-type cells are arrested in metaphase by depletion of the APC activator Cdc20 (Figure 6E). As mentioned earlier, exposing the ndc80-14D mutation also results in a metaphase arrest. When kinetochore breathing was analysed in these cells, the percentage of separated sister kinetochores was indistinguishable from that in wild-type cells arrested by Cdc20 depletion (Figure 6E). Thus, the interaction of ndc80-14D kinetochores and microtubules is stable enough to allow wild-type-like bipolar attachment. To support this, we investigated the kinetochore localization of Dam1, a component of the Dam1 complex, that is dependent on kinetochore–microtubule interaction and thus on a functional Ndc80 complex (Janke et al, 2002; Li et al, 2002) by ChIP. Whereas Ndc80 depletion per se resulted in a severe Dam1 localization defect no such effect was observed for ndc80-14D within the error range of the experiment (Figure 6F). In summary, we therefore conclude that ndc80-14D kinetochores maintain an active SAC even when they have achieved bipolar attachment, the cellular prerequisite for SAC inactivation.
Figure 6.
ndc80-14D kinetochores are functional. (A, B) Kinetochore localization of Ndc80-14D. (A) Strain YSK1106 (NDC80-RedStar2; NDC80-GFP) and strain YSK1124 (NDC80-RedStar2; ndc80-14D-GFP) were grown to mid-log phase and analysed by fluorescence microscopy. Bar: 5 μm. (B) Strain YMK1319 (ndc80-14D-Cherry; PGAL1-Ubi-R-NDC80-9Myc; , TetO∷CEN5, TetR-GFP) was depleted of Ndc80 and synchronized by α factor treatment in YPD for 3 h. Cells were analysed 120 min after the release into YPD by fluorescence microscopy. CEN5-GFP marks kinetochore localization. Bar: 2 μm. (C, D) Ndc801−257-14D binds to microtubules in vitro. (C) Taxol-stabilized microtubules at the indicated tubulin dimer concentrations were incubated with purified 1 μM His10-Ndc801−257 (WT) and His10-Ndc801−257-14D (14D) and subsequently centrifuged. About 30% of the pellet (P) and 10% of the supernatant (S) were analysed by SDS–PAGE and Coomassie staining. BSA served as a negative-binding control. (D) His10-Ndc801−257 and His10-Ndc801−257-14D at the indicated concentrations were incubated with or without taxol-stabilized microtubules at 1 μM Tubulin dimer concentration. After centrifugation, 30% of the pellets were subjected to SDS–PAGE plus Coomassie staining and the His10-Ndc801−257 or His10-Ndc801−257-14D bands were quantified. The values for bound His10-Ndc801−257 or His10-Ndc801−257-14D shown in the graph represent the pelleted amount in the presence of microtubules minus the pelleted amount in the absence of microtubules. AU, arbitrary units. (E) ndc80-14D cells exhibit wild-type-like bipolar attachment. Strains YMS412 (NDC80, pGAL-CDC20, TetO∷CEN5, TetR-GFP) and YSK1173 (ndc80-14D, pGAL-Ubi-R-NDC80, TetO∷CEN5, TetR-GFP) were synchronized as in (B). CEN5-GFP was visualized by fluorescence microscopy 120 min after the release. The percentage of cells with one or two CEN5-GFP signals (as shown by DIC/GFP overlays) was quantified (n>100). Bar: 5 μm. (F) Dam1 localization at ndc80-14D kinetochores. Strain YSK1170 (ndc80-14D; PGAL1-Ubi-R-NDC80-9myc), strain YMS412 (NDC80, PGAL1-CDC20) and strain YSK1155 (PGAL1-Ubi-R-NDC80-9Myc) (‘Δndc80') were synchronized by α factor treatment in YPD for 3 h and released into YPD. Cells were subjected to ChIP analysis with anti-Dam1 antibody 120 min after the release. The percentage of CEN3 DNA recovered in the IP is shown in respect to the input. Error bars represent the standard deviation of two independent experiments. Note that under these conditions, YMS412 arrests in metaphase (due to Cdc20 depletion) and, thus, provides the appropriate control for YSK1170 that arrests in metaphase due to the ndc80-14D mutation.
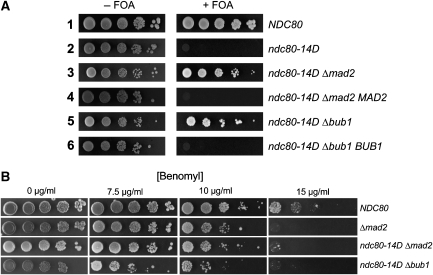
Elimination of SAC signalling rescues the ndc80-14D defect
If simulating constitutive phosphorylation of the Ndc80 N-terminal domain maintains SAC activation, irrespective of the fact that mitotic events have progressed to a state that permits and requires SAC inactivation, then the ndc80-14D mutant would be inviable as a result of aberrant SAC activation and not because of kinetochore defects that affect kinetochore–spindle interaction. To test this hypothesis, we eliminated SAC signalling in the ndc80-14D mutant by disrupting MAD2 or BUB1 and tested for cell growth. As mentioned earlier, the ndc80-14D mutant is inviable (as revealed by counter selecting against a plasmid-based wild-type copy of NDC80 with FOA). In contrast to this, Δmad2, ndc80-14D or Δbub1, ndc80-14D double mutants were viable (Figure 7A) and conversely, re-introduction of either MAD2 or BUB1 into the respective double mutant resulted in nonviable cells. For unknown reasons, the double mutants grew slower than Δmad2 and Δbub1 cells in liquid media (doubling time of 115 versus 90 min at logarithmic growth). Importantly, however, the loss rate of an artificial chromosomal fragment (see Materials and methods) in the Δbub1, ndc80-14D double mutant (7.2 × 10−3 per cell division) and in Δbub1 cells (9.1 × 10−3 per cell division) was very similar. Furthermore, double mutants and Δmad2 cells exhibited the same sensitivity towards benomyl (Figure 7B). Thus, ndc80-14D kinetochores function reliably once SAC signalling is eliminated. To our knowledge, there is currently no case known where a lethal kinetochore mutation can be rescued by eliminating SAC. In the contrary, disrupting SAC components in viable kinetochore mutants is frequently synthetic lethal. Therefore, we conclude that disruption of MAD2 or BUB1 in the ndc80-14D mutant allows the cells to circumvent an aberrant SAC induced cell-cycle arrest and let them perform sufficiently reliable chromosome segregation. Taken together, our data suggest that phosphorylation of the Ndc80 N-terminal domain, putatively dependent on Mps1, is required and sufficient to promote SAC activation.
Figure 7.
Elimination of SAC signalling rescues ndc80-14D cells. (A) Deletion of MAD2 or BUB1 rescues ndc80-14D mutants. All strains harboured an episomal copy of NDC80 on a CEN/URA3 plasmid. Δmad2 MAD2 or Δbub1 BUB1 cells harboured the wild-type gene copy on a CEN/LEU2 plasmid. All other strains harboured an empty CEN/LEU2 plasmid. Serial dilutions of cells were tested for growth on plates lacking leucine in the absence or presence of FOA. FOA counter selects for the plasmid-based copy of NDC80. (B) Serial dilutions of cells with the specified genotype were tested for growth on YPD plates containing the indicated concentrations of benomyl.
Role of Ipl1 phosphorylation sites
Ipl1-dependent phosphorylation of kinetochore proteins is considered to weaken nonbipolar kinetochore–microtubule interactions (Biggins and Murray, 2001; Tanaka et al, 2002). Also, phosphorylation by Aurora B kinase/Ipl1 has been reported to weaken the interaction between human (Ciferri et al, 2008) or Caenorhabditis elegans (Cheeseman et al, 2006) Ndc80 complexes and microtubules in vitro. Therefore, we wondered how mimicking phosphorylation at Ipl1 sites would correlate/contribute to the ndc80-14D phenotype. Cells that had only the three Ipl1 sites of Ndc80 (T54, T74, S100) mutated to aspartate (ndc80-3D, Supplementary Table SI) grew like wild-type cells indicating that Ipl1 phosphorylation is not sufficient to produce the ndc80-14D effect. Changing the one mutated Ipl1 site in ndc80-14D (T74D) back to wild type produced inviable cells (ndc80-13D, Supplementary Table SI) that could be rescued by MAD2 deletion (not shown). Thus, they behaved like ndc80-14D in this respect, indicating that mimicking phosphorylation at Ipl1 sites is not essential for the ndc80-14D phenotype. Finally, ndc80-14D** (Supplementary Table SI) a strain that is identical to ndc80-14D with the exception that it carries T54D plus S100D instead of T79D plus T82D (and thus has all three Ipl1 sites mutated) was inviable but could be rescued by MAD2 depletion (not shown), indicating that Ipl1 and Mps1 phosphorylation of Ndc80 might function additively in SAC activation.
All aspartate mutants analysed (Supplementary Table SI) harbouring a subset of the ndc80-13D mutations were viable. Thus, it appears that introducing a certain quantity of negative charge is an essential factor in establishing the ndc80-14D phenotype. On the other hand, some mutations appear to contribute more than others to the phenotype. ndc80-14D* (Supplementary Table SI) that carries 14 aspartate mutations, like ndc80-14D, but harbours mutations at positions 54, 56 and 58 instead of mutations at positions 74, 79 and 82 was viable.
Mps1 function is also required downstream of Ndc80 phosphorylation
Mps1 overexpression results in Ndc80 hyperphosphorylation and in constitutive SAC activation. If this is the sole function of Mps1 in SAC signalling, SAC activation should occur independent of Mps1 in the ndc80-14D mutant. To test this possibility, we constructed an ndc80-14D strain that allowed simultaneous depletion of a wild-type Ndc80 as well as Mps1. Mps1 is required for spindle pole body assembly as well as SAC function. In order not to interfere with spindle pole body duplication, we first arrested the cells by hydroxy urea (when spindle pole body duplication is completed) before we depleted Ndc80 and Mps1. Then we released them from the arrest into nocodazole media. Nocodazole treatment produces a SAC- dependent cell-cycle arrest if Mps1 is present. Consistent with Mps1's essential SAC function, Mps1-depleted NDC80 cells did not arrest, as revealed by DNA re-replication to 4N and re-budding (Supplementary Figure S3, not shown). Mps1-depleted ndc80-14D cells also re-replicated their DNA and re-budded, indistinguishable from wild-type cells. Thus, the SAC defect caused by the absence of Mps1 cannot be compensated by an ndc80-14D kinetochore. Consequently, Mps1 function is also required downstream of Ndc80 phosphorylation for SAC signalling.
Discussion
Mps1-dependent phosphorylation of Ndc80
We found that Mps1 phosphorylates Ndc80 in vitro and promotes Ndc80 phosphorylation in vivo. Upon in vitro phosphorylation, 25 phosphorylated serine/threonine residues, that is about half the available sites, were identified within the Ndc80 N-terminal domain (Ndc801−257). Similar to published Mps1 phosphorylation data (for example, Shimogawa et al, 2006; Jelluma et al, 2008), no Mps1 consensus site could be deduced from the Ndc80 phosphorylation sites. This indicates that the active centre of Mps1 accepts a broad variety of amino-acid sequences. On the other side, Mps1 phosphorylated Ndc80 preferentially over Nuf2, Spc24 and Spc25, when the Ndc80 complex was provided as a substrate. Mps1 also interacts with Ndc801−257 in vitro. Although it is currently unclear which Mps1 region is involved, the interaction of Ndc80 with Mps1 sequences other than the active centre can be one explanation for the observed substrate selectivity. A second explanation may be related to the fact that 18 of the identified phosphorylation sites reside in the unstructured N-terminal tail of S. cerevisiae Ndc80 (Supplementary Figure SI). Nuf2, Spc24 and Spc25 lack long unstructured terminal tails and, therefore, may be weaker Mps1 substrates.
Ndc80 phosphorylation and SAC activation
Several lines of evidence support that Mps1-dependent phosphorylation of Ndc80 is an integral step in SAC activation. (1) Changing 11 or 14 Ndc80 phosphorylation sites to alanine as in ndc80-11A or ndc80-14A resulted in sensitivity to microtubule destabilizing drugs. Furthermore, nocodazole treated ndc80-14A failed to maintain high Pds1 levels over the period of time that nocodazole treated wild-type cells do. These data are in agreement with the concept that interfering with Ndc80 phosphorylation at the targeted sites compromises SAC function. Although the Pds1 degradation kinetics of nocodazole treated ndc80-14A cells does not resemble that of wild-type cells, it is also not equivalent to that of Δmad2 mutants where SAC is completely abolished (unpublished data), indicating that ndc80-14A has residual SAC activity. However, only about half of the identified Mps1 phosphorylation sites are eliminated in this strain and many possible mutant variations have not been analysed. Particularly, mutating more than 14 phosphorylation sites to alanine might exhibit a stronger phenotype than ndc80-11A and ndc80-14A. Furthermore, Mps1-dependent phosphorylation of kinetochore proteins in the vicinity of Ndc80 (such as Nuf2) might contribute to SAC activation. (2) Changing the sites mutated in ndc80-11A to aspartate produced cells (ndc80-11D) that exhibit higher benomyl tolerance than wild-type cells. This is in agreement with the idea that ndc80-11D is better fitted to cope with a microtubule poison due to a particularly robust SAC activation. (3) ndc80-14D, in contrast to the equivalent alanine mutant ndc80-14A is inviable. Upon depletion of a background wild-type copy, ndc80-14D arrests as large budded cells with a short metaphase spindle and a 2N DNA content. (4) ndc80-14D kinetochores achieve wild-type-like bipolar attachments. Despite this, ndc80-14D cells maintain high Pds1 levels and exhibit significant amounts of Mad2 at the two clusters of kinetochores that are the result of bipolar kinetochore attachment. This is reminiscent of the Bub3 kinetochore localization in cells that overexpress Mps1 (Kerscher et al, 2003). (5) Most importantly ndc80-14D cells can be rescued by disruption of SAC signalling. This is in agreement with constitutive SAC activation in ndc80-14D and may reflect cell lethality and rescue respectively upon Mps1 overexpression in wild-type and Δmad2 strains (Hardwick et al, 1996).
Kinetochore function in ndc80-14D cells
Mps1 is known to regulate kinetochore bi-orientation (Maure et al, 2007) and kinetochore positioning to microtubule plus ends through Dam1 phosphorylation (Shimogawa et al, 2006). Furthermore, as mentioned earlier, the introduction of negative charge into the Ndc80 N-terminal domain as in ndc80-14D might disrupt kinetochore–microtubule attachment, and this would consequently activate SAC per se.
Several lines of evidence support that ndc80-14D kinetochores are functional and in particular that kinetochore–microtubule interaction is not severely affected. (1) Ndc801−257-14D binds to microtubules in vitro. In comparison to wild-type Ndc801−257, the binding efficiency only decreased (by a factor of 1.5) when the Ndc801−257-14D concentration approached the tubulin dimer concentration in the assay. The reason for this is unclear, but it is questionable whether this reflects a physiological situation. (2) In vivo, ndc80-14D cells arrest with bipolar attached kinetochores experiencing tension indistinguishable from wild-type cells that have been arrested in metaphase by APC inactivation. (3) Dam1, a subunit of the Dam1 complex localizes efficiently to ndc80-14D kinetochores. This indicates proficient kinetochore–microtubule interaction as kinetochore localization of the Dam1 complex depends on Ndc80 and on kinetochore–microtubule interaction. (4) The kinetochore–microtubule attachment in ndc80-14D cells suffices to support cell viability and reliable chromosome segregation when SAC signalling is disrupted.
The apparent discrepancy that the in vitro interaction between the Ndc80 complex and microtubules is affected by altering the charge or Ipl1-dependent phosphorylation state of the metazoan Ndc80 and Nuf2 N-terminal domains (see above), whereas Ndc80-14D exhibits no strong interaction defect and performs satisfactory in vivo could be explained in several ways. (1) Phosphorylating Ndc80 (by Ipl1) may not be the principal step to regulate kinetochore–microtubule interaction in S. cerevisiae. Instead, phosphorylating components of the Dam1 complex, that has no counterpart in higher eukaryotes, may fulfill this function in S. cerevisiae (Cheeseman et al, 2002). Consistent with this assumption is that mimicking phosphorylation at the three Ipl1 phosphorylation sites of Ndc80 has no affect on cell growth in S. cerevisiae. (2) Phosphorylation of Ndc80 might resolve syntelic or merotelic kinetochore–microtubules attachments but allow bipolar attachments when kinetochore–microtubule interaction is sustained by the applied tension. (3) A binding defect observed in vitro might be attenuated in vivo through the action of unaltered Nuf2. Interestingly, yeast cells that solely express a truncated Ndc80 that lacks amino acids 1–116 grow like wild-type cells (Supplementary Figure S4), although the N-terminal tail of Ndc80, as shown for the human homologue (Wei et al, 2007), is important for microtubule binding in vitro. Alternatively, the principals postulated for the interaction of the metazoan Ndc80/Nuf2 heterodimer with microtubules might be altered in S. cerevisiae.
Models for the role of Mps1-dependent phosphorylation of Ndc80 in SAC activation
Taken together, our data indicate that the requirements for SAC inactivation have been satisfied in metaphase arrested ndc80-14D cells but despite this, ndc80-14D kinetochores create a strong SAC signal. Therefore, we postulate that Mps1-dependent phosphorylation of Ndc80 is important for SAC activation possibly by introducing negative charge into the N-terminal domain and removal of these phosphate residues is required to inactivate SAC. There are several possibilities (that are not exclusive) how this might be orchestrated (Supplementary Figure S5). Mps1 might recognize Ndc80 only at kinetochores lacking bipolar attachment. Once Mps1 has left the bipolar attached kinetochore, a constitutively active phosphatase would dephosphorylate Ndc80 (Supplementary Figure S5A). This may be in agreement with the observed kinetochore localization of Mps1. Conversely, Mps1 might constitutively phosphorylate free or kinetochore Ndc80 but only Ndc80 at a bipolar attached kinetochore would be recognized by a phosphatase counteracting Mps1 activity (Supplementary Figure S5B). Finally, Ndc80 might predominantly get phosphorylated off the kinetochore and dephosphorylated at the kinetochore. A rapid exchange of Ndc80 at the kinetochore before metaphase might guarantee that there is sufficient phosphorylated Ndc80 at the kinetochore to activate the SAC. Upon bipolar attachment, the exchange rate of kinetochore proteins including Ndc80 significantly drops (Joglekar et al, 2006), which would lead to predominantly dephosphorylated Ndc80 at the kinetochore (Supplementary Figure S5C). Furthermore, Mps1 is inactivated by APC in anaphase (Palframan et al, 2006).
ndc80-14D kinetochores do not promote SAC activation in the absence of Mps1. This might raise the question whether Mps1's role in SAC signalling involves Ndc80 phosphorylation. On the other hand, it is quite possible that Mps1 performs at least one more signalling step downstream of Ndc80 phosphorylation. Hyperphosphorylation of Mad1 has been described upon Mps1 overexpression (Hardwick et al, 1996) and thus could be this step. Having SAC functions downstream of Ndc80 phosphorylation might also be the reason why Mps1 overexpression still leads to SAC activation in ndc10-1 cells (Fraschini et al, 2001) that presumably lack any kinetochores. Alternatively, Mps1 overexpression might restore SAC signalling from few partially functional kinetochores left in these cells.
The observation that the presence of specific phosphoepitopes (3F3/2) correlates with the absence of tension at the kinetochore exists for quite a time (Nicklas et al, 1995). Recently, it was reported that polo kinase is responsible for these phosphorylation events in vertebrate cells (Ahonen et al, 2005). In S. cerevisiae, the existence of kinetochore-based 3F3/2 epitopes has not been described. Also, no role in SAC activation is currently known for polo kinase in S. cerevisiae. In agreement with this, the Ndc80 phosphorylation sites identified here do not fit the 3F3/2 or polo kinase motif (Nakajima et al, 2003). It will be of interest whether Mps1-dependent phosphorylation of Ndc80 occurs in vertebrates. Otherwise, it might represent an alternative strategy of S. cerevisiae to label nonbipolar attached kinetochores by phosphate groups equivalent to the establishment of 3F3/2 phosphoepitopes or the Aurora B/Ipl1 phosphorylation events in higher eukaryotes.
Materials and methods
Mps1 kinase assay
Recombinant 10His-Ndc801−257 (about 1.25 pmol) or tandem affinity purified Ndc80 complex (about 1.9 pmol Ndc80) were incubated with tandem affinity purified Mps1 (1.2 pmol), 10 μCi [γ-32P]-ATP (specific activity: 3000 Ci/mmol) and 10 μM cold ATP in 80 μl kinase assay buffer (50 mM Tris–HCl 7.5, 10 mM MgCl2, 100 mM NaCl, 0.5 mM DTT, 1 mM PMSF) at 30°C for 60 min. Reactions were terminated by the addition of 10% TCA (final concentration) on ice and the precipitate was subjected to SDS–PAGE. The incorporation of 32P was visualized using a phosphorimager and the QuantityOne software from Biorad. For the identification of phosphorylation sites by mass spectrometry, about 25 pmol 10His-Ndc801−257 was incubated with 1.2 pmol Mps1 in kinase assay buffer containing 10 mM ATP at 30°C for 2 h.
Supplementary Material
Supplementary Figures S1
Supplementary Figures S2
Supplementary Figures S3
Supplementary Figures S4
Supplementary Figures S5
Supplementary Table S1
Supplementary Table S2
Supplementary Information
Acknowledgments
We thank Eileen Dietzel, Petra Ihrig and Jürgen Reichert for excellent technical assistance, Carsten Jäger for work regarding Mps1 and Ndc80 complex co-purification and Sue Biggins for providing plasmids. This work was supported by a grant from the Deutsche Forschungsgemeinschaft.
References
- Ahonen LJ, Kallio MJ, Daum JR, Bolton M, Manke IA, Yaffe MB, Stukenberg PT, Gorbsky GJ (2005) Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr Biol 15: 1078–1089 [DOI] [PubMed] [Google Scholar]
- Biggins S, Murray AW (2001) The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev 15: 3118–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo AR, Meehl JB, Morgan G, Schutz-Geschwender A, Winey M (2002) The yeast protein kinase Mps1p is required for assembly of the integral spindle pole body component Spc42p. J Cell Biol 156: 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan GK, Liu ST, Yen TJ (2005) Kinetochore structure and function. Trends Cell Biol 15: 589–598 [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR III, Chan CS, Drubin DG, Barnes G (2002) Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111: 163–172 [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A (2006) The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127: 983–997 [DOI] [PubMed] [Google Scholar]
- Ciferri C, De Luca J, Monzani S, Ferrari KJ, Ristic D, Wyman C, Stark H, Kilmartin J, Salmon ED, Musacchio A (2005) Architecture of the human ndc80-hec1 complex, a critical constituent of the outer kinetochore. J Biol Chem 280: 29088–29095 [DOI] [PubMed] [Google Scholar]
- Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, Salek M, Rappsilber J, Moores CA, Salmon ED, Musacchio A (2008) Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 133: 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED (2006) Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127: 969–982 [DOI] [PubMed] [Google Scholar]
- DeLuca JG, Howell BJ, Canman JC, Hickey JM, Fang G, Salmon ED (2003) Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr Biol 13: 2103–2109 [DOI] [PubMed] [Google Scholar]
- Fraschini R, Beretta A, Lucchini G, Piatti S (2001) Role of the kinetochore protein Ndc10 in mitotic checkpoint activation in Saccharomyces cerevisiae. Mol Genet Genomics 266: 115–125 [DOI] [PubMed] [Google Scholar]
- Gillett ES, Espelin CW, Sorger PK (2004) Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast. J Cell Biol 164: 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Yanagida M (2000) Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell 100: 619–633 [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW (1996) Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science 273: 953–956 [DOI] [PubMed] [Google Scholar]
- Janke C, Ortiz J, Lechner J, Shevchenko A, Magiera MM, Schramm C, Schiebel E (2001) The budding yeast proteins Spc24p and Spc25p interact with Ndc80p and Nuf2p at the kinetochore and are important for kinetochore clustering and checkpoint control. EMBO J 20: 777–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Ortiz J, Tanaka TU, Lechner J, Schiebel E (2002) Four new subunits of the Dam1–Duo1 complex reveal novel functions in sister kinetochore biorientation. EMBO J 21: 181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelluma N, Brenkman AB, van den Broek NJ, Cruijsen CW, van Osch MH, Lens SM, Medema RH, Kops GJ (2008) Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell 132: 233–246 [DOI] [PubMed] [Google Scholar]
- Joglekar AP, Bouck DC, Molk JN, Bloom KS, Salmon ED (2006) Molecular architecture of a kinetochore-microtubule attachment site. Nat Cell Biol 8: 581–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MH, Huneycutt BJ, Pearson CG, Zhang C, Morgan G, Shokat K, Bloom K, Winey M (2005) Chemical genetics reveals a role for Mps1 kinase in kinetochore attachment during mitosis. Curr Biol 15: 160–165 [DOI] [PubMed] [Google Scholar]
- Kerscher O, Crotti LB, Basrai MA (2003) Recognizing chromosomes in trouble: association of the spindle checkpoint protein Bub3p with altered kinetochores and a unique defective centromere. Mol Cell Biol 23: 6406–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauze E, Stoelcker B, Luca FC, Weiss E, Schutz AR, Winey M (1995) Yeast spindle pole body duplication gene MPS1 encodes an essential dual specificity protein kinase. EMBO J 14: 1655–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bachant J, Alcasabas AA, Wang Y, Qin J, Elledge SJ (2002) The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Genes Dev 16: 183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Lluesma S, Stucke VM, Nigg EA (2002) Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 297: 2267–2270 [DOI] [PubMed] [Google Scholar]
- Maure JF, Kitamura E, Tanaka TU (2007) Mps1 kinase promotes sister-kinetochore bi-orientation by a tension-dependent mechanism. Curr Biol 17: 2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleland ML, Gardner RD, Kallio MJ, Daum JR, Gorbsky GJ, Burke DJ, Stukenberg PT (2003) The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev 17: 101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED (2007) The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 8: 379–393 [DOI] [PubMed] [Google Scholar]
- Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E (2003) Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J Biol Chem 278: 25277–25280 [DOI] [PubMed] [Google Scholar]
- Nicklas RB, Ward SC, Gorbsky GJ (1995) Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J Cell Biol 130: 929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palframan WJ, Meehl JB, Jaspersen SL, Winey M, Murray AW (2006) Anaphase inactivation of the spindle checkpoint. Science 313: 680–684 [DOI] [PubMed] [Google Scholar]
- Sandall S, Severin F, McLeod IX, Yates JR III, Oegema K, Hyman A, Desai A (2006) A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell 127: 1179–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawa MM, Graczyk B, Gardner MK, Francis SE, White EA, Ess M, Molk JN, Ruse C, Niessen S, Yates JR III, Muller EG, Bloom K, Odde DJ, Davis TN (2006) Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase. Curr Biol 16: 1489–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K (2002) Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108: 317–329 [DOI] [PubMed] [Google Scholar]
- Vigneron S, Prieto S, Bernis C, Labbe JC, Castro A, Lorca T (2004) Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol Biol Cell 15: 4584–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei RR, Al-Bassam J, Harrison SC (2007) The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat Struct Mol Biol 14: 54–59 [DOI] [PubMed] [Google Scholar]
- Wei RR, Sorger PK, Harrison SC (2005) Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc Natl Acad Sci USA 102: 5363–5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E, Winey M (1996) The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol 132: 111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Goetsch L, Baum P, Byers B (1991) MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J Cell Biol 114: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong OK, Fang G (2005) Plx1 is the 3F3/2 kinase responsible for targeting spindle checkpoint proteins to kinetochores. J Cell Biol 170: 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1
Supplementary Figures S2
Supplementary Figures S3
Supplementary Figures S4
Supplementary Figures S5
Supplementary Table S1
Supplementary Table S2
Supplementary Information