Abstract
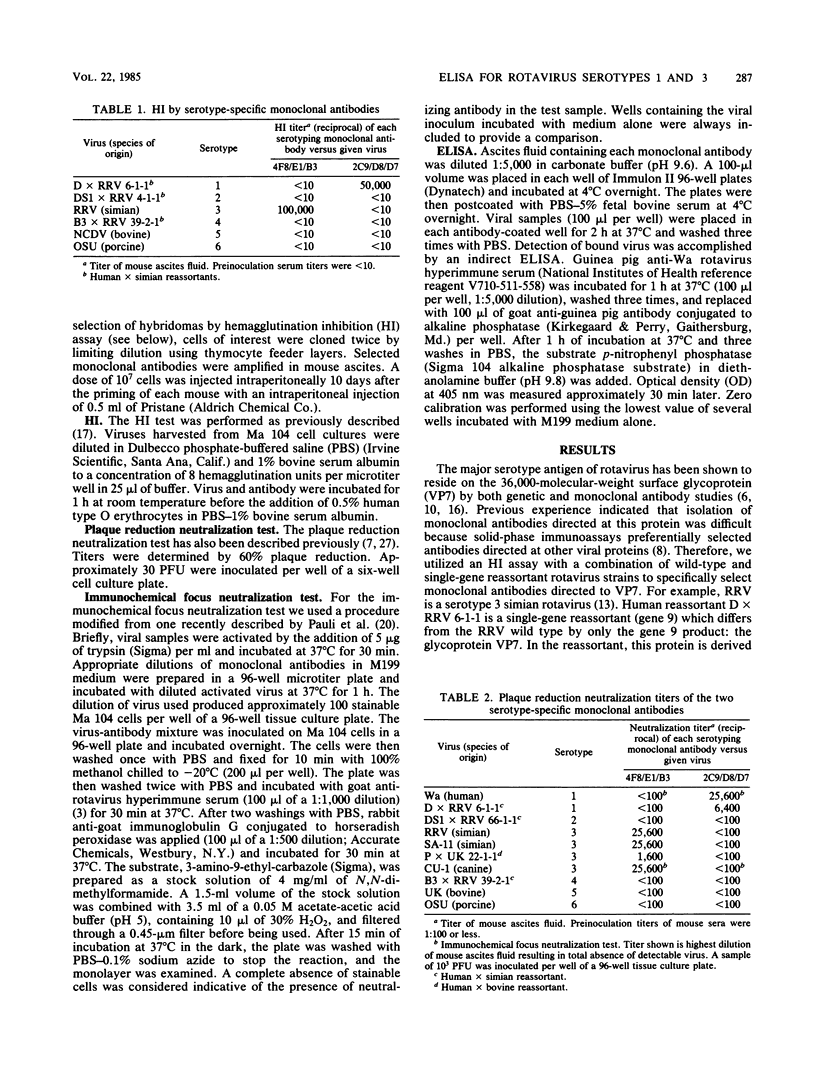
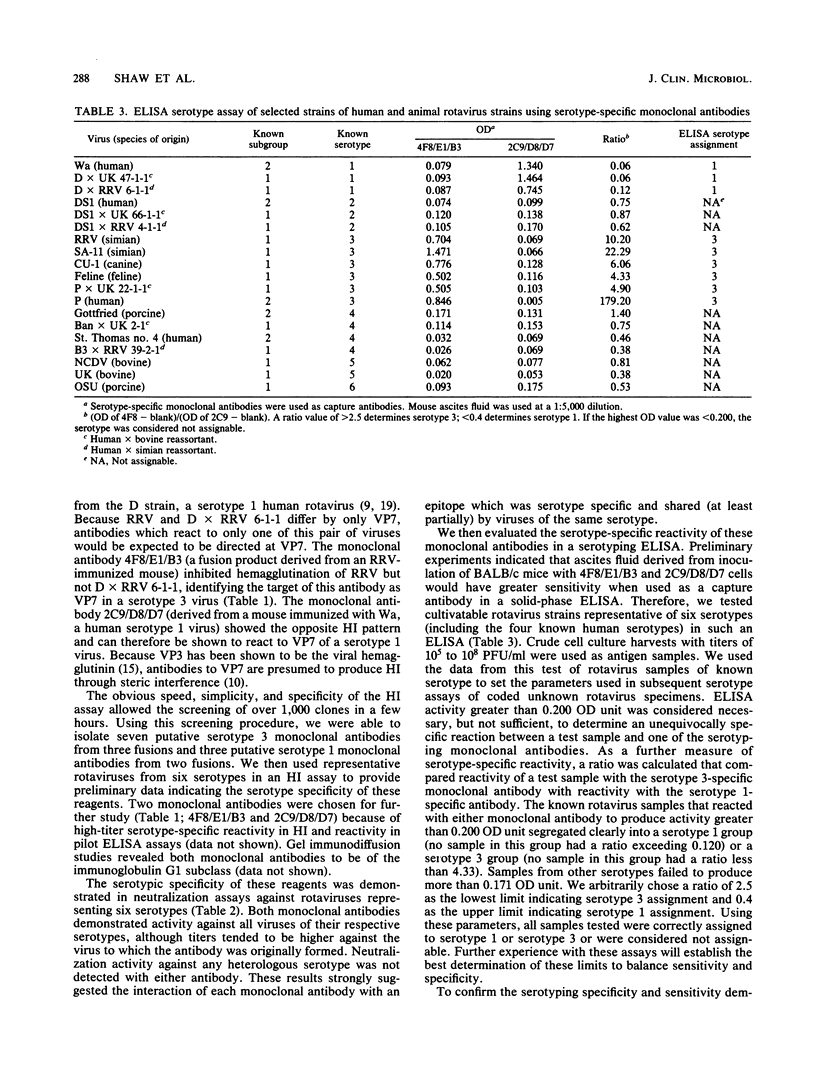
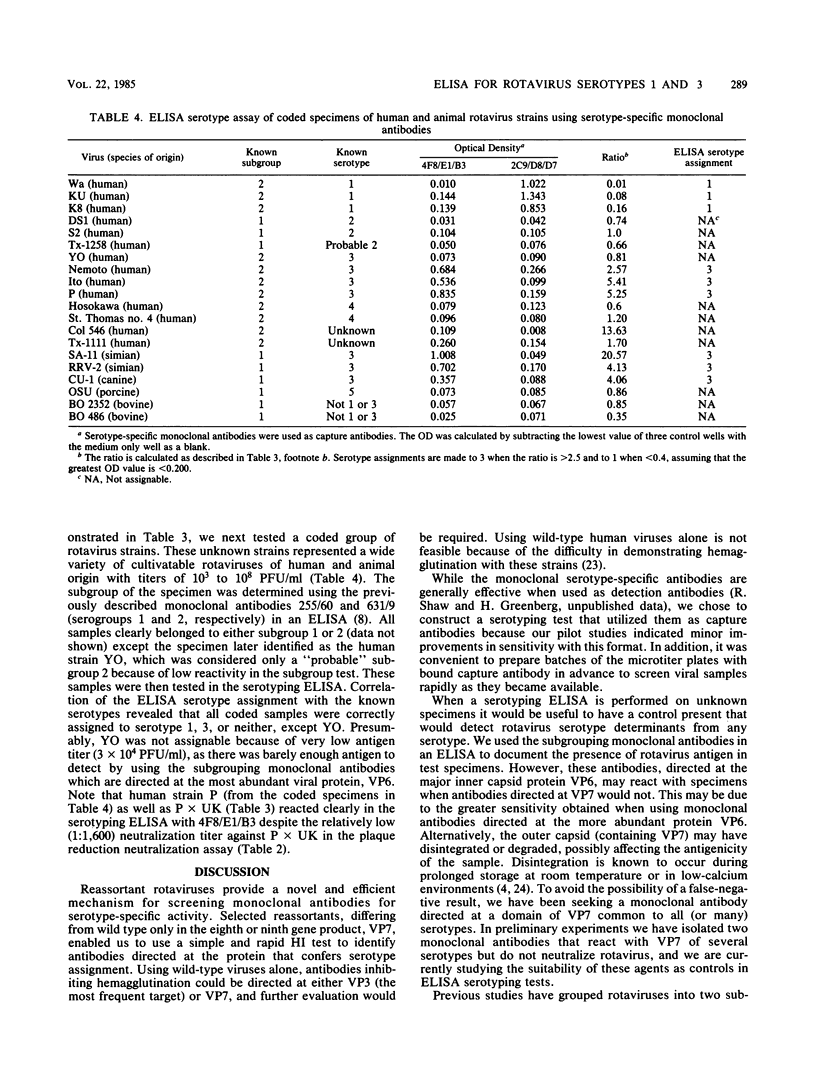
We prepared monoclonal antibodies against two serotypically distinct rotavirus strains: Wa, a serotype 1 virus of human origin, and rhesus rotavirus, a simian serotype 3 virus. Monoclonal antibodies which react specifically with VP7 of each serotype were identified by hemagglutination inhibition tests, plaque reduction neutralization studies, and solid-phase immunoassays which used wild-type and reassortant strains of rotavirus. An enzyme-linked immunoassay was designed which utilizes two of these antibodies to correctly identify serotype 1 and serotype 3 viruses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards G. M., Flewett T. H. Serological characterisation of human rotaviruses propagated in cell cultures. Brief report. Arch Virol. 1984;80(2-3):231–237. doi: 10.1007/BF01310663. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Kim H. W., Rodriguez W. J., Thomas L., Yolken R. H., Arrobio J. O., Kapikian A. Z., Parrott R. H., Chanock R. M. Comparison of direct electron microscopy, immune electron microscopy, and rotavirus enzyme-linked immunosorbent assay for detection of gastroenteritis viruses in children. J Clin Microbiol. 1981 May;13(5):976–981. doi: 10.1128/jcm.13.5.976-981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. Rotavirus stability and inactivation. J Gen Virol. 1979 May;43(2):403–409. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Kalica A. R., Wyatt R. G., Jones R. W., Kapikian A. Z., Chanock R. M. Rescue of noncultivatable human rotavirus by gene reassortment during mixed infection with ts mutants of a cultivatable bovine rotavirus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):420–424. doi: 10.1073/pnas.78.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Wyatt R. G., Kapikian A. Z., Kalica A. R., Flores J., Jones R. Rescue and serotypic characterization of noncultivable human rotavirus by gene reassortment. Infect Immun. 1982 Jul;37(1):104–109. doi: 10.1128/iai.37.1.104-109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Hung T., Chen G. M., Wang C. G., Yao H. L., Fang Z. Y., Chao T. X., Chou Z. Y., Ye W., Chang X. J., Den S. S. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet. 1984 May 26;1(8387):1139–1142. [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., James J. D., Jr, Kapikian A. Z. Hemagglutination by simian rotavirus. J Clin Microbiol. 1978 Mar;7(3):314–315. doi: 10.1128/jcm.7.3.314-315.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka S., Suzuki H., Numazaki T., Sato T., Konno T., Ebina T., Ishida N., Nakagomi O., Nakagomi T. Hemagglutination by human rotavirus strains. J Med Virol. 1984;13(3):215–222. doi: 10.1002/jmv.1890130303. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Lee S. H., Faulkner R. S., Ethier J., Young C. H. Rotavirus infection in a geriatric population. Arch Intern Med. 1982 Feb;142(2):313–316. [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli G., Gregersen J. P., Ludwig H. Plaque/focus immunoassay: a simple method for detecting antiviral monoclonal or other antibodies and viral antigens in cells. J Immunol Methods. 1984 Nov 30;74(2):337–344. doi: 10.1016/0022-1759(84)90301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Gilchrist J. E., Hudson G. R., Babiuk L. A. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J Virol. 1985 Jan;53(1):58–66. doi: 10.1128/jvi.53.1.58-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inaba Y., Shinozaki T., Fujii R., Matumoto M. Isolation of human rotavirus in cell cultures: brief report. Arch Virol. 1981;69(2):155–160. doi: 10.1007/BF01315159. [DOI] [PubMed] [Google Scholar]
- Shirley J. A., Beards G. M., Thouless M. E., Flewett T. H. The influence of divalent cations on the stability of human rotavirus. Arch Virol. 1981;67(1):1–9. doi: 10.1007/BF01314596. [DOI] [PubMed] [Google Scholar]
- Thouless M. E., Beards G. M., Flewett T. H. Serotyping and subgrouping of rotavirus strains by the ELISA test. Arch Virol. 1982;73(3-4):219–230. doi: 10.1007/BF01318076. [DOI] [PubMed] [Google Scholar]
- Urasawa T., Urasawa S., Taniguchi K. Sequential passages of human rotavirus in MA-104 cells. Microbiol Immunol. 1981;25(10):1025–1035. doi: 10.1111/j.1348-0421.1981.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Bishop C. A., Townsend T. R., Bolyard E. A., Bartlett J., Santos G. W., Saral R. Infectious gastroenteritis in bone-marrow-transplant recipients. N Engl J Med. 1982 Apr 29;306(17):1010–1012. doi: 10.1056/NEJM198204293061701. [DOI] [PubMed] [Google Scholar]


