Abstract
Tetrahydrobiopterin (BH4), a cofactor of inducible nitric-oxide synthase (iNOS), is an important post-translational regulator of NO bioactivity. We examined whether treatment of cardiac allograft recipients with sepiapterin [S-(-)-2-amino-7,8-dihydro-6-(2-hydroxy-1-oxopropyl)-4-(1H)-pteridinone], a precursor of BH4, inhibited acute rejection and apoptosis in cardiac transplants. Heterotopic cardiac transplantation was performed in Wistar-Furth donor to Lewis recipient strain rats. Recipients were treated daily after transplantation with 10 mg/kg sepiapterin. Grafts were harvested on post-transplant day 6 for analysis of BH4 (high-performance liquid chromatography), expression of inflammatory cytokines (reverse transcription- and real-time polymerase chain reaction), iNOS (Western blots), and NO (Griess reaction and NO analyzer). Histological rejection grade was scored, and graft function was determined by echocardiography. Apoptosis, protein nitration, and oxidative stress were determined by immunohistochemistry. Treatment of allografts with sepiapterin increased cardiac BH4 levels by 3-fold without changing protein levels of GTP cyclohydrolase, the enzyme that regulates de novo BH4 synthesis. Sepiapterin decreased inflammatory cell infiltrate and significantly inhibited histological rejection scores and apoptosis similar in magnitude to cyclosporine. Sepiapterin also decreased nitrative and oxidative stress. Sepiapterin caused a smaller increase in left ventricular mass versus untreated allografts but without improving fractional shortening. Sepiapterin did not alter tumor necrosis factor-α and interferon-γ expression, whereas it decreased interleukin (IL)-2 expression. Sepiapterin did not change total iNOS protein or monomer levels, or plasma and tissue NO metabolites levels. It is concluded that the mechanism(s) of antirejection are due in part to decreased apoptosis, protein nitration, and oxidation of cardiomyocytes, which seems to be mediated at the immune level by limiting inflammatory cell infiltration via decreased IL-2-mediated T-lymphocyte expansion.
Inducible nitric-oxide synthase (iNOS) is up-regulated in response to various inflammatory stimuli. In most cases, iNOS produces large amounts of NO. The production of NO by iNOS is critically influenced by the availability of the cofactor tetrahydrobiopterin (BH4). Using purified iNOS protein, it is well established that BH4 regulates NO production by stabilizing iNOS protein and facilitating iNOS homodimerization, thereby establishing its catalytic production of NO from arginine (Baek et al., 1993). The biological impact of this regulation was first demonstrated in BH4-deficient NIH3T3 cells, which were unable to support NO production after retroviral infection to overexpress iNOS (Tzeng et al., 1995). It was found that increases in NO production from iNOS occurred after supplementing cells with BH4 or its precursor sepiapterin [S-(-)-2-amino-7,8-dihydro-6-(2-hydroxy-1-oxopropyl)-4-(1H)-pteridinone]. This finding showed for the first time the crucial role of increased BH4 synthesis in regulating NO production via iNOS in a cellular setting. GTP cyclohydrolase (GTPCH) is considered to be the rate-limiting step in de novo BH4 synthesis. The simultaneous up-regulation of both GTPCH and iNOS is understood to coordinate the increased synthesis of BH4 levels at a sufficiently high level to facilitate enzymatic NO production from iNOS protein (Gross and Levi, 1992; Geller et al., 2000).
Cardiac allograft rejection is associated with the robust increase in iNOS in the heart. Expression of iNOS in this setting has been proposed to be linked with contractile dysfunction and heart failure (for review, see Pieper and Roza, 2008). In the past, there has been a primary focus on iNOS mRNA and/or protein expression in defining the role of iNOS in acute cardiac rejection. Information regarding the actual NO bioactivity deriving from iNOS in acute cardiac rejection is noticeably absent. Previous studies from our laboratory suggest that GTPCH is also up-regulated in cardiac allografts but that at later stages of rejection the synthesis of biopterin was insufficient to support NO production seen at earlier time periods (Pieper et al., 2005). This suggested that the progression to full rejection of the graft involves a BH4-deficient state. This happened to coincide with protein nitration and onset of graft dysfunction. With this background, it is presumed that increasing intragraft BH4 levels might decrease rejection. In the present study, we tested the hypothesis that treatment of allograft recipients with sepiapterin as a means to increase intragraft BH4 levels would inhibit acute cardiac rejection and apoptosis. Furthermore, we examined whether this effect was related to improving NO levels and iNOS homodimerization or to some other action on immune activation.
Materials and Methods
Transplantation. All animal procedures were approved by the local institutional animal care and use committee. All animals received humane care in compliance with the Principles of Laboratory Animal Care and the Institute of Laboratory Animal Resources (1996). Lewis (Lew:RT1l) and Wistar-Furth (WF:RT1u) rat strains (Harlan, Indianapolis, IN) were chosen for genetic disparity at both the major and minor histocompatibility loci for donor-to-recipient combination of Lewis→Lewis rats (for isografts) or Wistar-Furth→Lewis rats (for allografts). Isogeneic or allogeneic heterotopic cardiac transplantation was performed aseptically in pentobarbital (50 mg/kg i.p.)-anesthetized animals as described previously (Pieper et al., 2000). Donor hearts were arrested in ice-cold University of Wisconsin preservation solution and transplanted into anesthetized (50 mg/kg pentobarbital i.p.) recipient animals. To increase intragraft BH4 levels, allograft recipients were treated with sepiapterin (10 mg/kg/day i.p.). Drug treatments were given to recipient animals starting on the day of transplantation at 1 h before donor heart transplantation and continued daily until harvesting of grafts on postoperative day 6.
Grafts were arrested in pentobarbital-anesthetized animals at postoperative day 6 by flushing with ice-cold preservation solution (University of Wisconsin), and portions were frozen in liquid N2 for various analysis. Other portions of the graft were taken and evaluated for histological rejection as described below. Sometimes, plasma was also taken to document changes in plasma and cardiac NO metabolite levels using a Griess reaction assay and/or by chemiluminescence using a NO analyzer.
Histological Rejection Scoring. Tissue was fixed in 10% phosphate-buffered formalin. Paraffin-embedded sections were stained with hematoxylin and eosin. Histological rejection was scored blinded using criteria established by the International Society for Heart and Lung Transplantation as modified to a linear score system to allow statistical analysis as described in detail previously (Pieper et al., 2002). Initial scoring was determined blinded, with the average scoring of two individuals used for analysis.
Immunohistochemistry. Apoptosis was measured by the TUNEL assay using ApopTag technology (Millipore Bioscience Research Reagents, Temecula, CA) according to the manufacturer's instructions and as established previously (Pieper et al., 2008). Sections were counterstained for visualization of apoptotic nuclei. Apoptotic nuclei were counted from at least four section fields for each graft sample, averaged, and the mean for each experimental group was determined.
Nitration of tyrosine residues on graft protein was determined by immunohistochemistry with and without counterstaining similar to that described previously (Pieper et al., 2004). Sections of hearts were incubated with 1:50 dilution of anti-nitrotyrosine antibody (Millipore, Billerica, MA). To determine oxidative stress at the protein level, we examined 4-hydroxy-2-nonenal (4-HNE) protein adduct formation. Tissue sections were incubated with 1:100 anti-4-HNE antibody (Calbiochem, San Diego, CA). After antibody exposure, sections were incubated with horseradish peroxidase-conjugated secondary antibody. Reactivity was revealed using 3,3′-diaminobenzidine tetrahydrochloride, and sections were counterstained with either hematoxylin alone or hematoxylin with eosin for examination by light microscopy. Nitrotyrosine and 4-HNE levels were scored as follows: 0, complete absence of immunoreactivity; 1, a single foci or weak areas of staining; 2, moderate staining; 3, intense staining in one third or more myocytes; and 4, intense staining and/or multifocal intense staining. Individual sections from individual grafts were scored independently by two individuals, and the values were averaged.
Echocardiography. Echocardiography was performed using a Vivid 7 electrocardiograph (General Electric, Waukesha, WI) and an 11-MHz M12L linear array transducer. The parasternal short-axis mid-left ventricular view was used with the papillary muscles as anatomic landmarks. Image depth was 2 to 2.5 cm; frame rates of 234 to 256 frames per second and second harmonic imaging were used. The images were sent to a separate workstation for analysis using EchoPAC software with Q analysis (General Electric).
LV size [internal diameter in diastole (LVIDd) and systole (LVIDs)] and thickness (anterior and inferior diastolic thickness) were measured using anatomical M-mode of the two-dimensional B-mode images through the plane of the anterior and inferior segments. Fractional shortening (FS) was calculated using the formula FS = (LVIDd - LVIDs)/LVIDd × 100%. LV ejection fraction was calculated using the Teichholz method (Teichholz et al., 1976). LV mass was derived using the formula 0.8[1.04{(inferior diastolic thickness + left ventricular internal diameter + anterior diastolic thickness)3 - left ventricular internal diameter3}] + 0.6 (Devereux et al., 1986). Mid-left ventricular myocardial area was derived by subtracting the endocardial area by the epicardial area at mid-left ventricle during diastole. Diastolic measurements were performed at the R wave of electrocardiographic gating that corresponded to the donor heart rhythm in cases in which two distinct R waves were seen (i.e., from the native and heterotopically transplanted donor heart). In cases in which the donor heart rhythm could not be distinguished from the native heart on electrocardiography, diastolic measurements were done at the largest diameter of the cardiac cycle.
Radial strain and circumferential strain were measured as published previously (Migrino et al., 2007). In brief, the mid-left ventricle was divided into six segments as defined by the American Society of Echocardiography (Lang et al., 2005). The beginning of the cardiac cycle was defined as the R wave of the donor heart if electrocardiographic gating could distinguish native and donor heart rhythm, or the time of the cardiac cycle with the greatest LV diameter followed on successive frames by reduction in size, in cases in which donor heart rhythm could not be distinguished. The endocardial border was traced, and the outer border was adjusted to fit the epicardial contour. The software automatically selected acoustic objects within the myocardium to track and computed radial strain in the segments of the mid-ventricle throughout the cardiac cycle. End systolic strain was obtained for each segment, and the average was used to compute global radial or circumferential strain. Using the same software, average peak systolic circumferential strain rate from the six segments was calculated and represents the highest systolic change in circumferential strain by time. This parameter is more closely related to myocardial contractility than ejection fraction (Stoylen et al., 1999).
Western Blot. The distribution of iNOS monomers and dimers was analyzed by Western blots under reducing conditions as described previously (Vásquez-Vivar et al., 2008). Briefly samples were harvested and freshly homogenized in lysis buffer in the presence of protease inhibitors. β-Mercaptoethanol was omitted from the Laemmli sample buffer. Total protein concentration for each sample was determined using a DC protein assay (Bio-Rad Laboratories, Hercules, CA). Proteins were resolved on 7.5% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. A dilution of 1:1000 of iNOS antibody (Cayman Chemical, Ann Arbor, MI; and Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used overnight at 4°C. Immunoblots were developed with SuperSignal West Femto maximum sensitivity substrate kit (Pierce Chemical, Rockford, IL). Other Western blots were performed under reducing conditions (i.e., boiled in the presence of β-mercaptoethanol) and probed at 4°C overnight with antibodies to GTPCH (1:5000 dilution) or GAPDH (1:1000 dilution). Immunoreactivity for iNOS protein was effectively blocked in both reducing and nonreducing samples in the presence of blocking peptide (data not shown).
PCR Analysis. Total cellular RNA was isolated from graft biopsies by using 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA) per 100 μg of heart tissue according to the manufacturer's protocol. Genomic DNA was digested by treatment with RNase-free DNase (Ambion, Austin, TX), and RNA concentration was determined spectrophotometrically. Complementary DNA was synthesized from 1 μg of total RNA and random hexamer primers by using SuperScript III first-strand synthesis system for RT-PCR (Invitrogen) according to the manufacturer's directions. Primers and conditions used for these analyses are summarized in Table 1. PCR reactions were performed in a 25-μl volume containing 1 μl of cDNA, 25 pmol of sequence-specific primers, and 22.5 μl of Platinum PCR SuperMix High Fidelity (Invitrogen). A 10-μl aliquot of the PCR product was resolved by 1% Tris acetate-EDTA-agarose gel electrophoresis, and densitometric analysis of specific bands was performed using Alpha Imager (Alpha Innotech, San Leandro, CA). Additional studies on cytokine gene expression were performed using real-time quantitative PCR. Primer sequences and conditions are described in Table 2. Relative mRNA level was determined as 2[(Ct/β-actin - Ct/gene of interest)]. The results are presented as -fold expression normalized to β-actin.
TABLE 1.
Primers and conditions used for RT-PCR analyses
| Gene | Primer Sequence (Forward/Reverse) | Size | Annealing Temp./No. Cycles |
|---|---|---|---|
| base pairs | °C | ||
| GTPCHI | GGATACCAGGAGACCATCTCA | 148 | 57/27 |
| TAGCATGGTGCTAGTGACAGT | 372 | 60/40 | |
| iNOS | CACCTTGGAGTTTCACCCAGT | 328 | 60/30 |
| TGTTTGTAGCGCTGTGTGTCA | |||
| ARG1 | CCAAGCCAAAGCCCATAGAGATTA | 419 | 60/30 |
| CCCGTGCAGATTCCCAGAGC | |||
| TNF-α | TCAGCCTCTTCTCATTCCTGC | 203 | 58/32 |
| TTGGTGGTTTGCTACGACGTG | |||
| IFN-γ | CGCCGCGTCTTGGTTTTG | 452 | 58/30 |
| CGACTCCTTTTCCGCTTCCTTAG | |||
| GAPDH | AGTTCAACGGCACAGTCAAG | 148 | 57/27 |
| GTGGTGAAGACGCCAGTAGA |
ARG1, arginase 1
TABLE 2.
Primers and conditions used for real-time PCR analyses of inflammatory cytokines
| Gene | Primer Sequence (Forward/Reverse) | Size | Annealing Temp. |
|---|---|---|---|
| base pairs | °C | ||
| TNF-α | GCCCAGACCCTCACACTC | 99 | 60 |
| CCACTCCAGCTGCTCCTCT | |||
| IFN-γ | TTTTGCAGCTCTGCCTCAT | 109 | 60 |
| AGCATCCATGCTACTTGAGTTAAA | |||
| IL-2 | CTGCAAAGCAAAACAGCAG | 96 | 59 |
| TGGGGAGTTTCAGATTCTTGTAAT | |||
| β-Actin | CCCGCGAGTACAACCTTCT | 97 | 60 |
| CGTCATCCATGGCGAACT |
Tetrahydrobiopterin and 7,8-Dihydrobiopterin Analysis. Quantification of BH4 and 7,8-dihydrobiopterin (BH2) was performed by HPLC with electrochemical detection. Tissue samples were homogenized in 300 μl of 50 mM phosphate buffer, pH. 2.6, containing 0.1 mM diethylenetriaminepentaacetic acid and freshly added 1 mM dithiothreitol. Samples were centrifuged at 12,500 rpm for 10 min at 4°C, and supernatants were loaded onto Centricon filters (10,000 molecular weight cut-off). Filtrates were analyzed on an HPLC system (models 582 and 542, CoulArray system; ESA Inc., Chelmsford, MA) using an analytical Polar-RP column eluted with argon saturated 50 mM phosphate buffer, pH 2.6, as described previously (Whitsett et al., 2007). Calibration curves were made by summing up the peak areas collected at 0 and 150 mV for BH4 and 280 and 365 mV for BH2. Intracellular concentrations were calculated using authentic BH4 and BH2 (10–100 nM) standards, and concentrations were normalized to respective protein content of test samples.
Plasma and Tissue NOx Analysis. Blood was collected in EDTA, and plasma was separated by centrifugation. Plasma samples were deproteinized by filtering through 10,000 molecular weight cut-off filters. To measure NO metabolites in heart, approximately 40 mg of heart tissue was homogenized in Dulbecco's phosphate-buffered saline, pH 7.4. Samples were centrifuged at 14,000 rpm at 4°C for 30 min. Supernatants were collected and filtered through prewet 10,000 molecular weight cut-off Microcon filters (Millipore). A 20-μl aliquot of each sample or standard was used to inject into a NO analyzer (Sievers Instruments, Inc., Boulder, CO), and NOx levels were normalized to total protein. In addition to the NO analyzer, some samples were also processed for NO metabolites using the Griess reaction assay (Cayman Chemical).
Data Analysis. All values are expressed as mean ± S.E.M. Statistical analysis was performed by one-way analysis of variance with Student-Newman-Keuls test for multiple comparisons of multiple group means or with Student's t test for comparisons between two group means. Statistical significance was set at P < 0.05.
Results
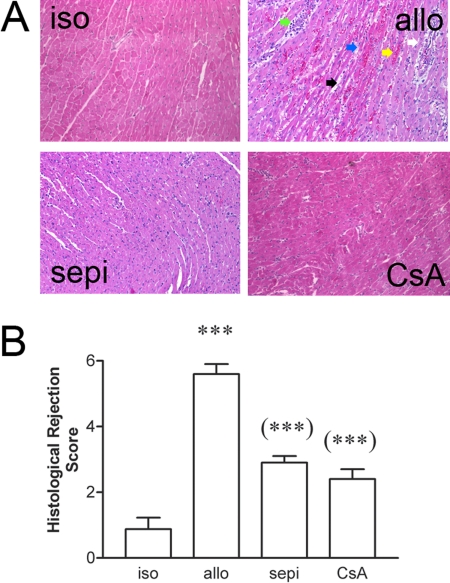
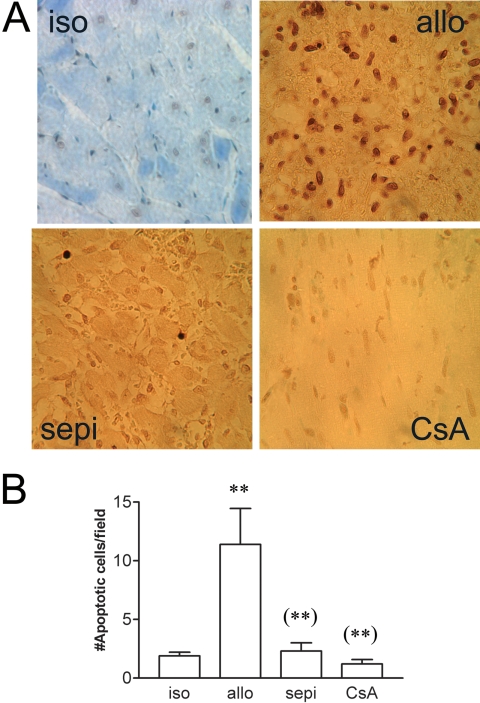
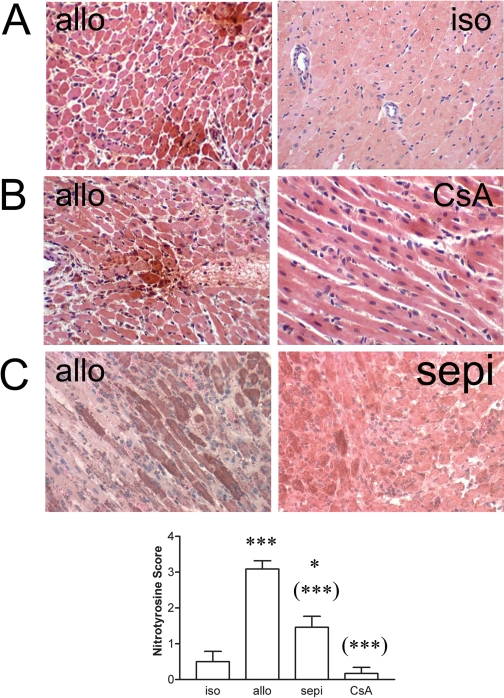
Histological rejection was not evident in isografts (Fig. 1A). In contrast in untreated allografts, there were areas of inflammatory cell infiltrate, necrosis, vasculitis, hemorrhage, and interstitial edema that were decreased in allografts treated with sepiapterin (Fig. 1A). Histological rejection scores were significantly (P < 0.001) increased in allografts versus isograft controls (Fig. 1B). Treatment of allograft recipients with sepiapterin significantly (P < 0.001) decreased histological rejection scores compared with that observed in untreated allografts. For comparison, the level of protection achieved by treatment with sepiapterin was similar to that achieved by treatment with cyclosporine at a dose determined previously to prolong graft survival to at least 111 days when given continuously (Khanna et al., 2004). Acute rejection of untreated allografts was also associated with enhanced apoptosis versus isograft controls (Fig. 2A). The number of TUNEL-positive, apoptotic cells was significantly (P < 0.01) decreased in recipients treated with sepiapterin (Fig. 2B). The degree of decrease in apoptosis was equivalent to that seen in recipients treated with cyclosporine (Fig. 2).
Fig. 1.
A, histological evidence of rejection in untreated allografts (allo) versus isografts (iso) and sepiapterin (sepi)-treated allografts or cyclosporine (CsA)-treated allograft recipients. Rejection is characterized by inflammatory cell infiltrate (blue arrow), necrosis (green arrow), interstitial edema (black arrow), vasculitis (white arrow), and hemorrhage (yellow arrow). B, decreased histological rejections scores by treatment with sepiapterin or CsA. ***, P < 0.001 versus iso; (***), P < 0.001 versus untreated allo.
Fig. 2.
A, examples of TUNEL staining showing increased apoptosis in untreated allografts (allo) versus isograft (iso) controls and decreased apoptosis in sepiapterin (sepi)-treated allografts versus untreated allo. B, treatment with sepiapterin decreased the number of apoptotic cells similar to that achieved by treatment with cyclosporine (CsA) for comparison. **, P < 0.01 versus iso; (**), P < 0.01 versus allo.
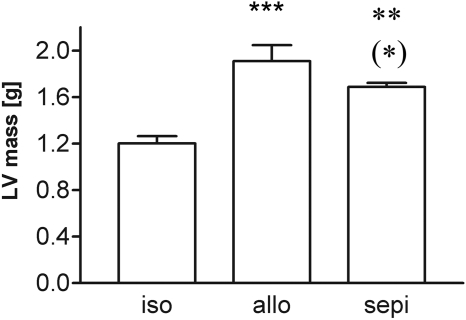
Echocardiography revealed an increase in LV mass of 58.1% in allografts compared with isograft controls. The increase in LV mass was 40.4% in sepiapterin-treated allografts versus isograft controls. This represents a significant (P < 0.05) reversal of 30% in sepiapterin-treated allografts from untreated allografts (Fig. 3). Both untreated and sepiapterin-treated allografts displayed similar levels of systolic dysfunction (i.e., low ejection fraction, fractional shortening, and global radial and circumferential strain) (Table 3).
Fig. 3.
Increased LV mass as shown by echocardiography in allograft (allo) recipients versus isograft (iso) controls and attenuation by treatment with sepiapterin (sepi) (n = 5–7 each group). ***, P < 0.001 allo versus iso; **, P < 0.01 sepi versus iso; (*), P < 0.05 allo versus sepi.
TABLE 3.
Echocardiography of untreated and sepiapterin-treated rat cardiac allografts
| Isograft (n = 5) | Untreated Allograft (n = 7) | Sepiapterin-Treated Allograft (n = 7) | Significance | |
|---|---|---|---|---|
| IVSd (cm) | 0.172 ± 0.022 | 0.429 ± 0.038 | 0.453 ± 0.025 | P < 0.001 iso vs. allo |
| P < 0.001 iso vs. sepi | ||||
| LVIDd (cm) | 0.552 ± 0.050 | 0.333 ± 0.082 | 0.164 ± 0.025 | P < 0.05 iso vs. allo |
| P < 0.01 iso vs. sepi | ||||
| LVPWd (cm) | 0.240 ± 0.035 | 0.409 ± 0.050 | 0.481 ± 0.023 | P < 0.01 iso vs. allo |
| P < 0.01 iso vs. sepi | ||||
| LVIDs (cm) | 0.468 ± 0.038 | 0.304 ± 0.081 | 0.126 ± 0.020 | P < 0.01 iso vs. sepi |
| P < 0.01 allo vs. sepi | ||||
| EDV (cm) | 0.436 ± 0.099 | 0.191 ± 0.093 | 0.017 ± 0.007 | P < 0.05 iso vs. sepi |
| P < 0.05 iso vs. allo | ||||
| ESV (cm) | 0.266 ± 0.055 | 0.159 ± 0.085 | 0.007 ± 0.004 | P < 0.05 iso vs. sepi |
| EF (%) | 36.19 ± 5.31 | 29.51 ± 5.77 | 44.76 ± 10.68 | n.s. |
| FS (%) | 14.98 ± 2.61 | 11.74 ± 2.47 | 20.55 ± 5.66 | n.s. |
| Radial strain (%) | 13.642 ± 7.048 | 1.183 ± 0.301 | 1.199 ± 0.247 | P < 0.05 iso vs. allo, P < 0.05 iso vs. sepi |
| Circumferential strain (%) | –4.169 ± 2.504 | –1.224 ± 0.341 | –1.217 ± 0.297 | n.s. |
| Peak circumferential strain rate (1/s) | –1.55 ± 0.59 | –0.38 ± 0.10 | –0.40 ± 0.10 | P < 0.05 iso vs. allo |
IVSd, interventricular septal thickness in diastole; LVPWd, left ventricular posterior wall diameter in diastole; EDV, end-diastolic volume; ESV, end-systolic volume; EF, left ventricular ejection fraction, iso, isografts; allo, allografts, sepi, sepiapterin
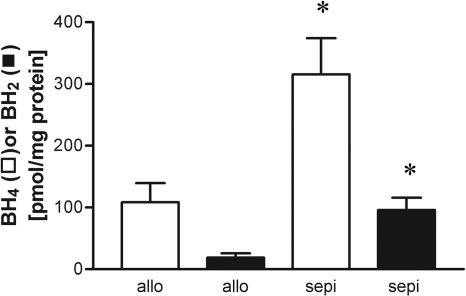
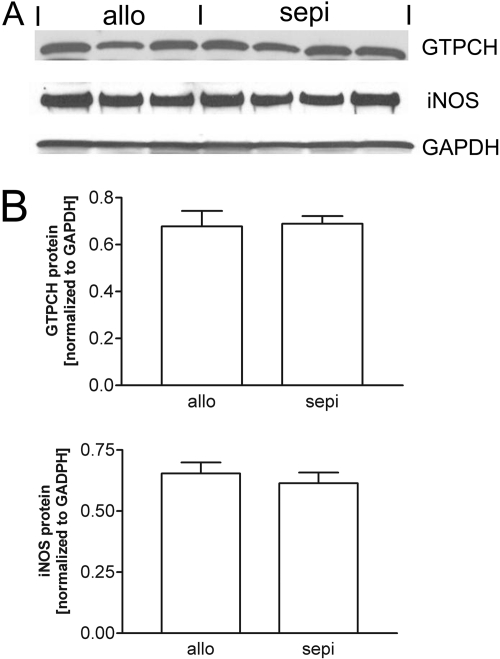
To determine whether treatment with sepiapterin altered intragraft biopterin levels, we performed HPLC on graft samples at postoperative day 6 for BH4 and BH2. Comparison of values in samples frozen in liquid N2 and stored versus analysis of freshly harvested, unfrozen samples yielded similar values (data not shown). The levels of BH4 were enhanced by 3-fold in recipients treated with the BH4-precursor sepiapterin compared with untreated allografts (Fig. 4). Treatment with sepiapterin also significantly increased intragraft levels of BH2 (Fig. 4). Treatment with sepiapterin did not alter the levels of GTPCH protein, indicating that the increase in BH4 due to sepiapterin cannot be due to up-regulation in GTPCH expression (Fig. 5).
Fig. 4.
HPLC analysis with electrochemical detection showing that treatment of allograft recipients with sepiapterin (sepi) increased cardiac allograft levels of BH4 (□) and BH2 (▪). *, P < 0.05 versus untreated allografts (allo).
Fig. 5.
Western blot (A) and densitometry (B) showing that treatment with sepiapterin (sepi) did not alter protein expression for GTPCH or iNOS normalized to GAPDH.
We next examined functional effects of treatment with sepiapterin on iNOS and NO levels. Plasma levels of NO metabolites measured by the Griess assay revealed increased levels of NO production in allografts versus isografts that were not altered by treatment of recipients with sepiapterin (Fig. 6A). Treatment with sepiapterin did not alter the cardiac content of NO in allografts determined by chemiluminescence using a NO analyzer (Fig. 6B). The lack of further increase in intracardiac NO levels by treatment with sepiapterin was additionally confirmed using a Griess reaction assay (data not shown). These findings were consistent with the finding that sepiapterin did not alter iNOS protein levels (Fig. 5).
Fig. 6.
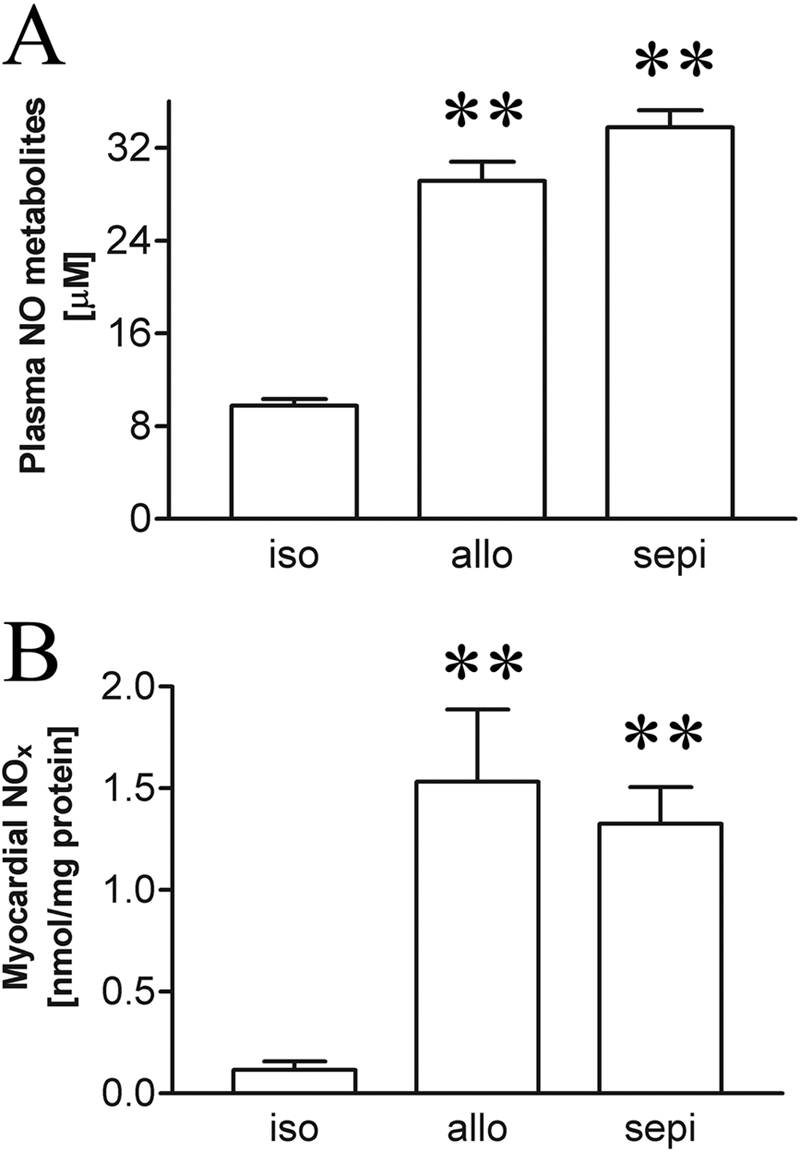
Plasma (n = 5–9 each) (A) and myocardial (B) NOx levels (n = 5–6 each) in isografts (iso) versus untreated allografts (allo) and allograft recipients receiving treatment with sepiapterin (sepi). Results show that treatment with sepiapterin did not alter NO levels. **, P < 0.01 allo or sepi versus iso.
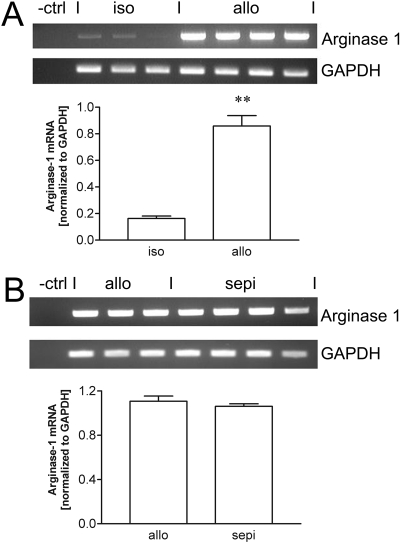
Because the level of arginase expression may indirectly alter the detection of NO levels and might be differentially expressed under treatment conditions, we next examined arginase 1 expression in isografts, untreated allografts, and allografts treated with sepiapterin. Arginase 1 expression was weakly expressed in isografts but significantly (P < 0.01) up-regulated in allograft recipients (Fig. 7A). Treatment of allograft recipients with sepiapterin did not significantly alter arginase 1 expression compared with expression in untreated allografts (Fig. 7B).
Fig. 7.
A, increase in arginase 1 mRNA expression normalized to GAPDH in allografts (allo) compared with isografts (iso). B, arginase 1 mRNA is not altered by treatment of recipients with sepiapterin (sepi). **, P < 0.01 versus isograft.
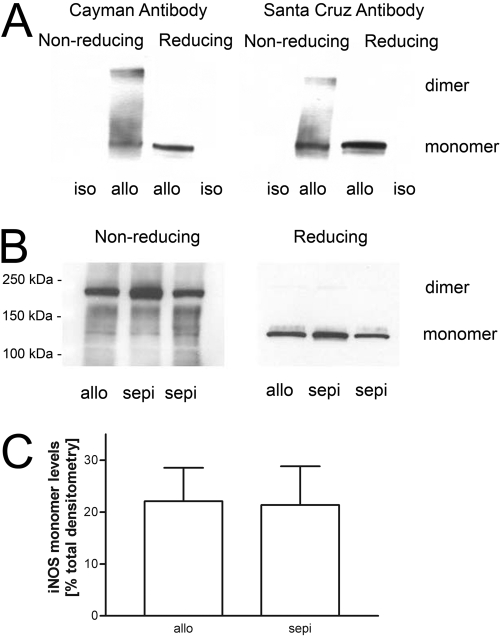
The alloimmune-induced increases in the protein levels of iNOS in allografts versus isograft controls were not altered by treatment with sepiapterin (Fig. 5). Using tissue samples that were frozen and stored in liquid N2 and processed under reducing conditions, iNOS was detected only in allografts, but not in isografts, and only as iNOS monomers (i.e., at 130 kDa) (Fig. 8A). Under nonreducing conditions, both iNOS monomers and higher molecular weight species, herein termed “iNOS dimers,” were detected, and the pattern was similar using two different commercial anti-iNOS antibodies (Fig. 8A). In these and other tests, the intensity of the monomer band was always equal to or larger than the upper iNOS dimer band. To determine whether freeze-thawing might influence dissociation of iNOS dimer, we also performed similar studies from freshly harvested samples (Fig. 8B). In this case, both iNOS monomers and dimers were present in cardiac allograft samples, but the proportion attributed to monomers was lower than that observed using frozen allograft samples. To avoid this complication, subsequent blots were performed only on freshly harvested, but unfrozen, samples from recipient grafts. Here, both iNOS monomers and dimers were also present in sepiapterin treatment; however, compared with untreated allografts, sepiapterin did not change the levels of iNOS monomers (Fig. 8, B and C).
Fig. 8.
A, individual Western blots of iNOS monomers and dimers in allografts (allo) but not isografts (iso) in samples taken from frozen, stored cardiac graft samples but processed under nonreducing and reducing conditions. Results show similar findings using two different commercial anti-iNOS antibodies. B, example showing Western blot analysis using anti-iNOS antibody (Santa Cruz Biotechnology, Inc.) of freshly harvested graft samples from rat cardiac allografts run under reducing and nonreducing conditions and effects of sepiapterin (sepi) treatment. C, densitometry of three different Western blots for iNOS monomers in rat cardiac allograft recipients without and with treatment with sepiapterin (n = 3–4 each group).
Nitration of proteins develops during acute cardiac allograft rejection. In the samples examined in this study, we observed immunohistochemical evidence for increased formation of nitrotyrosine during rejection in untreated allografts versus isograft controls (Fig. 9A). Staining in allografts was prominent throughout many cardiomyocytes and occasionally in blood vessels and infiltrating cells. In general, treatment with sepiapterin decreased both the proportion of cardiomyocytes staining for nitrotyrosine as well as the intensity of focal areas of staining for nitrotyrosine. Semiquantitative analysis indicated that treatment with sepiapterin significantly (P < 0.001) decreased nitrotyrosine levels relative to untreated allografts (Fig. 9C). This decrease in sepiapterin-treated allografts, although significant, was still elevated above isograft controls in contrast to the ablation in nitration using cyclosporine shown previously (Pieper et al., 2004) and in the present study using cyclosporine (Fig. 9B).
Fig. 9.
A, immunohistochemistry showing staining for nitrotyrosine in allografts (allo) versus isograft (iso) controls (400×). B and C, decreased nitrotyrosine staining in cyclosporine (CsA)-treated (600×) and sepiapterin (sepi)-treated allografts (400×) versus untreated allografts. D, increase in nitrotyrosine intensity score in allo versus iso controls by sepi or CsA (n = 3 for iso; n = 6–7 for each allograft group). *, P < 0.05 versus iso; ***, P < 0.001 versus iso; (***), P < 0.001 versus allo.
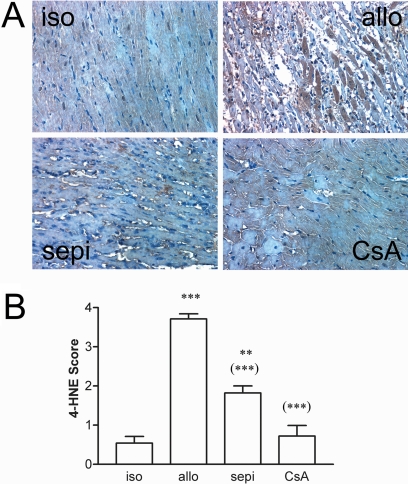
To characterize changes in oxidative stress due to treatment with sepiapterin, we also examined 4-HNE adducts in cardiac grafts by immunohistochemistry. In general, staining for 4-HNE was not observed in isografts, whereas there was prominent staining in a many cardiomyocytes of untreated allografts (Fig. 10). Most of the staining was confined to strong staining in cardiomyocytes. Occasionally, staining for 4-HNE could be seen in blood vessels, but this was not always the case. Treatment with sepiapterin and cyclosporine decreased both the intensity and frequency of 4-HNE staining in cardiomyocytes of allograft recipients. The greatest decrease in 4-HNE was seen in the cyclosporine-treated recipients.
Fig. 10.
A, immunoreactivity for 4-HNE indicative of oxidative stress is increased in cardiac allografts (allo) versus isograft (iso) controls (400×) and is decreased by treatment with sepiapterin (sepi) or cyclosporine (CsA). B, increase in 4-HNE intensity score was decreased by treatment by both treatments (n = 3 iso; n = 5–6 each for all other groups). ***, P < 0.001 versus iso, **, P < 0.01 versus iso, and (***), P < 0.001 versus allo.
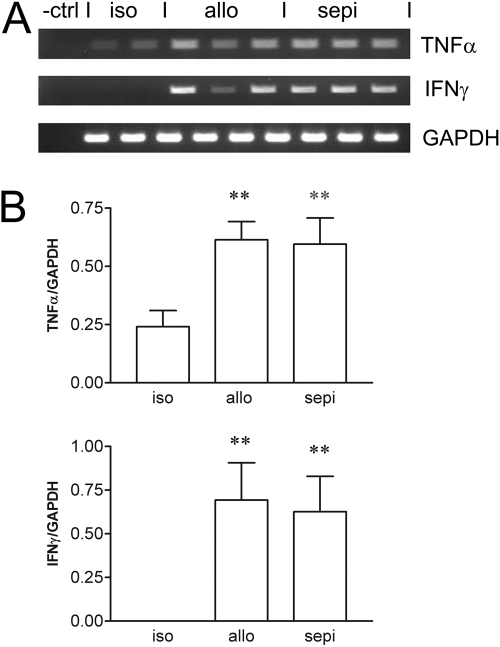
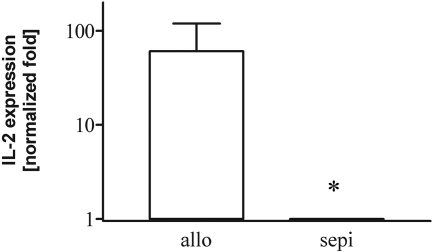
Along with up-regulation of iNOS expression, alloimmune activation increases expression of several inflammatory cytokines. Thus, we first examined gene expression by RT-PCR for the inflammatory cytokines TNF-α and IFN-γ, which are known to be increased in cardiac allograft rejection. TNF-α and IFN-γ, normalized to GAPDH, were significantly (P < 0.01) up-regulated in allografts compared with isograft controls (Fig. 11). Treatment with sepiapterin did not alter the increased expression of these cytokines versus that seen in untreated allografts. Similar analysis using real-time PCR confirmed these initial findings (data not shown). To determine whether sepiapterin might have some effect on T-cell function, we performed additional analysis using real-time PCR of the expression of IL-2, an important T-lymphocyte activation product and stimulator of T-cell proliferation and expansion. Expression of IL-2 was decreased by treatment with sepiapterin (Fig. 12).
Fig. 11.
RT-PCR (A) and densitometry (B) normalized to GAPDH housekeeping gene showing that treatment with sepiapterin (sepi) did not alter the allo-immune-induced up-regulation of cytokine gene expression for TNF-α and IFN-γ in cardiac allografts (allo) versus isografts (iso). **, P < 0.01 versus iso.
Fig. 12.
Intragraft IL-2 expression in allografts (allo) is decreased in allograft recipients treated with sepiapterin (sepi). *, P < 0.05 versus allo; allo (n = 3) and sepi (n = 4).
Discussion
We showed that sepiapterin significantly decreased histological rejection. The mechanism for antirejection was due in part to decreased IL-2 expression and was associated with decreased apoptosis, nitration, and oxidation of protein in cardiomyocytes. The magnitude of protection was comparable with that achieved by using low-dose cyclosporine. To our knowledge, this is the first study to report the protective effects of multiple-day treatment with sepiapterin in a model of cardiac allograft rejection.
Cardiac graft rejection in humans is associated with increased LV mass (Kawauchi et al., 1992; Mondillo et al., 2008). In the present study, we showed that sepiapterin significantly attenuated the increase in LV mass. Interpretation of conventional echocardiographic measurement of LV function in the heterotopically transplanted heart is challenging due to, in part, mechanical unloading. Thus, values for systolic function (i.e., fractional shortening or ejection fraction) are lower in isografts compared with normal hearts (Zhou et al., 2007). This may explain the inability to discriminate systolic function between isografts and untreated allografts. Measurements of strain and peak systolic strain rate are a novel application to assessing function in this model. These parameters measure deformation and are better suited for estimation of systolic function (Migrino et al., 2007, 2008). Although these better discriminate graft dysfunction in allografts versus isografts, measurements were not sufficiently sensitive to discriminate improvements using sepiapterin. In contrast the benefits of sepiapterin on LV mass are more consistent with this as a functional measure of rejection. Our finding using sepiapterin is consistent with observations that authentic BH4 did not improve contractile function assessed by subjective, graded scoring derived by external graft palpation in mouse transplants (Brandacher et al., 2006). Collectively, these findings support the notion that histological rejection and graft dysfunction do not always correlate (de Groot-Kruseman et al., 2002) and may arise from different etiologies.
BH4 levels are critical to optimal production of NO from iNOS. We reported previously that cardiac allografts displayed early transient increases in total biopterin that regressed at later stages, suggesting the development of a BH4 deficit (Pieper et al., 2005). We surmised that this arose from a critical switch in iNOS expression initially from the inflammatory cell infiltrate and later in cardiomyocytes. In this regard, unlike neonatal cardiomyocytes, we recently documented the low efficiency of adult cardiomyocytes to synthesize BH4 in response to inflammatory stimuli (Kalivendi et al., 2005; Vásquez-Vivar et al., 2008).
For inflammatory cells, the addition of sepiapterin to macrophages transfected to overexpressing iNOS supported iNOS homodimerization from inactive iNOS monomers and increased NO production (Sakai et al., 2006). This was one of the first cell-based studies to demonstrate the biological feasibility of augmenting intracellular BH4 to reproduce the pterin-facilitated iNOS homodimerization demonstrated using purified iNOS protein. In a previous study, authentic BH4 was shown not to alter plasma NO or cardiac graft iNOS mRNA levels in mouse heart transplants (Brandacher et al., 2006). However, the effects on NO levels in cardiac grafts per se were not examined. In our study, we found that sepiapterin did not increase intracardiac NO levels. In contrast, sepiapterin was shown to increase tissue NO in renal transplants (Huisman et al., 2002). The conflicting results may be explained by differences in experimental design, NO detection methods, or intrinsic differences in metabolism of sepiapterin in different organs. Indeed, renal NO was determined 24 h after transplantation; therefore, a consequence of treatment on early post-transplant reperfusion injury. We determined cardiac graft NO levels several days after daily treatment with sepiapterin, which reflects more the effects on alloimmune activation and rejection.
Under certain conditions, arginase can compete with iNOS for arginine, thereby limiting NO production (de Bono et al., 2007; Romero et al., 2008). In consideration of this possibility, we confirmed that arginase 1 is inducible in cardiac allografts. Arginase 1 expression in the heart probably derives from noncardiomyocyte cells (e.g., fibroblasts, endothelial and vascular smooth muscle cells). This conclusion comes from our recent findings that arginase is absent and not induced in adult cardiomyocytes stimulated with a variety of cytokine stimuli (Ionova et al., 2008). However, this cannot explain the ineffectiveness of sepiapterin to increase intracardiac NO levels because arginase 1 expression was unaltered by sepiapterin.
Despite the studies concerning BH4 and the relationship to endothelial NOS homodimerization in diseased blood vessels and myocardium (Schmidt and Alp, 2007), published studies on homodimerization of iNOS in heart are extraordinarily rare, consisting of a single publication in a model of ventricular hypertrophy (Zhang et al., 2007). Using nonreducing Western blot analysis, we confirmed the presence of the expected 130-kDa inactive iNOS monomers in acutely rejecting cardiac allografts. Our unexpected finding that sepiapterin treatment did not alter iNOS monomer levels is consistent with the data showing that cardiac NO content was also unchanged. This finding; however, is consistent with our studies showing that the addition of authentic BH4 (Vásquez-Vivar et al., 2008) or sepiapterin (Ionova et al., 2008) to isolated rat cardiomyocytes stimulated with inflammatory cytokines failed to increase NO production from iNOS or to decrease iNOS monomer levels. Thus, the findings in the in vivo cardiac model alloimmune inflammation complement and extend the findings seen in cytokine-stimulated cardiomyocytes.
Of significant importance to this understanding were our findings that adult rat cardiomyocytes lack both mRNA and protein expression for dihydrofolate reductase (Ionova et al., 2008). Dihydrofolate reductase is a critical step that is necessary for conversion back to BH4 of the BH2 formed after transport of both BH4 and sepiapterin into the cell (Sawabe et al., 2004). Lack of dihydrofolate reductase would be predicted to cause intracellular buildup of BH2 after administration of sepiapterin. Indeed, we found that there was a large increase in intracardiac BH2 levels that might be expected to offset any benefits on increased NO production and iNOS homodimerization due to increased BH4. The finding of increase in BH2 in the present in vivo study is similar to that found previously by our laboratory in isolated cardiomyocytes treated with sepiapterin (Ionova et al., 2008). Our findings do not exclude the possibility that sepiapterin may be increasing NO in other cell types that express dihydrofolate reductase in vivo (e.g., endothelial cells). However, if this occurred in endothelial cells, any overall increase in intragraft content of NO due to sepiapterin may not be detected because endothelial cells contribute only a small proportion of total graft NO levels.
Although sepiapterin clearly increased intragraft BH4 levels, the benefits to decreasing rejection and apoptosis occurred independently of any changes in alloimmune-induced levels of intracardiac iNOS protein, iNOS homodimerization, or NO levels. This finding would not be expected by conventional theory derived from other tissues or cells but could be explained if a large part of the increased intracardiac BH4 levels produced via sepiapterin occurred in noncardiomyocyte cells such as cardiac fibroblasts rather than in cardiomyocytes, which is a prime location of increased iNOS expression in this model. This indicates a unique mechanism of action of sepiapterin in vivo that is more complex than simply increasing NO bioactivity.
The findings in the present study of rat cardiac allografts are consistent with the antirejection activity of authentic BH4 or its 4-amino derivative in a murine model of acute cardiac rejection (Brandacher et al., 2001, 2006). Of interest is that the 4-amino analog, which inhibits catalytic activity of purified iNOS protein, unlike BH4, also had an antirejection effect in the mouse model. Together with the present findings using sepiapterin, the results suggest that these derivatives have antirejection actions via some other process independently of increased intracellular BH4 and/or NO production.
Previous studies have shown that genetic and pharmacological strategies that limit nitration also improve rejection (Szabolcs et al., 2001, 2002; Nilakantan et al., 2005; Pieper et al., 2005). In the present study, our findings using sepiapterin are consistent with the notion that protein nitration and rejection are linked. Regarding oxidative stress, the present study is the first to show oxidative stress in acute cardiac allograft rejection using 4-HNE. Our findings suggest that sepiapterin decreased rejection in part by decreasing oxidative stress. Because of the inherit deficiencies in BH4 synthesis and NO production via iNOS in adult cardiomyocytes per se, we suggest that the decrease in nitrative and oxidative stress due to sepiapterin treatment is probably more related to an external effect of recipient immune cells on cardiomyocytes in this model.
The mechanism of protection by sepiapterin did not involve changes in expression for the two major inflammatory cytokines TNF-α and IFN-γ, in agreement with findings using authentic BH4 in mouse cardiac transplants (Brandacher et al., 2006). This does not exclude the possibility that sepiapterin improved rejection by some other redundant gene pathway. An intriguing possibility for the antirejection mechanism of sepiapterin is via an effect on T-cell function (Thoeni et al., 2005). In this context, we showed that sepiapterin decreased IL-2 expression, an important product of T-cell activation and a growth factor for T cells. Thus, the decrease in rejection by sepiapterin could be explained at the immune cell level by inhibiting IL-2-mediated T-cell proliferation and expansion.
Acknowledgments
We acknowledge the technical assistance of Gail Hilton and Chao-Ying Chen.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL078937, HL067244] (to G.M.P. and J.V.V., respectively).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.148569.
ABBREVIATIONS: iNOS, inducible nitric-oxide synthase; BH4, tetrahydrobiopterin; GTPCH, GTP cyclohydrolase 1; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; 4-HNE, 4-hydroxy-2-nonenal; LV, left ventricular; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; FS, fractional shortening; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PCR, polymerase chain reaction; RT, reverse transcription; BH2, dihydrobiopterin; HPLC, high-performance liquid chromatography; NOx, total sum of nitrite + nitrate; TNF, tumor necrosis factor; IFN, interferon; IL, interleukin.
References
- Baek KJ, Thiel BA, Lucas S, and Stuehr DJ (1993) Macrophage nitric oxide synthase subunits. Purification, characterization and role of prosthetic groups and substrate I regulating their association into dimeric enzyme. J Biol Chem 268 21120-21129. [PubMed] [Google Scholar]
- Brandacher G, Maglione M, Schneeberger S, Obrist P, Thoeni G, Wrulich OA, Werner-Felmayer G, Margreiter R, and Werner ER (2006) Tetrahydrobiopterin compounds prolong allograft survival independently of their effect on nitric oxide synthase activity. Transplantation 81 583-589. [DOI] [PubMed] [Google Scholar]
- Brandacher G, Zou Y, Obrist P, Steurer W, Werner-Felmayer G, Margreiter R, and Werner ER (2001) The 4-amino analogue of tetrahydrobiopterin efficiently prolongs murine cardiac allograft survival. J Heart Lung Transplant 20 747-749. [DOI] [PubMed] [Google Scholar]
- de Bono JP, Warrick N, Bendall JK, Channon KM, and Alp NJ (2007) Radiochemical HPLC detection of arginine metabolism: measurement of nitric oxide synthesis and arginase activity in vascular tissue. Nitric Oxide 16 1-9. [DOI] [PubMed] [Google Scholar]
- de Groot-Kruseman HA, Baan CC, Hagman EM, Mol WM, Niesters HG, Maat AP, Vantrimpont PJ, Zondervan PE, Weimar W, and Balk AH (2002) Sequential monitoring of intragraft cytokine mRNA expression in relation to diastolic left ventricular wall thickness and function early after heart transplantation. Clin Transplant 16 433-441. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, and Reichek N (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57 450-458. [DOI] [PubMed] [Google Scholar]
- Geller DA, Di Silvio M, Billiar TR, and Hatakeyama K (2000) GTP cyclohydrolase I is coinduced in hepatocytes stimulated to produce nitric oxide. Biochem Biophys Res Commun 276 633-641. [DOI] [PubMed] [Google Scholar]
- Gross SS and Levi R (1992) Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem 267 25722-25729. [PubMed] [Google Scholar]
- Huisman A, Vos I, van Faassen EE, Joles JA, Gröne HJ, Martasek P, van Zonneveld AJ, Vanin AF, and Rabelink TJ (2002) Anti-inflammatory effects of tetrahydrobiopterin on early rejection in renal allografts: modulation of inducible nitric oxide synthase. FASEB J 16 1135-1137. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC.
- Ionova IA, Vásquez-Vivar J, Whitsett J, Herrnreiter A, Medhora M, Cooley BC, and Pieper GM (2008) Deficient BH4 production via de novo and salvage pathways regulates NO responses to cytokines in adult cardiac myocytes. Am J Physiol Heart Circ Physiol 295 H2178-H2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivendi S, Hatakeyama K, Whitsett J, Konorev E, Kalyanaraman B, and Vásquez-Vivar J (2005) Changes in tetrahydrobiopterin levels in endothelial cells and adult cardiomyocytes induced by LPS and hydrogen peroxide. A role for GFRP? Free Radic Biol Med 38 481-491. [DOI] [PubMed] [Google Scholar]
- Kawauchi M, Boucek MM, Gundry SR, Kanakriyeh MS, de Begona JA, Razzouk AJ, and Bailey LL (1992) Changes in left ventricular mass with rejection after heart transplantation in infants. J Heart Lung Transplant 11 99-102. [PubMed] [Google Scholar]
- Khanna AK, Plummer MS, Hilton G, Pieper GM, and Ledbetter S (2004) Anti-transforming growth factor antibody at low but not high doses limits cyclosporine-mediated nephrotoxicity without altering rat cardiac allograft survival: potential of therapeutic application. Circulation 110 3822-3829. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18 1440-1463. [DOI] [PubMed] [Google Scholar]
- Migrino RQ, Aggarwal D, Konorev E, Brahmbhatt T, Bright M, and Kalyanaraman B (2008) Early detection of doxorubicin cardiomyopathy using two-dimensional strain echocardiography. Ultrasound Med Biol 34 208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migrino RQ, Zhu X, Pajewski N, Brahmbhatt T, Hoffmann R, and Zhao M (2007) Assessment of segmental myocardial viability using regional 2-dimensional strain echocardiography. J Am Soc Echocardiogr 20 342-351. [DOI] [PubMed] [Google Scholar]
- Mondillo S, Maccherini M, and Galderisi M (2008) Usefulness of transthoracic echocardiography in heart transplantation recipients. Cardiovasc Ultrasound 6 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilakantan V, Halligan NL, Nguyen TK, Hilton G, Khanna AK, Roza AM, Johnson CP, Adams MB, Griffith OW, and Pieper GM (2005) Post-translational modification of manganese superoxide dismutase in acutely rejecting cardiac transplants: role of inducible nitric oxide synthase. J Heart Lung Transplant 24 1591-1599. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Cooper M, Johnson CP, Adams MB, Felix CC, and Roza AM (2000) Reduction of myocardial nitrosyl complex formation by a nitric oxide scavenger prolongs cardiac allograft survival. J Cardiovasc Pharmacol 35 114-120. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Khanna AK, Kampalath BN, Felix CC, Hilton G, Johnson CP, Adams MB, and Roza AM (2004) Inhibition of nitrosylation, nitration, lymphocyte proliferation, and gene expression in acute and delayed cardiac allograft rejection by an orally active dithiocarbamate. J Cardiovasc Pharmacol 43 522-530. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Nilakantan V, Halligan NL, Khanna AK, Hilton G, and Vásquez-Vivar J (2005) Nitric oxide formation in acutely rejecting cardiac allografts correlates with GTP cyclohydrolase I activity. Biochem J 391 541-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper GM, Nilakantan V, Nguyen TK, Hilton G, Roza AM, and Johnson CP (2008) Reactive oxygen and reactive nitrogen as signaling molecules for caspase 3 activation in acute cardiac transplant rejection. Antioxid Redox Signal 10 1031-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper GM and Roza AM (2008) The complex role of iNOS in acutely rejecting cardiac transplants. Free Radic Biol Med 44 1536-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper GM, Roza AM, Adams MB, Hilton G, Johnson M, Felix CC, Kampalath B, Darkes M, Wanggui Y, Cameron B, et al. (2002) A ruthenium (III) polyaminocarboxylate complex, a novel nitric oxide scavenger, enhances graft survival and decreases nitrosylated heme protein in models of acute and delayed cardiac transplant rejection. J Cardiovasc Pharmacol 39 441-448. [DOI] [PubMed] [Google Scholar]
- Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, and Caldwell RW (2008) Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res 102 95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Suzuki H, Oda H, Akaike T, Azuma Y, Murakami T, Sugi K, Ito T, Ichinose H, Koyasu S, et al. (2006) Phosphoinositide 3-kinase in nitric oxide synthesis in macrophage. Critical dimerization of inducible nitric-oxide synthase. J Biol Chem 281 17736-17742. [DOI] [PubMed] [Google Scholar]
- Sawabe K, Wakasugi KO, and Hasegawa H (2004) Tetrahydrobiopterin uptake in supplemental administration: elevation of tissue tetrahydrobiopterin in mice following uptake of the exogenously oxidized product 7,8-dihydrobiopterin and subsequent reduction by an anti-folate-sensitive process. J Pharmacol Sci 96 124-133. [DOI] [PubMed] [Google Scholar]
- Schmidt TS and Alp NJ (2007) Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci 113 47-63. [DOI] [PubMed] [Google Scholar]
- Stoylen A, Heimdal A, Bjornstad K, Torp HG, and Skjaerpe T (1999) Strain rate imaging by ultrasound in the diagnosis of regional differences of the left ventricle. Echocardiography 16 321-329. [DOI] [PubMed] [Google Scholar]
- Szabolcs MJ, Ma N, Athan E, Zhong J, Ming M, Sciacca RR, Husemann J, Albala A, and Cannon PJ (2001) Acute cardiac allograft rejection in nitric oxide synthase-2-/- and nitric oxide synthase-2+/+ mice. Effects of cellular chimeras on myocardial inflammation and cardiomyocyte damage and apoptosis. Circulation 103 2514-2520. [DOI] [PubMed] [Google Scholar]
- Szabolcs MJ, Sun J, Ma N, Albala A, Sciacca RR, Philips GB, Parkinson J, Edwards N, and Cannon PJ (2002) Effects of selective inhibitors of nitric oxide synthase-2 dimerization on acute cardiac allograft rejection. Circulation 106 2392-2396. [DOI] [PubMed] [Google Scholar]
- Teichholz LE, Kreulen T, Herman MV, and Gorlin R (1976) Problems in echocardiographic-angiographic correlations in the presence or absence of synergy. Am J Cardiol 37 7-11. [DOI] [PubMed] [Google Scholar]
- Thoeni G, Stoitzner P, Brandacher G, Romani N, Heufler C, Werner-Felmayer G, and Werner ER (2005) Tetrahydro-4-aminobiopterin attenuates dendritic cell-induced T cell priming independently from inducible nitric oxide synthase. J Immunol 174 7584-7591. [DOI] [PubMed] [Google Scholar]
- Tzeng E, Billiar TR, Robbins PD, Loftus M, and Stuehr DJ (1995) Expression of human inducible nitric oxide synthase in a tetrahydrobiopterin (H4B)-deficient cell line: H4B promotes assembly of enzyme subunits into an active dimer. Proc Natl Acad Sci U S A 92 11771-11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez-Vivar J, Whitsett J, Ionova I, Konorev E, Zielonka J, Kalyanaraman B, Shi Y, and Pieper GM (2008) Cytokines and lipopolysaccharides induce inducible nitric oxide synthase but not enzyme activity in adult rat cardiomyocytes. Free Radic Biol Med 45 994-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett J, Picklo MJ Sr, and Vasquez-Vivar J (2007) 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler Thromb Vasc Biol 27 2340-2347. [DOI] [PubMed] [Google Scholar]
- Zhang P, Xu X, Hu X, van Deel ED, Zhu G, and Chen Y (2007) Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res 100 1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YQ, Bishay R, Feintuch A, Tao K, Golding F, Zhu W, West LJ, and Henkelman RM (2007) Morphological and functional evaluation of murine heterotopic cardiac grafts using ultrasound biomicroscopy. Ultrasound Med Biol 33 870-879. [DOI] [PubMed] [Google Scholar]