Abstract
Auditory afferent fibre activity in mammals relies on neurotransmission at hair cell ribbon synapses. Developmental changes in the Ca2+ sensitivity of the synaptic machinery allow inner hair cells (IHCs), the primary auditory receptors, to encode Ca2+ action potentials (APs) during pre-hearing stages and graded receptor potentials in adult animals. However, little is known about the time course of these changes or whether the kinetic properties of exocytosis differ as a function of IHC position along the immature cochlea. Furthermore, the role of afferent transmission in outer hair cells (OHCs) is not understood. Calcium currents and exocytosis (measured as membrane capacitance changes: ΔCm) were measured with whole-cell recordings from immature gerbil hair cells using near-physiological conditions. The kinetics, vesicle pool depletion and Ca2+ coupling of exocytosis were similar in apical and basal immature IHCs. This could indicate that possible differences in AP activity along the immature cochlea do not require synaptic specialization. Neurotransmission in IHCs became mature from postnatal day 20 (P20), although changes in its Ca2+ dependence occurred at P9–P12 in basal and P12–P15 in apical cells. OHCs showed a smaller ΔCm than IHCs that was reflected by fewer active zones in OHCs. Otoferlin, the proposed Ca2+ sensor in cochlear hair cells, was similarly distributed in both cell types despite the high-order exocytotic Ca2+ dependence in IHCs and the near-linear relation in OHCs. The results presented here provide a comprehensive study of the function and development of hair cell ribbon synapses.
Sensory cells of hearing organs transduce sound into electrical responses. Two different sensory cells are present in the mammalian cochlea: inner hair cells (IHCs) and outer hair cells (OHCs). IHCs, the primary sensory receptors, respond to sound stimuli with sustained and graded receptor potentials. These responses are relayed to the brain with temporal precision (Fuchs, 2005) via the coordinated release of vesicles from IHC ribbon synapses onto type I spiral ganglion neurons (Glowatzki et al. 2008). Pre-hearing IHCs (≤P12 in most rodents) do not respond to sound but instead generate spontaneous Ca2+-dependent action potentials (APs: Marcotti et al. 2003). Intracellular Ca2+ signalling associated with APs could regulate a variety of cellular responses involved in the cell's functional differentiation and/or maturation (Berridge et al. 2000; Spitzer et al. 2000), as proposed for the exocytotic machinery (Johnson et al. 2007). Neurotransmitter release from pre-and post-hearing IHCs is controlled by Ca2+ influx through L-type CaV1.3 Ca2+ channels (Platzer et al. 2000; Brandt et al. 2003), known to cluster at the cell's presynaptic site (Roberts et al. 1990; Tucker & Fettiplace, 1995). In contrast to IHCs, mature OHCs provide electromechanical amplification of the cochlear partition (Dallos, 1992) that is modulated by efferent fibres representing the majority of OHC synaptic connections (Guinan, 1996). However, during early postnatal development OHCs are transiently innervated by the same afferent fibres as IHCs (Pujol et al. 1998), express CaV1.3 Ca2+ channels (Michna et al. 2003) and show exocytotic responses (Beurg et al. 2008), indicating the possible presence of functional afferent synapses.
The synaptic machinery at mammalian hair cell ribbon synapses undergoes morphological (Sobkowicz et al. 1982) and biophysical (Beutner & Moser, 2001; Johnson et al. 2005) change with development in order to transmit the different physiological responses generated by pre-(spontaneous APs) and post-hearing (graded receptor potentials) cells. One example of functional maturation is the steeper exocytotic Ca2+ dependence of immature IHCs (Ca2+ cooperativity of about 4) compared to that observed in adult cells (mouse: Johnson et al. 2005; gerbils: Johnson et al. 2008). Although this Ca2+ dependence was similar in IHCs along the immature cochlea, tonotopic differences in the kinetics and Ca2+ sensitivity of exocytosis were present in adult gerbil IHCs (Johnson et al. 2008), which could enhance the signalling of receptor potentials in the low-frequency (phasic) and high-frequency (tonic) cells. Despite the investigations on gerbils and mice, there remains a shortage of information regarding the developmental time course for changes in the exocytotic Ca2+ sensitivity in IHCs as a function of their position along the mammalian cochlea. Moreover, very little is known about the kinetic properties and topographic organization of the synaptic machinery in immature hair cells. Therefore, the aims of the present study were to determine whether tonotopic differences in the kinetics of exocytosis and its Ca2+ coupling exist in immature IHCs and to study the maturation of the synaptic Ca2+ sensitivity. Finally, we investigated whether exocytosis in apical and basal immature OHCs differs from that of IHCs. The information presented provides the first biophysical and tonotopic correlation of hair cell ribbon synapse functional development before and at around the onset of hearing in mammals. All recordings, apart from those designed to investigate OHCs, were performed in near physiological conditions (body temperature and using 1.3 mm extracellular Ca2+) to ensure a more realistic estimation of exocytosis at mammalian hair cell ribbon synapses.
Methods
The methods have been described fully before (Johnson et al. 2008) but important details are specified below.
Electrophysiology
Tissue preparation
Apical-and basal-coil IHCs (n= 78) from gerbils were studied in acutely dissected cochleae from postnatal day 5 (P5) to P16, where the day of birth is P0. This includes 13 IHCs (apical n= 6, P5–P6; basal: n= 7, P6–P7), used to determine the kinetics of exocytosis in Fig. 1C and D, that have been imported from Johnson et al. (2008) where they were only used to measure the Ca2+ dependence of vesicle release. The gerbil was preferred to the more commonly used mouse because of its extended low-frequency hearing range would emphasize any tonotopic differences (adult gerbil: ∼0.1–60 kHz; adult mouse: ∼2–100 kHz: Greenwood, 1990; Müller, 1996). A few P3 OHCs from gerbils (n= 9), C57B mice (apical-coil: n= 6) and Wistar rats (apical-coil: n= 6) were also investigated. All animals used for electrophysiology and some of those used for immunolabelling experiments were killed by cervical dislocation in accordance with UK Home Office regulations. The cochleae were dissected using the following extracellular solution (in mm): 135 NaCl, 5.8 KCl, 1.3 CaCl2, 0.9 MgCl2, 0.7 NaH2PO4, 5.6 d-glucose, 10 Hepes-NaOH, 2 sodium pyruvate. Amino acids and vitamins were added from concentrates (Eagle's minimal essential medium). The pH was adjusted to 7.5. The approximate position of apical and basal hair cells was measured as a fractional distance from the cochlear apex (for details see Johnson et al. 2008).
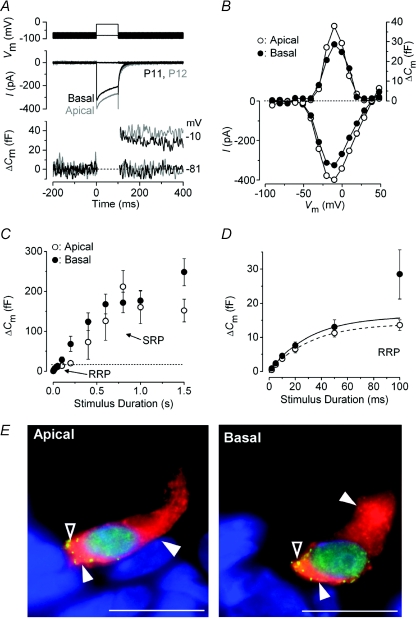
Figure 1. Neurotransmitter release in immature IHCs.
A, ICa (middle panel) and ΔCm (bottom panel) responses recorded from apical (grey traces) and basal (black traces) IHCs of the gerbil cochlea at around the onset of hearing. Recordings were obtained in response to 100 ms voltage steps from the holding potential of −81 mV to near −10 mV. The voltage protocol is shown in the top panel. B, current–voltage (I–V, bottom panel) and capacitance–voltage (ΔCm–V, top panel) curves from the IHCs shown in A. Recordings were obtained in response to 10 mV depolarizing voltage steps (100 ms) from −81 mV. C, average ΔCm responses from six apical (P5–P6) and ten basal (P6–P7) IHCs. Recordings were obtained by applying voltage steps from 2 ms to 1.5 s (to around −11 mV) in order to elicit both the RRP and SRP. D, expanded version of the first 100 ms shown in C where data up to 50 ms have been approximated with single exponential functions (apical: maximum ΔCm= 14.1 ± 2.7 fF, τ= 30.7 ± 12.5 ms; basal: maximum ΔCm= 16.3 ± 4.2 fF, τ= 30.9 ± 17.2 ms). The RRP consisted of 400 ± 45 (apical: n= 6, P5–P6) and 510 ± 96 (basal: n= 10, P6–P7) synaptic vesicles using a conversion factor of 37 aF per vesicle (Lenzi et al. 1999). Unless otherwise stated, in this and the following figures recordings were obtained at body temperature and using physiological 1.3 mm extracellular Ca2+. E, immunolocalization of synaptic ribbons (CtBP2: green dot-like pattern indicated by open arrowheads) and otoferlin (red: filled arrowheads) in apical and basal P4 IHCs. Nuclei were stained with DAPI (blue). Scale bars represent: 10 μm.
Patch clamp recording
All patch clamp recordings were performed near body temperature (34–37°C) using an Optopatch amplifier (Cairn Research Ltd, Faversham, UK). Patch pipettes were made from soda glass capillaries (2–3 MΩ) and coated with surf wax. The patch pipette filling solution contained (in mm): 106 caesium glutamate, 20 CsCl, 3 MgCl2, 1 EGTA-CsOH, 5 Na2ATP, 0.3 Na2GTP, 5 Hepes-CsOH, 10 Na2-phosphocreatine (pH 7.3). For some experiments BAPTA (0.1 mm, 0.6 mm, 1 mm) was used instead of 1 mm EGTA. Command protocols were applied and data acquired using pCLAMP software and a Digidata 1322A (Molecular Devices, Sunnyvale, CA, USA) and analysed with Origin (OriginLab Corp., Northampton, MA, USA). Synaptic vesicle exocytosis was measured as an increase in cell membrane capacitance (ΔCm) that is generally interpreted as a sign of neurotransmitter release from presynaptic cells (Parsons et al. 1994; Moser & Beutner, 2000). Real-time ΔCm was measured as previously described (Johnson et al. 2005). Briefly, a 4 kHz sine wave of 13 mV RMS was applied to cells from −81 mV and was interrupted for the duration of the voltage step. The capacitance signal from the Optopatch was amplified (×50), filtered at 250 Hz, sampled at 5 kHz and measured by averaging the Cm traces after the voltage step (around 200 ms) and subtracting from pre-pulse baseline. Experiments were performed during the superfusion of 30 mm TEA and 15 mm 4-AP to block most of the K+ currents. Apamin (300 nm) and linopirdine (80–100 μm) were also added to the solution in order to block specifically ISK and IK,n, respectively. Calcium currents were corrected off-line for the linear leak conductance (gleak) typically calculated between −81 mV and −71 mV (P5–P16 IHCs: 2.3 ± 0.1 nS, n= 77; P3 gerbil OHCs: 0.7 ± 0.1 nS, n= 9; P3 mouse OHCs: 1.0 ± 0.2 nS, n= 6; P3 rat OHCs: 1.0 ± 0.1 nS, n= 6). Membrane potentials were corrected for the voltage drop across the uncompensated series resistance (P5–P16 IHCs: 5.8 ± 0.1 MΩ, n= 78; gerbil OHCs: 6.5 ± 0.6 MΩ, n= 9; mouse OHCs: 6.7 ± 0.5 MΩ, n= 6; rat OHCs: 7.3 ± 0.2 MΩ, n= 6) and for a liquid junction potential (−11 mV). IHC membrane capacitance (Cm) was 8.8 ± 0.2 pF (P5–P16: n= 78). OHC Cm was 6.2 ± 0.3 pF in gerbils (n= 9), 6.0 ± 0.2 pF in mice (n= 6) and 6.9 ± 0.8 pF in rats (n= 6).
Statistical comparisons of means were made by Student's two-tailed t-test or, for multiple comparisons, analysis of variance, one-way ANOVA followed by Tukey's test. Two-way ANOVA, followed by Bonferroni's test, was used to compare data sets from apical and basal IHCs. Means are quoted ±s.e.m. and P < 0.05 indicates statistical significance.
Immunocytochemistry
Immunostaining of gerbil IHCs was performed using otoferlin and CtBP2 antibodies as previously described (Schug et al. 2006; Johnson et al. 2008). Animals not processed in the UK were killed by CO2 asphyxiation in accordance with the ethical guidelines approved by the University of Tübingen and the Tierschutzgesetz (Germany). Sections were viewed using an Olympus AX70 microscope equipped with epifluorescence illumination and motorization in the z-axis. Images were acquired using a CCD camera and the imaging software Cell∧F (OSIS GmbH, Münster, Germany). For ribbon counts, cryo-sectioned cochleae were imaged over a distance of 8 μm with the complete coverage of the IHC nucleus and beyond in an image-stack along the z-axis (z-stack). Typically the z-stack consisted of 30 layers with a z-increment of 0.276 μm, for each layer one image per fluorochrome was acquired. To perform ribbon counts, z-stacks were three-dimensionally deconvoluted using Cell∧F's RIDE module with the Nearest Neighbour algorithm and VoxelViewer. Figures 1E and 6D are representative single-layer images at the hair-cell nuclear plane. CtBP2 immunopositive ribbons and otoferlin staining were from apical and mid-basal immature IHCs from three gerbils (P4, P7 and P8). For each age tested between two and nine IHCs and one and five OHCs were analysed.
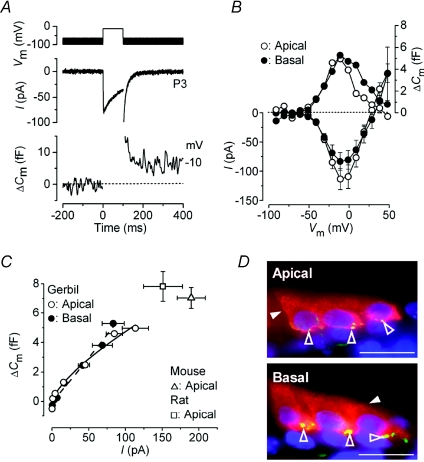
Figure 6. Neurotransmitter release in immature gerbil OHCs.
A, ICa and ΔCm responses recorded from an apical P3 gerbil OHC. Voltage protocol as described in Fig. 1A but using 5 mm extracellular Ca2+. Traces are the average of three protocol repetitions. B, average peak I–V and ΔCm–V curves from apical and basal OHCs (P3, n= 4 for each) using depolarizing voltage steps in 10 mV increments from −81 mV. The ΔCm values do not have s.e.m. since they were directly measured from the averaged traces of pooled cells. C, synaptic transfer functions for apical and basal OHCs. Average ΔCm was plotted against the corresponding ICa, between −71 mV and near −10 mV, from the I–V and ΔCm–V curves shown in B. Fits to the data points are according to eqn (1). The open triangle and square indicate the peak responses obtained from C57B mouse (n= 6) and rat (n= 6) apical OHCs, respectively. D, immunolocalization of active zones (green: open arrowheads) and otoferlin (red: closed arrowheads) in apical and basal gerbil OHCs (P4). The number of active zones in OHCs was: 1st row 8.9 ± 1.1 (n= 8), 2nd row 8.6 ± 1.4 (n= 8), 3rd row 9.0 ± 1.3 (n= 5); in basal OHCs was: 1st row 9.3 ± 1.0 (n= 8), 2nd row 10.9 ± 0.7 (n= 9), 3rd row 11.0 ± 0.7 (n= 8).
Results
Neurotransmitter release from pre-hearing gerbil IHCs
Tonotopic differences in the kinetic properties of synaptic vesicle release have recently been shown in adult gerbil IHCs (Johnson et al. 2008). Therefore, in this study we first investigated whether such kinetic differences were already present during pre-hearing stages (≤P12). A typical example of Ca2+ currents (ICa) and the corresponding ΔCm recorded from immature apical-and basal-coil gerbil IHCs is shown in Fig. 1A and B. Responses were obtained using a 100 ms depolarizing voltage step (10 mV nominal increments) from −81 mV. The kinetics of neurotransmitter release in pre-hearing IHCs (P5–P7) was investigated by measuring ΔCm near the peak ICa by varying the stimulus duration (from 2 ms to 1.5 s), which allowed the investigation of synaptic vesicle pool emptying (Fig. 1C). While relatively short stimuli revealed the number of vesicles in the readily releasable pool (RRP), longer steps induced the release of vesicles from a secondarily releasable pool (SRP) that is located further away from the Ca2+ channels (Moser & Beutner, 2000). Voltage steps of up to about 100 ms in apical and 50 ms in basal IHCs seemed to recruit mainly the RRP since ΔCm responses could be approximated with a single exponential (Fig. 1D). The higher ΔCm measured at 100 ms from some basal IHCs is likely to be caused by the earlier recruitment of the SRP. This was responsible for the apparently higher Ca2+ efficiency of exocytosis in early postnatal basal IHCs compared to age-matched apical cells (Johnson et al. 2008). The available RRP consisted of 400 and 510 synaptic vesicles in apical and basal IHCs, respectively, and had a similar initial release rate (Table 1). In order to determine whether the single ribbon synapse vesicle release rate varied as a function of IHC position along the cochlea, we performed ribbon counts on immature gerbil IHCs (Fig. 1E) using an antibody against the presynaptic ribbon component RIBEYE (CtBP2: Schmitz et al. 2000). The number of synaptic ribbons per IHC was similar in both regions of the cochlea (apical IHCs: 36.4 ± 1.2, n= 15; basal: 37.3 ± 0.8, n= 16) giving a release rate per ribbon of 355 and 360 vesicles s−1 in apical and basal IHCs, respectively, significantly different from that measured in basal adult cells only (Table 1: P < 0.005). The estimated number of readily releasable vesicles per ribbon was 10 in apical and 14 in basal immature IHCs, assuming that each active zone contributes equally to transmitter release. The size of the SRP (Fig. 1C) was also similar among immature IHCs and showed signs of saturation within the 1.5 s tested, not observed in adult IHCs (Johnson et al. 2008). An estimation of the SRP release rate was obtained by fitting the linear range of the data points (Fig. 1C, apical: 200–800 ms; basal: 100–600 ms). Linear regression indicated slopes of 216 vesicles s−1 (apical IHCs) and 214 vesicles s−1 (basal) per ribbon.
Table 1.
Synaptic ribbon properties in gerbil hair cells
| Immature OHCs (3rd row) | Immature IHCs | Adult IHCs | ||||
|---|---|---|---|---|---|---|
| Apical | Basal | Apical | Basal | Apical | Basal | |
| Ca2+ dependence of exocytosis (power N) | 0.7 | 1.1 | 3.6 | 3.9 | 2.2 | 1.0 |
| Ribbon no. | 18 | 22 | 36 | 37 | 21 | 22 |
| Vesicles in the total RRP | 135 | 143 | 400 | 510 | 534 | 619 |
| RRP vesicles per ribbon | 8 | 7 | 11 | 14 | 26 | 28 |
| RRP release rate (vesicles s−1) | — | — | 12 911 | 13 425 | 10 449 | 14 883 |
| RRP release rate per ribbon (vesicles s−1) | — | — | 355 | 360 | 507 | 664 |
| SRP release rate (vesicles s−1) | — | — | 8208 | 7933 | 2335 | 1695 |
| SRP release rate per ribbon (vesicles s−1) | — | — | 216 | 214 | 111 | 77 |
| SRP depletion time constant (s) | — | — | 8.6 | 4.8 | 12.0 | 21.3 |
All numbers reported are mean values. s.e.m. and cell numbers for immature data are given in Results. Adult values are obtained or calculated from Johnson et al. 2008. For immature OHCs, recording were only performed from the outer row (3rd row) of cells. The ribbon number and RRP vesicles per ribbon in immature OHCs are based on the estimation of two ribbons per active zone (see Results). OHC exocytotic kinetics were not measured. We did not observe any RRP depletion in both immature and adult IHCs using 50 ms voltage steps (at about 7 Hz).
Figure 1E also shows the distribution of otoferlin, the proposed Ca2+ sensor of exocytosis in cochlear hair cells (Roux et al. 2006; Beurg et al. 2008). Otoferlin was detected throughout the cytoplasm of immature IHCs with a higher expression level at the active zones (in the same region as RIBEYE) and usually below the cell's cuticular plate, where synaptic vesicle internalization is likely to occur (Griesinger et al. 2005). Although some variability in staining intensity was observed among preparations, we could not detect any consistent difference in the expression of otoferlin between apical and basal IHCs (judged from the qualitative observations of more than 50 sections). This labelling pattern was also similar to that previously described for IHCs of adult gerbils (Johnson et al. 2008), rats and mice (Schug et al. 2006).
Vesicle pool depletion in apical and basal immature IHCs
Immature IHCs are known to produce trains of spontaneous Ca2+ action potentials (Marcotti et al. 2003), the frequency of which could increase along the length of the cochlea up to a maximum of just below 10 Hz in basal cells (Johnson & Marcotti, 2008). The ability of these IHCs to maintain bouts of vesicle release was investigated by using repetitive stimuli of different durations (designed to release predominantly RRP or SRP: Fig. 2A and B, respectively). These experiments were carried out in order to determine whether vesicle pool refilling could become rate limiting for vesicle release in these cells. Following repeated 50 ms steps (stimulus frequency about 7 Hz), which induce RRP exocytosis in IHCs and correspond to about the width of an action potential (Marcotti et al. 2003), the cumulative ΔCm showed a near linear increase (Fig. 2C). This indicates that both apical and basal IHCs were able to replenish their RRP following each step. The overall individual ΔCm (Fig. 2E) was found to be similar between apical (7.2 ± 0.4 fF, n= 6) and basal (7.9 ± 0.3 fF, n= 11) immature IHCs. Following repetitive long-lasting (1 s) voltage steps (200 ms interstep intervals), the cumulative ΔCm showed saturation of SRP release in both apical and basal IHCs (Fig. 2D), which was also evident from the individual ΔCm (Fig. 2F). The depletion time constant was 8.6 s and 4.8 s in apical and basal IHCs, respectively. The absence of the initial potentiation of SRP exocytosis in pre-hearing IHCs, which was found in adult cells (Johnson et al. 2008), is likely to be due to the recruitment of the entire pool in response to the first step. Potentiation was evident by P16 in IHCs from both cochlear regions (data not shown). Although long-lasting (1 s) protocols are of less physiological relevance for immature spiking IHCs, it allowed us to compare the properties of SRP refilling to adult IHCs (Table 1).
Figure 2. Depletion of synaptic vesicle pools in immature IHCs.
A and B, ΔCm responses from basal-coil IHCs (P7) elicited using repetitive voltage steps to near −11 mV. The duration of the voltage step was selected in order to elicit the RRP (A: 50 ms) or the SRP (B: 1 s), with an interstep-interval of 100 ms or 200 ms, respectively. For clarity, only the first few steps are shown. The voltage protocol used is shown above the traces. C and D, average cumulative ΔCm values elicited using 50 (RRP) and 35 (SRP) steps from P7–P10 apical (RRP: n= 6; SRP: n= 7) and basal (RRP: n= 11; SRP: n= 10) IHCs, respectively. E and F, individual ΔCm values measured following each voltage step from panels C and D, respectively, in both apical and basal IHCs. Fits in F are according to a first-order exponential equation.
Developmental changes in the Ca2+ sensitivity of exocytosis
The Ca2+ sensitivity of neurotransmitter release in gerbil IHCs changes between immature and adult stages (Johnson et al. 2008). However, the time course of this transition is still unknown. In order to present a more comprehensive picture of the development in exocytotic Ca2+ sensitivity, values from immature (apical: P5–P6; basal: P6–P7) and adult (>P20) IHCs used in Figs 3 and 4C (see below) are derived from cells included in Johnson et al. (2008). Figure 3A shows the average size of ICa, measured near the peak of the I–V curves (see Fig. 1B), as a function of postnatal age and IHC position in the cochlea. In order to obtain a better representation of the change in ICa during the main stages of IHC functional development, values were pooled into three age groups (Fig. 3A and B: immature, intermediate and adult). Apical and basal IHCs showed a significant decrease in the size of ICa with development (Fig. 3B, overall P < 0.0001). However, during the same period, no significant difference in ΔCm was found between IHCs from both cochlear regions (Fig. 3C and D). The difference between the development of ICa and ΔCm was indicative of an increased Ca2+ efficiency of exocytosis (defined as ΔCm/ICa) with maturation as shown in Fig. 3E and F (overall P < 0.0001 for both apical and basal IHCs).
Figure 3. Changes in Ca2+ efficiency of exocytosis in developing gerbil IHCs.
A, size of ICa from near the peak of individual I–V curves (see Fig. 1B) as function of postnatal day. B, average size of ICa grouped into the three main stages of cochlear development shown in panel A: Immature, Intermediate and Adult. One-way ANOVA with Tukey's post hoc test indicates P < 0.001 for all combinations apart from immature vs. intermediate basal cells, which was not significant. C and D, development of ΔCm. E and F, development of the Ca2+ efficiency of exocytosis. In panel F the post hoc test indicates P < 0.01 or higher apart from immature vs. intermediate cells, which was not significant. Numbers of cells in panels A–F: apical (P5–P34): 2, 7, 1, 1, 4, 4, 5, 5, 6, 4, 3, 2, 3, 5, 6, 1; basal (P6–P30): 3, 6, 3, 4, 4, 4, 5, 5, 2, 4, 5, 3, 4, 3, 2, 1.
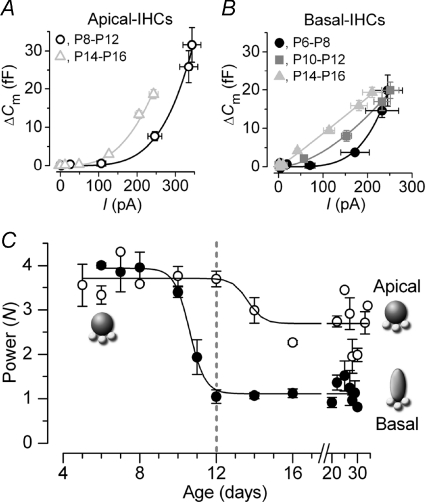
Figure 4. Changes in Ca2+ dependence of exocytosis in gerbil IHCs.
A and B, examples of synaptic transfer functions obtained by plotting average ΔCm against the corresponding ICa from I–V and ΔCm–V curves (as in Fig. 1B: voltage range –71 mV to −11 mV) of apical and basal IHCs, respectively. Data were grouped into age ranges at around the onset of hearing at P12. Fits are according to eqn (1). Values of N were: apical 4.3 (P8–P12, n= 12) and 3.0 (P14–P16, n= 10); basal 4.2 (P6–P8, n= 4), 1.7 (P10–P12, n= 12) and 1.1 (P14–P16, n= 10). C, developmental changes in N from both apical and basal IHCs as a function of days. Data points were obtained by averaging N values from fits to synaptic transfer functions of single cells using eqn (1). Note that basal IHCs start to mature a few days earlier than apical IHCs. The fits to the averaged data points are according to eqn (2). Numbers of cells: apical (P5–P34): 2, 7, 1, 1, 4, 4, 5, 5, 2, 1, 2, 3, 5, 2, 1; basal (P6–P30): 3, 6, 3, 4, 4, 4, 5, 5, 2, 2, 3, 2, 2, 2, 1.
The Ca2+ dependence of synaptic vesicle release in immature IHCs (Fig. 4), defined as the change in ΔCm as a function of ICa, was measured using the synaptic transfer curve (Augustine et al. 1985; Johnson et al. 2008). This was obtained by plotting ΔCm values against the corresponding ICa between −71 mV and −11 mV (see Fig. 1B). Data were approximated using a power function:
| (1) |
where c is a scaling coefficient and the power is N. For this comparison the peak ICa was used, instead of its time integral, to avoid possible errors due to the presence of the residual unblocked K+ current as previously described (Johnson et al. 2005, 2008). Figure 4A and B shows averaged transfer functions from apical and basal IHCs that have been grouped into pre-and post-hearing stages (see Fig. 4C for a better overview of the age range selected). For basal IHCs, an additional group showing the transfer function at the onset of hearing is included. A detailed representation of the developmental change in the exocytotic Ca2+ dependence as a function of days after birth is shown in Fig. 4C. Fits to the data in Fig. 4C are according to a sigmoidal logistic growth curve:
| (2) |
where k is a slope factor, thalf is the age where N is halfway between the maximal (Nmax) and minimal (Nmin) power. The fits gave values for thalf and k of P13.7 and 2.6 day−1 in apical and P10.6 and 2.5 day−1 in basal IHCs. The linearization in Ca2+ dependence occurred very rapidly from around the beginning of the second postnatal week in basal IHCs and was complete by the onset of hearing at P12. Contrary to basal IHCs, the high Ca2+ dependence of exocytosis in apical cells was only slightly reduced from just after the onset of hearing.
Functional coupling between Ca2+ channels and the RRP in pre-hearing IHCs
The developmental changes in the exocytotic Ca2+ dependence in apical and basal IHCs (Fig. 4) could arise from variations in the coupling between Ca2+ influx and the release-ready pool (RRP) of vesicles. In adult gerbil IHCs Ca2+ channels are localized within a nanodomain of the synaptic machinery (Johnson et al. 2008). Therefore, we investigated whether the higher exocytotic Ca2+ dependence in pre-hearing IHCs resulted from a weaker (microdomain) coupling to Ca2+ channels. The distance between Ca2+ channels and presynaptic release sites was estimated by measuring exocytosis in both apical and basal IHCs whilst using either intracellular EGTA (1 mm) or different concentrations of the faster Ca2+ chelator BAPTA (Fig. 5A–D), which is capable of buffering Ca2+ elevations closer to their source (Neher, 1998). For these experiments we used a similar voltage protocol to that described in Fig. 1D (voltage steps between 5 ms and 100 ms) in order to recruit mainly the RRP. The release of the RRP was unaffected when pre-hearing IHCs were buffered with 0.1 and 0.6 mm BAPTA (Fig. 5E). However, 1 mm BAPTA almost completely blocked RRP exocytosis in both cochlear regions (apical: P < 0.01; basal: P < 0.001; relative to recordings in 1 mm EGTA). This indicates a similar distance between Ca2+ channels and release sites in immature IHCs positioned at each end of the cochlea (in the range of 40 nm: Naraghi & Neher, 1997).
Figure 5. Coupling between Ca2+ channels and the RRP in immature gerbil IHCs.
A and B, ΔCm recordings from apical (A) and basal (B) immature IHCs in response to a 100 ms voltage step (from −81 mV to around −11 mV) using 0.1 mm, 0.6 mm and 1 mm intracellular BAPTA. C and D, average ΔCm from apical and basal IHCs, respectively, in response to voltage steps between 5 ms and 100 ms using the above BAPTA concentrations. E, average ΔCm (top panel) and peak ICa (bottom panel) at 50 ms, in order to measure the isolated RRP, from data shown in C and D. ΔCm values obtained in EGTA from Fig. 1C were also included. Number of cells in EGTA (1 mm) and BAPTA (0.1, 0.6, 1 mm) are: apical: 6, 3, 3, 3 (P5–P8); basal: 10, 1, 4, 4 (P6–P8).
Neurotransmitter release in immature OHCs
A recent investigation has shown that pre-hearing OHCs from Otofoferlin (Otof) control mice exhibit robust neurotransmitter release (Beurg et al. 2008). This led us to look for possible position-dependent differences in OHC exocytosis using immature P3 gerbils. Initially, we performed experiments using physiological 1.3 mm extracellular Ca2+. Under these experimental conditions ΔCm was very small and could not be accurately measured. Therefore, ICa and ΔCm were recorded from OHCs in 5 mm extracellular Ca2+ (Fig. 6A). Despite this, ΔCm was still relatively small and in order to obtain reliable measurements, especially for small ICa, traces recorded from all OHCs within each cochlear region were averaged. Higher Ca2+ concentrations could not be tested because they rapidly cause the preparation to deteriorate when working at body temperature (Johnson et al. 2005). The size of both the peak ICa (apical OHCs: −113 ± 18 pA, n= 4; basal OHCs: −83 ± 15 pA, n= 4) and maximal ΔCm (apical: 5.0 fF; basal: 5.3 fF), elicited using 100 ms steps, did not vary significantly along the cochlea (Fig. 6B). This indicates that the Ca2+ efficiency of exocytosis was similar between apical and basal OHCs. Although the size of ICa was comparable to that recently reported in Otof control OHCs (Beurg et al. 2008), ΔCm was surprisingly about 9 times smaller. At first we thought that this could be down to the different animal species used for the two studies (control Otof mice and gerbils), so we performed additional experiments in P3 OHCs from normal C57B mice and Wistar rats. Despite their larger peak ICa (mouse: −190 ± 19 pA, n= 6; rat: −151 ± 26 pA, n= 6), the maximal size of ΔCm remained relatively small (mouse: 7.0 ± 0.7 fF; rat: 7.8 ± 1.0 fF) and similar to that of gerbil OHCs (Fig. 6C). For the results obtained from gerbil OHCs, synaptic transfer functions (see above) were used to measure the Ca2+ dependence of exocytosis (Fig. 6C). The power values were 0.7 ± 0.1 (n= 4) in apical and 1.1 ± 0.1 (n= 4) in basal OHCs (Fig. 6C), indicating a near linear relation between Ca2+ entry and exocytosis as previously described (mouse Otof control OHCs: Beurg et al. 2008). Although we did not measure directly the release kinetics of the RRP (the very small ΔCm in OHCs limited the accuracy of recording responses to very short stimuli) we estimated its overall size using a 100 ms voltage step. This is likely to be a reliable measure since the same stimulus almost completely isolated the RRP in mouse OHCs (Beurg et al. 2008). Using this method, the RRP in gerbil OHCs consisted of about 135 vesicles and 143 vesicles in apical and basal cells, respectively. In agreement with the findings in IHCs (Fig. 1E), the number of CtBP2 positive dot-like spots per OHC (i.e. active zones) was similar along the cochlea (Fig. 6D: 3rd row OHCs: apical 9; basal 11). The size of these spots were usually larger than that seen in IHCs (see also Beurg et al. 2008) most likely reflecting multiple synaptic ribbons at OHC active zones (Shnerson et al. 1982). Considering that on average two synaptic ribbons could be present at each active zone, the number of vesicles per ribbon in the RRP in immature OHCs is likely to be about eight. The intensity and distribution of otoferlin labelling was similar among OHCs (Fig. 6D).
Discussion
In the present study we have investigated the biophysical properties of neurotransmitter release at ribbon synapses of immature mammalian hair cells using capacitance measurements. Recordings were performed using body temperature and physiological extracellular Ca2+. We found that, in contrast to adult animals (Johnson et al. 2008), the kinetic properties of exocytosis in immature IHCs were similar between apical and basal cells. However, the developmental time course of the exocytotic Ca2+ dependence differed in IHCs from the two cochlear regions. We have also provided an indication of the coupling between Ca2+ channels and release sites in immature IHCs. Finally, ΔCm in immature OHCs was much smaller compared to that of IHCs, consistent with there being fewer synaptic active zones in OHCs.
Neurotransmitter release at ribbon synapses of immature hair cells
Immature apical and basal gerbil IHCs showed a similar size of ΔCm and at least two kinetic components of synaptic vesicle release could be isolated. The initial fast component (RRP: Moser & Beutner, 2000) exhibited a release time constant of about 31 ms that was comparable to adult gerbil IHCs (42–52 ms: Johnson et al. 2008). However, the larger number of synaptic ribbons in pre-hearing IHCs (Table 1) means that their release rate per ribbon is actually about half that of adult cells. The reduction in ribbon number with maturation is likely to be a consequence of the pruning of immature branched afferent endings to reach their characteristic unbranched configuration with adult IHCs (Pujol et al. 1998). The larger pool size and greater release rate of the RRP per ribbon in adult IHCs (Table 1) could promote the coincidence of release events, which seems to be required for accurate temporal coding of sound (Moser et al. 2006, Wittig & Parsons, 2008). The refilling of the RRP in pre-hearing IHCs is unlikely to become rate limiting for vesicle release for frequencies up to at least 7 Hz, which is in the range of the predicted frequency of spontaneous Ca2+ action potentials intrinsic to these cells (<10 Hz: Johnson & Marcotti, 2008). In contrast to the RRP, the SRP release rate per ribbon in immature IHCs was faster than that of adult cells (Table 1). This is unlikely to be a consequence of the larger ICa in immature IHCs because it cannot explain their comparatively lower RRP release rate. The inverse relation of the RRP and SRP release rates between immature and adult IHCs appears to be correlated with the cell's different exocytotic Ca2+ dependence (Fig. 4C), especially considering that the rates for adult apical cells lie in between (Table 1). The lower demand for maintained exocytosis in immature spiking IHCs could also explain the faster depletion of the SRP compared to the more sustained activity of adult cells (Table 1).
The above findings indicate that the synaptic machinery of pre-hearing IHCs lacks any position-dependent biophysical specialization that has been described in the adult gerbil cochlea (Johnson et al. 2008). This could indicate that in pre-hearing IHCs, possible differences in signalling characteristics such as the frequency and/or pattern of spontaneous action potentials along the gerbil cochlea (Johnson & Marcotti, 2008) do not require specialized synaptic machinery. This is in contrast to more mature IHCs where the receptor characteristics of low-and high-frequency cells are different, with basal cells responding to sound with graded receptor potentials (Russell & Sellick, 1978) while apical cells show an additional phasic component representing the sound frequency (Dallos, 1985).
Although the role of exocytosis in immature OHCs is less clear, they express CaV1.3 Ca2+ channels (Michna et al. 2003), appear to show robust exocytosis (Beurg et al. 2008) and have presynaptic specializations opposite to type I afferent fibres (Shnerson et al. 1982), which are only transiently present during early postnatal stages (Pujol et al. 1998). This suggests that OHCs could potentially contribute to the refinement of synaptic connections within the immature cochlea, a role only suggested for electrically active IHCs (Kros et al. 1998).
In the present study we have shown that early postnatal OHCs from wild-type rodents (gerbils, C57B mice and Wistar rats) exhibited relatively small exocytotic responses, which could be about an order of magnitude smaller than that of IHCs if OHCs were to be recorded under similar conditions (physiological 1.3 mm Ca2+). This came as a surprise considering the large RRP size recently described in OHCs from Otoferlin control mice (Beurg et al. 2008). Moreover, Otof control OHCs fired spontaneous action potentials that are absent in wild-type rodents when working in near-physiological Ca2+ concentrations (CD-1 mice: Marcotti & Kros 1999; rats: Oliver et al. 1997). Together these observations suggest that the biophysical properties of OHCs in the mixed Otof background mouse could be somehow different to those of other wild-type animals. Moreover, the linear exocytotic Ca2+ dependence reported in OHCs of both mice (Beurg et al. 2008) and gerbils (Fig. 6C) is unlikely to be suitable for encoding the spiking activity (on–off events) since the release of neurotransmitter may also occur in the intervals between spikes.
Despite the relatively small ΔCm responses of OHCs, the estimated size of the RRP at each active zone was comparable to that of immature IHCs, consisting of 15 and 13 vesicles in apical and basal cells, respectively. These numbers correlate with those obtained at IHC ribbon synapses using morphological observations (≤30 vesicles per active zone in mouse: Khimich et al. 2005), electrophysiological recordings (∼12 vesicles in rat: Goutman & Glowatzki, 2007) and also at ribbon synapses in the retina (22 vesicles: von Gersdorff et al. 1996). Although the release of single quanta is likely to trigger action potential activity in the afferent fibres (IHCs: Glowatzki & Fuchs, 2002), it remains unclear how immature OHCs drive afferent activity since in wild-type rodents they are not spontaneously active.
The exocytotic Ca2+ sensitivity at gerbil IHC synapses changes with development
The onset of hearing in most rodents occurs at around P10–P12. While immature IHCs exhibit intrinsic spontaneous Ca2+-dependent action potential activity (Glowatzki & Fuchs, 2002; Marcotti et al. 2003), adult cells respond to sound vibration with rapid and graded receptor potentials (Russell & Sellick, 1978). It has been suggested that developmental and tonotopic differences in the exocytotic Ca2+ dependence are important for IHC function, allowing them to encode these different receptor potentials (mouse: Johnson et al. 2005; gerbil: Johnson et al. 2008). Our findings indicate that the transition between a high-order (immature) and a near linear (adult) relation is achieved in 2–3 days just before the onset of hearing in high-frequency cells. Although low-frequency IHCs maintain an overall higher order exocytotic Ca2+ dependence, its moderate decline occurred a few days later (Fig. 5: between P12 and P16), reflecting the delayed development of the apical coil (Pujol et al. 1998). In contrast to the Ca2+ dependence, the exocytotic Ca2+ efficiency was not fully mature until ≥P20, due to the size of ICa reaching a steady level at around this time. The high Ca2+ efficiency of exocytosis in adult IHCs is likely to drive spontaneous activity in auditory afferents, the frequency of which gradually increases only from around the onset of hearing (Woolf & Ryan, 1985). These results also suggest that some caution should be taken when investigating and comparing the biophysical properties of exocytosis at intermediate stages (P13–P18) with either immature or adult stages.
Functional Ca2+ coupling of gerbil IHC ribbon synapses
Although the basic biophysical development of IHC synapses is now better understood, very little information is available on the factors responsible for the developmental changes in the exocytotic Ca2+ dependence. It has been suggested that a close coupling between Ca2+ channels and release sites (creating a Ca2+ nanodomain) could be responsible for the linear Ca2+ dependence of exocytosis in adult IHCs (Brandt et al. 2005). Under this scenario, high Ca2+ cooperativity in immature IHCs should be under the control of a Ca2+ microdomain. However, we found that the distance between Ca2+ channels and release sites in pre-hearing IHCs positioned at either end of the cochlea was in the range of 40 nm, which is comparable and even slightly closer to that found in adult cells (about 50 nm: Johnson et al. 2008). Therefore, exocytosis in gerbil IHCs is under a Ca2+ nanodomain control irrespective of age or position along the cochlea, suggesting that something other than Ca2+ channel localization is likely to be responsible for the modulating the Ca2+ sensitivity.
The different exocytotic Ca2+ dependence observed before and after the onset of hearing (Johnson et al. 2005) and as a function of tonotopic position in adult gerbils (Johnson et al. 2008) is likely to be intrinsic to the synaptic machinery of IHCs. Therefore, corresponding differences in the expression of Ca2+ sensing molecules at IHC ribbon synapses has to be considered. So far, the proposed candidate to fulfil this role in auditory but not in vestibular hair cells is otoferlin (Roux et al. 2006; Beurg et al. 2008). Since otoferlin appears to be similarly distributed in immature (Figs 1E and 6D) and adult gerbil hair cells (Johnson et al. 2008; for mouse see Roux et al. 2006), it is unlikely that it alone could control both the high-order (immature IHCs N≈ 4; low-frequency adult cells N≈ 2–3: Fig. 4C) and linear (high-frequency adult IHCs: Fig. 4C; immature OHCs: Fig. 6C) Ca2+ dependence of exocytosis in mammalian cochlear hair cells. Therefore, additional as yet unknown Ca2+ sensing proteins or promoters of exocytosis (Jahn et al. 2003), which are differentially expressed as a function of development and cochlear position, could be involved.
Acknowledgments
This work was supported by Wellcome Trust, Deafness Research UK and Royal Society grants to W.M. and by a Baden-Württemberg research grant to M.K. W.M. is a Royal Society University Research Fellow. We would like to thank M. J. Palmer for her very constructive comments on an earlier version of the manuscript and M. Cardwell and A. Davids for their excellent assistance with the animals. We thank N. Blin and M. Pfister (University of Tübingen, Germany) for their generous supply of the otoferlin antibody.
Glossary
Abbreviations
- APs
action potentials
- ΔCm
membrane capacitance changes
- ICa
calcium current
- IHCs
inner hair cells
- OHCs
outer hair cells
- P
postnatal day
- RRP
readily releasable pool
- SRP
secondarily releasable pool
Author contributions
S.L.J. collected and analysed the electrophysiological data (in the UK) and helped write the paper. C.F. performed the immunolabelling experiments in Germany. M.K. supervised and analysed immunolabelling experiments. All authors contributed to the design of the research. W.M. conceived and coordinated the study, supervised the experiments, participated in data collection and analysis and wrote the paper. All authors discussed the results, commented on the manuscript and approved the version to be published.
References
- Augustine GJ, Charlton MP, Smith SJ. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Beurg M, Safieddine S, Roux I, Bouleau Y, Petit C, Dulon D. Calcium-and otoferlin-dependent exocytosis by immature outer hair cells. J Neurosci. 2008;28:1798–1803. doi: 10.1523/JNEUROSCI.4653-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Moser T. The presynaptic function of mouse cochlear inner hair cells during development of hearing. J Neurosci. 2001;21:4593–4599. doi: 10.1523/JNEUROSCI.21-13-04593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J Neurosci. 2005;25:11577–11585. doi: 10.1523/JNEUROSCI.3411-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. Response characteristics of mammalian cochlear hair cells. J Neurosci. 1985;5:1591–1608. doi: 10.1523/JNEUROSCI.05-06-01591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. The active cochlea. J Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA. Time and intensity coding at the hair cell's ribbon synapse. J Physiol. 2005;566:7–12. doi: 10.1113/jphysiol.2004.082214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Glowatzki E. Time course and calcium dependence of transmitter release at a single ribbon synapse. Proc Natl Acad Sci U S A. 2007;104:16341–16346. doi: 10.1073/pnas.0705756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Grant L, Fuchs PA. Hair cell afferent synapses. Curr Opin Neurobiol. 2008;18:389–395. doi: 10.1016/j.conb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species-29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Griesinger CB, Richards CD, Ashmore JF. Fast vesicle replenishment allows indefatigable signalling at the first auditory synapse. Nature. 2005;435:212–215. doi: 10.1038/nature03567. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr . Physiology of olivocochlear efferents. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer; 1996. pp. 435–502. [Google Scholar]
- Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol. 2005;563:177–191. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Adelman JP, Marcotti W. Genetic deletion of SK2 channels in mouse inner hair cells prevents the developmental linearization in the Ca2+ dependence of exocytosis. J Physiol. 2007;583:631–646. doi: 10.1113/jphysiol.2007.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W. Biophysical properties of CaV1.3 calcium channels in gerbil inner hair cells. J Physiol. 2008;586:1029–1042. doi: 10.1113/jphysiol.2007.145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Forge A, Knipper M, Münkner S, Marcotti W. Tonotopic variation in the calcium dependence of neurotransmitter release and vesicle pool replenishment at mammalian auditory ribbon synapses. J Neurosci. 2008;28:7670–7678. doi: 10.1523/JNEUROSCI.0785-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cells synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rüsch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Runyeon JW, Crum J, Ellisman MK, Roberts WM. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. J Neurosci. 1999;19:119–132. doi: 10.1523/JNEUROSCI.19-01-00119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Kros CJ. Developmental expression of the potassium current IK,n contributes to maturation of mouse outer hair cells. J Physiol. 1999;520:653–660. doi: 10.1111/j.1469-7793.1999.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Rüsch A, Kros CJ. Sodium and calcium currents shape action potentials in immature mouse inner hair cells. J Physiol. 2003;552:743–761. doi: 10.1113/jphysiol.2003.043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna M, Knirsch M, Hoda JC, Muenkner S, Langer P, Platzer J, Striessnig J, Engel J. Cav1.3 (α1D) Ca2+ currents in neonatal outer hair cells of mice. J Physiol. 2003;553:747–758. doi: 10.1113/jphysiol.2003.053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Neef A, Khimich D. Mechanisms underlying the temporal precision of sound coding at the inner hair cell ribbon synapse. J Physiol. 2006;576:55–62. doi: 10.1113/jphysiol.2006.114835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. The cochlear place-frequency map of the adult and developing Mongolian gerbil. Hear Res. 1996;94:148–156. doi: 10.1016/0378-5955(95)00230-8. [DOI] [PubMed] [Google Scholar]
- Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Oliver D, Plinkert P, Zenner HP, Ruppersberg JP. Sodium current expression during postnatal development of rat outer hair cells. Pflugers Arch. 1997;434:772–778. doi: 10.1007/s004240050464. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Pujol R, Lavignr-Rebillard M, Lenoir M. Development of sensory and neural structures in the mammalian cochlea. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System. New York: Springer; 1998. pp. 146–192. [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990;10:3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Sellick PM. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug N, Braig C, Zimmermann U, Engel J, Winter H, Ruth P, Blin N, Pfister M, Kalbacher H, Knipper M. Differential expression of otoferlin in brain, vestibular system, immature and mature cochlea of the rat. Eur J Neurosci. 2006;24:3372–3380. doi: 10.1111/j.1460-9568.2006.05225.x. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Königstorfer A, Südhof TC. RIBEYE, a component of synaptic ribbons: a protein's journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Shnerson A, Devigne C, Pujol R. Age-related changes in the C57BL/6J mouse cochlea. II. Ultrastructural findings. Dev Brain Res. 1982;2:77–88. doi: 10.1016/0165-3806(81)90060-2. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GE, Slapnick SM. Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. J Neurosci. 1982;2:942–957. doi: 10.1523/JNEUROSCI.02-07-00942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC, Lautermilch NJ, Smith RD, Gomez TM. Coding of neuronal differentiation by calcium transients. BioEssays. 2000;22:811–817. doi: 10.1002/1521-1878(200009)22:9<811::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005;28:20–29. doi: 10.1016/j.tins.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Tucker T, Fettiplace R. Confocal imaging of calcium microdomains and calcium extrusion in turtle hair cells. Neuron. 1995;15:1323–1335. doi: 10.1016/0896-6273(95)90011-x. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron. 1996;16:1221–1227. doi: 10.1016/s0896-6273(00)80148-8. [DOI] [PubMed] [Google Scholar]
- Wittig JH, Parsons TD. Synaptic ribbon enables temporal precision of hair cell afferent synapse by increasing the number of readily releasable vesicles: a modeling study. J Neurophysiol. 2008;100:1724–1739. doi: 10.1152/jn.90322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NK, Ryan AF. Ontogeny of neural discharge patterns in the ventral cochlear nucleus of the Mongolian gerbil. Dev Brain Res. 1985;17:131–147. doi: 10.1016/0165-3806(85)90138-5. [DOI] [PubMed] [Google Scholar]