Abstract
Introduction
The purpose of this 3-way crossover study was to identify the effective dose of soy protein isolate enriched with isoflavones for suppressing bone resorption in postmenopausal women using a novel, rapid assessment of antibone resorbing treatments.
Methods
Thirteen postmenopausal women (≥6 yr since menopause) were predosed with 41Ca iv. After a 200-d baseline period, subjects were given 43 g soy protein/d that contained 0, 97.5, or 135.5 mg total isoflavones in randomized order. The soy protein isolate powder was incorporated into baked products and beverages. Each 50-d intervention phase was preceded by a 50-d pretreatment phase for comparison. Serum isoflavone levels and biochemical markers were measured at the end of each phase. Twenty-four-hour urine samples were collected approximately every 10 d during each phase for 41Ca/Ca analysis by accelerator mass spectrometry.
Results
Serum isoflavone levels reflected the amount of isoflavones consumed in a dose-dependent manner. None of the isoflavone levels had a significant effect on biochemical markers of bone turnover, urinary cross-linked N teleopeptides of type I collagen and serum osteocalcin, or bone turnover as assessed by urinary 41Ca/Ca ratios.
Conclusions
Soy protein with isoflavone doses of up to 135.5 mg/d did not suppress bone resorption in postmenopausal women. This is the first efficacy trial using the novel technique of urinary 41Ca excretion from prelabeled bone.
Estrogen is antiresorptive on the skeleton (1). Estrogen hormone therapy (HT) has been a mainstay of osteoporosis prevention in postmenopausal women until the estrogen plus progestin arm of the Women’s Health Initiative study found that health risks of stroke, embolism, and breast cancer exceeded health benefits from reduced fracture and colorectal cancer (2). The negative side effects of estrogen therapy have led to a search for alternatives to HT. A popular dietary alternative is soy isoflavones that are structurally similar to physiological estrogens that bind to the estrogen receptors (ERs) α and β. Although isoflavones bind with lower affinity than physiological estrogens to ERα and ERβ, they are more available than estrogens to bind to ER because they are less likely to be bound to SHBG in serum by 10-fold. However, studies of their benefits on bone have been inconclusive. Several short-term clinical trials have shown that soy isoflavones increase bone mineral content (BMC) and bone mineral density (BMD) (3, 4), whereas others showed no benefit (5, 6). Daily consumption of 99 mg isoflavones for 12 months in postmenopausal women did not improve BMD, except for a possible effect on intertrochanter BMD region of the hip (7). A large multisite trial found that not only was ipriflavone, a synthetic isoflavone, not effective in reducing bone loss over 3 yr, it also resulted in lymphocytopenia in postmenopausal women (8). On the other hand, soy consumption has been associated with a decreased risk of fracture in a large cohort of Shanghai women (9).
Because effects on bone mass of antiresorption treatment take 6 months to several years to measure, biomarkers of bone resorption have been suggested (10) to screen more rapidly for effective treatments and to establish their dose response. A novel approach using 41Ca offers a more sensitive [10−18 mol 41Ca are required for detection by accelerator mass spectrometry (AMS)] and direct measure of early response (first few weeks) of bone resorption to treatment than conventional biomarkers that do not directly measure resorption of bone mineral (11). The signal from 41Ca is less variable than for biochemical markers. Excretion of urinary 41Ca reflects bone resorption in a skeleton that has been labeled with 41Ca, a rare isotope (41Ca:40Ca natural abundance of 10−14) (12). Importantly, the long half-life of 41Ca (~105 yr) and the ability of AMS to detect very low concentrations in the urine allow continuous measurements of bone resorption for the lifetime of the subject with the administered doses. The method also allows study of the efficacy of a series of interventions on bone turnover in the same subjects. There are limited reports of use of 41Ca to monitor Ca metabolism in end stage renal dialysis (13) and a case study of a suppression of bone resorption with a bisphosphonate (14).
The purpose of this randomized, double-blind, crossover study was to evaluate the dose response of isolated soy protein with naturally occurring isoflavones on calcium absorption and bone turnover in postmenopausal women using a novel, sensitive, method for detecting early (days to weeks) changes in bone resorption. We hypothesized that reduced bone resorption, as measured by urinary 41Ca/Ca ratio, is inversely related to soy isoflavone dose in postmenopausal women. This is the first reported intervention trial using 41Ca.
Subjects and Methods
Subjects
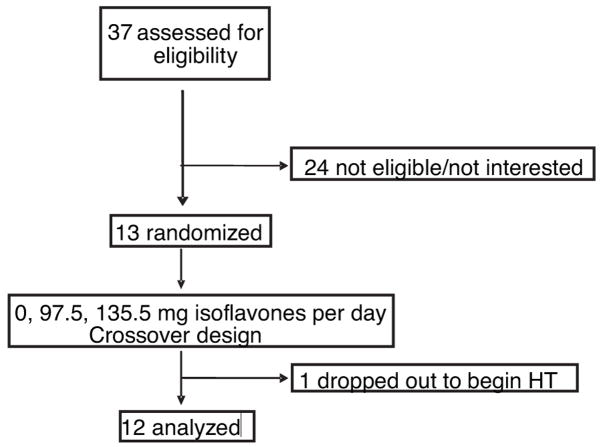
Figure 1 shows the study profile. Thirteen healthy, community-dwelling postmenopausal women were recruited after telephone screening was conducted to identify eligible subjects. Exclusion criteria included being less than 4 yr postmenopause; using hormone replacement therapy, antiresorptive drugs, nonprescription drugs, corticosteroids, drugs for the treatment of bone disease, thiazide diuretics, and thyroid medication; having a history of a disease of the gut, kidney, liver or bones; or soy allergies. No racial or ethnic groups were excluded.
Fig. 1.
Study profile.
Study design
The study design was a blinded, randomized, crossover intervention trial to evaluate the effect of soy isoflavones on bone turnover in postmenopausal women who did not habitually consume soy products. Subjects were dosed iv with either 100 nCi or, in the case of six subjects who were in a previous study, 1 μCi 41Ca. The dose was reduced when we found that adequate analytical detection was possible with 10-fold lower doses, which conserves supply of 41Ca. Subjects began interventions more than 200 d postdose when the changes between 41Ca:Ca values were relatively small. For the interventions, subjects were given approximately 43 g protein/d that contained 0.0, 97.5, or 135.5 mg total isoflavones [aglycones (without sugars) units] in 58 grams soy isolate (Protein Technologies International, St. Louis, MO) in randomized order. The isoflavone content of the soy protein products is shown in Table 1. When expressed as a percentage of total isoflavones, both the 97.5- and 135.5-mg isoflavone soy protein isolates contained approximately 63% as genistin, malonyl genistin, acetyl genistin, or genistein, and approximately 33% as daidzin, malonyl daidzin, or acetyl daidzin. The soy protein isolates were incorporated into baked products and beverages. Each 50-d intervention period was preceded by a 50-d pretreatment period. All subjects received all three soy interventions. Subjects were given 500 mg/d calcium and 500 IU/d vitamin D in the form of supplements throughout the study to minimize fluctuations in calcium intake and vitamin D status. Furthermore, a metaanalysis showed that calcium intakes more than 1 g/d were associated with enhancing effects of estrogen on bone (15).
TABLE 1.
Isoflavone content of soy protein isolatesa
| Isoflavone intervention (mg/d) |
|||
|---|---|---|---|
| 0.0 μg aglycone units/g product | 97.5 μg aglycone units/g product | 135.5 μg aglycone units/g product | |
| Daidzin | 0 | 199 | 292 |
| Glycitin | 0 | 53 | 38 |
| Genistin | 0 | 281 | 332 |
| Malonyl daidzin | 0 | 264 | 368 |
| Malonyl glycitin | 0 | 41 | 40 |
| Malonyl genistin | 0 | 487 | 695 |
| Acetyl daidzin | 0 | 86 | 132 |
| Acetyl glycitin | 0 | 0 | 0 |
| Acetyl genistin | 0 | 114 | 167 |
| Daidzein | 0 | 0 | 0 |
| Glycitein | 0 | 0 | 0 |
| Genistein | 0 | 173 | 271 |
| Total | 0 | 1698b | 2335c |
Average of triplicate analysis of soy protein isolates used in baking and beverages. Soy protein isolates were incorporated into the diets at 58 g/d.
Daily total isoflavone consumption of 97.5 mg aglycone units was lower than the value of 103.6 mg determined using the method of the Association of Official Analytical Chemists performed by Nestle Purina Analytical Lab (St. Louis, MO).
Daily total isoflavone consumption of 135.5 mg aglycone units was lower than the value of 152.4 mg determined using the method of the Association of Official Analytical Chemists performed by Nestle Purina Analytical Lab.
Twenty-four-hour urine samples were collected approximately every 10 d during all intervention and pretreatment periods. Each 24-h urine collection period started with the second void and continued through the first void of the following day. Fasting blood and urine samples were obtained at the end of each intervention and pretreatment period to measure serum isoflavone concentrations and biochemical markers of bone turnover. Serum isoflavone concentrations were used as an indicator of compliance. Biochemical markers of bone turnover included urinary collagen type I cross-linked N teleopeptides, urinary free deoxypyridinoline, and serum bone alkaline phosphatase (BAP). In addition, serum equol (a metabolite of soy isoflavones), PTH, 25-hydroxy vitamin D, and 1,25-dihydroxy vitamin D were measured at the end of each intervention.
At the end of baseline (first pretreatment period) and each intervention, a calcium absorption test was performed. Fasting blood and the second void urine were collected after breakfast, which consisted of calcium-free bread (~90 g) and a mocha drink (13 g). For the interventions, 14.5-g soy protein isolates with the appropriate isoflavone level for that test period were added to the mocha drink. Calcium triphosphate was added to the mocha drink and to bring the total calcium content of the test meal excluding the stable isotope to 250 mg calcium, a standard load for calcium absorption tests. Midway through the breakfast, the subjects consumed a capsule containing 15.2 mg 44Ca as CaCO3. Subjects were instructed to ingest at least 8 ounces deionized water 2 h after the dose. Relative calcium absorption fraction was determined as: (5-h Ca enrichment0.92373) × [0.3537 × (height (meters)0.52847) × (weight (kilograms)0.37213)]. We use the term relative for two reasons. This equation has not yet been verified for stable isotope enrichment that may differ from specific activity of 45Ca determined in the original equation (16). Furthermore, the isotope was not thoroughly mixed with the test meal as was the case for validation of the 5-h blood draw (16); insufficient exchange between the tracer and the calcium in the test meal may alter the results.
BMC and BMD of the lumbar spine, proximal femur, and total body were measured by dual-energy x-ray absorptiometry (Lunar DPX IQ, Madison, WI) at the beginning of the study. The subjects completed a 3-d food record during each intervention and pretreatment period to assess their usual dietary pattern. Nutrient intakes were calculated by using The Nutrition Data System for Research (version 4.04/32). Approval for the study was obtained from the Purdue University and Indiana University Purdue University Institutional Review Boards. All the subjects provided written informed consent.
Chemical analysis
Urine samples were collected in acid-washed containers. The 24-h urine collection was measured for volume and recorded. After the urine samples were mixed thoroughly, approximately 10 ml ammonium hydroxide was added to every 1 liter of urine samples and mixed well. Next, the pH of the mixture was checked to ensure that it was at least 10. This was followed by the addition of 50 ml saturated ammonium oxalate to the sample and mixed well. The samples were allowed to stand overnight so that calcium was separated from 24-h urine samples by precipitation as calcium oxalate (CaC2O4). The supernatant was decanted, leaving the precipitate and some urine, which were then filtered. The precipitate and filter paper were completely dried, the precipitate dissolved in 50 ml of approximately 0.25 M HNO3 solution, and chromatographically purified by cation exchange using Bio-Rad AG 50W-X8 resin (Bio-Rad, Hercules, CA). Calcium fluoride (CaF2) was precipitated with hydrofluoric acid, washed, dried in a vacuum oven, loaded into aluminum sample holders, and inserted into the ion source of the AMS to obtain the 41Ca/Ca ratio of the samples (11).
Serum isoflavone concentrations were analyzed (17) using reverse-phase HPLC-electrospray ionization and a PE-Sciex API III triple quadrupole mass spectrometer (Sciex, Concord, Canada). For measurement of isoflavone content in dietary samples, 80% aqueous methanol extraction at 4 C of the isoflavones from the freeze-dried dietary sample was followed by reverse-phase HPLC analysis (17). RIAs, immunoradiometric assay, and enzyme immunoassays were used to measure hormones and biochemical markers of bone turnover. The interassay percent coefficient of variation (CV) for urinary N telopeptide cross-links is 7.1 and 12.6% for serum PTH. Serum 25-hydroxyvitamin D (10.5% CV) and 1,25 (OH)2 vitamin D (10.5% CV) were measured by using RIAs (DiaSorin Inc., Stillwater, MN). Serum osteocalcin was measured (9% CV) by using a RIA that was developed at the Indiana University General Clinical Research Center.
Statistical analysis
Means and SD values of subject characteristics and outcome measures were determined. Biochemical measures of response to interventions were compared by ANOVA. The natural logarithm of the 41Ca to Ca ratio was analyzed using a general linear model that included terms for each 50-d treatment and pretreatment period. Covariates included terms that allowed the estimation of distinct linear relationships with time for each subject, thereby accounting for initial dose differences as well as other subject characteristics. Treatment effects are estimated as contrasts between the treatment period and the corresponding pretreatment control period. Results are transformed to relative resorption (RR) by exponentiating the estimates and the corresponding confidence limits. In this scale, a value of RR = 1.0 corresponds to no resorption reduction, whereas RR = 0.8 corresponds to a 20% reduction. The statistical model with programs and sample data are available in the supplemental data published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org. A priori power calculations were based on a change in slope of the log 41Ca to Ca ratio vs. time relationship and indicated that a sample size of 10 would be sufficient to have an 80% chance of detecting a change of the size seen in preliminary studies (14). SAS software (version 9.0, SAS Institute, Cary, NC) was used for all computations.
Results
Table 2 shows the baseline characteristics of the subjects. Although no race was intentionally excluded, all subjects who expressed interest in the study were white. Twelve women had natural menopause, and one woman had surgical menopause. Twelve women completed the study, whereas one subject dropped out due to starting estrogen therapy. Compared with non-Hispanic white U.S. women of similar age from the 1988 –1994 National Health and Nutrition Examination Survey III (15), the subjects were comparable in height but heavier. BMDs at both the spine and femur were within the reference range. Total BMD of the subjects in our study was 1.2 ± 0.1 g/cm2 with a mean T score of 0.3. The average habitual calcium intake was 955 ± 343 mg/d.
TABLE 2.
Baseline characteristics of the subjects
| Characteristics | Study populationa (n ± 13) | Reference populationb |
|---|---|---|
| Age (yr) | 62.2 ± 3.6 (54–67) | |
| Height (m) | 1.62 ± 0.06 (1.5–1.7) | 1.61–1.62 |
| Weight (kg) | 83 ± 13 (63–112) | 71–74 |
| Body mass index (kg/m2) | 31.6 ± 4.4 (23.0–39.3) | 27.3–28.2 |
| Time after menopause (yr) | 16.4 ± 7.2 (7–31) | |
| BMD (g/cm2) | ||
| Spine | 1.25 ± 0.23 (0.87–1.57) | 0.31–1.45 |
| Total femur | 0.947 ± 0.148 (0.65–1.18) | 0.47–1.07 |
| Total body | 1.15 ± 0.11 (0.936–1.33) | |
| Total BMC (g) | 2719 ± 586 (1961–3370) | |
Mean ± SD and (range).
The reference ranges for height, weight, and BMI were taken from the Third National Health and Nutrition Examination Survey database (1988–1994) representing non-Hispanic white women aged 50–69 yr (18). The age-adjusted reference total hip BMD was from Looker et al. (19) and for spine BMD from Kleerkoper et al. (20).
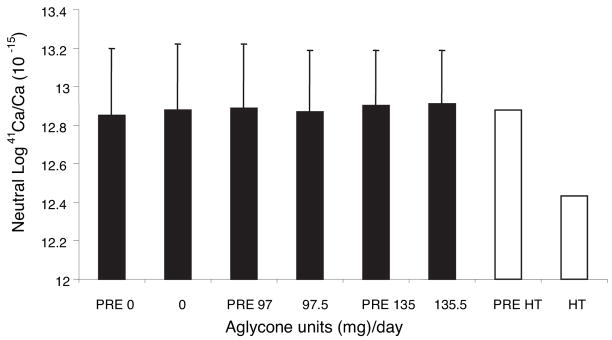
Figure 2 shows the estimated means of log urinary 41Ca/Ca ratios for each soy isoflavone intervention and pretreatment period. The SE was 0.3 for each intervention, indicating the small variance about the mean. There were no differences among interventions (P > 0.05) or between each intervention and its corresponding pretreatment period (P > 0.05). The range in mean urinary 41Ca/Ca ratios was 13.0–13.4. The subject who discontinued because of estrogen treatment continued to collect urine samples for 41Ca/Ca analysis and the 41Ca/Ca ratio markedly decreased with estrogen. Expressed as RR (Table 3), there was no significant reduction with isoflavone enrichment over the soy protein isolate in which isoflavones had been removed because the 95% confidence intervals included 1. An example of the urinary 41Ca/Ca ratios over time for one subject appears in Fig. 3.
Fig. 2.
Effect of isoflavone content in soy protein isolates on urinary 41Ca/Ca in postmenopausal women in a crossover design (least squares mean ± SE). The means ± SE are based on five urine collections per subject using a model that adjusts for subject and time. Open bars, Means of pretreatment and HT treatment in a subject who dropped out. Data points before the first intervention phase were not used in analysis.
TABLE 3.
Suppression of bone resorption of soy protein isolates enriched with isoflavones compared to control soy protein isolate in postmenopausal women
| Soy intervention (isoflavones in mg/d) | RR | 95% Confidence interval | P |
|---|---|---|---|
| 0 | 1.03 | 0.90–1.18 | 0.67 |
| 97.5 | 0.98 | 0.86–1.12 | 0.75 |
| 135.5 | 1.01 | 0.88–1.16 | 0.85 |
Fig. 3.
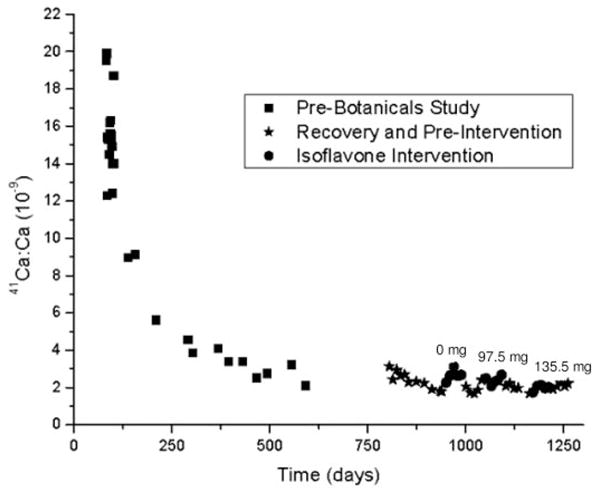
Mean ± SD of AMS measurement of urinary 41Ca/Ca time course for one subject. The mechanism uncertainty of the AMS measurement is 5.5%.
At baseline, biochemical markers of bone turnover were within the reference range for postmenopausal women (21). There were no significant changes in biochemical markers or serum PTH, 25-hydroxyvitamin D, or 1,25-dihydroxyvitamin D with intervention (Table 4). Serum isoflavones concentrations corresponded to isoflavone content of each intervention (Fig. 4), indicating good compliance.
TABLE 4.
Biomarkers of bone turnover and calcium regulating hormones at baseline and at the end of each intervention in postmenopausal women
| Variable | Baseline | Soy intervention (mg isoflavones/d) |
||
|---|---|---|---|---|
| 0.0 | 97.5 | 135.5 | ||
| Serum BAP (ng/ml) | 15.4 ± 4.2 | 15.8 ± 4.0 | 15.1 ± 2.8 | 15.3 ± 5.0 |
| Serum osteocalcin (ng/ml) | 12.4 ± 4.6 | 11.6 ± 5.3 | 12.3 ± 5.3 | 12.4 ± 4.7 |
| Urinary NTx (nmol BCE/mmol creatinine) | 54.2 ± 38.3 | 49.9 ± 37.9 | 40.2 ± 19.8 | 34.3 ± 23.4 |
| Serum PTH (pg/ml) | 42.3 ± 12.0 | 44.7 ± 10.0 | 43.4 ± 14.9 | 42.6 ± 8.5 |
| Serum 25(OH)D (ng/ml) | 32.1 ± 5.6 | 34.4 ± 10.0 | 32.2 ± 7.7 | 34.6 ± 8.3 |
| Serum 1,25(OH)2D (pg/ml) | 43.0 ± 17.0 | 50.4 ± 29.5 | 37.0 ± 11.7 | 39.2 ± 14.6 |
Mean ± SD, n = 12. Values in the same row were not significantly different from each other. NTx, Cross-linked N teleopeptides of type I collagen; BCE, bone collagen equivalent; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Fig. 4.
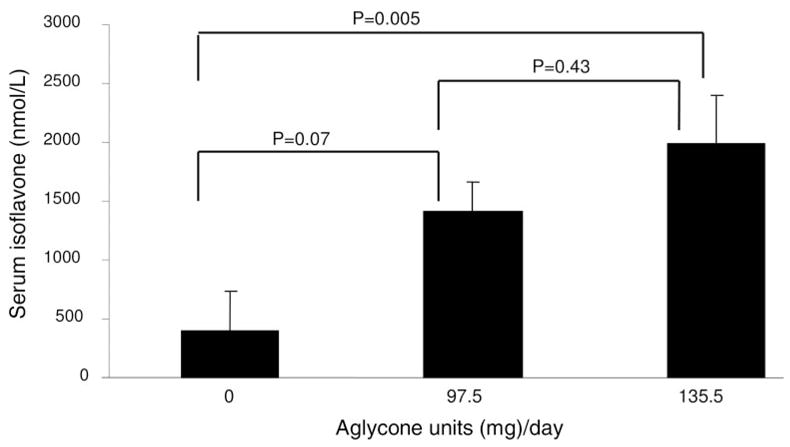
Dose response of serum isoflavone levels to isoflavone intake after 50 d in postmenopausal women (mean ± SE, n = 12).
Soy isoflavones had no effect on fractional calcium absorption. Relative fractional calcium absorption for 0-, 97.5-, and 135.5-mg soy isoflavones was 0.18 ± 0.10, 0.19 ± 0.09, and 0.15 ± 0.05, respectively. These values were not significantly different from baseline relative fractional calcium absorption of 0.20 ± 0.06.
Discussion
In this double-blind, crossover study, isoflavone supplementation up to 135.5 mg total aglycones/d in a soy protein preparation did not impact bone resorption or calcium absorption in postmenopausal women. Furthermore, serum and urinary biochemical markers of bone formation and resorption were unaffected by isoflavone intervention; however, the study was not powered to show differences in these markers, given their large variation (22). This finding of a lack of effect of soy protein with isoflavones on bone loss is consistent with some other human and animal studies (6, 23–25) but not others (3, 4, 26, 27). There is emerging evidence to suggest that equol producers are more responsive to isoflavones than nonproducers. In the Lydeking-Olsen et al. study (27), BMD and BMC changed by +0.6 and +0.3%, respectively, in equol (a biologically active metabolite of the soy isoflavone daidzein) nonproducers compared with a significant change of +2.4 and +2.8% in equol producers. Four subjects in our study were equol producers. These subjects had no change in urinary 41Ca excretion with intervention, but this study was not powered to explore this effect. Perimenopausal women may also be more responsive to isoflavones than postmenopausal women (4, 7). In our study, only women more than 4 yr postmenopausal were included, which is past the early rapid loss of bone at menopause.
Intestinal calcium absorption declines with ovarian hormone deficiency and is significantly negatively correlated with years since menopause (28), ultimately contributing to bone loss. Phytoestrogens might increase calcium absorption efficiency given that 17 β-estradiol administration to rats increased intestinal calcium absorption (29). However, none of the isoflavone levels that we tested had a significant effect on calcium absorption in postmenopausal women, nor did the approximately 1.7% phytate content (estimated by the supplier) of the isolated soy protein suppress relative fractional calcium absorption.
Approaches to evaluate the early onset of response of bone to interventions are needed for screening effective therapies. Changes in certain biochemical markers of bone turnover in response to interventions can be detected as early as 1 month (30). However, traditional biochemical markers have limitations due to their inherent large variability and lack of specificity (21). 41Ca as a biomarker is less variable than biochemical markers, and it reflects an earlier response of bone resorption to antiresorptive drug therapies than bone density (14). For our study, the residual variation used to assess treatment differences (the mean squared error for the treatment by subject interaction term in the model) expressed as a CV (SE values divided by the mean) is 2%. This compares to reported day-to-day variation of traditional biochemical markers of bone turnover of within subject averages of 28.1% for urinary pyridinoline and 27.7% for deoxypyridinoline (31). To our knowledge, this is the first reported intervention trial using 41Ca technology. Although the intervention was ineffective in suppressing bone resorption, we had an opportunity to observe the effect of estrogen in one subject. The marked reduction of about 37% in urinary 41Ca/Ca response due to estrogen therapy in the subject who dropped out strongly suggests that 41Ca is a sensitive and rapid response marker to bone resorption. Based on these limited data, it is reasonable to assume that an effective treatment may decrease the 41Ca/Ca ratio by 15–25%, a difference that could be detected in 12 subjects with 90% power. Recently, we found in a study of a similar design that urinary 41Ca/Ca was significantly suppressed by approximately 25% by estradiol plus progesterone in four postmenopausal women and by risedronate in six postmenopausal women (Reinwald, S., B. R. Martin, G. P. McCabe, J. R. Nolan, G. P. Jackson, M. Peacock, and C. M. Weaver, unpublished data). This large suppression of bone resorption assessed by 41Ca/Ca reflects the early remodeling transient. This magnitude for change is similar to changes in traditional biomarkers but larger than the 1.2–4.7% long-term benefit to bone loss reflected in trials on estrogen or risedronate with BMD as an outcome (32–34).
Strengths of this study include application of a sensitive method to detect early effects of treatment aimed at suppressing bone resorption. The sensitivity of the method and use of a crossover design with subjects serving as their own control increased the power to detect potential differences. The isoflavones were administered through diet, which is the normal route for nutrient acquisition, digestion, and absorption. A limitation of this study consisted of a convenience sample that may not have been representative of the general population. A leaner population with lower BMD is more vulnerable to bone loss, although the response of bone turnover to an intervention may not differ with initial BMD. We could not assess long-term impact of isoflavone on bones in this short-term study.
In conclusion, the results of this randomized, crossover study do not support the hypothesis that isoflavones up to 135.5 mg/d from soy protein have beneficial effects on early bone metabolism in postmenopausal women using 41Ca, a novel, sensitive, and rapid assessment of bone resorption.
Supplementary Material
Acknowledgments
Soy products were supplied by Protein Technologies International (St. Louis, MO).
This work was supported by the National Institutes of Health Center Grants P50 AT00477 and M01-RR-750.
Abbreviations
- AMS
Accelerator mass spectrometry
- BAP
bone alkaline phosphatase
- BMC
bone mineral content
- BMD
bone mineral density
- CV
coefficient(s) of variation
- ER
estrogen receptor
- HT
hormone therapy
- RR
relative resorption
Footnotes
The abstract was presented at American Society for Bone and Mineral Research 2004, Seattle, Washington.
The authors have nothing to declare.
JCEM is published monthly by The Endocrine Society (http://www.endo-society.org), the foremost professional society serving the endocrine community.
References
- 1.Raisz LG, Wiita B, Artis A, Bowen A, Schwartz S, Trahiotis M, Shoukri K, Smith J. Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab. 1996;81:37–43. doi: 10.1210/jcem.81.1.8550780. [DOI] [PubMed] [Google Scholar]
- 2.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1998;68(suppl):1375S–1379S. doi: 10.1093/ajcn/68.6.1375S. [DOI] [PubMed] [Google Scholar]
- 4.Alekel DL, Germain AS, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000;72:844–852. doi: 10.1093/ajcn/72.3.844. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JJB, Chen X, Boass A, Symons M, Kohlmeier M, Renner JB, Garner SC. Soy isoflavones: no effects on bone mineral content and bone mineral density in healthy, menstruating young adult women after one year. J Am Coll Nutr. 2002;21:388–393. doi: 10.1080/07315724.2002.10719240. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher JC, Satpathy R, Rafferty K, Haynatzka V. The effect of soy protein isolate on bone metabolism. Menopause. 2004;11:290–298. doi: 10.1097/01.gme.0000097845.95550.71. [DOI] [PubMed] [Google Scholar]
- 7.Kreijkamp-Kaspers S, Kok L, Grobbee DE, de Haan EHF, Aleman A, Lampe JW, van der Schouw YT. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- 8.Alexandersen P, Toussaint A, Christiansen C, Devogelaer JP, Roux C, Fechtenbaum J, Gennari C, Reginster JY. Ipriflavone in the treatment of postmenopausal osteoporosis: a randomized controlled trial. JAMA. 2001;285:1482–1488. doi: 10.1001/jama.285.11.1482. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Shu X, Li H, Yang G, Li Q, Gao Y, Zheng W. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. 2005;165:1890–1895. doi: 10.1001/archinte.165.16.1890. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava AK, Vliet EL, Lewiecki EM, Maricic M, Abdelmalek A, Gluck O, Baylink DJ. Clinical use of serum and urine bone markers in the management of osteoporosis. Curr Med Res Opin. 2005;21:1015–1026. doi: 10.1185/030079905X49635. [DOI] [PubMed] [Google Scholar]
- 11.Jackson GS, Weaver CM, Elmore D. Use of accelerator mass spectrometry for studies in nutrition. Nutr Res Rev. 2001;14:317–334. doi: 10.1079/NRR200129. [DOI] [PubMed] [Google Scholar]
- 12.Rink D, Klein J, Middleton R. 41Ca: past, present and future. Nucl Instrum Methods Phys Res B. 1990;52:572–582. [Google Scholar]
- 13.Fitzgerald RL, Hillegonds DJ, Burton DW, Griffin TL, Mullaney S, Vogel JS, Deftos LJ, Harold DA. 41Ca and accelerator mass spectrometry to monitor calcium metabolism in end stage renal disease patients. Clin Chem. 2005;51:2095–2102. doi: 10.1373/clinchem.2005.049650. [DOI] [PubMed] [Google Scholar]
- 14.Freeman SPHT, Beck B, Bierman JM, Caffee MW, Heaney RP, Holloway L, Marcus R, Southon JR, Vogel JS. The study of skeletal calcium metabolism with 41Ca and 45Ca. Nucl Instrum Methods Phys Res B. 2000;172:930–933. [Google Scholar]
- 15.Nieves JW, Koniar L, Cosman F, Lindsay R. Calcium potentiates the effect of estrogen and calcitonin on bone mass: review and analysis. Am J Clin Nutr. 1998;67:18–24. doi: 10.1093/ajcn/67.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Heaney RP, Recker RR. Estimating true fractional calcium absorption. Ann Int Med. 1988;108:905–906. doi: 10.7326/0003-4819-108-6-905_2. [DOI] [PubMed] [Google Scholar]
- 17.Barnes S, Coward L, Kirk M, Sfakianos J. HPLC-mass spectrometry analysis of isoflavones. Exp Biol Med. 1998;217:254–262. doi: 10.3181/00379727-217-44230. [DOI] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. Third National Health and Nutrition Examination Survey (NHANES III) [Accessed September 28];Anthropometric Reference Data, 1988–1994 National Center for Health Statistics. 2005 Available at: http://www.cdc.gov/nchs/about/major/nhanes/Anthropometric%20Measures.htm Tables 6, 9, 15.
- 19.Looker AC, Wahner HW, Dunn WL, Calvo MS, Marris TB, Heyse SP, Johnston CC, Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 20.Kleerkoper M, Nelson DA, Peterson EL, Flynn MJ, Pawluska AS, Jacobsen G, Wilson P. Reference data for bone mass, calciotropic hormones, and biochemical markers of bone remodeling in older (55–78) postmenopausal white and black women. J Bone Miner Res. 1994;9:1267–1276. doi: 10.1002/jbmr.5650090817. [DOI] [PubMed] [Google Scholar]
- 21.Favus MJ. Primer on the metabolic bone diseases and disorders of mineral metabolism. 5. Philadelphia: Lippincott Williams, Wilkins; 2003. Laboratory values related to calcium metabolism/metabolic bone disease; pp. 546–550. [Google Scholar]
- 22.Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with Risedronate. J Bone Miner Res. 2003;18:1051–1056. doi: 10.1359/jbmr.2003.18.6.1051. [DOI] [PubMed] [Google Scholar]
- 23.Wong WW. Effects of soy isoflavones on blood lipids, blood pressure and biochemical markers of bone metabolism in postmenopausal women. J Nutr. 2000;130(686S) (Abstract) [Google Scholar]
- 24.Cai DJ, Zhao Y, Glasier J, Cullen D, Barnes S, Turner CH, Wastney M, Weaver CM. Comparative effect of soy protein, soy isoflavones, and 17β-estradiol on bone metabolism in adult ovariectomized rats. J Bone Miner Res. 2005;20:828–839. doi: 10.1359/JBMR.041236. [DOI] [PubMed] [Google Scholar]
- 25.Spence LA, Lipscomb ER, Cadogan J, Martin B, Wastney ME, Peacock M, Weaver CM. The effect of soy protein and soy isoflavones on calcium metabolism in postmenopausal women: a randomized crossover study. Am J Clin Nutr. 2005;81:916–922. doi: 10.1093/ajcn/81.4.916. [DOI] [PubMed] [Google Scholar]
- 26.Wangen KE, Duncan AM, Merz-Demlow BE, Xu X, Marcus R, Phipps WR, Kurzer MS. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85:3043–3048. doi: 10.1210/jcem.85.9.6787. [DOI] [PubMed] [Google Scholar]
- 27.Lydeking-Olsen E, Beck-Jensen J-E, Setchell KDR, Holm-Jensen T. Soymilk or progesterone for prevention of bone loss: a 2 year randomized, placebo-controlled trial. Eur J Nutr. 2004;43:246–257. doi: 10.1007/s00394-004-0497-8. [DOI] [PubMed] [Google Scholar]
- 28.Devine A, Prince RL, Kerr DA, Dick IM, Criddle RA, Kent GN, Price RI, Webb PG. Correlates of intestinal calcium absorption in women 10 years past the menopause. Calcif Tissue Int. 1993;52:358–360. doi: 10.1007/BF00310199. [DOI] [PubMed] [Google Scholar]
- 29.Arjmandi BH, Hollis BW, Kalu DN. In vivo effect of 17 β-estradiol on intestinal calcium absorption in rats. Bone Miner. 1994;26:181–189. doi: 10.1016/s0169-6009(08)80062-1. [DOI] [PubMed] [Google Scholar]
- 30.Chailurkita L, Ongphiphadhanakul B, Piaseu N, Saetung S, Rajatanavin R. Biochemical markers of bone turnover and response of bone mineral density to intervention in early postmenopausal women: an experience in a clinical laboratory. Clin Chem. 2001;47:1083–1088. [PubMed] [Google Scholar]
- 31.Eastel R, Colwell A, Hampton L, Reev J. Biochemical markers of bone resorption compared with estimates of bone resorption from radiotracer kinetic studies in osteoporosis. J Bone Miner Res. 1997;12:59–65. doi: 10.1359/jbmr.1997.12.1.59. [DOI] [PubMed] [Google Scholar]
- 32.Villareal DT, Binder EF, Williams DB, Schechitman KB, Yarasheski KE, Kohrt WM. Bone mineral density response to estrogen replacement in frail elderly women. JAMA. 2001;286:815–820. doi: 10.1001/jama.286.7.815. [DOI] [PubMed] [Google Scholar]
- 33.Leung JYY, Ho AYY, IP TP, Lee G, Kung AWC. The efficacy and tolerability of risedronate on bone mineral density and bone turnover markers in osteoporotic Chinese women: a randomized placebo-controlled study. Bone. 2005;36:358–364. doi: 10.1016/j.bone.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Harris ST, Ericksen EF, Davidson M, Ettinger MP, Moffett AH, Jr, Baylink DJ, Crusan CE, Chines AA. Effect of combined residronate and hormone replacement therapies on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2001;86:1890–1897. doi: 10.1210/jcem.86.5.7505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.