Abstract
Inhibition of soluble epoxide hydrolase (SEH), the enzyme responsible for degradation of vasoactive epoxides, protects against cerebral ischemia in rats. However, the molecular and biological mechanisms that confer protection in normotension and hypertension remain unclear. Here we show that 6 weeks of SEH inhibition via 2 mg/day of 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA) in spontaneously hypertensive stroke-prone (SHRSP) rats protects against cerebral ischemia induced by middle cerebral artery occlusion, reducing percent hemispheric infarct and neurodeficit score without decreasing blood pressure. This level of cerebral protection was similar to that of the angiotensin-converting enzyme inhibitor, enalapril, which significantly lowered blood pressure. SEH inhibition is also protective in normotensive Wistar-Kyoto (WKY) rats, reducing both hemispheric infarct and neurodeficit score. In SHRSP rats, SEH inhibition reduced wall-to-lumen ratio and collagen deposition and increased cerebral microvessel density, although AUDA did not alter middle cerebral artery structure or microvessel density in WKY rats. An apoptosis mRNA expression microarray of brain tissues from AUDA-treated rats revealed that AUDA modulates gene expression of mediators involved in the regulation of apoptosis in neural tissues of both WKY and SHRSP rats. Hence, we conclude that chronic SEH inhibition protects against cerebral ischemia via vascular protection in SHRSP rats and neural protection in both the SHRSP and WKY rats, indicating that SEH inhibition has broad pharmacological potential for treating ischemic stroke.
Epoxyeicosatrienoic acids (EETs), lipid metabolites produced from arachidonic acid by CYP450 enzymes, are novel mediators that antagonize the sequela of hypertension,1 match cerebral blood flow to increased neural activity and metabolic demand, promotes angiogenesis,2 and protect against ischemia.3,4 Because ischemic stroke occurs with loss of cerebral blood flow and is strongly associated with hypertension, modulation of epoxide degradation has potential in managing ischemic stroke. Unfortunately, pharmacological utility of exogenous EETs is impractical because the epoxides are rapidly degraded by the soluble epoxide hydrolase (SEH) into their less active diol, dihydroxyeicosatrienoic acids.5 In fact, human SEH polymorphisms are linked to the incidence of ischemic stroke,6 and this association could be related to modifications in SEH activity, and thus epoxide catabolism.7 An alternative strategy that has been used to increase EETs systemically is SEH inhibition.1
We previously showed that the SEH inhibitor 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA) protects against cerebral ischemia in spontaneously hypertensive stroke-prone (SHRSP) rats, an animal model of essential hypertension.8 Interestingly, chronic AUDA treatment in SHRSP rats effectively decreased infarct size induced by middle cerebral artery occlusion (MCAO) without decreasing blood pressure.8 Although blood pressure is an important variable in controlling infarct size in the SHRSP, the cerebrovasculature is also a key determinant of increased sensitivity to cerebral ischemia and thus infarct size.9,10 Our study provided preliminary evidence that AUDA protection in the SHRSP involved changes in vascular structure.8 More recently, a report suggested that administration of AUDA-butyl ester protects against cerebral ischemic reperfusion injury in normotensive mice by mechanisms that may involve neural protection rather than vascular protection.11 As a result, the contribution of vascular and neural protection to the cerebral protective effects of SEH inhibition during states of hypertension and normotension are unclear and require further investigation. The present study tested the hypothesis that SEH inhibition provides cerebral protection via different mechanisms in normotensive WKY and hypertensive SHRSP rats.
Materials and Methods
Animals
All male animals were housed and fed a normal rat chow (Tekads 8604; Harlan, Indianapolis, IN) in the animal care facility at the Medical College of Georgia approved by the American Association for the Accreditation of Laboratory Animal Care and all protocols were approved by the institutional animal care and use committee at this institution. All drug treatments were administered via drinking water. Six- to seven-week-old SHRSP rats (Charles River, Wilmington, MA) were divided into four treatment groups as follows: 6 weeks of vehicle (500 mg/L of cyclodextrin and 0.075% of ethanol), 6 weeks of AUDA (2 mg/day via drinking water, 50 mg/L, dissolved using vehicle to aid in solubilization), 6 weeks of enalapril (2.5 mg/day via drinking water dissolved in vehicle), and 5 weeks of AUDA (2 mg/day via drinking water dissolved via vehicle followed by a 7- to 12-day washout period). In addition, a group of 12- to 13-week-old SHRSPs was treated with trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (tAUCB), an SEH inhibitor,12,13 2 mg/day for 7 to 12 days via drinking water (50 mg/L) prepared using vehicle to aid in solubulization. Six- to seven-week-old WKY (Harlan) were treated with either 6 weeks of vehicle or 6 weeks of AUDA, 2 mg/day. Blood pressure was monitored by tail plethysmography (IITC Life Science, Woodland Hills, CA) during the experimental period in acclimatized conscious rats. At the end of the treatment, all animals were anesthetized with pentobarbital (50 mg/kg) for middle cerebral artery occlusion (MCAO) experiments and tissue collection.
Plasma and Brain AUDA and Metabolite Measurements
AUDA levels were measured in homogenized brain, and AUDA metabolites were assessed in plasma samples by reverse phase high performance liquid chromatography followed by negative mode electron spray ionization and tandem mass spectroscopy as previously described.11
MCAO
MCAO was conducted as previously described by Longa and colleagues.14 In brief, anesthetized rats with body temperature maintained at 37°C had a 3–0 dermalon monofilament with a rounded tip inserted cranially until a drop in the Laser Doppler (Perimed, North Royalton, OH) attached to the skull 2 mm down and 5 mm lateral to bregma confirmed MCA occlusion.8 Physiological parameters (PO2, PCO2, hematocrit, and blood glucose) were measured via blood sampling with a femoral arterial line using a blood gas analyzer (GemPremier 3000; Instrumentation Laboratory, Lexington, MA). There were no differences in physiological parameters measured during the MCAO at baseline between vehicle and AUDA treatment (see Supplemental Table S1 at http://ajp.amjpathol.org).
Six hours after MCAO, neurodeficit was assessed using a combination of a modified form of the Bederson and colleagues15 score and an abbreviated adaptation of the modified Neurological Severity Score described by Chen and colleagues.16 The neurodeficit score for WKY animals consisted of a tail suspension test (score range, 1 to 4) to assess postural reflexes and a pad walk to assess motility (range, 1 to 3) that were combined for an overall neurodeficit score. SHRSP neurodeficit score also included an additional spontaneous activity score (1 to 4) to assess the more dramatic deficits displayed in the SHRSP rats, assessing general condition (calm to aggressive) and abnormal movements (curvilinear walk to seizure-like activity).
Next, brains were collected and sliced into 2-mm sections coronally from the frontal pole. Noninfarcted hemisphere, infarcted hemisphere, and infarct were measured using the National Institutes of Health (Bethesda, MD) Image software on digitized images of the slices stained with 2% triphenyltetrazolium chloride (TTC). Percent hemisphere infarct was calculated using the Swanson equation to account for swelling.17
Histology of MCA
Animals were perfused with a vasodilator cocktail (papaverine 0.3 mmol/L/L, adenosine 0.2 mmol/L, diltiazem 0.2 mmol/L/L) prepared in phosphate-buffered saline. A section of the MCA, 4 mm away from the Circle of Willis, was embedded. Serial sections of 5 μm in thickness were taken equidistantly along 650 μm, totaling 16 measurements per vessel. Outer and inner perimeters were measured via by a blinded reviewer using Axiovision 4.0 software (Axio Vision Rel.4.6.3; Carl Zeiss, Thornwood, NY). These measurements were used to calculate wall thickness and wall to lumen (W:L) ratio using the equation of a circle. The MCA was also stained for collagen with the Masson’s trichrome stain and picrosirius red stain. The MCA was scored on a scale of 1 to 10 by two blinded observers.
Microvessel Density
Because the reduction in infarct size evident in SEH knockout mice was associated with increased perfusion in the cortex and striatum during MCA occlusion and early reperfusion18 and we previously found that AUDA treatment reduced infarcted tissue in cortex and striatum,8 we measured microvessel density in the cortex and the striatum of treated and control rats. To measure the microvessel density, the brains were collected and sliced into five 2-mm slices in the same manner used for TTC staining and infarct quantification. The slices are flash-frozen in a methylbutane dry-ice bath. Serial sections, 5 μm thick, were cut of each slice. After fixation with 10% formalin and blocking in 10% normal goat serum, frozen 5-μm serial sections of brain were incubated overnight with von Willebrand factor antibody (1:250, F3520; Sigma, St. Louis, MO) at 4°C, totaling five sections per animal. After washing with phosphate-buffered saline and incubating with secondary antibody, Cy3 goat anti-rabbit IgG conjugate (1:200; Zymed, South San Francisco, CA), slides were mounted with a coverslip using Prolong Antifade Reagent mounting media (Molecular Probes, Carlsbad, CA). Fluorescence was visualized with a Zeiss LSM 510 Meta confocal laser-scanning microscope (Thornwood, NY 543 nm wavelength). Twenty-five pictures of the cortex and striatum per animal at ×200 magnification were analyzed using Axiovision 5.2 (Carl Zeiss, Thornwood, NY) by two blinded reviewers. Data were reported as area (mm2) of fluorescence per area of field (mm2).
Real-Time Polymerase Chain Reaction (PCR) Apoptosis Gene Expression Array
As previously described,19 total RNA was extracted from 50 mg of whole brain from male rats using the RNeasy lipid mini kit and DNase digestion (Qiagen, Valencia, CA) according to the manufacturer’s protocol and RNA concentrations were determined using absorbance at 260 nm. Using RT2 PCR array first strand kit (SuperArray Bioscience, Frederick, MD), 1 μg of RNA was converted to cDNA. The cDNA was then incubated with RT2 real-time SYBR Green PCR mastermix (SuperArray Bioscience) into a 96-well PCR array plate, one sample per plate (three samples per experimental group). Thermal cycling and real-time detection via a Bio-Rad iCycler (Bio-Rad Laboratories, Hercules, CA) was conducted following the instructions of apoptosis array (Superarray Bioscience). The Superarray RT2 PCR arrays are equipped with controls for genomic DNA contamination, reverse transcription, and PCR. Threshold cycle (Ct) values were normalized to β-actin. The significance analysis of microarrays (SAM) software (Stanford University, Stanford, CA) was used to determine the significance of changes in gene expression in the apoptosis microarray20 with a median false discovery rate of 17% and q-value (comparable with P value) of 10%.
Statistics
All data are expressed as mean ± SEM. Differences were assessed using analysis of variance and Student’s t-tests with P values <0.05 being statistically significant.
Results
Brain AUDA and Plasma AUDA Metabolite Levels
AUDA is metabolized by β oxidation into an inactive metabolite 12-(3-adamantyl-ureido)-butyl acid (AUBA). AUBA levels are used as an indication of AUDA exposure. Because AUDA reversibly and competitively inhibits the SEH enzyme, measuring SEH activity in the tissue to determine the degree of SEH inhibition is not feasible.13,21,22 Plasma levels of AUBA reached 4 ng/ml in the SHRSP rats and 13 ng/ml in the WKY rats after 6 weeks of SEH inhibition. In the brain, AUDA levels reached 2 μmol/g in the SHRSP rats and 3 μmol/g in the WKY rats. These levels suggest that treatment was sufficient to inhibit SEH enzymatic activity.
AUDA Decreases Infarct Size Without Preventing Hypertension
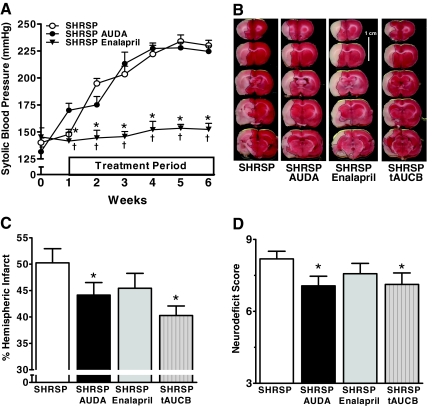
AUDA treatment in the SHRSP rats did not prevent the development of hypertension in the 12-week-old SHRSP rats (Figure 1) consistent with our previous findings.8 To determine whether the protective effects of AUDA were independent of blood pressure lowering, additional SHRSP rats were treated with an angiotensin-converting enzyme inhibitor and served as a blood pressure control group for the SHRSP AUDA-treated rats. As expected, administration of the angiotensin-converting enzyme inhibitor enalapril (2.5 mg/day) for 6 weeks was effective at preventing the development of hypertension in the SHRSP rats. Despite the absence of an effect on blood pressure, the reduction in infarct size and neurodeficit achieved with AUDA was similar to that achieved by 6 weeks of enalapril treatment (Figure 1, A–D).
Figure 1.
AUDA, enalapril, and tAUCB treatment decrease infarct size and neurodeficit in SHRSP. A: Systolic blood pressure in AUDA- and enalapril-treated SHRSP. †P < 0.05 relative to SHRSP enalapril, *P < 0.05 relative to SHRSP AUDA. B: AUDA, enalapril, and tAUCB decrease infarct size after MCAO in SHRSP as demonstrated by the representative TTC-stained coronal slices arranged caudally starting at the frontal pole from each treatment group (black line delineates infarct from viable tissue). C: Quantification of percent hemispheric infarct size in SHRSP groups. *P < 0.05 relative to SHRSP. D: Quantification of neurodeficit score in SHRSP groups. *P < 0.05 relative to SHRSP. SHRSP, n = 16; SHRSP AUDA, n = 16; SHRSP enalapril, n = 14; and SHRSP tAUCB, n = 9.
Previous studies have suggested that high concentrations of AUDA activate the orphan nuclear receptor PPAR-α.23,24 Thus, we treated a separate set of SHRSP rats with tAUCB, a potent SEH inhibitor12,13 that lacks potential for PPAR agonistic activity (Figure 1). As expected, tAUCB protected against cerebral ischemia in the SHRSPs, reducing infarct size to 43 ± 3%, P < 0.05, hemispheric infarct size and neurodeficit score to 6.3 ± 0.7, n = 9, P < 0.05. These results indicate that the protective properties of AUDA are not attributable to PPAR agonistic activity but are the result of SEH inhibition.
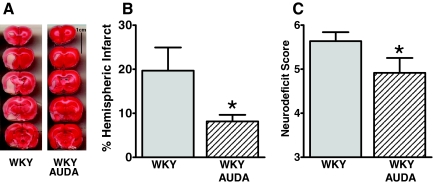
Like the SHRSPs, AUDA treatment in the WKY did not alter blood pressure in the 12-week-old WKY rats (143 ± 1 mmHg versus 141 ± 3 mmHg). However, we still found a protective effect against cerebral ischemia in the WKY animals treated with AUDA. SEH inhibition significantly reduced percent infarct size 40% and neurodeficit 13% (Figure 2, A–C).
Figure 2.
AUDA treatment decreases infarct size and neurodeficit in WKY rats. A: AUDA decreases infarct size after MCAO in WKY rats as demonstrated by the representative TTC-stained coronal slices arranged caudally starting at the frontal pole from each treatment group (black line delineates infarct from viable tissue). B: Quantification of percent hemispheric infarct size in WKY groups. *P < 0.05 relative to WKY. C: Quantification of neurodeficit score in WKY groups. *P < 0.05 relative to WKY. WKY, n = 11; and WKY AUDA, n = 12.
AUDA Provides Vascular Protection in the SHRSP Rats
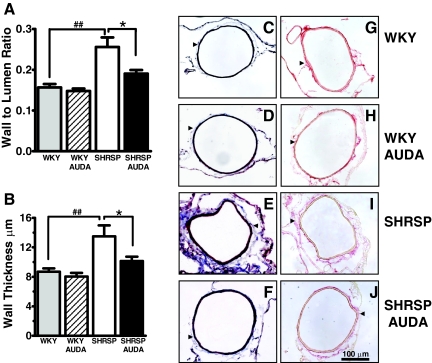
Previous studies have suggested that AUDA may mediate cerebral protection by increasing MCA compliance.8 However, direct evidence implicating AUDA in vascular structure and remodeling is lacking. Thus, we analyzed blood vessel structure in the MCA in the SHRSP and WKY rats. The wall thickness and W:L ratio, two measures of vascular remodeling, and collagen deposition around the MCA were increased in the adult SHRSP in comparison with the adult WKY animals. Treatment with AUDA attenuated remodeling of MCA in SHRSP rats, manifest as a reduction in wall thickness (26%) and the W:L ratio (27%) (Figure 3, A and B). In addition, there was an appreciable reduction in collagen deposition around the MCA of SHRSP AUDA-treated animals as determined by the Masson’s trichrome stain and picrosirius red staining (Figure 3, C–J). Semiquantitative scoring by blinded reviewers determined that untreated SHR-SP had a collagen score of 7.8 ± 0.3 and that this significantly decreased to 4.6 ± 1.3 in AUDA-treated SHRSP. In contrast, we found that MCA wall thickness and W:L ratio of the WKY rats treated with AUDA was not altered (Figure 3, A and B). There was also no noticeable reduction in collagen deposition around the MCAs of AUDA-treated WKYs (Figure 3, C–J).
Figure 3.
Middle cerebral artery (MCA) remodeling and collagen deposition is attenuated by AUDA treatment in SHRSP. A: AUDA attenuates the increase in wall to lumen ratio in the SHRSP. ##P < 0.05 relative to WKY, *P < 0.05 relative to SHRSP. B: AUDA attenuates the increase in wall thickness in the SHRSP. ##P < 0.05 relative to WKY, *P < 0.05 relative to SHRSP. C–F: Staining the MCAs for collagen (pointed to by the black arrowhead) via Masson’s trichrome stain. G–J: Staining the MCAs for collagen via picrosirius red stain (collagen red). Stainings demonstrate that AUDA attenuates the increase in collagen deposition around the MCA as depicted by representative images taken at ×200 magnification. WKY (C and G), AUDA-treated WKY (D and H), SHRSP (E and I), AUDA-treated SHRSP (F and J). WKY, n = 4; WKY AUDA, n = 5; SHRSP, n = 6; and SHRSP AUDA, n = 6.
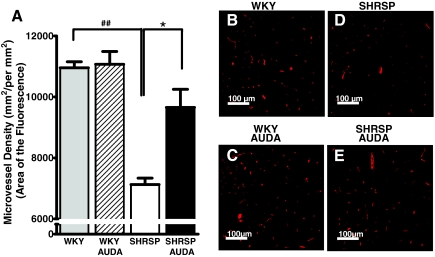
To further investigate the impact of AUDA on the vasculature, we assessed cerebral microvessel density [area (mm2) of fluorescence per area of field (mm2)]. Assessment of immunofluorescently labeled von Willebrand factor in the brain parenchyma of SHRSP and WKY rats at the end of the treatment period revealed that the area of microvessel density in the adult SHRSP was 33% lower than the adult WKY rats (Figure 4, A, B, and D). AUDA was again protective by increasing the microvessel density by 20% in the SHRSP rats (Figure 4, A, D, and E). This supports the notion that SEH inhibition could be providing cerebral protection by reducing the area at risk to ischemia in the SHRSP rats. WKY rats did not exhibit any change in cerebral microvessel density in the WKY animals treated with AUDA (Figure 4, A–C).
Figure 4.
Cerebral microvascular density is increased by AUDA treatment in SHRSP. A: The area of fluorescently labeled von Willebrand factor (mm2) per area of the field (mm2) in the WKY (n = 4), WKY AUDA (n = 4), SHRSP (n = 4), and SHRSP AUDA (n = 5). ##P < 0.05 relative to WKY, *P < 0.05 relative to AUDA. B–E: Representative von Willebrand factor-labeled immunofluorescent images (red). Original magnifications, ×200.
To determine whether the protection achieved by chronic AUDA treatment could be sustained after discontinuation of treatment, a separate group of SHRSP rats were treated with AUDA, 2 mg/day, for 5 weeks followed by 7 to 12 days of AUDA withdrawal. The area under the curve (AUC) of orally administered AUDA is 0.4 × 104nmol/L.minute,12 and the half-life for AUDA after oral gavage is 7.3 hours.29 As a result, the withdrawal period should be sufficient to exclude the effects of the plasma presence of the drug AUDA. As anticipated, a trend for protection was still evident after the withdrawal period (46 ± 9% hemispheric infarct, n = 5, P < 0.26 and 7.6 ± 0.8 neurodeficit, n = 5, P < 0.20) further supporting the notion that chronic AUDA treatment protection from ischemic damage was in part attributable to structural changes in the SHRSP cerebrovasculature.
Apoptosis
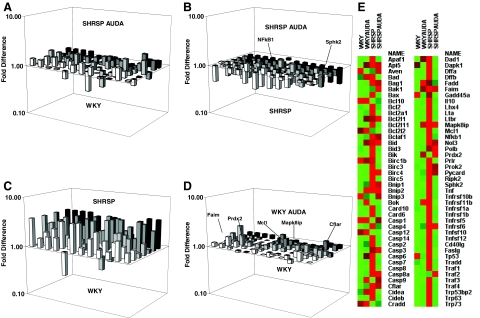
EETs and CYP overexpression reportedly antagonize intrinsic and extrinsic apoptosis.30,31,32,33 Also, EETs and SEH inhibition have been shown to promote cell survival during hypoxic reperfusion injury.34 As a result, we undertook a global approach to assess expression of apoptotic genes (see Supplemental Table S2 at http://ajp.amjpathol.org for gene description of the 84 genes analyzed in the PCR plate) in brain samples collected from controls and AUDA-treated SHRSP and WKY rats using an apoptosis PCR microarray (Figure 5 and Table 1). We have identified 57 genes up-regulated ≥1.5-fold and 25 ≥2-fold in the SHRSP in comparison with the WKY encoding tumor necrosis factor (TNF) ligands and receptors, members of the Bcl-2 family, caspases, nuclear factor (NF)-κB1, and factors involved in DNA damage-induced apoptosis (Table 1 and Figure 5, A and E). In contrast, AUDA-treated SHRSP had only 1 gene up-regulated ≥2-fold and 11 ≥1.5-fold in comparison with WKY (Figure 5, C and E; and Table 1). Furthermore, AUDA reduced gene expression of 39 apoptotic factors ≥1.5-fold and 32 ≥2-fold versus SHRSP vehicle-treated rats (Figure 5, B and E). AUDA down-regulated genes in the SHRSP rats encoding NF-kB1, sphingosine kinase 2 (Sphk2), TNF ligands (CD40L, Lta, Lbtr, Tnf, FasL), and TNF receptors (1a, 1b, and 11b), mediators of DNA damage-induced apoptosis (Dad1, Cidea, Cideb, Dffb, Trp53bp2, Trp63, and Trp73), members of the Bcl-2 family (Bc12, Bid3, Bax, Bcl2l11, Mcl1, and Bik), caspases, and additional mediators listed in Table 1. In the WKY, AUDA up-regulated seven genes ≥1.5-fold as listed in Table 1 (Figure 5, D and E). Interestingly, AUDA increased anti-apoptotic mediators that have also been shown to be neurotrophic and/or neuroprotective, including mitogen-activated protein kinase 8 interacting protein (Mapk8ip),35,36,37,38 Fas apoptotic inhibitory molecule (Faim),39,40 caspase 8, and FADD-like apoptosis regulator/Fas-associated death domain-like interleukin-1β-converting enzyme inhibitory protein (Cflar/Flip),41,42 myeloid cell leukemia sequence 1 (Mcl-1),43 and an antioxidant peroxiredoxin 2 (Prdx2) in the WKY animals (Table 1 and Figure 5E).
Figure 5.
Expression of apoptotic genes in neural tissue was compared with mRNA PCR microarray. n = 3 per experimental group. A: Gene expression of 25 apoptotic factors increased more than or equal to twofold in SHRSP control versus WKY. B: AUDA down-regulated 32 apoptotic factors more than or equal to twofold in SHRSP, including nuclear factor (NF)-kB1 and sphingosine kinase 2 (Sphk2). C: Difference in gene expression in AUDA-treated SHRSP versus WKY. D: Gene expression of seven apoptotic factors increased ≥1.5-fold in AUDA-treated WKY versus WKY. E: Heat map of gene expression profiles of each treatment group and relative changes in expression of the genes between the four experimental groups (green lowest expression to bright red highest expression between the four experimental groups).
Table 1.
Using PCR Microarray, N-Fold Changes in mRNA Expression Levels Were Determined of Genes Encoding Mediators Involved in the Apoptotic Pathway (n = 3 Per Experimental Group)
| Fold up- or down-regulation apoptotic genes
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | SHRSP/WKY | WKY AUDA/WKY | SHRSP AUDA/WKY | SHRSP AUDA/SHRSP | Gene | SHRSP/WKY | WKY AUDA/WKY | SHRSP AUDA/WKY | SHRSP AUDA/SHRSP |
| Apaf1 | 1.5 | 1.0 | 1.1 | −1.1 | Dad1 | 1.4* | 1.2* | 1.0 | −1.2* |
| Api5 | 2.3* | 1.5* | 1.7* | −1.1 | Dapk1 | 1.3 | −1.2 | −1.1 | −1.3 |
| Aven | 1.4* | −1.2 | −1.0 | 1.1 | Dffa | 2.5 | −1.0 | 1.2 | −1.7 |
| Bad | 1.4* | 1.0* | −1.1 | −1.1 | Dffb | 3.5* | 1.1 | 1.3 | −2.1* |
| Bag1 | 2.2* | 1.1 | 1.5 | −1.1 | Fadd | 1.8 | 2.2* | 1.5* | −1.1 |
| Bak1 | 1.4 | 1.0 | 1.7* | 1.3 | Faim | 2.4* | 1.5* | 1.6* | −1.1 |
| Bax | 3.8* | 1.2 | 1.4 | −2.1* | Gadd45a | 1.2 | −1.2 | −1.4 | −1.2 |
| Bcl10 | −2.9* | −1.0 | −1.4 | 1.4 | Il10 | 3.8* | 1.2* | 1.4 | −2.1* |
| Bcl2 | 1.8* | −1.1 | 1.1 | −1.3* | Lhx4 | 2.9* | −1.1 | 1.1 | −2.1* |
| Bcl2a1 | 1.8* | 1.1 | 1.0 | −1.3 | Lta | 3.8* | 1.2* | 1.4 | −2.1* |
| Bcl2l1 | 1.6* | 1.2* | 1.4 | 1.1 | Ltbr | 3.6* | 1.1 | 1.3 | −2.1* |
| Bcl2l11 | 3.8* | 1.2 | 1.4 | −2.1* | Mapk8ip | 2.1* | 1.4* | 1.5* | 1.0 |
| Bcl2l2 | 1.5* | 1.2* | −1.3 | −1.2 | Mcl1 | 3.8* | 1.2* | 1.4 | −2.1* |
| Bclaf1 | 1.7* | 1.2* | 1.3 | −1.1 | Nfkb1 | 3.3* | −1.0 | 1.3 | −1.5* |
| Bid | 1.9* | 1.3* | 1.5 | 1.1 | Nol3 | 2.7* | 1.0 | 1.4 | −1.1 |
| Bid3 | 1.9 | −1.2 | −1.1 | −1.8* | Polb | 1.3 | 1.1 | 1.4 | 1.1 |
| Bik | 2.6 | −1.1 | 1.0 | −2.0* | Prdx2 | 1.2 | 1.1* | 1.1 | 1.1 |
| Birc1b | 1.1 | −1.2 | −2.4 | −2.2* | Prlr | 3.8* | 1.1 | −1.0 | −1.6 |
| Birc3 | 3.5* | 1.1 | 1.3 | −2.1* | Prok2 | 2.5* | 1.3 | 1.5* | −1.3 |
| Birc4 | 1.7* | 1.0 | 1.4 | 1.0 | Pycard | 3.2* | 1.5* | 2.0* | −1.2 |
| Birc5 | 3.5* | 1.1 | 1.3 | −2.1* | Ripk2 | 1.6* | 1.1* | 1.1 | −1.3* |
| Bnip1 | 2.4* | 1.3* | 1.5* | −1.1 | Sphk2 | 2.1* | −1.1 | 1.1 | −2.3* |
| Bnip2 | 1.9* | 1.5* | 1.5 | −1.0 | Tnf | 3.6* | 1.1 | 1.3 | −2.1* |
| Bnip3 | −1.0 | −1.1 | −1.0 | 1.1 | Tnfrsf10b | 3.8* | 1.2* | 1.4 | −2.1* |
| Bok | 1.2 | 1.4* | −1.0 | 1.0 | Tnfrsf11b | 1.8* | 1.5* | −1.1 | −1.7* |
| Card10 | 2.1 | 1.1 | 1.2 | −1.4 | Tnfrsf1a | 2.3 | −1.4 | −1.1 | −2.1* |
| Card6 | 2.6* | −1.2 | 1.2 | −1.8* | Tnfrsf1b | 2.1* | 1.0 | 1.2 | −1.7* |
| Casp1 | −1.5 | −1.0 | −1.2 | 1.5 | Tnfrsf5 | 3.2* | 1.1 | 1.2 | −2.2* |
| Casp4 | −2.3* | 1.2* | −2.1 | 1.1 | Tnfrsf6 | 1.9* | 1.1* | 1.3 | −1.0 |
| Casp12 | 3.8* | 1.3* | 1.4 | −2.1* | Tnfsf10 | 2.4* | 1.1 | 1.1 | −1.9* |
| Casp14 | 1.3 | 1.2* | 1.1 | −1.3 | Tnfsf12 | 3.8* | 1.2* | 1.4 | −2.1* |
| Casp2 | 2.2* | 1.2* | 1.8* | 1.2 | Cd40lg | 3.8* | 1.2* | 1.4 | −2.1* |
| Casp3 | 1.7 | 1.2* | 1.4 | −1.4 | Faslg | 3.2* | 1.0 | −1.1 | −2.3* |
| Casp6 | 3.1* | 1.6* | 1.1 | −2.0* | Tp53 | 1.6 | −1.2 | −1.3 | −1.3 |
| Casp7 | 2.8* | 1.2* | 1.3 | −1.8* | Tradd | 1.7* | −1.5 | −1.2 | −2.0* |
| Casp8 | 3.7* | 1.1* | 1.1 | −2.0* | Traf1 | 3.3* | 1.1 | 1.2 | −2.1* |
| Casp8ap2 | 1.7* | 1.2* | 1.9* | 1.0 | Traf2 | 1.3 | 1.0 | 1.2 | 1.1 |
| Casp9 | −1.0 | 1.3* | 1.2 | 1.2 | Traf3 | 3.2* | 1.1 | 1.2 | −2.1* |
| Cflar | 2.2* | 1.4* | 1.8* | −1.1 | Traf4 | 3.8* | 1.2* | 1.4 | −2.1* |
| Cidea | 2.2* | 1.3* | −1.4 | −2.0* | Trp53bp2 | 3.8* | 1.2* | 1.4 | −2.1* |
| Cideb | 3.8* | 1.2* | 1.4 | −2.1* | Trp63 | 3.8* | 1.2* | 1.4 | −2.1* |
| Cradd | −1.3 | 1.2* | −1.2 | 1.4 | Trp73 | 3.8* | 1.2* | 1.4 | −2.1* |
Fold up- or down-regulation (N-fold Δ in expression level) in between the compared groups identified in each column is given.
Factors significantly up- or down-regulated are in bold.
Discussion
In this study, we investigated vascular and nonvascular mechanisms by which AUDA can protect against cerebral ischemia in normotension and hypertension. Although chronic SEH inhibition via AUDA protects SHRSP rats via vascular and neural protection, only neural protection was observed in WKY rats.
Chronic AUDA treatment of SHRSP rats protected through vascular protection by attenuating the hypertrophic remodeling and collagen deposition that occurs in the large cerebral vessel of the SHRSP rats.9,10 Several reports suggest that EETs and SEH inhibition may attenuate vascular remodeling by modulating intracellular signaling pathways in vascular smooth muscle cells44 and fibroblasts.45 Our laboratory has previously shown that SEH inhibition with 1-cyclohexyl-3-dodecyl-urea (CDU) decreased collagen deposition in kidneys of angiotensin-infused hypertensive rats and decreased renal vascular remodeling.1 Furthermore, we have previously found increased MCA compliance in SHRSP rats chronically treated with AUDA.8 This corroborates our current findings of reduced W:L ratio, wall thickness, and collagen deposition in the SHRSP.
In addition to its effects on the MCA, AUDA treatment increased cerebral microvessel density in the SHRSP rats. Jesmin and colleagues27,28 reported that SHRSP rats have reduced regional cerebral blood flow, angiogenic factors, and cerebral microvessel density in the cerebral cortex at 6 weeks of age in comparison with age-matched WKY rats.28 In our study, vehicle-treated SHRSP rats also demonstrated reduced cerebral microvessel density at 12 weeks of age, the time of occlusion, in comparison with the WKY rats to a degree similar to the previous report. EETs have been shown to induce angiogenesis in several in vivo models and on co-culture of astrocytes and endothelial cells.2 Because hypoxia and increased metabolic demand are potential triggers for astrocyte EET-mediated angiogenesis,46 we speculate that SEH inhibition resulted in increased cerebral microvessel density by countering the deficiencies present in the SHRSP rats. Interestingly, there was a trend for maintenance of the cerebral protection in SHRSP rats after AUDA was withdrawn for 7 to 12 days. Taken together, these results suggest that cerebral protective effects of AUDA in SHRSP rats were in part attributable to structural changes in the vasculature.
Unlike the effects of AUDA on vascular remodeling and microvessel density, SEH inhibition provided neural protection in both normotensive and hypertensive rat strains by modulating gene expression of mediators involved in the regulation of apoptosis in the brain tissue of both WKY and SHRSP rats. We propose that the up-regulation of the anti-apoptotic mediators and neuroprotectants in the WKY and dampening of overexpression of pro-apoptotic mediators in the SHRSP rats may set the stage for increased tolerance of cellular stress. Although the studies conducted here were on tissue pre-ischemic insult and therefore do not allow for the determination of the specific genes that may be involved in providing protection from cerebral ischemia, they do show that SEH inhibition causes a global change in the expression of mediators involved in the apoptotic pathway. The range of fold changes in expression levels we detected are in accordance with prior reports31 of apoptotic gene expression and protein expression pre-insult, which report smaller inductions pre-insult that prime the cells for a dramatic up-regulation on induction of apoptosis, and thus promotion of cell survival.31 However, because of the limitations of our study, we cannot determine the cell type or specific genes that could be attributed to possible neuroprotection. The SAM statistical analysis is limited by the occurrence of false-positives, and we have not confirmed changes in gene expression by cellular localization, subcellular localization, or protein analysis.
The changes that we report with SEH inhibition do however corroborate previous findings of the anti-apoptotic properties of EETs, CYP overexpression, and SEH inhibition. EETs and CYPs reportedly inhibit apoptosis induced by Fas ligand,30 TNF-α induced,31,32 serum deprivation,30 etoposide, arachidonic acid, ceramide production, inhibition of reactive oxygen species,33 and hypoxic reperfusion possibly by antagonizing reactive oxygen species.34 Our data are also in agreement with a previous report that MAPK and PI3/Akt signaling pathways protect endothelial cells from TNF-α induced apoptosis.31 Closely related, a recent study linked human SEH polymorphisms to cortical neuronal sensitivity to oxygen-glucose deprivation. Overexpression of SEH and human polymorphisms associated with variations in SEH activity increased cell death induced by oxygen-glucose deprivation.47 To the best of our knowledge, our study is the first to provide a possible link to the neuroprotection achieved via SEH inhibition with AUDA in hypertension and normotension with a shift in the balance in the gene expression of pro- and anti-apoptotic mediators, producing possible ischemic tolerance.
Previous reports indicate that AUDA has PPAR-α activity at high concentrations most probably attributable to its long alkyl chain.23,24 Its structural similarity to fatty acids makes the compound lipophilic, which increases its ability to bind to the hydrophobic ligand-binding domain of PPAR-α.23,24 Although we cannot exclude the possibility of PPAR-α, the AUBA levels achieved in the present study were in the nanomolar range, which would indicate that AUDA levels were not sufficient to induce PPAR-α activity. We also found protection with a nonalkanoic acid SEH inhibitor, tAUCB. Not only does tAUCB lack the long fatty acid chain required for PPAR-α binding, tAUCB is more polar than AUDA.12,13 As a result, tAUCB lacks potential for binding to the hydrophobic PPAR-α binding domain.48,49 Moreover, mice that had the gene responsible for SEH production deleted also were protected from cerebral ischemic reperfusion injury.50 Based on our current findings and published findings in the Ephx2−/− mice, we conclude that the protection from cerebral ischemia with AUDA treatment is most likely to be attributable to SEH inhibition.
In our study, the SHRSP and WKY animals were treated with AUDA in the drinking water, as in our prior study.8 In our prior study, the AUDA and AUBA plasma levels were sufficient to show adequate inhibition of the SEH enzyme as demonstrated by an increase in the urinary epoxide to diol ratio. In the current study, we do not have confirmatory data to show the level of SEH inhibition via the epoxide to diol ratio in the brain or plasma. However, the plasma and brain AUDA metabolite and AUDA drug levels are similar to that previously established as therapeutic in our prior study.8 In addition, measuring the degree of SEH inhibition directly is more difficult because AUDA reversibly and competitively inhibits the SEH enzyme.13,21,22 In the current study, the AUDA plasma levels were variable between the WKY and SHRSP animals, and could be attributable to differences in the drinking water intake between the strains. We cannot rule out possible differences in pharmacokinetics and AUDA metabolism between the rat strains. However, the brain tissue levels of AUDA were very similar between the two groups in our study. The reason for similar brain AUDA levels is not known but could be related to the blood brain barrier or to brain compartmentalization of AUDA. We did not measure brain AUDA levels in the prior study. As a result, we cannot determine relative differences in between dose and brain tissue levels of AUDA in this study in comparison with our prior study. Yet, the doses of the SEH inhibitor were similar to those also shown to be protective against LPS challenge in mice.12 In addition, the plasma levels in both studies were above the rat IC50 for AUDA (8 nmol/L).12
Moreover, in our study we did not investigate the other potential protective effects of the ACE inhibitor enalapril or its possible effects in the WKY rats. Here, we used enalapril as a blood pressure-lowering agent to provide a blood pressure control in the SHRSP rats and therefore did not treat the normotensive WKY rats with enalapril. However, it is important to recognize that ACE inhibitors such as enalapril have the potential to protect against cerebral ischemia by mechanisms that may not be directly related to blood pressure lowering.
In the present study, we evaluated the cerebral protective effects of SEH inhibition in normotensive and hypertensive animals because both presentations are clinically relevant. Conducting these studies in an animal model with hypertension is essential for addressing the main patient population affected by ischemic stroke.51 Based on the TOAST study subclassifications, 36 to 56.6% of ischemic strokes are reportedly related to vascular disease associated with hypertension, such as large vessel atherosclerosis and small artery disease.52,53 Moreover, human SEH polymorphisms have been found to alter SEH activity7 and not only affect the incidence of ischemic stroke but also hypertension and atherosclerosis.1,6 Smoking, another risk factor for ischemic stroke, synergistically increases the prevalence of hypertension and atherosclerotic disease with the SEH polymorphisms.1 Because SEH polymorphisms are linked to both ischemic stroke and its modifiable risk factors, modulating SEH enzymatic activity may be beneficial in decreasing the incidence and severity of ischemic stroke in high-risk patients. It is also important to address the nonhypertensive population and the patients without evidence of vascular disease that have cerebral ischemic events. The ability of SEH inhibition to protect in the absence of hypertension and vascular disease implicates that SEH inhibition may also be beneficial in treating the nontraditional ischemic stroke patients. This hypothesis is further confirmed by the epidemiological association of increased risk for ischemic stroke with Ephx2 gene polymorphisms in patients with large artery disease and patients lacking the traditional etiology for ischemic stroke.54
In summary, we show that chronic SEH inhibition is protective during cerebral ischemia in hypertensive and nonhypertensive rats. SEH inhibition normalized cerebrovascular structure in hypertensive animals and provided neural protection in normotensive and hypertensive rats. Overall, the ability of AUDA to provide protection in the presence or absence of hypertension is clinically relevant because ischemic stroke occurs in hypertensive and nonhypertensive patients. The TOAST subclassifications demonstrates the heterogeneity of the ischemic stroke patient population,52,53 and as a result, using a treatment that can act through multiple mechanisms of protection under varying clinical presentations further increases the patient population that can be effectively treated. As a result, SEH inhibition has broad pharmacological potential for ischemic stroke management, and its utility should be further investigated.
Supplementary Material
Acknowledgments
We thank Jeffrey Quigley and Dr. David Stepp for technical assistance.
Footnotes
Address reprint requests to John D. Imig, Department of Pharmacology and Toxicology, Medical College of Wisconsin, 8701 Watertown Plank Rd., Milwaukee, WI 53226. E-mail: jdimig@mcw.edu.
Supported by the American Heart Association (established investigator to J.D.I.) and the National Institutes if Health (grants HL59699, NS053002, and F31 HL087723-01).
Disclosures: B.D.H. is the founder and J.D.I. is a member of the Scientific Advisory Board of Arête Therapeutics, a developer of SEH inhibitors for the treatment of hypertension.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev. 2006;24:169–188. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Fleming I. Vascular cytochrome p450 enzymes: physiology and pathophysiology. Trends Cardiovasc Med. 2008;18:20–25. doi: 10.1016/j.tcm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Liu M, Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab. 2005;25:939–948. doi: 10.1038/sj.jcbfm.9600085. [DOI] [PubMed] [Google Scholar]
- Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99:442–450. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Fornage M, Lee CR, Doris PA, Bray MS, Heiss G, Zeldin DC, Boerwinkle E. The soluble epoxide hydrolase gene harbors sequence variation associated with susceptibility to and protection from incident ischemic stroke. Hum Mol Genet. 2005;14:2829–2837. doi: 10.1093/hmg/ddi315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla-Zawislak BD, Srivastava PK, Vazquez-Matias J, Mohrenweiser HW, Maxwell JE, Hammock BD, Bradbury JA, Enayetallah AE, Zeldin DC, Grant DF. Polymorphisms in human soluble epoxide hydrolase. Mol Pharmacol. 2003;64:482–490. doi: 10.1124/mol.64.2.482. [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2005;46:842–848. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle P. Dorsal cerebral collaterals of stroke-prone spontaneously hypertensive rats (SHRSP) and Wistar Kyoto rats (WKY). Anat Rec. 1987;218:40–44. doi: 10.1002/ar.1092180108. [DOI] [PubMed] [Google Scholar]
- Coyle P, Jokelainen PT. Differential outcome to middle cerebral artery occlusion in spontaneously hypertensive stroke-prone rats (SHRSP) and Wistar Kyoto (WKY) rats. Stroke. 1983;14:605–611. doi: 10.1161/01.str.14.4.605. [DOI] [PubMed] [Google Scholar]
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–1940. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50:225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Zhang W, Kooper D, Alkayed NJ. Soluble epoxide hydrolase gene deletion is associated with increased cbf and reduced stroke damage. Stroke. 2006;37:683. [Google Scholar]
- Knight SF, Quigley JE, Yuan J, Roy SS, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension. 2008;51:352–359. doi: 10.1161/HYPERTENSIONAHA.107.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JW, Denton DL, Morisseau C, Koger CS, Wheelock CE, Hinton DE, Hammock BD. Evaluation of fish models of soluble epoxide hydrolase inhibition. Environ Health Perspect. 2001;109:61–66. doi: 10.1289/ehp.0110961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf NM, Morisseau C, Jones PD, Hock B, Hammock BD. Development of a high-throughput screen for soluble epoxide hydrolase inhibition. Anal Biochem. 2006;355:71–80. doi: 10.1016/j.ab.2006.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Hu S, Watanabe T, Weintraub NL, Snyder GD, Yao J, Liu Y, Shyy JY, Hammock BD, Spector AA. Activation of peroxisome proliferator-activated receptor alpha by substituted urea-derived soluble epoxide hydrolase inhibitors. J Pharmacol Exp Ther. 2005;314:260–270. doi: 10.1124/jpet.105.085605. [DOI] [PubMed] [Google Scholar]
- Ng VY, Morisseau C, Falck JR, Hammock BD, Kroetz DL. Inhibition of smooth muscle proliferation by urea-based alkanoic acids via peroxisome proliferator-activated receptor alpha-dependent repression of cyclin D1. Arterioscler Thromb Vasc Biol. 2006;26:2462–2468. doi: 10.1161/01.ATV.0000242013.29441.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New DI, Chesser AM, Thuraisingham RC, Yaqoob MM. Structural remodeling of resistance arteries in uremic hypertension. Kidney Int. 2004;65:1818–1825. doi: 10.1111/j.1523-1755.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- Izzard AS, Graham D, Burnham MP, Heerkens EH, Dominiczak AF, Heagerty AM. Myogenic and structural properties of cerebral arteries from the stroke-prone spontaneously hypertensive rat. Am J Physiol. 2003;285:H1489–H1494. doi: 10.1152/ajpheart.00352.2003. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Togashi H, Mowa CN, Ueno K, Yamaguchi T, Shibayama A, Miyauchi T, Sakuma I, Yoshioka M. Characterization of regional cerebral blood flow and expression of angiogenic growth factors in the frontal cortex of juvenile male SHRSP and SHR. Brain Res. 2004;1030:172–182. doi: 10.1016/j.brainres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Togashi H, Sakuma I, Mowa CN, Ueno K, Yamaguchi T, Yoshioka M, Kitabatake A. Gonadal hormones and frontocortical expression of vascular endothelial growth factor in male stroke-prone, spontaneously hypertensive rats, a model for attention-deficit/hyperactivity disorder. Endocrinology. 2004;145:4330–4343. doi: 10.1210/en.2004-0487. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Schulz D, Morisseau C, Hammock BD. High-throughput pharmacokinetic method: cassette dosing in mice associated with minuscule serial bleedings and LC/MS/MS analysis. Anal Chim Acta. 2006;559:37–44. doi: 10.1016/j.aca.2005.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol. 2006;291:H517–H531. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, Lu ZY, Wang DW, Zeldin DC. Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways. Am J Physiol. 2007;293:H142–H151. doi: 10.1152/ajpheart.00783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JG, Chen CL, Card JW, Yang S, Chen JX, Fu XN, Ning YG, Xiao X, Zeldin DC, Wang DW. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65:4707–4715. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- Chen JK, Capdevila J, Harris RC. Cytochrome p450 epoxygenase metabolism of arachidonic acid inhibits apoptosis. Mol Cell Biol. 2001;21:6322–6331. doi: 10.1128/MCB.21.18.6322-6331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Graham L, Dikalov S, Mason RP, Falck JR, Liao JK, Zeldin DC. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol. 2001;60:310–320. doi: 10.1124/mol.60.2.310. [DOI] [PubMed] [Google Scholar]
- Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL, Davis RJ. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277:693–696. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Lee KW, Park BY, Lee JK, Park J, Choi IY, Eom SJ, Chang TS, Kim MJ, Yeom YI, Chang SK, Lee YD, Choi EJ, Han PL. Molecular cloning of multiple splicing variants of JIP-1 preferentially expressed in brain. J Neurochem. 1999;72:1335–1343. doi: 10.1046/j.1471-4159.1999.721335.x. [DOI] [PubMed] [Google Scholar]
- Becker AJ, Gillardon F, Blumcke I, Langendorfer D, Beck H, Wiestler OD. Differential regulation of apoptosis-related genes in resistant and vulnerable subfields of the rat epileptic hippocampus. Brain Res Mol Brain Res. 1999;67:172–176. doi: 10.1016/s0169-328x(99)00060-1. [DOI] [PubMed] [Google Scholar]
- Harding TC, Xue L, Bienemann A, Haywood D, Dickens M, Tolkovsky AM, Uney JB. Inhibition of JNK by overexpression of the JNL binding domain of JIP-1 prevents apoptosis in sympathetic neurons. J Biol Chem. 2001;276:4531–4534. doi: 10.1074/jbc.C000815200. [DOI] [PubMed] [Google Scholar]
- Segura MF, Sole C, Pascual M, Moubarak RS, Perez-Garcia MJ, Gozzelino R, Iglesias V, Badiola N, Bayascas JR, Llecha N, Rodriguez-Alvarez J, Soriano E, Yuste VJ, Comella JX. The long form of Fas apoptotic inhibitory molecule is expressed specifically in neurons and protects them against death receptor-triggered apoptosis. J Neurosci. 2007;27:11228–11241. doi: 10.1523/JNEUROSCI.3462-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole C, Dolcet X, Segura MF, Gutierrez H, Diaz-Meco MT, Gozzelino R, Sanchis D, Bayascas JR, Gallego C, Moscat J, Davies AM, Comella JX. The death receptor antagonist FAIM promotes neurite outgrowth by a mechanism that depends on ERK and NF-kappa B signaling. J Cell Biol. 2004;167:479–492. doi: 10.1083/jcb.200403093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth AH, Bermpohl D, Webb TE, Darwish R, Fiskum G, Qiu J, McCarthy D, Moskowitz MA, Whalen MJ. Expression of cellular FLICE inhibitory proteins (cFLIP) in normal and traumatic murine and human cerebral cortex. J Cereb Blood Flow Metab. 2005;25:1030–1040. doi: 10.1038/sj.jcbfm.9600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumori Y, Northington FJ, Hong SM, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Reduction of caspase-8 and -9 cleavage is associated with increased c-FLIP and increased binding of Apaf-1 and Hsp70 after neonatal hypoxic/ischemic injury in mice overexpressing Hsp70. Stroke. 2006;37:507–512. doi: 10.1161/01.STR.0000199057.00365.20. [DOI] [PubMed] [Google Scholar]
- Mori M, Burgess DL, Gefrides LA, Foreman PJ, Opferman JT, Korsmeyer SJ, Cavalheiro EA, Naffah-Mazzacoratti MG, Noebels JL. Expression of apoptosis inhibitor protein Mcl1 linked to neuroprotection in CNS neurons. Cell Death Differ. 2004;11:1223–1233. doi: 10.1038/sj.cdd.4401483. [DOI] [PubMed] [Google Scholar]
- Davis BB, Thompson DA, Howard LL, Morisseau C, Hammock BD, Weiss RH. Inhibitors of soluble epoxide hydrolase attenuate vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA. 2002;99:2222–2227. doi: 10.1073/pnas.261710799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves D, Moreno JJ. Epoxyeicosatrienoic acids induce growth inhibition and calpain/caspase-12 dependent apoptosis in PDGF cultured 3T6 fibroblast. Apoptosis. 2007;12:1979–1988. doi: 10.1007/s10495-007-0123-3. [DOI] [PubMed] [Google Scholar]
- Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33:2957–2964. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, Alkayed NJ. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci. 2007;27:4642–4649. doi: 10.1523/JNEUROSCI.0056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronet P, Petersen JF, Folmer R, Blomberg N, Sjoblom K, Karlsson U, Lindstedt EL, Bamberg K. Structure of the PPARalpha and -gamma ligand binding domain in complex with AZ 242; ligand selectivity and agonist activation in the PPAR family. Structure. 2001;9:699–706. doi: 10.1016/s0969-2126(01)00634-7. [DOI] [PubMed] [Google Scholar]
- Xu HE, Lambert MH, Montana VG, Plunket KD, Moore LB, Collins JL, Oplinger JA, Kliewer SA, Gampe RT, Jr, McKee DD, Moore JT, Willson TM. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 2001;98:13919–13924. doi: 10.1073/pnas.241410198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, Debarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., III Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Han SW, Kim SH, Lee JY, Chu CK, Yang JH, Shin HY, Nam HS, Lee BI, Heo JH. A new subtype classification of ischemic stroke based on treatment and etiologic mechanism. Eur Neurol. 2007;57:96–102. doi: 10.1159/000098059. [DOI] [PubMed] [Google Scholar]
- Gschwendtner A, Ripke S, Freilinger T, Lichtner P, Muller-Myhsok B, Wichmann HE, Meitinger T, Dichgans M. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with an increased risk of ischemic stroke in white Europeans. Stroke. 2008;39:1593–1596. doi: 10.1161/STROKEAHA.107.502179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.