Abstract
Cell-penetrating peptide (TATp) was attached to the distal tips of polyethylene glycol (PEG) moieties of polyethyleneglycol-phosphatidylethanolamine (PEG-PE) micelles loaded with paclitaxel (PCT). The TATp-modified micelles demonstrated an increased interaction with cancer cells compared to non-modified micelles resulting in a significant increase of the in vitro cytotoxicity to different cancer cells. TATp-modified PCT-loaded micelles were administered intratumorally in mice and the induction of apoptosis in tumor cells was studied after 48h with the Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) assay using free PCT and TATp-free PCT-loaded PEG-PE micelles as controls. A significant apoptotic cell death was observed in tumors treated with PCT-loaded micelles modified with TATp, while the treatment with free PCT or with non-modified PCT-loaded micelles resulted in much smaller number of TUNEL-positive cells within tumors.
Keywords: Intracellular drug delivery, Polymeric micelles, Polyethyleneglycol-phosphatidylethanolamine, Cell-penetrating peptide, TAT peptide, Intratumoral drug administration, Paclitaxel
1. Introduction
One of the popular nanocarrier systems for the delivery of poorly soluble drugs, including anticancer drugs, is the polymeric micelles (Kataoka et al., 2001; Torchilin 2007). Amongst different types of polymeric micelles reported, micelles composed of polyethyleneglycol-phosphatidylethanolamine (PEG-PE) conjugate draw a lot of attention because of their small size (10–100 nm), good solubilization efficiency, and high stability (Torchilin 2001a; Krishnadas et al., 2003; Lukyanov et al., 2004; Torchilin 2007). The small size of micelles allows for their efficient accumulation in pathological tissues with leaky vasculature, such as tumors and infarcts, via the enhanced permeability and retention (EPR) effect (Maeda et al., 2000; Maeda et al., 2008). Many efforts have been made to increase the delivery of the micellar nanocarriers to tumor cells by using monoclonal antibodies (mAb) (Torchilin et al., 2003b) or other tumor-specific ligands (Bae et al., 2006) attached to the micelle surface.
In order to facilitate the delivery of anticancer drug not only to the surface of cancer cells, but also inside those cells for an enhanced therapeutic effect, a promising approach for the intracellular delivery that is under development over the last decade - the use of cell-penetrating peptides (CPPs) (Schwarze et al., 1999; Snyder et al., 2004). In particular, the CPP derived from the human immunodeficiency virus-1 transactivator protein (TATp) has attracted much interest. TATp-mediated cytoplasmic uptake of polymers, plasmid DNA (Astriab-Fisher et al., 2002; Nguyen et al., 2008), nanoparticles (Lewin et al., 2000; Zhao et al., 2002; Rao et al., 2008), liposomes (Torchilin et al., 2001b; Levchenko et al., 2003; Fretz et al., 2004) and micelles (Sethuraman et al., 2007; Sawant et al., 2008) has been reported. Various uptake mechanisms appear to be involved in different systems, and in some cases, the mechanism is cell-type or cargo-specific (Zorko et al., 2005). Smaller molecules attached to TATp seem to transduce directly into cells by the less energy independent electrostatic interactions and hydrogen bonding (Vives et al., 2003; Rothbard et al., 2004), while the larger cargos seem to get into cells by the energy-dependent macropinocytosis pathway (Wadia et al., 2004). One of the major obstacles in using TATp-assisted intracellular delivery of pharmaceutical nanocarriers is the lack of selectivity of TATp. This non-selectivity provides concern about drug-induced toxic effects towards normal tissues. Thus intratumoral administration of TATp containing nanocarriers may serve as good solution to this problem for delivery of anticancer drugs at least in certain cases (Fujita et al., 2008).
Here, we prepared and studied paclitaxel (PCT)-loaded TATp-modified PEG-PE micelles. The potential of these micelles for the induction of apoptosis was further evaluated in vivo after the intratumoral injection in tumor-bearing mice.
2. Materials and Methods
2.1 Materials
TAT-cysteine peptide (TATp-Cys) (12-mer: CysTyrGlyArgLys-LysArgArgGlnArgArgArg) was synthesized at the Tufts University Core Facility (Boston, MA, USA). PCT was purchased from Sigma (St. Louis, MO, USA). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (poly(ethylene glycol))-750] (PEG750-PE), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Rh-PE), were from Avanti Polar Lipids (Alabaster, AL). 3-(4,5-dimethylthia-zol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was from Promega (Madison, WI, USA). Cancer cell lines were purchased from the American Type Culture Collection (Rockville, MD, USA). Cell culture media, Dulbecco’s Modified Eagle’s Medium (DMEM), heat-inactivated fetal bovine serum (FBS), and concentrated solutions of sodium pyruvate and penicillin/streptomycin stock solutions were purchased from Cellgro (Herndon, VA, USA). All other chemicals were analytical grade preparations from Sigma (St. Louis, MO, USA).
2.2 Synthesis of TATp-PEG-PE conjugates
TATp-PEG1000-PE was synthesized using a heterobifunctional NHS-PEG1000-Mal derivative, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine and TATp-Cys as in (Kale et al., 2007a, b).
2.3 Preparation of PCT-loaded micelles
PCT solubilized in micelles were prepared by adding PCT (1 mg/mL in methanol) to a PEG750-PE solution in chloroform. The organic solvents were removed by rotary evaporation to form a thin film of drug/micelle material mixture. This film was further dried under high vacuum overnight to remove traces of remaining solvents. Drug-loaded micelles were formed by resuspending the film in 5 mM 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES)-buffered saline (HBS), pH 7.4. The mixture was incubated in a water bath at 37°C for 10 min. Excess non-incorporated drug was separated by centrifugation (13,000g) before characterization. For preparation of TATp-containing micelles, 2.5 mol % of TATp-PEG1000-PE in chloroform was added to the above preparation during micelle preparation. For cell interaction study, 0.5 mol% of the fluorescent probe, Rh-PE, was added to the micelle composition during micelle preparation.
2.4 Characterization of micelles
2.4.1 Size analysis and morphology
The micelle size (hydrodynamic diameter) was measured by the dynamic light scattering (DLS) using a N4 Plus Submicron Particle System (Coulter Corporation, Miami, FL, USA). The micelle suspensions were diluted with the deionized, distilled water until the concentration providing a light scattering intensity of 5 × 104 to 1 × 106 counts/s was achieved. The particle size distribution of all samples was measured in triplicate.
2.4.2 Drug solubilization efficiency
The amount of drug in the micellar phase was measured by reversed phase-HPLC. The clear aqueous dispersion was diluted with the mobile phase prior to applying onto the HPLC column (since the mobile phase contains acetonitrile, micelles are disrupted and free drug is determined). The D-7000 HPLC system equipped with a diode array and fluorescence detector (Hitachi, Japan) and Spherisorb ODS2 column, 4.6 mm × 250 mm (Waters, Milford, MA, USA) was used. The column was eluted with acetonitrile/water (65:35, v/v) at 1.0 mL/min. PCT was detected at 227 nm. Injection volume was 50 μL; all samples were analyzed in triplicate.
2.4.3 Cell cultures
The MCF7 (human breast adenocarcinoma) and 4T1 (murine mammary carcinoma), cells were maintained in DMEM cell culture medium at 37 °C, 5% CO2. DMEM media were supplemented with 10% FBS, 1 mM Na-pyruvate, 50 U/mL penicillin, and 50 μg/mL streptomycin.
2.4.4 Interaction of micelles with cells
The 4T1 cells were grown on cover slips placed in 6-well tissue culture plates. After the cells reached a confluence of 60–70%, the plates were washed with DMEM and the cells were treated with various Rh-PE-labeled micelle (0.2 mg/mL of the micelle-forming material) samples (with and without TATp) in DMEM. After 1 h, the medium was removed and the plates washed with sterile PBS, pH 7.4. Individual cover slips were mounted cell-side down onto fresh glass slides with PBS. Cells were viewed with a Nikon Eclipse E400 microscope under bright light or under epifluorescence with a rhodamine/TRITC filter.
2.4.5 Cytotoxicity assay
Cells were plated at a 5×103 cells per well density in 96-well plates (Corning, Inc., Corning, NY, USA). After 24 h incubation at 37°C, 5% CO2, the medium was replaced with medium containing free drug dissolved in DMSO or drug loaded micelles with or without TATp for 48h. After the incubation, each well was washed twice with Hank’s buffer and cell survival was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) method. The absorbance of the degraded MTT was measured at 492 nm (the measure of the cytotoxicity) using a Labsystems Multiskan MCC/340 microplate reader (Labsystems and Life Sciences International, UK).
2.4.6 Tumor growth and intratumoral administration of PCT-loaded micelles
Approximately 105 4T1 cells were inoculated in 6–8 weeks old female Balb/C mice by the subcutaneous injection into the left flank. Fourteen days after tumor inoculation, the mice were injected intratumorally with different PCT formulations equivalent to 5 mg/kg of PCT. After 48 h, the tumors were harvested, embedded in tissue freezing media and stored at −80°C. Cryostat sections were made, stained with the Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) assay and examined under a microscope.
3. Results and Discussion
For an efficient interaction of the micelle-attached TATp with the cell, the TATp moiety should be located “above” the carrier surface (Sawant et al. 2008) to allow for the unhindered interaction of TATp moieties with the cell surface (Torchilin et al., 2003a). With this in mind, micelles were prepared using PEG750-PE as the main micelle-forming component with the addition 2.5 mol % TATp-PEG1000-PE (see the schematics in Figure 1). Such micelles were loaded with PCT. At 5 mM concentration of PEG750-PE, with or without TATp, micelles were produced containing 151±2.78 μg PCT per mL of micelle formulation. The average micelle size of all formulations was in the range of 8- to-25 nm.
Figure 1.
Schematic representation of the micelles used in this study.
The in vitro cell interaction of the TATp-bearing PEG-PE micelles was confirmed by fluorescence microscopy with 4T1 cells. The interaction of different micelle preparations with 4T1 cells is shown in Figure 2. Plain micelles composed of PEG750-PE demonstrated a very limited interaction with the cells (Figure 2, A), however, the use of the TATp-bearing PEG-PE micelles resulted in the expected strong interaction with the cells (Figure 2, B).
Figure 2.
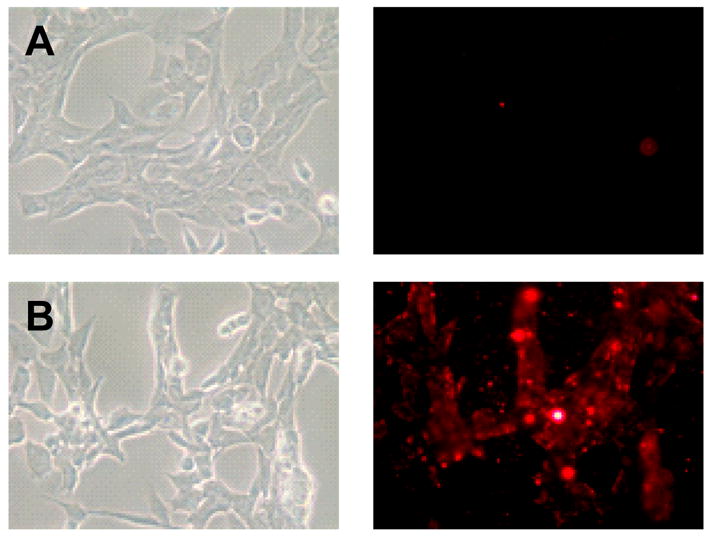
Left panel shows the bright field and right panel shows the fluorescent microscopy of 4T1 cells treated with Rh-PE:PEG750-PE micelles (A), Rh-PE: PEG750-PE: TATp-PEG1000-PE micelles. Magnification ×40 objective.
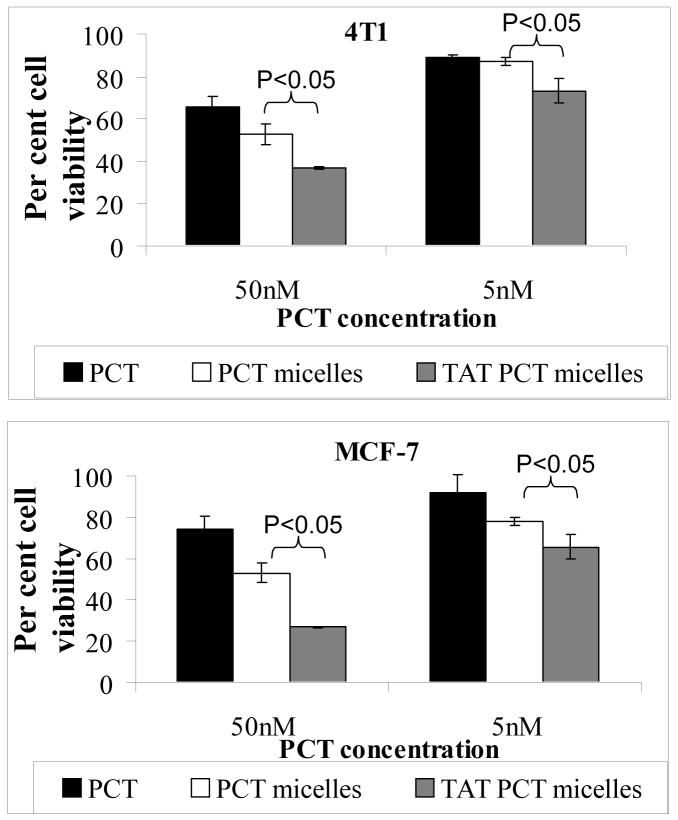
The next question was, whether this strong interaction would help to increase the delivery of the drug (PCT in PCT-loaded TATp-bearing micelles) into cells compared to the free PCT or PCT-loaded micelles without TATp. The in vitro cytotoxicity of different formulations was investigated using MCF7 and 4T1 cells. The cells were incubated with free drug or with different drug-loaded micellar formulations for 2 days and analyzed for their survival using the MTT colorimetric assay for the dehydrogenase activity of viable cells. As seen in the Figure 3, there is a significantly higher cytotoxicity with PCT-loaded TATp-bearing micelles compared to PCT-loaded micelles without TATp or free PCT at both tested PCT concentrations (5 and 50 nM). This could be explained by the increased interaction of TATp-bearing micelles with cells compared to micelles without TATp. Drug-free, plain or TATp-modified micelles, as well as the TATp itself were not toxic to cells at the concentrations used (data not shown).
Figure 3. In vitro.
cytotoxicity of various PCT formulations towards 4T1 and MCF-7 cells.
In in vivo studies in tumor-bearing, to avoid any unwanted distribution of PCT-loaded TATp-micelles, those were injected intratumorally, and tumors were harvested after 48h. The nuclear DNA fragmentation in tumor sections (to confirm the presence of the apoptosis process) was followed by the TUNEL assay using the DNA fragmentation kit, Fluorescein FragEL™ according to the manufacturer’s instructions. The TUNEL assay enables the differentiation of apoptosis from necrosis because DNA fragments produced by the apoptosis are exclusively labeled with the TUNEL method (Gold et al., 1994). The results of the TUNEL staining of tissue sections are shown in Figure 4. In DAPI stained images, it is difficult to observe differences among groups since the cell nuclei of live cells represented bright blue fluorescence attributed to DAPI staining. However, the TUNEL assay images showed the very different results depending on the type of the treatment. The cell nuclei of non-apoptotic bodies did not exhibit the green fluorescence attributed to FITC-labeled TdT. Very few TUNEL-positive cells were observed in tumors injected with free PCT or with PCT-loaded micelles (Figure 4). Significant apoptotic cell death was observed in tumors treated with PCT-loaded TATp-bearing micelles (Figure 4). Thus, these in vivo results are in good agreement with our in vitro cytotoxicity data.
Figure 4.
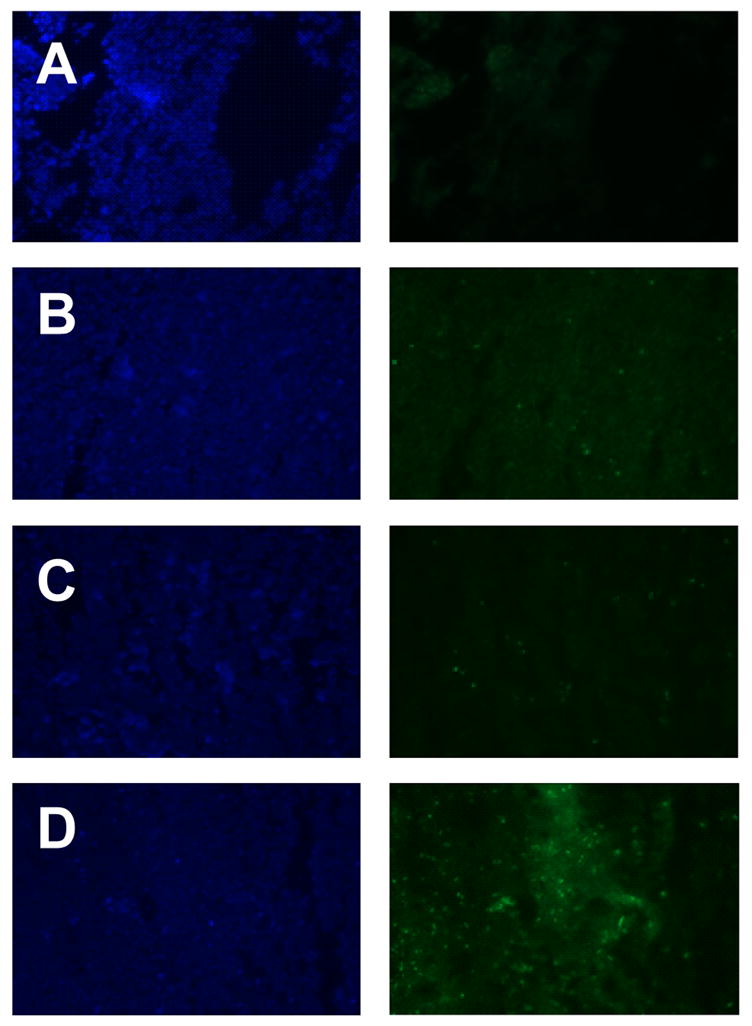
Detection of the apoptotic cells by the fluorescence microscopy in frozen tumor sections. Apoptosis determined by TUNEL. The left panel shows the sections stained with DAPI and the right panel shows TUNEL. Negative control (A), free PCT (B), PCT-loaded micelles without TATp (C), PCT-loaded micelles with TATp (D). Magnification ×20 objective.
4. Conclusion
Micelles bearing a CPP function, TATp, demonstrated strong interaction with cells in vitro. This enhanced interaction translated into the increased cytotoxicity both in vitro and in vivo when such micelles were loaded with the anticancer drug PCT. Such CPP-mediated drug-loaded pharmaceutical nanocarriers represent promising candidates for intratumoral administration.
Acknowledgments
This work was supported by the NIH grants RO1 EB001961 and RO1 CA121838 to Vladimir P. Torchilin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astriab-Fisher A, Sergueev D, Fisher M, Shaw BR, Juliano RL. Conjugates of antisense oligonucleotides with the Tat and antennapedia cell-penetrating peptides: effects on cellular uptake, binding to target sequences, and biologic actions. Pharm Res. 2002;19:744–754. doi: 10.1023/a:1016136328329. [DOI] [PubMed] [Google Scholar]
- Bae Y, Kataoka K. Significant enhancement of antitumor activity and bioavailability of intracellular pH-sensitive polymeric micelles by folate conjugation. J Control Release. 2006;116:e49–50. doi: 10.1016/j.jconrel.2006.09.044. [DOI] [PubMed] [Google Scholar]
- Fretz MM, Koning GA, Mastrobattista E, Jiskoot W, Storm G. OVCAR-3 cells internalize TAT-peptide modified liposomes by endocytosis. Biochim Biophys Acta. 2004;1665:48–56. doi: 10.1016/j.bbamem.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Fujita T, Furuhata M, Hattori Y, Kawakami H, Toma K, Maitani Y. High gene delivery in tumor by intratumoral injection of tetraarginine-PEG lipid-coated protamine/DNA. J Control Release. 2008;129:124–127. doi: 10.1016/j.jconrel.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest. 1994;71:219–225. [PubMed] [Google Scholar]
- Kale AA, Torchilin VP. Design, synthesis, and characterization of pH-sensitive PEG-PE conjugates for stimuli-sensitive pharmaceutical nanocarriers: the effect of substitutes at the hydrazone linkage on the ph stability of PEG-PE conjugates. Bioconjug Chem. 2007a;18:363–370. doi: 10.1021/bc060228x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale AA, Torchilin VP. Enhanced transfection of tumor cells in vivo using “Smart” pH-sensitive TAT-modified pegylated liposomes. J Drug Target. 2007b;15:538–545. doi: 10.1080/10611860701498203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- Krishnadas A, Rubinstein I, Onyuksel H. Sterically stabilized phospholipid mixed micelles: in vitro evaluation as a novel carrier for water-insoluble drugs. Pharm Res. 2003;20:297–302. doi: 10.1023/a:1022243709003. [DOI] [PubMed] [Google Scholar]
- Levchenko TS, Rammohan R, Volodina N, Torchilin VP. Tat peptide-mediated intracellular delivery of liposomes. Methods Enzymol. 2003;372:339–349. doi: 10.1016/s0076-6879(03)72019-9. [DOI] [PubMed] [Google Scholar]
- Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev. 2004;56:1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2008 doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- Nguyen J, Xie X, Neu M, Dumitrascu R, Reul R, Sitterberg J, Bakowsky U, Schermuly R, Fink L, Schmehl T, Gessler T, Seeger W, Kissel T. Effects of cell-penetrating peptides and pegylation on transfection efficiency of polyethylenimine in mouse lungs. J Gene Med. 2008;10:1236–1246. doi: 10.1002/jgm.1255. [DOI] [PubMed] [Google Scholar]
- Rao KS, Reddy MK, Horning JL, Labhasetwar V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials. 2008;29:4429–4438. doi: 10.1016/j.biomaterials.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J Am Chem Soc. 2004;126:9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- Sawant RR, Sawant RM, Kale AA, Torchilin VP. The architecture of ligand attachment to nanocarriers controls their specific interaction with target cells. J Drug Target. 2008;16:596–600. doi: 10.1080/10611860802230240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J Control Release. 2007;118:216–224. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EL, Dowdy SF. Cell penetrating peptides in drug delivery. Pharm Res. 2004;21:389–393. doi: 10.1023/B:PHAM.0000019289.61978.f5. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001a;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D’Souza GG. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc Natl Acad Sci USA. 2003a;100:1972–1977. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci USA. 2003b;100:6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci USA. 2001b;98:8786–8791. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives E, Richard JP, Rispal C, Lebleu B. TAT peptide internalization: seeking the mechanism of entry. Curr Protein Pept Sci. 2003;4:125–132. doi: 10.2174/1389203033487306. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- Zhao M, Kircher MF, Josephson L, Weissleder R. Differential conjugation of tat peptide to superparamagnetic nanoparticles and its effect on cellular uptake. Bioconjug Chem. 2002;13:840–844. doi: 10.1021/bc0255236. [DOI] [PubMed] [Google Scholar]
- Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]